Abstract
Objectives
We examined the 4 year trend in antimicrobial susceptibilities and prescribing across levels of care at two London teaching hospitals and their multisite renal unit, and for the surrounding community.
Methods
Laboratory and pharmacy information management systems were interrogated, with antimicrobial use and susceptibilities analysed between hospitals, within hospitals and over time.
Results
A total of 108 717 isolates from 71 687 patients were identified, with significant differences (at P < 0.05) in antimicrobial susceptibility between and within hospitals. Across the 4 years, rates of ESBL-/AmpC-producing Enterobacteriaceae ranged from 6.4% to 10.7% among community isolates, 17.8% to 26.9% at ward level and 25.2% to 52.5% in critical care. Significant variations were also demonstrated in glycopeptide-resistant enterococci (ward level 6.2%–17.4%; critical care 21.9%–56.3%), MRSA (ward level 18.5%–38.2%; critical care 12.5%–47.9%) and carbapenem-resistant Pseudomonas spp. (ward level 8.3%–16.9%; critical care 19.9%–53.7%). Few instances of persistently higher resistance were seen between the hospitals in equivalent cohorts, despite persistently higher antimicrobial use in Hospital 1 than Hospital 2. We found significant fluctuations in non-susceptibility year on year across the cohorts, but with few persistent trends.
Conclusions
The marked heterogeneity of antimicrobial susceptibilities between hospitals, within hospitals and over time demands detailed, standardized surveillance and appropriate benchmarking to identify possible drivers and effective interventions. Homogeneous antimicrobial policies are unlikely to continue to be suitable as individual hospitals join hospital networks, and policies should be tailored to local resistance rates, at least at the hospital level, and possibly with finer resolution, particularly for critical care.
Keywords: antibiograms, healthcare-associated infections, multidrug-resistant organisms, antimicrobial stewardship
Introduction
Antimicrobial resistance rates vary between countries,1 and between the community and hospitals.2 Variation within hospitals is also described; internationally, resistance rates are often highest in critical care,3,4 but in Europe this varies by organism1 and in the UK critical care reservoirs seem less apparent.5 Robust benchmarking is, however, lacking despite advocacy towards the standardized collection of cumulative antimicrobial susceptibility test data6 (the ‘antibiogram’).7
The identification of local variations in bacterial resistance between cohorts2,8 and over time9 enables informed decisions on empirical antimicrobial regimens and is becoming increasingly achievable as economic and political pressures create hospital networks where previously separate units, patient cohorts and their associated flora are now served by single large centralized laboratories. However, single antimicrobial policies are frequently adopted within these networks, often not adequately allowing for variations in bacterial resistance between and within the sites served. In this context, antimicrobial policies rarely account for the variations in carriage rates of resistant bacteria in relation to population structure and travel or migration patterns.10 Defining patient cohorts according to locale, level of care and other acknowledged risk factors for antimicrobial resistance, with subsequent detailing of resistance trends, may facilitate more appropriate antimicrobial prescribing. A further concern is that highly standardized antimicrobial policies may concentrate selection pressure on particular agents, sequentially eroding their utility, exactly as has occurred with anti-gonococcal treatments.11
This study analyses 4 years of bacterial susceptibility data and prescribing trends from two West London tertiary referral hospitals, their multisite renal unit and the surrounding community practices, all served by a single laboratory. The objectives were to identify and describe the fine-resolution variations in resistance rates and trends between the hospitals and within the hospitals at ward (NHS Level 0 and Level 1 beds) and critical care (NHS Level 2 and Level 3 beds)12 levels, and furthermore to explore the relationships between resistance patterns and antimicrobial use.
Methods
The laboratory information management system (LIMS; Sunquest®, Misys) was interrogated for the seven cohorts of interest: Teaching Hospital 1 (27 critical care beds; 388 ward beds), Teaching Hospital 2 (26 critical care beds; 453 ward beds), the multisite renal unit (84 inpatient beds and ∼3100 dialysis and transplant outpatients) and community specimens (from over 50 local primary care practices and from outpatients attending clinics at Hospitals 1 and 2). Hospital 1 includes general medicine, cardiology and tertiary referral haematology, cardiothoracic surgery and hepatobiliary surgery. Hospital 2 includes general medicine, general surgery, trauma and orthopaedics and tertiary referral oncology and neurosurgery. All patients at Hospital 1, Hospital 2 and the renal unit were over 16 years of age. A third hospital within the hospital network utilized a different LIMS at the time of this study and was excluded. Infection advice for all sites is provided by an integrated team of infection specialists, with an established overarching antimicrobial policy and an active antimicrobial stewardship programme for all hospitals in the network. Off-policy prescription can occur under the direction of infection specialists.
All samples submitted for culture for clinical indications (as indicated by the clinician submitting the sample) were identified covering the four fiscal years from 2009 to 2013 (in the UK the fiscal year runs from April to March). They included blood and CSF, respiratory and ear/nose/throat, tissue and wound, genital and urine samples. Samples submitted for the purposes of cross-infection screening and MRSA screening were excluded, as variations in screening practice existed between and within the hospitals. The clinical criteria and sampling protocols to obtain diagnostic specimens for culture were uniform across the two hospitals in the corresponding ward and critical care cohorts. Results were de-duplicated for organisms repeatedly isolated within a 7 day period from the same patient. Laboratory operating procedures followed national standards for microbiological investigation;13 isolates were identified using API® (bioMérieux) from 2009 to 2011 and by MALDI-TOF spectroscopy (Biotyper®, Bruker) from 2011 to 2013. Susceptibilities were determined by disc diffusion using BSAC criteria.14 AmpC- and ESBL-producing Enterobacteriaceae were identified by standard methods.13 Comparisons between sites and cohorts were carried out for glycopeptide-resistant enterococci (GRE), MRSA, Pseudomonas spp. and AmpC- and ESBL-producing Enterobacteriaceae (defined as including Citrobacter spp., Escherichia coli, Enterobacter spp., Hafnia spp., Klebsiella spp., Morganella spp., Pantoea spp., Proteus spp., Providencia spp., Raoultella spp., Serratia spp. and other lactose-fermenting coliforms, but excluding Salmonella spp. and Shigella spp.). Enterobacteriaceae were considered at family level rather than for each species separately as standard operating procedures stipulate identification to genus or species level only for isolates from specific sample types or with particular resistance patterns. Non-susceptibility (i.e. resistant and intermediate) proportions were calculated against the total number of isolates tested in each isolate group.
Antimicrobial usage data were sourced from the hospital network pharmacy system, and defined daily doses per 1000 occupied bed days (DDDs/1000 OBDs) were calculated.15 Antimicrobial usage data is based upon antimicrobials distributed to wards for inpatient and stock supply and was available to the hospital level for Hospitals 1 and 2 (inpatients only) and for the renal inpatient practice. All antibacterials were included; antiviral, antileprotic, antimycobacterial and antihelminthic medications were excluded. Antimicrobial guidelines were reviewed for the relevant time periods to identify any policy shifts.
Variable referral practice from local primary care centres precluded an estimation of population-attributable rates of infection and resistance (i.e. an extrapolation of the observed rates in the study cohorts out to the wider population), but isolate frequency was calculated for inpatients based upon OBDs. CIs for non-susceptibility were calculated using the Wilson method with continuity correction.16 Analysis was undertaken in Stata/SE Version 11®, with χ2 tests for inter-cohort comparison and for temporal trends, and with binomial regression analysis when these identified significant differences (P < 0.05, with the Bonferroni correction to account for multiple comparisons between years in each cohort).
Results
The LIMS extract yielded antimicrobial susceptibility results for 145 703 isolates. After excluding organisms not of interest to this study, 108 717 isolates from 105 319 samples and 71 687 patients remained.
Isolate frequency in relation to occupancy denominators
At ward level, little variation was observed between the two hospitals in terms of the frequency of isolation of the species groups reviewed (Table 1; expressed as isolates/1000 OBDs), with the exception of an upswing in Enterobacteriaceae isolates in the most recent year that was observed in both hospitals. The frequency of isolates from the renal inpatient cohort was comparable to that from the general ward areas. Marked year-by-year fluctuations were observed in isolate frequency in critical care but with a modest overall down-trend. Three features were notable: first, the high frequency of isolates of all species groups in Hospital 1 in critical care in 2009–10, not attributable to any discernible policy changes, case-mix or known outbreaks; second, a marked down-trend in the frequency of enterococci in both critical care units over the study period; and last, a year-on-year decrease in Enterobacteriaceae isolates from critical care patients at Hospital 2.
Table 1.
Isolates/1000 OBDs from secondary and tertiary care inpatients in West London, 2009–13
| Renal |
Hospital 1 |
Hospital 2 |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| inpatient |
wards |
critical care |
wards |
critical care |
||||||||||||||||
| 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | |
| Enterobacteriaceae (55 600 isolates) | 13 | 9 | 10 | 14 | 17 | 11 | 13 | 23 | 51 | 26 | 27 | 33 | 13 | 13 | 11 | 17 | 40 | 37 | 27 | 20 |
| Pseudomonas spp. (12 616 isolates) | 5 | 5 | 5 | 4 | 6 | 3 | 3 | 4 | 26 | 12 | 12 | 19 | 5 | 5 | 5 | 5 | 23 | 28 | 27 | 19 |
| Enterococci (13 643 isolates) | 7 | 4 | 3 | 4 | 5 | 4 | 3 | 4 | 20 | 13 | 9 | 10 | 4 | 3 | 3 | 4 | 12 | 13 | 6 | 5 |
| S. aureus (26 858 isolates) | 3 | 3 | 3 | 4 | 5 | 4 | 4 | 7 | 15 | 4 | 6 | 8 | 8 | 8 | 7 | 7 | 13 | 14 | 13 | 11 |
Sample types for isolates from the renal inpatient cohort comprised 11% blood cultures, 7% respiratory tract, 8% invasive tissue or fluid, 48% non-invasive wound swab and 26% urine. Sample types for isolates from Hospital 1 were 9% blood cultures, 13% respiratory tract, 10% invasive tissue or fluid, 37% non-invasive wound swab and 31% urine. Sample types for isolates from Hospital 2 were 5% blood cultures, 14% respiratory tract, 8% invasive tissue or fluid, 38% non-invasive wound swab and 35% urine.
Resistance in Enterobacteriaceae
A total of 55 600 Enterobacteriaceae were identified (Table 2). Significantly higher prevalence rates for Enterobacteriaceae with ESBL/AmpC phenotypes were seen in critical care versus general wards in Hospital 1 in three of the four years and in Hospital 2 in all four years (Table 3 and Figure 1a). The proportions of ESBL/AmpC phenotypes were ∼1 in 5 Enterobacteriaceae from general wards and up to 1 in 2 in critical care. Fluctuating rates of Enterobacteriaceae with ESBL/AmpC phenotypes were seen at the two hospitals, with significant differences in these rates between the critical care areas in three of the four years, but only in the two most recent years in general ward cohorts. ESBL/AmpC rates among Enterobacteriaceae from the renal outpatient cohort were as high as at hospital ward level and, among renal inpatients, were as high as in critical care, peaking at 51.5% in 2012–13. The difference in prevalence of ESBL-/AmpC-producing Enterobacteriaceae between renal inpatients and renal outpatients was significant in all four years.
Table 2.
Bacterial non-susceptibility to selected antimicrobials among 108 717 clinical isolates from primary, secondary and tertiary care patients in West London, 2009–13
| Renal |
Hospital 1 |
Hospital 2 |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Community |
outpatient |
inpatient |
wards |
critical care |
wards |
critical care |
||||||||||||||||||||||
| 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | |
| Enterobacteriaceae isolates (55 600 total) | 13 225 | 12 880 | 5055 | 6641 | 314 | 389 | 575 | 703 | 396 | 283 | 293 | 365 | 1229 | 831 | 965 | 1661 | 494 | 254 | 248 | 304 | 1995 | 1799 | 1508 | 2186 | 371 | 289 | 207 | 140 |
| ciprofloxacin non-susceptibility (%) | 9.6 | 11.3 | 12.0 | 11.5 | 36.0 | 29.6 | 36.7 | 42.2 | 59.1 | 54.1 | 54.9 | 60.8 | 23.7 | 26.2 | 25.7 | 23.4 | 24.3 | 18.1 | 18.5 | 23.7 | 20.1 | 22.5 | 20.8 | 20.7 | 19.9 | 25.3 | 19.8 | 24.3 |
| AmpC or ESBL phenotype (%) | 6.4 | 8.2 | 10.7 | 9.5 | 24.5 | 22.4 | 27.0 | 29.9 | 35.6 | 38.9 | 43.3 | 51.5 | 21.5 | 25.5 | 26.9 | 21.1 | 29.1 | 25.2 | 44.4 | 47.0 | 21.5 | 23.1 | 20.8 | 17.8 | 36.4 | 52.2 | 40.1 | 36.4 |
| Pseudomonas spp. isolates (12 616 total) | 1869 | 1954 | 745 | 1505 | 146 | 133 | 130 | 103 | 155 | 138 | 135 | 102 | 454 | 266 | 213 | 282 | 252 | 111 | 107 | 169 | 820 | 748 | 658 | 652 | 211 | 218 | 207 | 133 |
| ciprofloxacin non-susceptibility (%) | 12.3 | 14.3 | 12.8 | 11.1 | 26.7 | 23.3 | 15.4 | 19.4 | 40.6 | 45.7 | 25.9 | 27.5 | 25.6 | 33.8 | 20.7 | 22.7 | 32.5 | 29.7 | 34.6 | 28.4 | 26.6 | 20.1 | 18.4 | 15.8 | 35.1 | 26.6 | 30.0 | 39.8 |
| piperacillin/tazobactam non-susceptibility (%) | 1.0 | 2.4 | 2.3 | 1.9 | 2.7 | 3.8 | 5.4 | 2.9 | 19.4 | 23.2 | 3.7 | 6.9 | 6.8 | 12.4 | 8.0 | 7.8 | 8.3 | 27.0 | 15.0 | 13.0 | 7.7 | 8.2 | 5.6 | 4.4 | 17.1 | 17.0 | 17.4 | 14.3 |
| meropenem non-susceptibility (%) | 3.6 | 3.5 | 5.0 | 3.2 | 10.3 | 6.8 | 4.6 | 5.8 | 32.9 | 33.3 | 8.9 | 18.6 | 16.7 | 18.4 | 10.3 | 13.1 | 32.9 | 39.6 | 42.1 | 33.1 | 15.6 | 11.6 | 8.2 | 8.0 | 31.8 | 21.1 | 27.5 | 22.6 |
| Enterococci isolates (13 643 total) | 2689 | 3284 | 1555 | 1321 | 77 | 79 | 91 | 102 | 226 | 132 | 93 | 105 | 341 | 281 | 238 | 324 | 188 | 128 | 80 | 89 | 607 | 467 | 370 | 481 | 115 | 100 | 48 | 32 |
| amoxicillin non-susceptibility (%) | 1.5 | 1.0 | 2.1 | 2.6 | 15.6 | 15.2 | 8.8 | 13.7 | 58.8 | 56.8 | 55.9 | 59.0 | 43.7 | 35.6 | 31.5 | 34.0 | 57.4 | 71.9 | 61.3 | 71.9 | 11.0 | 21.6 | 16.5 | 20.6 | 67.8 | 67.0 | 56.3 | 43.8 |
| vancomycin non-susceptibility (%) | 0.3 | 0.4 | 1.4 | 1.1 | 22.1 | 17.7 | 14.3 | 12.8 | 56.2 | 59.8 | 64.5 | 55.2 | 17.3 | 17.4 | 11.3 | 15.4 | 41.5 | 56.3 | 25.0 | 34.8 | 9.2 | 9.6 | 6.2 | 10.0 | 55.7 | 49.0 | 37.5 | 21.9 |
| S. aureus isolates (26 858 total) | 5076 | 5495 | 4382 | 4658 | 67 | 86 | 111 | 95 | 100 | 79 | 96 | 104 | 402 | 319 | 336 | 517 | 142 | 42 | 52 | 72 | 1211 | 1144 | 941 | 932 | 116 | 112 | 96 | 75 |
| methicillin non-susceptibility (%) | 13.7 | 12.3 | 11.3 | 9.3 | 16.4 | 18.6 | 18.9 | 14.7 | 33.0 | 31.6 | 24.0 | 26.9 | 29.1 | 38.2 | 19.9 | 21.1 | 19.0 | 35.7 | 23.1 | 12.5 | 38.3 | 27.4 | 25.4 | 18.5 | 44.0 | 39.3 | 47.9 | 22.7 |
Table 3.
Statistical analysis of variation in non-susceptibility between cohorts and over time among 108 717 clinical isolates from primary, secondary and tertiary care patients in West London, 2009–13
| Between hospital critical care: Hospital 1 versus Hospital 2 |
Between hospital wards: Hospital 1 versus Hospital 2 |
Within Hospital 1: critical care versus wards |
Within Hospital 2: critical care versus wards |
Renal: inpatients versus outpatients |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enterobacteriaceae, ESBL/AmpC phenotype | 2009–10 | * | 2009–10 | NS | 2009–10 | *** | 2009–10 | *** | 2009–10 | ** |
| 2010–11 | *** | 2010–11 | NS | 2010–11 | NS | 2010–11 | *** | 2010–11 | *** | |
| 2011–12 | NS | 2011–12 | *** | 2011–12 | *** | 2011–12 | *** | 2011–12 | *** | |
| 2012–13 | * | 2012–13 | ** | 2012–13 | *** | 2012–13 | *** | 2012–13 | *** | |
| Enterobacteriaceae, ciprofloxacin non-susceptible | 2009–10 | NS | 2009–10 | * | 2009–10 | NS | 2009–10 | NS | 2009–10 | *** |
| 2010–11 | * | 2010–11 | * | 2010–11 | ** | 2010–11 | NS | 2010–11 | *** | |
| 2011–12 | NS | 2011–12 | ** | 2011–12 | * | 2011–12 | NS | 2011–12 | *** | |
| 2012–13 | NS | 2012–13 | * | 2012–13 | NS | 2012–13 | NS | 2012–13 | *** | |
| Pseudomonas spp., ciprofloxacin non-susceptible | 2009–10 | NS | 2009–10 | NS | 2009–10 | * | 2009–10 | * | 2009–10 | * |
| 2010–11 | NS | 2010–11 | *** | 2010–11 | NS | 2010–11 | * | 2010–11 | *** | |
| 2011–12 | NS | 2011–12 | NS | 2011–12 | ** | 2011–12 | *** | 2011–12 | * | |
| 2012–13 | * | 2012–13 | * | 2012–13 | NS | 2012–13 | *** | 2012–13 | NS | |
| Pseudomonas spp., piperacillin/tazobactam non-susceptible | 2009–10 | *** | 2009–10 | NS | 2009–10 | NS | 2009–10 | *** | 2009–10 | *** |
| 2010–11 | NS | 2010–11 | * | 2010–11 | *** | 2010–11 | *** | 2010–11 | *** | |
| 2011–12 | NS | 2011–12 | NS | 2011–12 | NS | 2011–12 | *** | 2011–12 | NS | |
| 2012–13 | NS | 2012–13 | * | 2012–13 | NS | 2012–13 | *** | 2012–13 | NS | |
| Pseudomonas spp., meropenem non-susceptible | 2009–10 | NS | 2009–10 | NS | 2009–10 | *** | 2009–10 | *** | 2009–10 | *** |
| 2010–11 | *** | 2010–11 | ** | 2010–11 | *** | 2010–11 | *** | 2010–11 | *** | |
| 2011–12 | * | 2011–12 | NS | 2011–12 | *** | 2011–12 | *** | 2011–12 | NS | |
| 2012–13 | * | 2012–13 | * | 2012–13 | *** | 2012–13 | *** | 2012–13 | ** | |
| Enterococcus spp., glycopeptide non-susceptible | 2009–10 | * | 2009–10 | *** | 2009–10 | *** | 2009–10 | *** | 2009–10 | *** |
| 2010–11 | NS | 2010–11 | ** | 2010–11 | *** | 2010–11 | *** | 2010–11 | *** | |
| 2011–12 | NS | 2011–12 | ** | 2011–12 | ** | 2011–12 | *** | 2011–12 | *** | |
| 2012–13 | NS | 2012–13 | ** | 2012–13 | *** | 2012–13 | * | 2012–13 | *** | |
| Enterococcus spp., amoxicillin non-susceptible | 2009–10 | NS | 2009–10 | *** | 2009–10 | ** | 2009–10 | *** | 2009–10 | *** |
| 2010–11 | NS | 2010–11 | *** | 2010–11 | ** | 2010–11 | *** | 2010–11 | *** | |
| 2011–12 | NS | 2011–12 | *** | 2011–12 | *** | 2011–12 | *** | 2011–12 | *** | |
| 2012–13 | ** | 2012–13 | *** | 2012–13 | *** | 2012–13 | ** | 2012–13 | *** | |
| S. aureus, methicillin non-susceptible | 2009–10 | *** | 2009–10 | *** | 2009–10 | * | 2009–10 | NS | 2009–10 | * |
| 2010–11 | NS | 2010–11 | *** | 2010–11 | NS | 2010–11 | ** | 2010–11 | * | |
| 2011–12 | ** | 2011–12 | * | 2011–12 | NS | 2011–12 | *** | 2011–12 | NS | |
| 2012–13 | NS | 2012–13 | NS | 2012–13 | NS | 2012–13 | NS | 2012–13 | * | |
NS, no significant difference.
*Significant at P < 0.05.
**Significant at P < 0.01.
***Significant at P < 0.001.
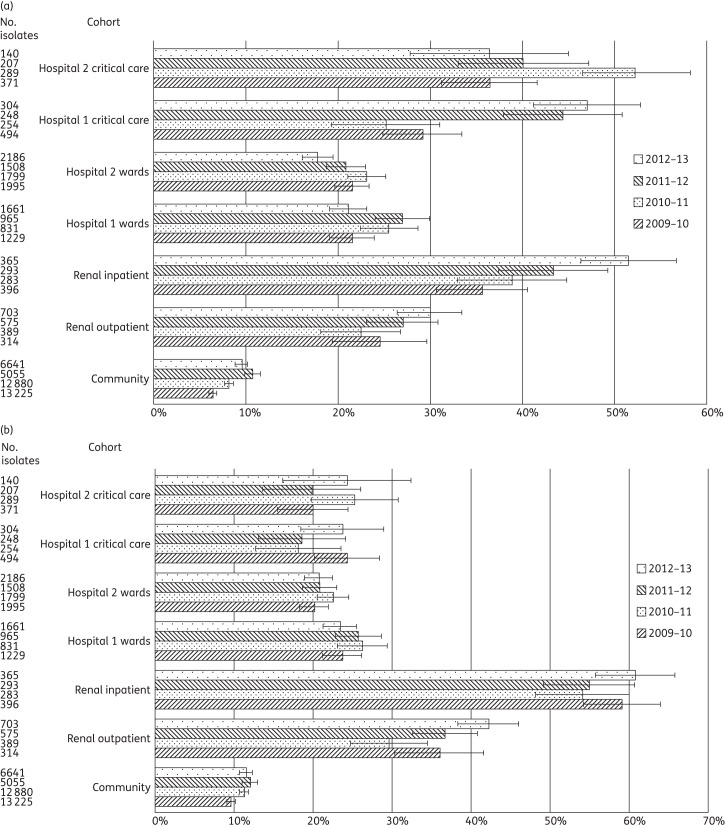
Figure 1.
(a) Proportion of Enterobacteriaceae from clinical samples displaying ESBL/AmpC resistance phenotypes among 55 600 isolates from primary, secondary and tertiary care patients in West London, 2009–13. Error bars indicate 95% CIs calculated by Wilson's method with continuity correction. (b) Proportion of Enterobacteriaceae from clinical samples resistant to ciprofloxacin among 55 600 isolates from primary, secondary and tertiary care patients in West London, 2009–13. Error bars indicate 95% CIs calculated by Wilson's method with continuity correction.
Binomial regression demonstrated a significant increase in the relative proportion of ESBL-/AmpC-producing Enterobacteriaceae from Hospital 1 ward inpatients from 2009–10 to 2010–11 of 18.8% (95% CI 1.4–39.1%, P = 0.03). Hospital 1 critical care also showed a significant, 1.8-fold, increase in the prevalence of these organisms from 2010–11 to 2011–12 (95% CI 1.4–2.3, P < 0.001). In Hospital 2, critical care saw a significant relative increase in the prevalence of ESBL/AmpC producers from 2009–10 to 2010–11 of 43.6% (95% CI 20.7–70.9%, P < 0.001), followed by a relative decrease from 2010–11 to 2011–12 of 23.3% (95% CI 6.3–37.1%, P = 0.009). The proportions of ESBL-/AmpC-producing Enterobacteriaceae in the community samples showed significant increases between 2009–10 and 2010–11 (P < 0.001) and 2010–11 and 2011–12 (P < 0.001) followed by a dip from 10.7% to 9.5% in 2012–13 (P = 0.03); the year-to-year variation was, however, small compared with the hospital cohorts.
Non-susceptibility to ciprofloxacin among the Enterobacteriaceae varied surprisingly little between or within the hospitals (Table 3), with non-susceptibility rates clustered from 18.1% to 25.7% (Figure 1b). However, the renal unit showed a significant variation in ciprofloxacin non-susceptibility between the inpatient and outpatient cohorts in all four years. For renal outpatients, the prevalence of ciprofloxacin non-susceptibility was almost double that among ward patients in Hospitals 1 and 2; that for renal inpatients neared triple those in Hospitals 1 and 2. There was no significant temporal variation in ciprofloxacin non-susceptibility in either hospital or in the renal unit. Non-susceptibility to ciprofloxacin in community isolates was approximately half that among inpatients.
We saw little carbapenem non-susceptibility in Enterobacteriaceae, precluding a robust temporal or inter-cohort analysis. Meropenem non-susceptibility was noted in fewer than 0.5% of all hospital Enterobacteriaceae, except for isolates from renal inpatients during 2009–10, when an outbreak due to OXA-48-carbapenemase producing Klebsiella pneumoniae was detected.17 Ertapenem non-susceptibility also was noted in 1%–2% of Enterobacteriaceae, predominantly Enterobacter species, and was attributed to the breakpoint determining a ‘tail’ of AmpC-derepressed isolates to be non-susceptible.
Resistance in Pseudomonas
A total of 12 616 Pseudomonas spp. were identified, 10 226 of them confirmed as P. aeruginosa (Table 2). Across both hospitals and the renal cohorts, Pseudomonas spp. comprised 75.3%–88.9% of all non-fermenters, with 63.0%–77.1% identified as P. aeruginosa. Non-susceptibility to ciprofloxacin (Figure 2a), piperacillin/tazobactam (Figure 2b) and meropenem (Figure 2c) was analysed.
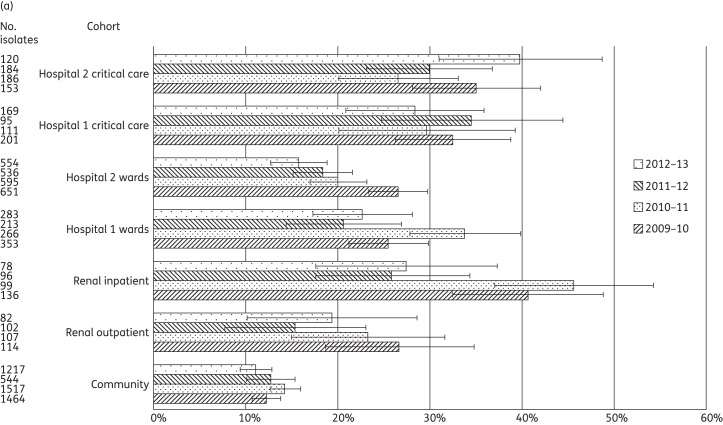
Figure 2.
(a) Proportion of Pseudomonas spp. from clinical samples displaying ciprofloxacin non-susceptibility among 12 616 isolates from primary, secondary and tertiary care patients in West London, 2009–13. Error bars indicate 95% CIs calculated by Wilson's method with continuity correction. (b) Proportion of Pseudomonas spp. from clinical samples displaying piperacillin/tazobactam non-susceptibility among 12 616 isolates from primary, secondary and tertiary care patients in West London, 2009–13. Error bars indicate 95% CIs calculated by Wilson's method with continuity correction. (c) Proportion of Pseudomonas spp. from clinical samples displaying meropenem non-susceptibility among 12 616 isolates from primary, secondary and tertiary care patients in West London, 2009–13. Error bars indicate 95% CIs calculated by Wilson's method with continuity correction.
There was a significant variation in ciprofloxacin non-susceptibility rates between the two hospitals in critical care in only one year, and at ward level in two years. Within-hospital comparisons demonstrated significant differences in all four years in Hospital 2, with the non-susceptibility rate in critical care almost double that in the general wards (Table 3). Temporal analysis found no significant variation in ciprofloxacin non-susceptibility in critical care, but showed significant falls at ward level in Hospital 2 between 2009–10 and 2010–11 (P = 0.002) and in Hospital 1 between 2010–11 and 2012–13 (P = 0.001). The renal inpatient cohort showed a significant fall (almost 50%) in ciprofloxacin non-susceptibility between 2010–11 and 2011–12 (P < 0.001). In the community, ciprofloxacin non-susceptibility among Pseudomonas spp. remained between 11.1% and 14.3% across the four years.
Meropenem non-susceptibility was more prevalent than piperacillin/tazobactam non-susceptibility in all years and cohorts (Table 2 and Figure 2b and c). Non-susceptibility to meropenem was significantly more prevalent (typically 2- to 3-fold) in critical care than in the general wards in all years at both hospitals (Table 3). Instances of significant between-hospital variation in meropenem non-susceptibility were noted between corresponding levels of care, with less variation for piperacillin/tazobactam non-susceptibility.
There was no statistically significant temporal variation in non-susceptibility to meropenem or piperacillin/tazobactam in the ward or critical care cohorts, in renal outpatients or in the community. In the renal inpatient cohort, by contrast, there were significant falls in the prevalence of non-susceptibility to both meropenem (33.3% to 8.9%; P < 0.001) and piperacillin/tazobactam (23.2% to 3.7%; P < 0.001) between 2010–11 and 2011–12.
Resistance in enterococci
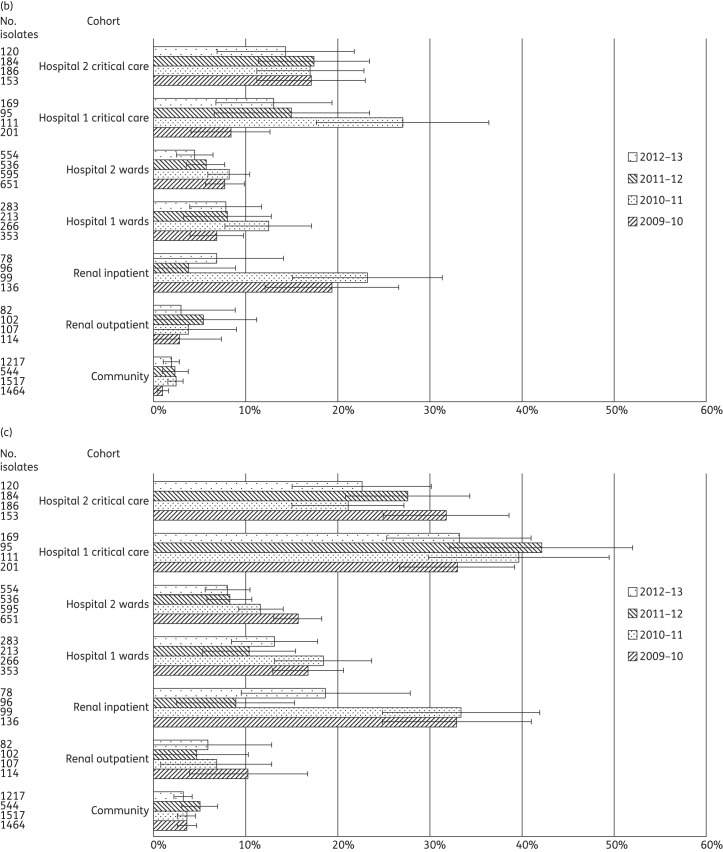
A total of 13 643 enterococci were identified (Table 2). Significant differences in GRE rates between ward and critical care areas were seen for all years in both hospitals (Table 3 and Figure 3a). A comparison between the hospitals demonstrated significant differences in the prevalence of GRE at ward level in all years but little significant variation between the critical care areas (with the exception of 2009–10). GRE were consistently 2–4 times more frequent among enterococci from renal inpatients than renal outpatients, and this was significant in all years. There was no significant year-on-year variation in the proportion of GRE in any cohort.
Figure 3.
(a) Proportion of enterococci from clinical samples displaying glycopeptide non-susceptibility among 13 643 isolates from primary, secondary and tertiary care patients in West London, 2009–13. Error bars indicate 95% CIs calculated by Wilson's method with continuity correction. (b) Proportion of enterococci from clinical samples displaying amoxicillin non-susceptibility among 13 643 isolates from primary, secondary and tertiary care patients in West London, 2009–13. Error bars indicate 95% CIs calculated by Wilson's method with continuity correction.
An analysis of amoxicillin-non-susceptible enterococci (i.e. presumptive Enterococcus faecium) demonstrated a significant variation between critical care and the general ward areas in both hospitals in all years (Table 3 and Figure 3b); specifically, the proportions of amoxicillin-non-susceptible enterococci in critical care were typically twice those in general wards in Hospital 1, and 3–6 times higher in Hospital 2. A significant variation was also demonstrated between renal inpatients and outpatients, with the former having amoxicillin-non-susceptible rates ∼4 times those for the latter. A comparison between the two hospitals demonstrated little significant variation in the proportion of amoxicillin-non-susceptible enterococci between the critical care cohorts (with the exception of 2012–13), but for general ward isolates Hospital 1 consistently had 1.5–4 times higher rates than Hospital 2. Amoxicillin non-susceptibility among community isolates was consistent and 10-fold below that of the other cohorts, at 1.0%–2.6%.
Resistance in Staphylococcus aureus
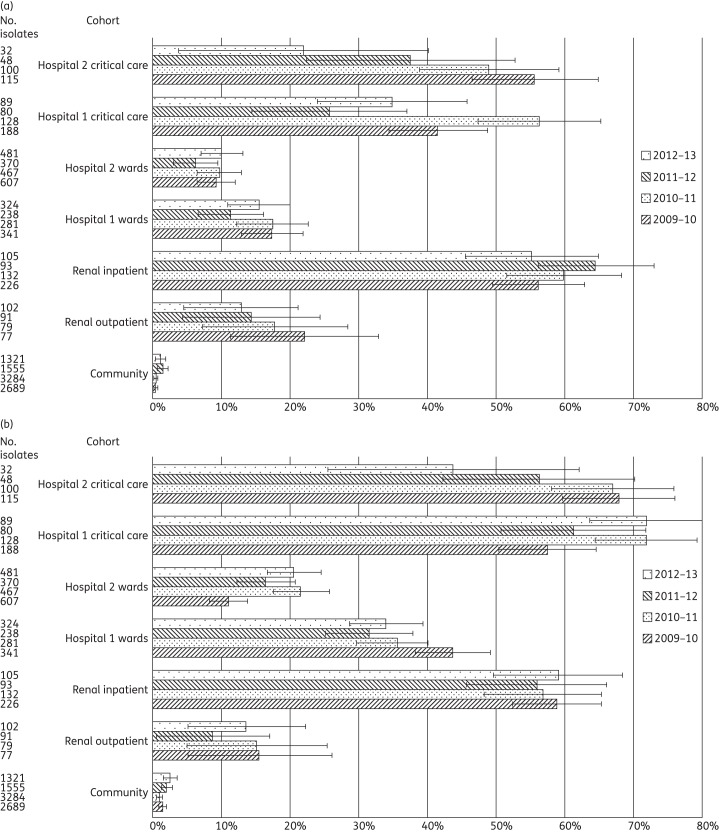
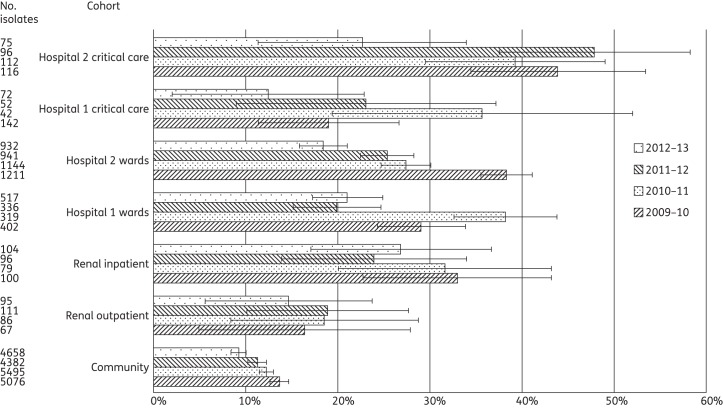
A total of 26 858 S. aureus isolates were identified, of which 4292 (16.0%) were MRSA (Table 2 and Figure 4). MRSA rates were significantly higher in critical care than in the general wards at Hospital 1 only in 2009–10 (Table 3). In Hospital 2, MRSA was more prevalent in critical care areas in two years, with its proportion peaking at almost twice that at ward level in 2011–12. A comparison between the hospitals at ward level demonstrated an alternating trend as to which had the higher MRSA rate; these differences were significant until 2012–13. In critical care, Hospital 2 had persistently higher MRSA rates than Hospital 1 across all years, and this was significant in two years. Among renal patients, the proportion of MRSA from the inpatient cohort was significantly higher than from the outpatient cohort in three of the four years, peaking at almost double in 2009–10.
Figure 4.
Proportion of S. aureus from clinical samples displaying methicillin non-susceptibility among 26 858 isolates from primary, secondary and tertiary care patients in West London, 2009–13. Error bars indicate 95% CIs calculated by Wilson's method with continuity correction.
An analysis over time showed a significant decrease in the proportion of MRSA at ward level in Hospital 1 between 2010–11 and 2011–12, from 38.2% to 19.9% (P < 0.001), with no significant subsequent rebound. A similar decrease at ward level was observed in Hospital 2 over a longer period, from 38.2% in 2009–10 to 27.4% in 2010–11 (P < 0.001). This was followed by a further decrease, from 25.4% in 2011–12 to 18.5% in 2012–13 (P < 0.001). In critical care, no significant temporal variations were observed in Hospital 1 but Hospital 2 saw a significant recent reduction in the proportion of MRSA, from 47.9% in 2011–12 to 22.7% in 2012–13 (P < 0.001). In the community, MRSA showed a downward trend, with significant falls between 2009–10 and 2010–11 (P = 0.02) and between 2011–12 and 2012–13 (P < 0.001).
Antimicrobial usage
Biannual point prevalence studies consistently indicated that 33.3%–41.9% of patients were on antimicrobials, with this proportion rising to 62.9%–71.4% in critical care and to 71.8%–80.4% among renal inpatients. Analysis as DDDs/1000 OBDs, for the four most commonly prescribed antimicrobials (ciprofloxacin, amoxicillin/clavulanate, piperacillin/tazobactam and meropenem) among all inpatient groups is shown in Table 4, demonstrating persistently higher use in Hospital 1 than Hospital 2. This differential was 23%–56% for ciprofloxacin, 26%–53% for amoxicillin/clavulanate, 53%–82% for piperacillin/tazobactam, 74%–117% for meropenem and 27%–40% for total antimicrobial use. Although the renal unit had a lower use of amoxicillin/clavulanate than either hospital, the use of ciprofloxacin was 159%–213% higher than for Hospital 2, piperacillin/tazobactam use 104%–147% higher and meropenem use 39%–264% higher. There was little variation over time in the proportion of prescribing accounted for by these top four antimicrobials, except for a spike in meropenem use in Hospital 1 in 2010–11, a reduction by half for meropenem consumption among renal inpatients between 2010–11 and 2011–12, and for ciprofloxacin, a fall of ∼33% during the study period in Hospital 1 and of 20% in the renal inpatient cohort.
Table 4.
DDDs/1000 OBDs of selected antimicrobials used in secondary and tertiary care patients in West London, 2009–13
| Renal inpatients |
Hospital 1 |
Hospital 2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | |
| Ciprofloxacin | 218 (18.1%) | 211 (15.7%) | 197 (14.5%) | 184 (12.9%) | 106 (7.1%) | 120 (7.3%) | 109 (6.4%) | 87 (4.9% | 70 (6.2%) | 77 (5.9%) | 73 (6.0%) | 71 (5.2%) |
| Amoxicillin/clavulanate | 127 (10.5%) | 138 (10.2%) | 177 (13.0%) | 158 (11.1%) | 305 (20.4%) | 354 (21.5%) | 394 (23.2%) | 384 (21.6%) | 243 (21.5%) | 248 (19.1%) | 257 (21.1%) | 286 (21.0%) |
| Piperacillin/tazobactam | 114 (9.5%) | 135 (10.0%) | 158 (11.6%) | 147 (10.3%) | 87 (5.8%) | 110 (6.7%) | 104 (6.1%) | 110 (6.2%) | 48 (4.2%) | 65 (5.0%) | 64 (5.2%) | 72 (5.3%) |
| Meropenem | 142 (11.8%) | 143 (10.7%) | 76 (5.6%) | 85 (6.0%) | 77 (5.2%) | 112 (6.8%) | 100 (5.9%) | 106 (6.0%) | 39 (3.5%) | 53 (4.1%) | 46 (3.8%) | 61 (4.5%) |
| Cumulativea | 49.8% (49.3–50.3) | 46.5% (46.1–47.0) | 44.6% (44.1–45.1) | 40.3% (39.8–40.8) | 38.5% (38.3–38.8) | 42.3% (42.0–42.6) | 41.6% (41.3–41.8) | 38.7% (38.5–39.0) | 35.2% (35.1–35.5) | 34.2% (34.0–34.4) | 36.2% (35.9–36.4) | 36.1% (35.8–36.3) |
Percentages in parentheses indicate the consumption of that agent as a proportion of all antimicrobials used.
aProportion as a percentage of all antimicrobials prescribed (95% CI).
A review of antimicrobial policies revealed only two major changes in the study period, both promoting the use of narrower-spectrum antimicrobials. The first, in 2010, was the introduction across the hospital network of an antimicrobial policy for infection in over-65-year-olds. This stipulated the use of narrow-spectrum antimicrobials, avoiding amoxicillin/clavulanate for urinary tract infections, peritonitis and pneumonia (advocating aminoglycosides, amoxicillin/metronidazole/gentamicin and amoxicillin, respectively). It aimed to reduce Clostridium difficile infections. The second major change related to antimicrobial stewardship in the renal cohort from 2009 onwards (see below).
Discussion
We found significant differences in antimicrobial non-susceptibility within and between the two hospitals for Enterobacteriaceae, enterococci, S. aureus and Pseudomonas spp. Furthermore, we found a substantial year-on-year fluctuation in non-susceptibility among most ‘drug–bug’ combinations but with few persistent trends. There was much less fluctuation in results for the community cohort, refuting the hypothesis that the variation in the hospital isolates represented a testing quality issue.
The data suggest a few instances where the cohort at one hospital had persistently higher non-susceptibility rates than the corresponding cohort at the other hospital. Notable examples include MRSA in Hospital 2 critical care, meropenem-non-susceptible Pseudomonas spp. in Hospital 1 critical care and amoxicillin-non-susceptible enterococci (i.e. E. faecium) in Hospital 1 ward areas. Nevertheless, the wider lack of consistency in relative rates or trends between the two hospitals suggests that short-term factors were a greater contributor to influencing the year-on-year variation. These short-term factors could potentially represent transmission, with many ‘mini-outbreaks’ among patients who were hospitalized or who had frequent healthcare contact. A concept of ‘mini-outbreaks’, particularly in the critical care areas, is additionally supported by the fluctuations in the frequency with which species were encountered within each cohort (Table 1). Further investigation with prospective large-scale typing is indicated and may be facilitated by the increasing availability of whole-genome sequencing.
One factor that may contribute to the variation seen between the two critical care units is the impact of the use of selective digestive decontamination (SDD)18 in Hospital 2 critical care but not in Hospital 1. This may help to explain why isolation rates of Enterobacteriaceae were generally lower in Hospital 2 critical care than Hospital 1. However, the proportion of Enterobacteriaceae displaying an ESBL/AmpC phenotype peaked at 52.2% in 2010–11 at Hospital 2 (twice that in Hospital 1 in the corresponding year). Over the succeeding 2 years, the rates of isolates with ESBL/AmpC phenotype converged despite there being no change in SDD practice. Elsewhere, data on the impact of SDD on multidrug-resistant organisms is conflicting, and long-term cluster-randomized controlled trials are needed.19,20
In contrast to the general lack of consistency in the differences in rates of resistance between the two hospitals, the results do suggest reasonably consistent ‘within-hospital’ variation, with higher resistance rates and a greater frequency of isolates in critical care versus ward areas. A consistent excess of resistance was also seen in the renal cohorts, where resistance rates in renal inpatients resembled those in critical care rather than those found at ward level; conversely, resistance rates in renal outpatients resembled those of ward inpatients rather than community patients. This differential between the proportions of resistant isolates in general versus specialist cohorts was demonstrated for most ‘drug–bug’ combinations, with the exception of methicillin resistance for S. aureus and ciprofloxacin non-susceptibility in Enterobacteriaceae, for which fairly uniform rates were noted across all cohorts. One of the most likely causes for these ‘within-hospital’ variations may be the greater frequency of antimicrobial use in critical care areas/renal inpatients than in general wards. In our 6-monthly point prevalence studies, antimicrobial usage in critical care was higher than the benchmarked national point prevalence finding of 60.8% for critical care patients,21 and usage in the renal inpatient cohort was higher even than in critical care. An increased use of devices and central intravenous cannulae in critical care and among renal inpatients may be driving the frequent empirical co-prescription of glycopeptides, selecting for GRE.22 Nevertheless, markedly higher antimicrobial prescribing in Hospital 1 than Hospital 2 was not reflected in higher overall resistance rates.
Beyond the levels of antimicrobial usage, one of the biggest drivers of variation both between and within hospitals may be the spectra of activity of the particular antimicrobials prescribed, and it is widely suggested that resistance itself is encouraging increasingly broad-spectrum empirical antimicrobial use, thereby driving the selection of further resistance. A possible example was seen with the spike in meropenem usage in Hospital 1 between 2009–10 and 2010–11, which was temporally associated with spikes in piperacillin/tazobactam resistance among Pseudomonas spp. at both ward and critical care levels. This rise in meropenem use was associated with a non-significant but possibly consequent rise in meropenem-non-susceptible Pseudomonas spp. in both Hospital 1 cohorts, persisting in critical care for the subsequent year. Addressing this feedback loop through antimicrobial stewardship is key, and advances in rapid microbiological diagnostics to facilitate de-escalation may help.23,24
One example of the successful interruption of such a feedback loop is demonstrated in the renal cohort data. Until and including 2010–11, meropenem use in this cohort was high. Following an outbreak of K. pneumoniae with an OXA-48-carbapenemase in 2008–10, carbapenem use was almost halved on the advice of infection specialists between 2010–11 and 2011–12. While causality cannot be directly attributed, a beneficial yet unintended impact over a relatively short time frame was a significant fall in the proportion of Pseudomonas spp. non-susceptible to meropenem. A marked and concordant fall in piperacillin/tazobactam resistance in Pseudomonas spp.—despite plateaued consumption in this cohort over this period—may reflect the fact that both meropenem and piperacillin/tazobactam are affected by, and putatively select for, the same efflux-based resistance mechanisms in P. aeruginosa.
A further facet of antimicrobial use may contribute to the high burden of antimicrobial non-susceptibility, specifically homogeneous use, concentrating the selection pressure on a fraction of the antimicrobial armamentarium.25,26 In our study, just four agents consistently represented 34%–50% of all antimicrobials prescribed in the hospitals and the renal inpatients, and two of these—amoxicillin/clavulanate and piperacillin/tazobactam—are closely related. While the damaging consequences of homogeneous antimicrobial policies are not proven for all antimicrobials,27 an argument for heterogeneity exists and might be achieved through antimicrobial cycling28,29 or mixing.30,31 The latter option is likely to be preferable, through offering broader choices of antimicrobials within policies and prospective monitoring to preserve the diversity of prescribing within those choices offered.
Critics of antibiogram-based surveillance data often cite inaccuracies in the sampling of selected patient populations and restricted geographical sampling.32 We largely evaded these problems by extracting all the data from a laboratory serving multiple cohorts across two hospitals and an associated community that shared an overarching antimicrobial policy and infection specialists. Antibiograms can also suffer in accuracy, and therefore utility, when there are marked changes in patient mix. There was some change in the patient population through this study period, with solid organ oncology being consolidated from both hospitals to Hospital 2 in 2010–11, and with the formation of a new heart attack centre at Hospital 1 in 2009–10. Changes in primary care in one part of West London between 2010–11 and 2011–12 led to in a decrease in the number of community samples. Changing hospital configurations such as these highlight the need for regular antibiogram review and relation to the patient cohorts. One limitation of this study is that the susceptibility rates were not stratified by sample type. When a comparison by sample type was attempted, meaningful analysis was precluded by low numbers. It is acknowledged that rates may potentially vary between sample types, not least because of sampling bias. However, using an all-sample approach to resistance surveillance, rather than restricting data to blood culture results, may better reflect the drivers towards antimicrobial prescribing in clinical practice. A further limitation is the inability to delineate whether clinical isolates from patients in one cohort were acquired in that cohort or elsewhere (e.g. community-acquired isolates detected during inpatient stays or hospital-acquired infections becoming manifest in the community). The impact of community antimicrobial use, over and above hospital use, on the variation in susceptibility rates seen between cohorts could not be assessed and would require longitudinal, linked, primary and secondary care patient-level data, not presently available.
Conclusions
The Annual Report of the Chief Medical Officer,33 the UK Five-Year Antimicrobial Resistance Strategy,34 the Chennai Declaration35 and the G8 Science Ministers Statement36 all highlight surveillance as key to addressing antimicrobial resistance. Aggregated regional or hospital level data are no longer adequate and analysis at a finer resolution, as here, is needed; however, challenges are posed by the need to retain sufficient statistical power to detect variation. A standardization of reporting parameters urgently needs to be agreed to enable useful surveillance and benchmarking.
Despite (i) overarching antimicrobial and infection control policies, (ii) standardized laboratory practice and (iii) integrated infection specialists with an active stewardship programme, this study found a significant variation in antimicrobial susceptibility in common organisms between different patient cohorts in the multisite hospital network. Variations in antimicrobial use and clinical practice may be responsible, but relationships are far from clear and random fluctuations, potentially due to numerous ‘mini-outbreaks’, may be short-term modulators. The marked heterogeneity in antimicrobial susceptibility moreover suggests that whole-hospital antimicrobial policies may not be appropriate in hospitals with multiple sites or where units have markedly different patient populations. Local policies, rapidly responsive to short-term fluctuations of both antimicrobial resistance and prescribing patterns, may be necessary and desirable. Policies should also be mindful of the potential unintended consequences of reactive prescribing.
Funding
This work was supported by the National Institute of Health Research Imperial Biomedical Research Centre and the UK Clinical Research Collaboration who fund the Centre for Infection Prevention and Management (UKCRC G0800777). The authors L. S. P. M., M. G. and A. H. H. are affiliated with the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infection and Antimicrobial Resistance at Imperial College London in partnership with PHE.
Transparency declarations
L. S. P. M. and A. H. H. have consulted for bioMérieux. M. J. G. has attended advisory boards for AstraZeneca and The Medicines Company. D. M. L. is partly self-employed and consults for numerous pharmaceutical and diagnostic companies, including Achaogen, Adenium, Allecra, Astellas, AstraZeneca, Bayer, Basilea, bioMérieux, Cubist, Curetis, Discuvra, Fedora, GSK, Merck, Meiji Seika, Pfizer, Roche, Shionogi, Tetraphase, VenatoRx and Wockhardt, holds grants or contracts from AstraZeneca, Cubist, Meiji Seika, Merck and Wockhardt, has received lecture honoraria or travel reimbursement from AstraZeneca, Bruker, Curetis, GSK, J&J, Merck, Novartis, Pfizer and Tetraphase, and holds shares in Dechra, Leo, GSK, Merck and Pfizer, collectively amounting to <10% of portfolio value. All other authors: none to declare.
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or PHE.
Acknowledgements
We would like to thank the contribution of Imperial College Healthcare NHS Trust Antimicrobial Review Group and Pharmacy Department, Mr Paul Jones Biomedical Scientist, and the Antimicrobial Resistance Monitoring and Reference Laboratory at PHE.
References
- 1.ECDC. Antimicrobial Resistance Surveillance in Europe 2011. http://www.ecdc.europa.eu/en/publications/publications/antimicrobial-resistance-surveillance-europe-2011.pdf .
- 2.Lamoth F, Wenger A, Prod'hom G, et al. Comparison of hospital-wide and unit-specific cumulative antibiograms in hospital- and community-acquired infection. Infection. 2010;38:249–53. doi: 10.1007/s15010-010-0033-0. [DOI] [PubMed] [Google Scholar]
- 3.Azim A, Dwivedi M, Rao PB, et al. Epidemiology of bacterial colonization at intensive care unit admission with emphasis on extended-spectrum β-lactamase- and metallo-β-lactamase-producing Gram-negative bacteria—an Indian experience. J Med Microbiol. 2010;59:955–60. doi: 10.1099/jmm.0.018085-0. [DOI] [PubMed] [Google Scholar]
- 4.Verhamme KMC, De Coster W, De Roo L, et al. Pathogens in early-onset and late-onset intensive care unit-acquired pneumonia. Infect Control Hosp Epidemiol. 2007;28:389–97. doi: 10.1086/511702. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds R, Maher K, Hope R on behalf of BSAC Working Party on Resistance Surveillance. ICU—always the hotbed of resistance? Clin Microbiol Infect. 2011;17(Suppl S4):S333 (P1231). [Google Scholar]
- 6.Zapantis A, Lacy MK, Horvat RT, et al. Nationwide antibiogram analysis using NCCLS M39-A guidelines. J Clin Microbiol. 2005;43:2629–34. doi: 10.1128/JCM.43.6.2629-2634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CLSI. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data–Third Edition: Approved Guideline M39-A3. Wayne, PA, USA: CLSI; 2009. [Google Scholar]
- 8.Binkley S, Fishman NO, LaRosa LA, et al. Comparison of unit-specific and hospital-wide antibiograms: potential implications for selection of empirical antimicrobial therapy. Infect Control Hosp Epidemiol. 2006;27:682–7. doi: 10.1086/505921. [DOI] [PubMed] [Google Scholar]
- 9.Aldeyab MA, Harbarth S, Vernaz N, et al. The impact of antibiotic use on the incidence and resistance pattern of extended-spectrum β-lactamase-producing bacteria in primary and secondary healthcare settings. Br J Clinical Pharmacol. 2012;74:171–9. doi: 10.1111/j.1365-2125.2011.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wickramasinghe NH, Xu L, Eustace A, et al. High community faecal carriage rates of CTX-M ESBL-producing Escherichia coli in a specific population group in Birmingham, UK. J Antimicrob Chemother. 2012;67:1108–13. doi: 10.1093/jac/dks018. [DOI] [PubMed] [Google Scholar]
- 11.Livermore DM. Minimising antibiotic resistance. Lancet Infect Dis. 2005;5:450–9. doi: 10.1016/S1473-3099(05)70166-3. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health. The Critical Care Minimum Dataset (CCMDS) 2006. http://www.isb.nhs.uk/documents/dscn/dscn2005/022005v3.pdf .
- 13.PHE. Standards for Microbiology Investigations. http://www.hpa.org.uk/ProductsServices/MicrobiologyPathology/UKStandardsForMicrobiologyInvestigations/TermsOfUseForSMIs/AccessToUKSMIs/
- 14.BSAC. BSAC Methods for Antimicrobial Susceptibility Testing Version 12. http://bsac.org.uk/wp-content/uploads/2012/02/Version-12-Apr-2013_final1.pdf .
- 15.WHO Collaborating Centre for Drug Statistic Methodology. Guidelines for ATC Classification and DDD Assignment 2013. http://www.whocc.no/filearchive/publications/1_2013guidelines.pdf .
- 16.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–72. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Thomas CP, Moore LSP, Elamin N, et al. Early (2008–2010) hospital outbreak of Klebsiella pneumoniae producing OXA-48 carbapenemase in the UK. Int J Antimicrob Agents. 2013;42:531–6. doi: 10.1016/j.ijantimicag.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 18.De Smet AMGA, Kluytmans JAJW, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360:20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- 19.Oostdijk EAN, de Smet AMGA, Blok HEM, et al. Ecological effects of selective decontamination on resistant Gram-negative bacterial colonization. Am J Respir Crit Care Med. 2010;181:452–7. doi: 10.1164/rccm.200908-1210OC. [DOI] [PubMed] [Google Scholar]
- 20.Daneman N, Sarwar S, Fowler RA, et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:328–41. doi: 10.1016/S1473-3099(12)70322-5. [DOI] [PubMed] [Google Scholar]
- 21.HPA. English National Point Prevalence Survey on Healthcare-associated Infections and Antimicrobial Use, 2011. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317134304594 .
- 22.Shime N, Kosaka T, Fujita N. The importance of a judicious and early empiric choice of antimicrobial for methicillin-resistant Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis. 2010;29:1475–9. doi: 10.1007/s10096-010-1024-x. [DOI] [PubMed] [Google Scholar]
- 23.Advisory Committee on Antimicrobial Resistance and Healthcare Associated Infection. Antimicrobial Stewardship: Start Smart—Then Focus. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215308/dh_131181.pdf .
- 24.Ashiru-Oredope D, Sharland M, Charani E, et al. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart–Then Focus. J Antimicrob Chemother. 2012;67(Suppl 1):i51–63. doi: 10.1093/jac/dks202. [DOI] [PubMed] [Google Scholar]
- 25.Livermore DM. Fourteen years in resistance. Int J Antimicrob Agents. 2012;39:283–94. doi: 10.1016/j.ijantimicag.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Sandiumenge A, Diaz E, Rodriguez A, et al. Impact of diversity of antibiotic use on the development of antimicrobial resistance. J Antimicrob Chemother. 2006;57:1197–204. doi: 10.1093/jac/dkl097. [DOI] [PubMed] [Google Scholar]
- 27.Ginn AN, Wiklendt AM, Gidding HF, et al. The ecology of antibiotic use in the ICU: homogeneous prescribing of cefepime but not tazocin selects for antibiotic resistant infection. PLoS One. 2012;7:e38719. doi: 10.1371/journal.pone.0038719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergstrom CT, Lo M, Lipsitch M. Ecological theory suggests that antimicrobial cycling will not reduce antimicrobial resistance in hospitals. Proc Natl Acad Sci USA. 2004;101:13285–90. doi: 10.1073/pnas.0402298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow K, Wang X, Curtiss R, et al. Evaluating the efficacy of antimicrobial cycling programmes and patient isolation on dual resistance in hospitals. J Biol Dyn. 2011;5:27–43. doi: 10.1080/17513758.2010.488300. [DOI] [PubMed] [Google Scholar]
- 30.Sandiumenge A, Lisboa T, Gomez F, et al. Effect of antibiotic diversity on ventilator-associated pneumonia caused by ESKAPE organisms. Chest. 2011;140:643–51. doi: 10.1378/chest.11-0462. [DOI] [PubMed] [Google Scholar]
- 31.Piper GL, Kaplan LJ. Antibiotic heterogeneity optimizes antimicrobial prescription and enables resistant pathogen control in the intensive care unit. Surg Infect (Larchmt) 2012;13:194–202. doi: 10.1089/sur.2012.121. [DOI] [PubMed] [Google Scholar]
- 32.Dalhoff A. Resistance surveillance studies: a multifaceted problem—the fluoroquinolone example. Infection. 2012;40:239–62. doi: 10.1007/s15010-012-0257-2. [DOI] [PubMed] [Google Scholar]
- 33.Davies SC. Annual Report of the Chief Medical Officer, Volume 2, 2011. Infections and the Rise of Antimicrobial Resistance. http://media.dh.gov.uk/network/357/files/2013/03/CMO-Annual-Report-Volume-2-20111.pdf . [DOI] [PubMed]
- 34.Davies SC, Gibbens N. UK Five Year Antimicrobial Resistance Strategy 2013 to 2018. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/244058/20130902_UK_5_year_AMR_strategy.pdf .
- 35.Ghafur A, Mathai D, Muruganathan A, et al. The Chennai Declaration: a roadmap to tackle the challenge of antimicrobial resistance. Indian J Cancer. 2013;50:71–3. doi: 10.4103/0019-509X.104065. [DOI] [PubMed] [Google Scholar]
- 36.Willetts D, Livanov D, Schütte G, et al. 2013. G8 Science Ministers Statementhttps://www.gov.uk/government/news/g8-science-ministers-statement .