Abstract
Background
In the U.S., Europe, and throughout the world, abdominal obesity prevalence is increasing. Depressive symptoms may contribute to abdominal obesity through the consumption of diets high in energy density.
Purpose
To test dietary energy density ([DED]; kilocalories/gram of food and beverages consumed) for an independent relationship with abdominal obesity or as a mediator between depressive symptoms and abdominal obesity.
Methods
This cross-sectional study included 87 mid-life, overweight adults; 73.6% women; 50.6% African-American. Variables and measures: Beck Depression Inventory-II (BDI-II) to measure depressive symptoms; 3-day weighed food records to calculate DED; waist circumference, an indicator of abdominal obesity. Hierarchical regression tested if DED explained waist circumference variance while controlling for depressive symptoms and consumed food and beverage weight. Three approaches tested DED as a mediator.
Results
Nearly three-quarters of participants had abdominal obesity, and the mean waist circumference was 103.2 (SD 14.3) cm. Mean values: BDI-II was 8.67 (SD 8.34) which indicates most participants experienced minimal depressive symptoms, and 21.8% reported mild to severe depressive symptoms (BDI-II ≥ 14); DED was 0.75 (SD 0.22) kilocalories/gram. Hierarchical regression showed an independent association between DED and waist circumference with DED explaining 7.0% of variance above that accounted for by BDI-II and food and beverage weight. DED did not mediate between depressive symptoms and abdominal obesity.
Conclusions
Depressive symptoms and DED were associated with elevated waist circumference, thus a comprehensive intervention aimed at improving depressive symptoms and decreasing DED to reduce waist circumference is warranted.
Keywords: depressive symptoms, dietary energy density, waist circumference, abdominal obesity
1. Introduction
Abdominal obesity, adipose tissue that is centrally distributed, is a cardiometabolic risk factor [1]. In the United States and Europe, the prevalence of abdominal obesity (defined as waist circumference > 102 cm for men and > 88 cm for women; [2, 3]) is increasing [4–7]. Obesity was once considered a problem primarily in high income countries, however, the prevalence is increasing worldwide, particularly in urban areas of low and middle income countries [8]. Chronic psychological stress accompanied by depressive symptoms and in combination with diets that are high in energy density, those high in fat, sugars, and starches, may contribute to abdominal obesity and cardiometabolic risk. A greater understanding of the behavioral mechanisms that mediate the relationship between depressive symptoms and abdominal obesity may lead to the creation of innovative, tailored weight loss interventions.
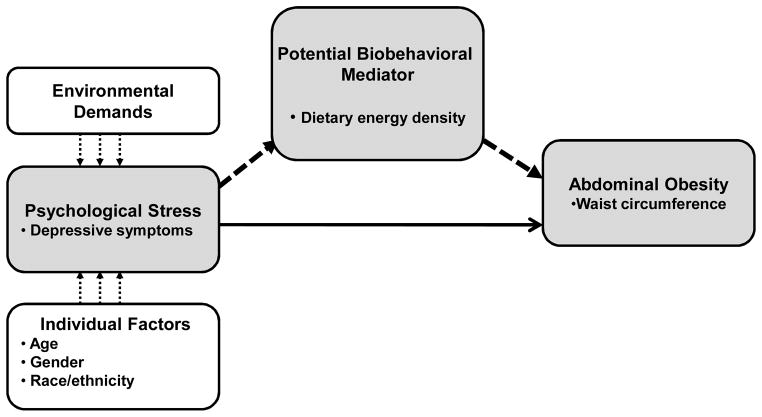
The conceptual basis of the study was adapted from Cohen, Kessler, and Gordon’s (1995) stress and coping theory that proposes that individual characteristics, such as age, gender, and race/ethnicity, and environmental demands may predispose individuals to psychological stress. Depending on the individual’s coping behaviors and available resources, these demands may contribute to psychological stress, particularly in susceptible individuals. This stress can precipitate or exacerbate depressive symptoms which may trigger behaviors that can mitigate or increase cardiometabolic disease risk [9]. Figure 1. Our conceptual model proposes that depressive symptoms are associated with unhealthy dietary patterns, and diets characterized by high dietary energy density (DED). Depressive symptoms may be accompanied by increased appetite, intake of high fat and high carbohydrate “sweet” energy dense foods, a low intake of fruits and vegetables, and excess food and alcohol intake [10–14] Although depressive symptoms do not necessarily confirm major depression, if symptoms persist the likelihood of depression is high. This is of concern because a meta-analysis of community-based studies showed depression increased the risk of developing obesity [15], and major depression is associated with abdominal obesity in men [16] and women [17].
Figure 1.
The Adapted Stress and Coping Model [8].
Note. The solid line represents the direct relationship between depressive symptoms and abdominal obesity. The heavy dotted line represents dietary energy density as a potential mediator linking depressive symptoms with abdominal obesity.
However, two recent studies highlight the inconsistent findings regarding the relationship between depressive symptoms and weight loss. In a longitudinal study of a weight loss intervention, obese women, ages 40–65 years, who reported an improvement in their depressive symptoms were more likely to lose 5 kilograms (kg) or more of body weight than women who did not report an improvement in depressive symptoms [18]. On the other hand, in another recently published weight loss intervention study, greater depressive symptoms at baseline did not predict weight loss in women [19].
DED, the ratio of energy (kilocalories; [kcal]) to weight (grams; [g]) of food and beverages consumed, is particularly relevant in examining how diets that are high in fat, sugars, and starches may modify cardiometabolic risk. Diets that are high in energy density may represent an important health risk due to the excess intake of energy, total and saturated fats, and the limited intake of micronutrient rich foods [20]. DED is linked to the water content of foods: water accounts for 85% of DED variance while fat and water together account for 99% of DED variance [21]. The amount of energy concentrated per gram of food is critical in determining overall energy intake [21, 22]. DED characterizes nutrient quality: as energy density increases, nutrient quality decreases [20, 23, 24]. High DED has been correlated with higher BMI in women, and high DED has been associated with elevated waist circumference in women and men [38].
Excess abdominal adipose tissue contributes to cardiometabolic disease risk: abdominal adipose tissue is metabolically active secreting proteins that promote inflammation, atherogenesis, and insulin resistance [25, 26]. As the adipose tissue expands, less adiponectin (a cardioprotective protein) is produced thus further contributing to cardiometabolic disease risk [27, 28].
It is not known if the association between depressive symptoms and abdominal obesity is mediated through behavioral mechanisms such as DED. Therefore, the purpose of this paper is to test DED for an independent association with abdominal obesity or as a mediator between depressive symptoms and abdominal obesity.
2. Methods
2.1. Design
A prospective, cross-sectional design examined associations between depressive symptoms, DED, and waist circumference, and data were collected to assess depressive symptoms, DED, and anthropometry. A priori power analysis, Power Analysis and Sample Size (PASS; Kaysville, Utah) 2005 software, showed 83 participants were needed to achieve a power of .80 with α of .05 based on a linear regression model with four predictor and four control variables. Adults ages 18–65 years with a body mass index (BMI = kg/m2; [29]) ≥ 25.0 kg/m2 (overweight; [2]) were enrolled. The exclusion criteria included: pregnant or breastfeeding, treated with steroids, valproic acid, phenothiazines, or antidepressants, a health history of diabetes, liposuction, bariatric or abdominal surgery, or participation in physical activity for ≥ 90 minutes per week.
Participants were recruited from community health fairs, local businesses, worksites, and neighborhood churches, and potential participants were screened to ensure they met the inclusion criteria. Data were collected at participants’ homes or the School of Nursing. The study was approved by the university Institutional Review Board, 2007, and the investigation conforms to the principles in the Declaration of Helsinki [30].
2.2. Variables and measures
Individual characteristics and health information were self-reported. The Beck Depression Inventory-II (BDI-II; [31]) measured depressive symptoms over the previous 2 weeks. The BDI-II scores range from 0–63 with higher scores indicating greater depressive symptoms, and scores ≥ 14 indicate mild to severe depressive symptoms [31]. The BDI-II has well established reliability and validity [25], and α for this study was .91.
A standardized 3-day weighed food record (gold standard in dietary assessment, [32]) assessed DED. Using the Soehnle 67000 digital food scale (Murrhardt, Germany), participants weighed, measured, and recorded their food and beverage intake for 3 consecutive days including 1 weekend day. Food records were analyzed by the research dietitian using Food Processor SQL Nutrition Analysis software (ESHA Research, Salem, Oregon) for caloric intake (kilocalories [kcal]) and weight (grams [g]) of food and beverages consumed.
One trained research nurse measured the height (centimeters [cm]), weight (kg), and waist circumference (cm) of each participant using standardized National Health and Nutrition Examination Survey (NHANES) procedures [33]. Height and weight were used to calculate BMI. Abdominal obesity was assessed by waist circumference measured three times with a standard steel tape, and the mean of the waist circumference measurements was used in the analyses. Intrarater reliability for the waist circumference measurement was .96.
2.3 Study procedure
Two study visits were conducted by one trained research nurse over a 1 week time period. During the first visit, standardized procedures were used to teach participants how to use a food scale and how to weigh, measure, and record their food and beverage intake on the standardized food record. To minimize measurement errors, participants were given a standardized instruction booklet which included illustrations, photographs and examples of serving sizes. Anthropometric measurements were obtained. During the 3 days at home, participants completed the study questionnaires and kept the food record. During the second visit, standardized procedures were used to review questionnaires and the food record for completeness and accuracy. A variety of measurement aids including photographs of serving sizes, measuring devices including cups, spoons, and rulers, and food models were used to ensure accurate estimations. After the second study visit, the research nurse scored the BDI-II questionnaires, and if the score indicated moderate or severe (BDI-II score ≥ 20) depressive symptoms, participants were referred to mental health services for further evaluation [34]. The food record was reviewed and analyzed by the study dietitian.
2.4. Data analysis
Data were analyzed using Statistical Program for Social Sciences-PC (SPSS; Chicago, Illinois) version 15.0 software. Frequency distributions, scatter plots, and statistical tests of normality were used to assess the normality of the distribution. Based on these assessments, the BDI-II and mean waist circumference values were skewed and underwent a square root transformation, and the transformed variables were used in regression and mediation models. Because the data did not meet the normality assumptions of parametric testing, the Mann-Whitney U test assessed significant differences between variables [35].
For the 3 days of reporting, the mean calories (kcal) and weight (g) of food and beverages consumed were calculated and used in the analyses. To assess underreporting of caloric intake, the daily energy requirement for each participant was calculated using the sex-specific Harris-Benedict equation [36]. The Harris-Benedict equation, which takes age, gender, height, weight, and activity level into account, was used to estimate energy expenditure. The estimated energy expenditure value reflects the caloric intake needed to maintain current body weight [31]. The estimated mean caloric intake from the three day food record was compared to the estimated energy expenditure value calculated using the Harris-Benedict equation. Participants with an estimated caloric intake equal to or greater than the daily energy requirement were considered to have reported adequate caloric intake. Participants were considered to have reported inadequate caloric intake if the caloric intake was less than the daily energy requirement.
Paralleling published procedures [37], the DED value was calculated as the ratio of daily caloric intake of food and beverages consumed (kcal) to daily weight of food and beverages consumed (g). The rationale for using this method of calculation is that it provided a comprehensive assessment of caloric intake without the need for special manipulation of dietary intake information [37]. The DED value was calculated for each study day, and the mean DED value for the three days was used in the analysis. Paralleling published procedures [38], the DED values were centered and standardized so that each unit of DED represented one standard deviation change, and these were used in the regression model.
Hierarchical linear regression models were constructed with waist circumference as the dependent variable. Independent variables in the full model with an α of .20 or below were entered into the reduced model and were retained if significant in the reduced model. The reduced models are presented here. Hierarchical linear regression determined if DED explained waist circumference variance above that accounted for by depressive symptoms and weight of food and beverages consumed. The mean consumed food and beverage weight was entered as a covariate paralleling published procedures [38]. Using the SPSS macro [39], Baron and Kenny [40], Sobel [41], and bootstrap sampling distribution [42] methods tested DED as a simple mediator between BDI-II scores and waist circumference.
3. Results
A non-random sample of 87 adults was enrolled. Most were women, about an equal number of African-Americans and Caucasians, and a mean age of 41.3 (SD 10.2) years. The mean BMI suggests that most participants were classified as obese with a BMI greater than or equal to 30 kg/m2. Participants were well educated, all were employed, and most reported a household annual income of less than $60,000 per year. The majority of participants reported no chronic health conditions. The most commonly reported health condition was hypertension. Table 1.
Table 1.
Demographic and health characteristics of participants (N = 87).
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Age (yrs) mean (SD) | 41.3 (10.2) |
| BMI (kg/m2) mean (SD) | 32.13 (6.09) |
| Men | 30.98 (4.54) |
| Women | 32.54 (6.54) |
| Female gender n (%) | 64 (73.6) |
| Race | |
| African-American | 44 (50.6) |
| White | 43 (50.6) |
| Total combined family income (US $) n (%) | |
| ≥ $60,000 | 35 (40.2) |
| Highest level of education | |
| High school graduate/technical school | 9 (10.3) |
| Completed some college | 27 (31.0) |
| College graduate | 27 (31.0) |
| College graduate/graduate or professional school | 24 (27.6) |
| Health history | |
| Hypertension | 18 (20.7) |
| High cholesterol | 13 (14.9) |
| Angina | 5 (5.7) |
| Stroke | 0 (0) |
| Chronic health conditions | 75 (86.2) |
| Depressive symptoms (BDI-II ≥ 14) | 19 (21.8) |
The median BDI-II score was 6.0 (range 0 – 42.0) with 21.8% (n = 19) reporting mild to severe depressive symptoms (BDI-II scores ≥ 14; [31]). The median BDI-II score for those with depressive symptoms (n = 19, Mdn = 19.0, range 14 – 42) was significantly higher than those without depressive symptoms (n = 68, Mdn = 4.5, range 0 – 13) (Mann-Whitney Z = – 6.657, p ≤ .01).
Among all participants, the mean caloric intake for food and beverages consumed was 2029.77 (SD 589.01) kilocalories, and the mean weight of food and beverages consumed was 2864.04 (SD 864.92) grams. The majority (n = 51, 58.6%) reported a caloric intake less than their daily energy requirement. Overall, the mean DED was 0.75 (SD 0.22) kcal/g, and the median was 0.74 (range 0.32 – 1.31) kcal/g. Men (Mdn = 0.77, range 0.93 – 1.25 kcal/g) and women (Mdn = 0.72, range 0.41 – 1.31) did not differ on DED (Mann-Whitney Z = –1.010, p = .31). Overall, the mean waist circumference was 103.2 (SD 14.3) cm, and the mean waist circumference was 103.4 (SD 12.7) and 103.2 (SD 14.9) cm for men and women, respectively. Overall, 73.6% (n = 64) of participants had abdominal obesity: 43.5% (n = 10) of men and 84.8% (n = 54) of women had waist circumference measurements > 102 and > 88 cm for men and women, respectively [2, 3].
Hierarchical regression showed that DED explained 7.0% of waist circumference variance above that accounted for by depressive symptoms and mean food and beverage weight. Together these variables explained 20.6% of waist circumference variance. Table 2. A post hoc power analysis showed 78% power to detect a Δ R2 of .07 attributed to DED while controlling for two independent variables. Collinearity diagnostics showed condition index < 30.0, variance inflation factor < 2.0, and tolerance > 0.5 indicating no collinearity within the data [43]. Results of the Baron and Kenny, Sobel, and bootstrap sampling distribution tests were not significant showing that DED was not a mediator between depressive symptoms and waist circumference. Table 3.
Table 2.
Hierarchical Regression Model of Waist Circumference.
| Variable | B | SE B | β |
|---|---|---|---|
| Step 1 – covariates | |||
| BDI-IIa | 0.15 | .05 | .31** |
| Food & beverage weight (g) | 2.3E−4 | 8.6E−5 | .28** |
| R2 = .135; F = 6.504** | |||
| Step 2 – DED | |||
| BDI-IIa | .14 | .05 | .29** |
| Food & beverage weight (g) | 3.5E−4 | 9.3E−5 | .42** |
| DED (kcal/g) | .21 | .08 | .30** |
| Δ R2 = .070; R2 = .206; F = 7.090** | |||
Note:
Square root transformed variable. BDI-II — Beck Depression Inventory II; B — beta coefficient.
p ≤ .01.
Table 3.
Evaluation of Dietary Energy Density as a Mediating Variable.
| Method of Analysis | ||||
|---|---|---|---|---|
| Test for direct and indirect effects using simple linear regression models (Baron and Kenny) | ||||
| Coefficient | Standard error | T-statistic | P-value | |
| b (WCa, BDI-IIa) | .12 | .05 | 2.33 | .02 |
| b(DED, BDI-IIa) | .02 | .02 | 1.46 | .15 |
| b(WCa, DED. BDI-IIa) | .35 | .34 | 1.03 | .31 |
| b(WCa, BDI-IIa. DED) | .11 | .05 | 2.15 | .03 |
| Test for indirect effect using the normal distribution (Sobel) | ||||
| Value | Standard error | Z-statistic | P-value | |
| Sobel | .01 | .01 | .73 | .46 |
| Test for indirect effect using the nonparametric bootstrap method | ||||
| Mean | Standard error | LL 95% CI | UL 95% CI | |
| Bootstrap | .01 | .01 | −.01 | .04 |
Note:
Square root transformed variable; BDI-II — Beck Depression Inventory II; DED — Dietary Energy Density; WC — Waist circumference; LL — Lower limit; UL — Upper limit; CI — Confidence interval.
4. Discussion
This sample reflected overweight adults with abdominal obesity at risk for cardiometabolic disease. Food cost has been identified as a factor that contributes to high DED [44, 45], and this may have been a factor for some participants since the majority reported earning less than $60,000 per year. Time pressures, limited time for meal preparation, and a demand for prepackaged and fast foods also contribute to poor quality diets [46]. For most participants, full time work responsibilities may have limited time for grocery shopping and meal preparation as well as created a demand for prepackaged and fast foods.
The overall median BDI-II value indicates that the majority experienced minimal depressive symptoms, yet more than a fifth of participants reported mild to severe depressive symptoms. Depressive symptoms were four times more common than is major depression in the US population which is estimated at 5.0% [47], and were similar to the 7.9 – 17.0% major depression prevalence associated with chronic medical conditions [48].
DED values were relatively low which may be due to many participants reporting a caloric intake less than their energy needs. The problem of reporting inadequate caloric intake is not unique: in a large sample of US adults, 18% and 28% of men and women, respectively, reported inadequate caloric intake, and among these, 47% were overweight [49].
The majority had waist circumference measurements greater than the sex specific cut points indicating increased relative risk of cardiovascular disease. More than a fifth of participants reported hypertension, roughly 15% reported high cholesterol, and 6% reported angina indicating many have risk for cardiovascular disease progression.
Hierarchical regression showed greater depressive symptoms and high DED were independently associated with larger waist circumference. Among overweight adults, mood needs to be monitored to identify individuals at risk of depression [50]. High DED is associated with cardiometabolic risk factors, such as elevated fasting insulin, and metabolic syndrome [38], therefore early identification and intervention for overweight individuals consuming high DED may reduce the development of cardiometabolic disease. Two potentially modifiable factors, depressive symptoms and DED, were identified as targets for interventions.
Understanding the behavioral pathways linking depressive symptoms and abdominal obesity remains elusive. Our findings did not support DED as a mediator between depressive symptoms and larger waist circumference in overweight adults. However, results from the first study to examine eating behaviors as a possible mediating pathway linking depressive symptoms with abdominal obesity were recently published [51]. Depressive symptoms were measured using the Center for Epidemiological Studies Depression Scale, emotional eating behaviors were measured using the Three-Factor Eating Questionnaire-R18, and anthropometric measurements were obtained during a health examination. Using structural equation modeling on cross sectional data, the researchers showed that among Finnish men (n = 2,312) and women (n = 2,674) aged 25–74 years, greater depressive symptoms were associated with a greater tendency to eat during negative emotions, and this emotional eating was associated with higher BMI and larger waist circumference [51].
In another study, Konttinen et al. (2010) showed that among Finnish men (n=1,679) and women (n=2,035) aged 25–64 years, emotional eating was related to a greater intake of sweet foods high in DED [52]. It could be that emotional eating is the factor linking depressive symptoms with the intake of sweet foods high in DED. Despite our null findings of DED as a mediating variable, the findings from studies conducted by Konttinen et al. (2010) suggest that dietary factors and emotional eating may be behavioral mechanisms that explain, in part, the link between depressive symptoms and abdominal obesity [51, 52].
4.1. Strengths and limitations
Enrolling a large number of African-Americans and women provided insight into the behavioral responses of a population at high risk of cardiometabolic disease and depression. Additionally, very little questionnaire data were missing ensuring efficient use of available data and minimizing biased estimates [53]. Analyzing DED provided an assessment of caloric intake and dietary quality as well as improved our understanding of the associations between depressive symptoms and cardiometabolic disease risk.
Limitations include the use of non-random sampling. The findings are biased toward well educated individuals with middle class and higher incomes. The study activities required highly motivated participants, and people who enrolled may be different from non-participants. Despite best efforts, it was difficult to enroll men. Of the men, most did not have abdominal obesity making it difficult to generalize these findings to men with abdominal obesity. Demographic, health, and depressive symptom data were self-reported, and these data may be subject to over or under reporting and social desirability bias. The cross-sectional nature of the data may preclude causality.
Although rigorous methods were used to obtain detailed food records, many participants reported inadequate caloric intake which may have masked significant associations. Reporting inadequate caloric intake may be due to multiple factors. First, participants may not have written down everything they ate and drank. Second, participants may have underestimated portion sizes. Estimating composite foods is particularly challenging and this may have resulted in underestimating quantities of multiple components. Third, participants may have under-consumed. Participants may have eaten less food and/or drank fewer caloric beverages during the three days of reporting. Finally, weighing and writing down all food and beverages consumed is time intensive and may have created a recording burden [32]. Self-reported food intake data are subject to bias, and a limitation is that these caloric intake data were not triangulated with an objective measure, such as the doubly labeled water method, to estimate total energy expenditure. In weight stable adults, caloric intake equals total energy expenditure. Therefore, comparing the reported caloric intake to an objective measure of total energy expenditure would have provided an assessment of the accuracy of reported caloric intake [54].
4.2. Recommendations for research
Depressive symptoms and DED were independently associated with abdominal obesity. Easily administered, reliable instruments to assess depressive symptoms need to be tested. To ensure that estimated DED values reflect true values, accurate methods to measure dietary intake need to be developed and tested. A comprehensive nursing intervention aimed at reducing depressive symptoms and decreasing DED needs to be developed and tested in diverse populations and among relatively healthy adults prior to the onset of cardiometabolic disease. A comprehensive intervention would incorporate strategies to assess and improve depressive symptoms (psychological stress), and reduce DED (biobehavioral predictor of waist circumference) with the goal of reducing abdominal obesity. Research is needed to identify potential mediators between depressive symptoms and abdominal obesity and to clarify the biobehavioral pathways involved.
4.3. Recommendations for clinical practice
Working as a team, advanced practice nurses, nurses, and allied professionals can play an important role in providing a comprehensive approach to the care of overweight adults. Nurses and allied professionals need to be aware that overweight adults with abdominal obesity may experience depressive symptoms. As part of the examination, advanced practice nurses can assess if a patient is experiencing depressive symptoms that warrant further evaluation, by asking the two questions recommended in The European Guidelines [55] and by the American Heart Association [56]: 1) “Do you feel down, depresssed, and hopeless?” 2) “Have you lost interest and pleasure in life?” [57]. Advanced practice nurses can use these questions to identify and refer individuals at risk of depression to psychiatric/mental health nurse specialists or other qualified mental health professionals for evaluation and treatment.
Nurses and allied professionals need to be aware of DED as a strategy to manage body weight and to potentially decrease abdominal obesity [58, 59]. Rolls and Barnett [59] have defined four categories of dietary energy density from high DED (values of 4.0–9.0 kcal/g) to very low DED (values of < .06 kcal/g) and classified common foods accordingly. (Table 4). Nurses and allied professionals can provide education and practical approaches to help overweight individuals decrease DED to reduce energy intake and improve nutrient quality. This is consistent with the European Guidelines [60] and can be accomplished by replacing high energy dense foods with high volume foods, such as fruits and vegetables, dairy products with low or nonfat dairy products, and caloric with non-caloric beverages.
Table 4.
Examples of foods by dietary energy density categories.
| Dietary energy density category | Ratio of kilocalories per gram | Examples of foods |
|---|---|---|
| High | 4.0 – 9.0 | Chocolate candies, cookies, full fat salad dressing, vegetable oil, margarine, butter |
| Medium | 1.5 – 4.0 | Lean, broiled meats, cheeses, low calorie margarine, light butter |
| Low | 0.6 – 1.5 | Beans and legumes, low fat salad dressings, rice, cereals with low fat milk |
| Very low | < 0.6 | Skim milk, broth based soups, fruits and vegetables |
Note. Rolls and Barnett [59] have defined these categories of dietary energy density and classified common foods accordingly.
Nurses can help overweight individuals identify emotional states that trigger unhealthy dietary patterns, identify and practice healthier coping mechanisms, and incorporate healthier dietary patterns into their daily life. Using behavioral coaching strategies, nurses can help patients understand the link between mood, diet, and cardiometabolic disease risk, and now to effectively manage these.
Nurses and allied professionals need to be aware of the deleterious effect of abdominal obesity on cardiometabolic health, and they can play an important role in screening for overweight and obesity. Carefully measuring height and weight is important to calculate an accurate BMI. Although BMI is a useful screening tool for assessing adiposity, it does not adequately discriminate cardiometabolic disease risk at the individual level. Waist circumference is a useful complement to BMI in that it provides additional information about body fat distribution. With training and practice in measuring waist circumference, advanced practice nurses will be prepared to measure waist circumference as part of the physical exam and to include this information in their assessment of abdominal obesity and cardiometabolic disease risk. The European Guidelines recommend measuring waist circumference midway between the lower rib margin and anterior superior iliac crest [55].
Advanced practice nurses can use anthropometric data to counsel patients regarding their risk of cardiometabolic disease and discuss strategies to reduce risk. Weight loss is an important risk reduction strategy, and nurses can play an important role in referring overweight individuals for weight loss treatment. The European Guidelines recommend weight loss for individuals with BMI ≥ 30 kg/m2, and men and women with waist circumference > 102 cm and > 88 cm [40].
To summarize, advanced practice nurses, in collaboration with nurses and allied professionals, can provide a comprehensive nursing intervention for overweight adults by: 1) identifying those individuals at risk for depression by screening with the two-item questionnaire and referring high risk individuals to psychiatric/mental health nurse specialists for further evaluation and treatment; 2) measuring height and weight to calculate BMI and measuring waist circumference to identify overweight individuals at greatest risk for cardiometabolic disease; 3) educating overweight adults about dietary changes to reduce DED; and 4) using behavioral coaching strategies to help overweight adults understand the link between diet, mood, and cardiometabolic disease risk, and to work together to help these individuals develop strategies to improve diet, mood and decrease cardiometabolic disease risk.
In conclusion, depressive symptoms were relatively common among overweight adults. The majority had waist circumference measurements indicative of abdominal obesity and increased relative risk of cardiovascular disease. Our findings showed depressive symptoms and DED were independently associated with abdominal obesity. The findings did not support DED as a mediator. A comprehensive nursing intervention aimed at detecting, managing, and decreasing depressive symptoms and DED as targets to reduce abdominal obesity and cardiometabolic risk is warranted.
Acknowledgments
This work was supported by funding from The National Institutes of Health, National Institute for Nursing Research, National Research Service Award; Nurses Educational Funds, Inc.; American Nurses Foundation; Southern Nurses Research Society; Sigma Theta Tau International, Alpha Epsilon Chapter; National Institutes of Health, National Center for Research Resources, PHS Grant UL1RR025008 Clinical and Translational Science Award program and PHS Grant MO1 RR0039 General Clinical Research Center program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Barry A, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2005;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. Bethesda, MD: U.S. Department of Health and Human Services: National Institutes of Health, National Heart Lung and Blood Institute; 1998. [Google Scholar]
- 3.Expert Panel on the Detection E. Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among U.S adults. Obesity. 2007;15:216–224. doi: 10.1038/oby.2007.505. [DOI] [PubMed] [Google Scholar]
- 5.Liese AD, Doring A, Hense HW, Keil U. Five year changes in waist circumference, body mass index and obesity in Augsburg, Germany. Eur J Nutr. 2001;40:282–288. doi: 10.1007/s394-001-8357-0. [DOI] [PubMed] [Google Scholar]
- 6.Morrell J, Fox K. Prevalence of abdominal obesity in primary care: The IDEA UK study. Int J Clin Pract. 2009;63:1301–1307. doi: 10.1111/j.1742-1241.2009.02126.x. [DOI] [PubMed] [Google Scholar]
- 7.Lahti-Koski M, Harald K, Mannisto S, Laatikainen T, Jousilahti P. Fifteen-year changes in body mass index and waist circumference in Finnish adults. Eur J Cardiovasc Prev Rehabil. 2007;14:398–404. doi: 10.1097/HJR.0b013e32800fef1f. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Obesity and overweight. 2006 Retrieved 12/08/2010, http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 9.Cohen, Kessler RC, Gordon LU. Measuring Stress: A Guide for Health and Social Scientists. New York: Oxford University Press; 1995. [Google Scholar]
- 10.Benton D. Carbohydrate ingestion, blood glucose and mood. Neurosci Biobehav Rev. 2002;26:293–308. doi: 10.1016/s0149-7634(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 11.Siegel JM, Hyg MS, Yancey AK, McCarthy WJ. Overweight and depressive symptoms among African American women. Prev Med. 2000;31:232–240. doi: 10.1006/pmed.2000.0712. [DOI] [PubMed] [Google Scholar]
- 12.Strine TW, Mokdad AH, Dube SR, Balluz LS, Gonzalez O, Berry JT, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry. 2008;30:127–137. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Konttinen H, Mannisto S, Sarlio-Lahteenkorva S, Silventoinen K, Haukkala A. Emotional eating, depressive symptoms and self-reported food consumption. A population- based study. Appetite. 2010;54:473–479. doi: 10.1016/j.appet.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Jeffery RW, Linde JA, Simon GE, Ludman EJ, Rohde P, Ichikawa LE, et al. Reported food choices in older women in relation to body mass index and depressive symptoms. Appetite. 2009;52:238–240. doi: 10.1016/j.appet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Pennix BW, et al. Overweight, obesity, and depression. A systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 16.Rosmond R, Bjorntorp P. Occupational status, cortisol secretory pattern, and visceral obesity in middle-aged men. Obes Res. 2000;8:445–450. doi: 10.1038/oby.2000.55. [DOI] [PubMed] [Google Scholar]
- 17.Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry. 1997;41:1140–1142. doi: 10.1016/S0006-3223(97)85394-2. [DOI] [PubMed] [Google Scholar]
- 18.Simon GE, Rohde P, Ludman EJ, Jeffery RW, Linde JA, Operskalski BH, et al. Association between change in depression and change in weight among women enrolled in weight loss treatment. Gen Hosp Psychiatry. 2010;32:583–589. doi: 10.1016/j.genhosppsych.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Presnell K, Pells J, Stout A, Musante G. Sex differences in the relation of weight loss self-efficacy, binge eating, and depressive symptoms to weight loss success in a residential obesity treatment program. Eat Behav. 2008;9:170–180. doi: 10.1016/j.eatbeh.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Ledikwe JH, Blanck HM, Kettel Khan L, Serdula MK, Seymour JD, Tohill BC, et al. Low-energy-density diets are associated with high diet quality in adults in the United States. J Am Diet Assoc. 2006;106:1172–1180. doi: 10.1016/j.jada.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Drewnowski A. The role of energy density. Lipids. 2003;38:109–115. doi: 10.1007/s11745-003-1039-3. [DOI] [PubMed] [Google Scholar]
- 22.Rolls BJ, Ello-Martin JA, Tohill BC. What can intervention studies tell us about the relationship between fruit and vegetable consumption and weight management? Nutr Rev. 2004;62:1–17. doi: 10.1111/j.1753-4887.2004.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 23.Marti-Henneberg C, Capdevila F, Arija V, Perez S, Cuco G, Vizmanos B, et al. Energy density of the diet, food volume and energy intake by age and sex in a healthy population. Eur J Clin Nutr. 1999;53:421–428. doi: 10.1038/sj.ejcn.1600770. [DOI] [PubMed] [Google Scholar]
- 24.Cuco G, Arija V, Marti-Henneberg C, Fernandez-Ballart J. Food and nutritional profile of high energy density consumers in an adult Mediterranean population. Eur J Clin Nutr. 2001;55:192–199. doi: 10.1038/sj.ejcn.1601144. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 26.Matias I, Cristino L, Di Marzo V. Endocannabinoids: Some like it fat (and sweet too) J Neuroendocrinol. 2008;20:100–109. doi: 10.1111/j.1365-2826.2008.01678.x. [DOI] [PubMed] [Google Scholar]
- 27.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 28.Meier U, Gressner AM. Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 29.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–343. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 30.Rickham PP. Human experimentation. Code of ethics of the World Medical Association. Declaration of Helsinki. Br Med J. 1964;2:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory (BDI-II) San Antonio, TX: The Psychological Association; 1996. [Google Scholar]
- 32.Gibson RS. Principles of Nutritional Assessment. New York: Oxford University Press; 2005. [Google Scholar]
- 33.National Center for Health Statistics. National Health and Nutrition Examination Survey: Anthropometry procedures manual. 2000. [Google Scholar]
- 34.Clark PC, Deaton C, Dunbar SB. Identifying possible depression in clinical research: Ethical and outcome considerations for the investigator/clinician. Appl Nurs Res. 2003;16:53–59. doi: 10.1053/apnr.2003.50003. [DOI] [PubMed] [Google Scholar]
- 35.Hintze JL. Power Analysis and Sample Size System (PASS) User’s Guide. Kaysville, Utah: NCSS; 2006. [Google Scholar]
- 36.Harris J, Benedict F. A Biometric Study of Basal Metabolism in Man. Washington, DC: Carnegie Institute of Washington; 1919. [Google Scholar]
- 37.Ledikwe JH, Blanck HM, Kettel Khan L, Serdula MK, Seymour JD, Tohill BC, et al. Dietary energy density determined by eight calculation methods in a nationally representative United States population. J Nutr. 2005;135:273–278. doi: 10.1093/jn/135.2.273. [DOI] [PubMed] [Google Scholar]
- 38.Mendoza JA, Drewnowski A, Christakis DA. Dietary energy density is associated with obesity and the metabolic syndrome in US adults. Diabetes Care. 2007;30:974–979. doi: 10.2337/dc06-2188. [DOI] [PubMed] [Google Scholar]
- 39.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods, Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 40.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 41.Sobel ME. Asymptotic intervals for indirect effects in structural equation models. In: Leinhard S, editor. Sociological Methodology. San Francisco: Jossey-Bass; 1982. pp. 290–312. [Google Scholar]
- 42.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 43.Field A. Discovering Statistics Using SPSS. London: Sage Publications; 2005. [Google Scholar]
- 44.Glanz K, Basil M, Maibach E, Goldberg J, Snyder D. Why Americans eat what they do: Taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J Am Diet Assoc. 1998;98:1118–1126. doi: 10.1016/S0002-8223(98)00260-0. [DOI] [PubMed] [Google Scholar]
- 45.Andrieu E, Darmon N, Drewnowski A. Low-cost diets: More energy, fewer nutrients. Eur J Clin Nutr. 2006;60:434–436. doi: 10.1038/sj.ejcn.1602331. [DOI] [PubMed] [Google Scholar]
- 46.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: Where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 47.Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50:85–94. doi: 10.1001/archpsyc.1993.01820140007001. [DOI] [PubMed] [Google Scholar]
- 48.Egede LE. Major depression in individuals with chronic medical disorders: Prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29:409–416. doi: 10.1016/j.genhosppsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. Dietary methods research in the third National Health and Nutrition Examination Survey: Underreporting of energy intake. Am J Clin Nutr. 1997;65:1203S–1209S. doi: 10.1093/ajcn/65.4.1203S. [DOI] [PubMed] [Google Scholar]
- 50.de Wit LM, Fokkema M, van Straten A, Lamers F, Cuijpers P, Penninx BW. Depressive and anxiety disorders and the association with obesity, physical, and social activities. Depress Anxiety. 2010;27:1057–1065. doi: 10.1002/da.20738. [DOI] [PubMed] [Google Scholar]
- 51.Konttinen H, Silventoinen K, Sarlio-Lahteenkorva S, Mannisto S, Haukkala A. Emotional eating and physical activity self-efficacy as pathways in the association between depressive symptoms and adiposity indicators. Am J Clin Nutr. 2010;92:1031–1039. doi: 10.3945/ajcn.2010.29732. [DOI] [PubMed] [Google Scholar]
- 52.Konttinen H, Mannisto S, Sarlio-Lahteenkorva S, Silventoinen K, Haukkala A. Emotional eating, depressive symptoms and self-reported food consumption. A population-based study. Appetite. 2010;54:473–479. doi: 10.1016/j.appet.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Fitzmaurice G. Missing data: implications for analysis. Nutrition. 2008;24:200–202. doi: 10.1016/j.nut.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 54.National Academy of Science, Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, D.C: The National Academies Press; 2005. [Google Scholar]
- 55.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: full text. Eur J Cardiovasc Prev Rehabil. 2007;14:S1–S113. doi: 10.1097/01.hjr.0000277983.23934.c9. [DOI] [PubMed] [Google Scholar]
- 56.Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lespérance F, et al. Depression and coronary heart disease. Recommendations for screening, referral, and treatment. Prog Cardiovasc Nurs. 2009;24:19–26. doi: 10.1111/j.1751-7117.2009.00028.x. [DOI] [PubMed] [Google Scholar]
- 57.Whooley MA, Simon GE. Managing depression in medical outpatients. N Engl J Med. 2000;343:1942–1950. doi: 10.1056/NEJM200012283432607. [DOI] [PubMed] [Google Scholar]
- 58.Research to Practice Series, No 5. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Nutrition, Physical Activity, and Obesity; Low-energy-dense foods and weight management: Cutting calories while controlling hunger. [Google Scholar]
- 59.Rolls B, Barnett RA. The volumetrics weight-control plan. New York: Harper Torch; 2000. [Google Scholar]
- 60.Tsigos C, Hainer V, Basdevant A, Finer N, Fried M, Mathus-Vliegen E, et al. Management of obesity in adults: European clinical practice guidelines. Obes Facts. 2008;1:106–116. doi: 10.1159/000126822. [DOI] [PMC free article] [PubMed] [Google Scholar]