Abstract
Objectives
Materials that are capable of releasing ions such as calcium and fluoride, that are necessary for remineralization of dentin and enamel, have been the topic of intensive research for many years. The source of calcium has most often been some form of calcium phosphate, and that for fluoride has been one of several metal fluoride or hexafluorophosphate salts. Fluoride-containing bioactive glass (BAG) prepared by the sol-gel method acts as a single source of both calcium and fluoride ions in aqueous solutions. The objective of this investigation was to determine if BAG, when added to a composite formulation, can be used as a single source for calcium and fluoride ion release over an extended time period, and to determine if the BAG-containing composite can be recharged upon exposure to a solution of 5,000 ppm fluoride.
Methods
BAG 61 (61% Si; 31% Ca; 4% P; 3% F; 1% B) and BAG 81 (81% Si; 11% Ca; 4% P; 3% F; 1% B) were synthesized by the sol gel method. The composite used was composed of 50/50 Bis-GMA/TEGDMA, 0.8% EDMAB, 0.4% CQ, and 0.05% BHT, combined with a mixture of BAG (15%) and strontium glass (85%) to a total filler load of 72% by weight. Disks were prepared, allowed to age for 24 h, abraded, then placed into DI water. Calcium and fluoride release was measured by atomic absorption spectroscopy and fluoride ion selective electrode methods, respectively, after 2, 22, and 222 h. The composite samples were then soaked for 5 min in an aqueous 5,000 ppm fluoride solution, after which calcium and fluoride release was again measured at 2, 22, and 222 h time points.
Results
Prior to fluoride recharge, release of fluoride ions was similar for the BAG 61 and BAG 81 composites after 2 h, and also similar after 22 h. At the four subsequent time points, one prior to, and three following fluoride recharge, the BAG 81 composite released significantly more fluoride ions (p<0.05). Both composites were recharged by exposure to 5,000 ppm fluoride, although the BAG 81 composite was recharged more than the BAG 61 composite. The BAG 61 composite released substantially more calcium ions prior to fluoride recharge during each of the 2 and 22 h time periods. Thereafter, the release of calcium at the four subsequent time points was not significantly different (p>0.05) for the two composites.
Significance
These results show that, when added to a composite formulation, fluoride-containing bioactive glass made by the sol-gel route can function as a single source for both calcium and fluoride ions, and that the composite can be readily recharged with fluoride.
Keywords: composite, bioactive glass, ion release
Introduction
Composites make up the largest percentage of materials used in restorative dentistry, largely because they have properties that make them both durable and esthetically pleasing. Recurrent caries is a major mode of failure in composite restorations [1,2] and efforts are constantly being made to formulate materials that will decrease its occurrence. Materials incorporating potential remineralizing and antibacterial agents are examples.
Calcium is crucial for the restoration of damaged tooth tissue since it is a primary component of hydroxyapatite in enamel and dentin. When these tissues are damaged, a readily available source of calcium ions is necessary for remineralization. Calcium hydroxide has, for example, been used for many years in pulp-capping agents because of its function in remineralizing the surrounding tooth tissue. [3,4,5] The resulting increase in pH, however, may cause irritation to surrounding soft tissue, thus limiting its applications. To overcome the negative effects of the hydroxide ion, calcium phosphates have been added to composite formulations as an alternative means to provide calcium ions. [6]
Several clinical studies [7,8,9] have demonstrated the cariostatic effects of fluoridated restorative materials. It has been reported that long-term exposure to fluoride with levels of 0.095 to 0.190 ppm fluoride in the saliva may be sufficient for cariostasis. [10] Fluoride acts to reduce caries by being a biocide [11,12] and by reducing the solubility of enamel and dentin through its incorporation into tooth tissue to form fluoroapatite [13,14,15]. In addition, it has been shown that fluoride acts to remineralize damaged tooth tissue following demineralization [13,14,16]. Furthermore, even though all fluoridated dental materials exhibit varying degrees of depletion of fluoride over time, even small amounts of fluoride provide some degree of cariostatic efficacy [17,18]. For these reasons, a large number of restorative materials and oral care products contain fluoride [16]; these include glass ionomer cements, resin-modified glass ionomer cements, composites, adhesives, varnishes, toothpastes, and mouth rinses. Fluoride is commonly incorporated into these materials by addition of either NaF, CaF2, SnF2, KPF6, YbF3, or fluoro-aluminosilicate glass. Because of their different solubilities, the rate of fluoride release and recharge for each substance is different. [19] In vitro studies of the restorative materials have shown that there is an initial rapid release of fluoride due to surface dissolution, and a much slower diffusion-controlled release over time [16,20,21,22]. These materials, therefore, serve as reservoirs of fluoride and can also be recharged with fluoride from a topical source [20,23]. A problem, however, is that when these salts dissolve to release fluoride, they leave behind a void in the matrix. [19]
Because carious lesions and further decay result from loss of calcium ions, it would be advantageous if a single additive to the composite formulation could act as a source of both fluoride and calcium ions. Bioactive glass (BAG) is such an additive; studies on BAG containing glass ionomer cements showed that BAG is a good source of both fluoride and calcium ions. [24] This characteristic, therefore, sets BAG apart from the metal fluoride salts commonly used in dental materials since they release only fluoride. Bioactive glass made by the sol-gel process produces a material that has surface areas hundreds of times larger than BAG made by the melt-quench method. [25] Because of its much greater surface area, sol-gel-derived BAG has the potential to more readily release these ions in composites, and be rechargeable when exposed to topical sources of calcium and fluoride. Furthermore, studies of composites formulated with BAG have shown that the mechanical properties are not significantly compromised by addition of 12 to 15 wt% BAG, [26,27] and the total material loss for a nonsilanated bioglass-contaning composite was not quite 1% after 60 days in DI water. [27]
In this study we test the hypothesis that the addition of fluoride-containing bioactive glass derived by the sol-gel method, will yield a material that releases both calcium and fluoride ions, and can be recharged with fluoride.
Materials and Methods
Synthesis of fluoride-containing bioactive glass
Methoxyethanol (MeOEtOH) (Me = CH3; Et = CH2CH3), tetraethyl orthosilicate (Si(OEt)4), triethyl phosphate (OP(OEt)3), and calcium metal were purchased from Acrõs Organics; boron trifluoride etherate (BF3OEt2) was purchased from Sigma-Aldrich. These reagents were used without further purification. Calcium methoxyethoxide (Ca(OEtOMe)2 was synthesized immediately prior to use. [28] In a typical reaction, stoichiometric quantities of Ca(OEtOMe)2, OP(OEt)3, and Si(OEt)4 were combined under N2 to yield a homogeneous solution. A solution (approx. 10%) of BF3OEt2 in MeOEtOH was then added dropwise over 20 min to the vigorously stirred solution. The resulting solution was stirred under N2 for 1 h, after which it was placed in an incubator at 37 °C and 100% humidity. A monolith formed after about 7 d; this was followed by addition of DI water to insure complete hydrolysis. After another 3 d, the H2O and excess MeOEtOH were decanted and the gel was rinsed with 100% EtOH. The gel was then heated to 600 °C in a muffle furnace (Thermo Scientific, series 650–750) whereupon small pieces of colorless transparent glass formed. This glass was ball-milled in 100% EtOH to yield a slurry which was then dried at 37 °C, sieved, and micronized (Sturtevant, Model OM2, Hanover, MA, USA) Average particle size ranged from 0.04 to 3.0 μm, as determined by laser particle size measurements (Beckman Coulter LS13 320, Brea, CA) In this way, the bioactive glasses BAG 81 (81% Si; 11% Ca; 4% P; 3% F 1% B) and BAG 61 (61% Si; 31% Ca; 4% P; 3% F; 1% B) (mole percents) were obtained. Surface areas of 316 and 129 m2·g−1, respectively, were determined by the BET method, [29] with the use of a Quantasorb Sorption System [30] (Quantachrome Corp., model QS 9, Greenvale, N.Y.). The amorphous character of the glass was established by X-ray diffraction, [31] and its bioactivity was established by ion release of Ca and F with the use of atomic absorption spectroscopy (Shimadzu, Japan) and fluoride ion specific electrode (Orion 9409BN), respectively, and by formation of hydroxyapatite upon immersion in simulated body fluid (infrared analysis: Thermo Scientific, Nicolet 6700, USA).
Preparation of composites
Resins were produced with 50:50 BisGMA:TEGDMA (Esstech), 0.4% CQ (Sigma-Aldrich), 0.8% EDMAB (Acrõs Organics), and 0.05% BHT (Acrõs Organics). Fillers composed of 57% by weight irregular 1–3 μm (average size) silane-treated-strontium glass (Bisco) mixed with 15 wt% fluoride-containing bioactive glass were added to the resin to obtain a total loading of 72 wt% filler. Control composites of identical composition contained aerosol silica filler (OX-50, Degussa) instead of BAG. The composite samples were packed into molds (4 mm diameter × 2 mm thick), light-cured (Demi, Kerr: 573 mW/cm2) for 40 s, lightly abraded (4,000 grit paper) on the top and bottom surfaces, and stored dry for 24 h prior to use.
Ion release from composites
Two disks in each of five vials, n=5, were immersed in 2 mL DI water. These five samples were used for all subsequent fluoride and calcium measurements. Ion releases were measured at 2, 22, and 222 h time points prior to fluoride recharge, and at 2, 22, and 222 h time points following fluoride recharge. The disks were removed after each time period, rinsed with DI water, and re-immersed in fresh DI water.
Fluoride release
Supernatants (1 mL) after each time period were combined with 1 mL low-level TISAB (Orion) and fluoride concentrations measured with a fluoride ion specific electrode (Orion 9409BN). The disks were then placed in a 5,000 ppm fluoride (NaF, Baker) solution for 5 min, rinsed and re-immersed in DI water. Fluoride was re-measured after 2, 22, and 222 h. All reported concentrations of the fluoride released were obtained by subtracting any contribution due to the control from the experimentally-measured values. Average rates of release were calculated by dividing the fluoride measured at each time point by the time interval (2, 20, and 200 h) over which it was released.
Calcium release
After each time period as described above, 0.50 mL of the supernatant was combined with 4.5 mL of a freshly-prepared 5% HNO3 (Fisher Scientific) solution, and 0.50 mL of a solution made by reacting 5.803 g La2O3 (Sigma-Aldrich) with 50.00 mL conc. HCl (Fisher Scientific) and diluting to 200 mL. The calcium ion concentration was determined by atomic absorption spectroscopy using standard methods [32].
Statistical analysis
The results for the average fluoride and calcium ion release per hour were analyzed by two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc comparison test (p<0.05).
Results
Fluoride release and recharge
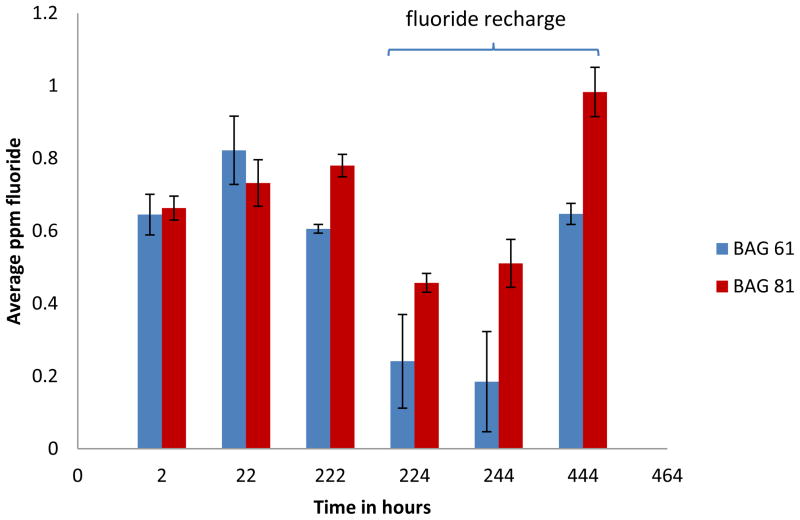
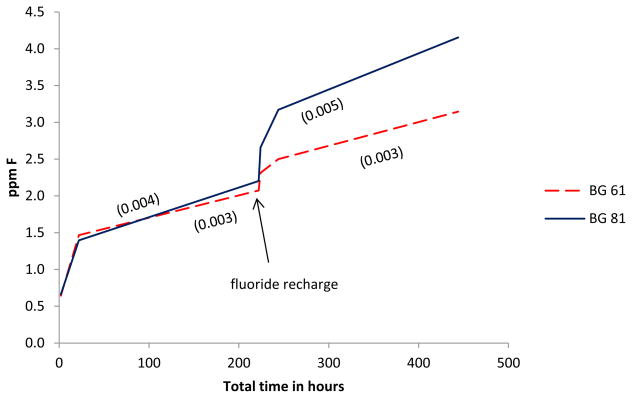
Both BAG 61 and BAG 81 composites continued to release fluoride ions over the entire time period of the experiment (Figure 1). The data in Figure 1 also shows that upon exposure to a 5,000 ppm fluoride solution, both composites were recharged and continued to release fluoride beyond what would have been expected in the absence of exposure to the 5,000 ppm fluoride solution. At all time points beyond 22 hours, the average release of fluoride from the BAG 81 composite was significantly greater (p<0.05) than that from the BAG 61 composite.
Figure 1.
Average concentrations of fluoride ion released for BAG 61 and BAG 81 composites both prior to and after F recharge (n=5); time is given as the total elapsed time, and the error bars represent standard deviation. The concentrations shown represent the difference between the measured values and the values obtained for the control samples. The values for the control samples were 0.147(0.039), 0.240(0.073), 0.226(0.063), 0.619(0.089), 0.089(0.006), and 0.101(0.000) for the 2, 22, 222, 224, 244, and 444 h time points, respectively.
The residual readings for the controls prior to fluoride recharge were 0.147(0.037), 0.243(0.073), and 0.226(0.063) ppm (standard deviation) for the 2, 22, and 222 h time points, respectively. That there was any level of fluoride detected was likely attributable to a systematic operating error at these low-level concentrations that resulted in unavoidable instrumental drift. The residual readings for the controls following fluoride recharge were 0.619(0.089). 0.089(0.006), and 0.101(0.000) ppm for the 2, 22, and 222 h time points, respectively.
Fluoride release prior to recharge
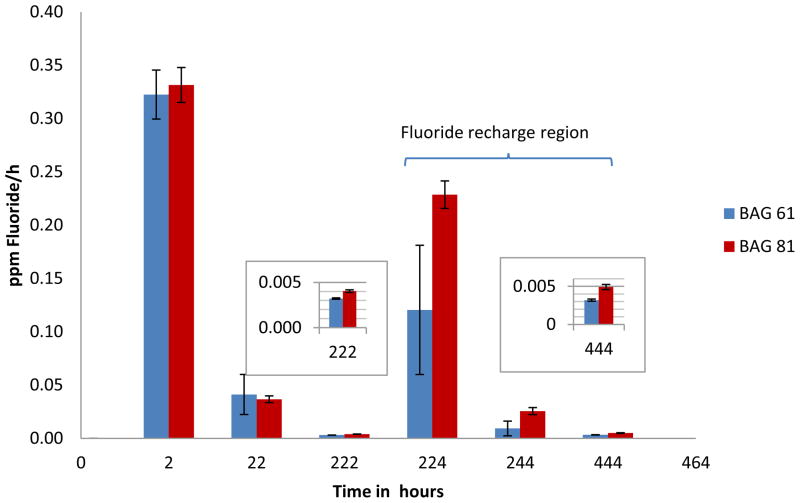
After an initial burst of fluoride released during the first 2 hours, there was a decline in the rate of fluoride released during the next 20 hours, and a further decline in the rate over the following 200 hours (Figure 2). The fluoride releases for the BAG 61 and BAG 81 composites were equivalent to each other at both the 2 and 20 hour time periods. It was only after a further 200 hours that there was a significant difference (p<0.05) in the fluoride ion released for BAG 81 [0.00390(0.00016) ppm/h] (standard deviation) compared with that for BAG 61 [0.00303(0.00001) ppm/h].
Figure 2.
Average rate (per hour) of fluoride ion release for BAG 61 and BAG 81 composites prior to and after recharging with 5,000 ppm fluoride ions (n=5); time is given as the total elapsed time, and the error bars represent standard deviation. Inserts are for the 22 and 444 h time points.
Fluoride release after recharge
After exposure of the composites to 5,000 ppm fluoride for 5 min, both BAG 61 and BAG 81 composites released an initial burst of fluoride in the 2 hours immediately following recharge, and as before recharge, the rate of fluoride released declined over subsequent time periods (Figure 2). Unlike the rates at the 2 hour time point prior to recharge, over the first two hours following fluoride ion recharge, the BAG 81 composite released fluoride ion at a significantly (p<0.05) greater rate [0.229(0.013) ppm/h] than did the BAG 61 composite [0.129(0.060) ppm/h]. This trend continued over the two following time periods. During the next 20 hours, the BAG 81 composite released fluoride ion at a rate of 0.0255(0.0033) ppm/h compared to 0.00927(0.00691) ppm/h for the BAG 61 composite, and rates of 0.00491(0.00034) ppm/h and 0.00324(0.00014) ppm/h over the following 200 hours, respectively.
Calcium release
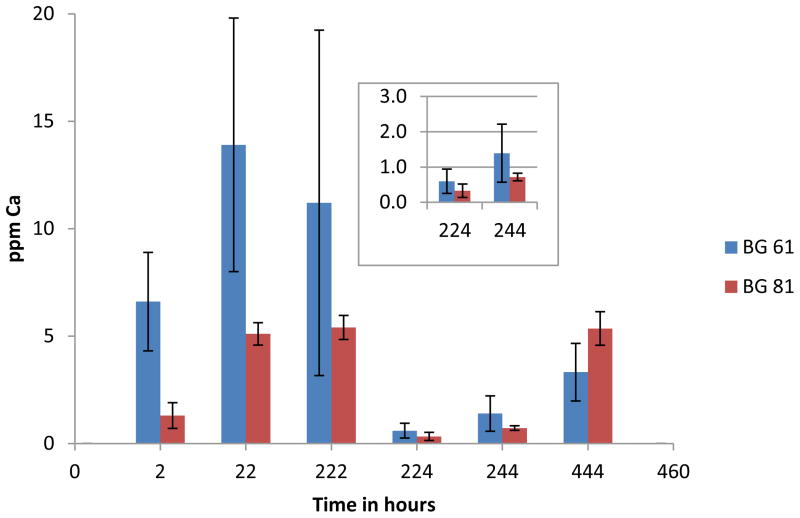
Both BAG 61 and BAG 81 composites continued to release calcium ions over the time period of the experiment (Figure 3). There was no detectable calcium released from the control composite at any time point.
Figure 3.
Average concentrations of calcium ions released for BAG 61 and BAG 81 composites at each time point, both prior to and after fluoride recharge (n=5); time is given as the total elapsed time, and the error bars represent standard deviation. Insert is for the 224 and 244 h time points.
Calcium release prior to fluoride recharge
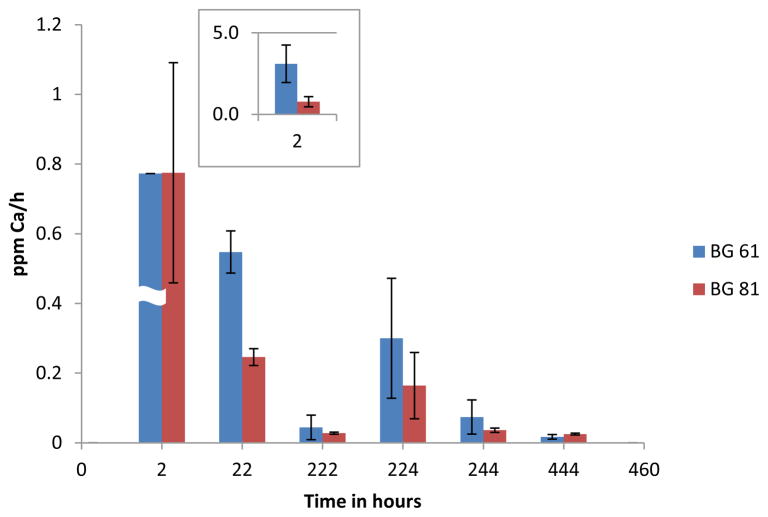
Over all three time periods prior to fluoride recharge, the release of calcium from the BAG 61 composite [6.64(2.30), 13.9(5.9) and 12.7(8.4) ppm] (standard deviation) was greater than that from the BAG 81 composite [1.58(0.77), 5.06(0.59), and 5.44(0.72) ppm] (Figure 3). After an initial burst of calcium ions over the 2 hour time period, the average rate per hour of calcium release for both composites declined over the following 20 and 200 hour time periods (Figure 4). During the initial 2 hour time period, the BAG 61 composite released calcium ions at an average rate of 3.32(1.15) ppm/h which was significantly greater (p<0.05) than that for the BAG 81 composite [0.78(0.32) ppm/h]. The rates of calcium ion release over the next 20 and 200 hours for the BAG 61 composite [0.548(0.060) and 0.056(0.040) ppm/h] compared to that of the BAG 81 composite [0.246(0.024) and 0.028(0.003) ppm/h] were not significantly different (p>0.05). For the BAG 61 composite, the rate of release of calcium at the 2 hour time point was significantly greater than that at subsequent time points, but there was no significant difference in the rate between the 20 and 200 hour time periods. For the BAG 81 composite, the rates of release of calcium ions at all three time points are not significantly different.
Figure 4.
Average rate (per hour) of calcium ions released for BAG 81 and BAG 61 composites prior to and after recharging with 5,000 ppm fluoride ions (n=5); time is given as the total elapsed time, and the error bars represent standard deviation. The insert is for the 2 h time point: the average rate of Ca released from the BAG 61 composite was 3.3 ppm/h.
Calcium release after fluoride ion recharge
The average rates of release of calcium for the BAG 61 composite were [0.299(0.172), 0.074(0.049) and 0.017(0.007) ppm/h; those for the BAG 81 composite were 0.164(0.095), 0.036(0.006), and 0.025(0.003) ppm/h (Figure 4). Both composites continued to release calcium over the 222 hour time period. These rates were, however, not significantly different (p>0.05) either between the two composite groups, or within the groups.
Discussion
Both calcium and fluoride ions are known to be beneficial for remineralization and strengthening of tooth tissue, and fluoride also functions as a biocide against S mutans in the oral environment. The usefulness of a filling material that is capable of supplying beneficial ions such as calcium and fluoride, may be in terms of its ability to release ions to adjacent tooth tissue, not in increasing the concentration of these ions in the saliva. That is, in order to provide the most benefit, the ions need to be located at the surface of the tooth which is vulnerable to decay. Studies have shown that fluoride concentrations in plaque adjacent to fluoride-releasing restorations was in the range of 7–21 μgF−/g plaque [33,34] compared to substantially lower levels of 1–5 μgF−/g plaque following use of fluoride-containing mouthwashes [35]. Thus, a material that is capable of releasing both fluoride and calcium ions would be expected to enhance the formation of caries-resistant fluoroapatite on the surface of the tooth which has been shown to make the major contribution to caries prevention. [36] Given the magnitude of the use of composites as dental restoratives, composites that contain available sources of these ions may have substantial advantages compared to those that do not release these ions. As is evident from Figures 1 and 3, both of the BAG composites in this study were found to release calcium and fluoride ions prior to and following fluoride recharge.
Fluoride release occurred over the entire 444 hour period for the BAG 61 and 81 composites, and the BAG composites were rechargeable. A graph showing the cumulative release of fluoride over the 444 h time period is shown in Figure 5. The cumulative release of 1.40–1.47 ppm fluoride in 22 hours and the measured release of 0.61–0.78 ppm in 222 hours for BAG 61 and 81 composites can be compared to releases of 2.3–3.3 ppm (24 h) and 0.2–1.05 ppm (226 h) for Wave and Dyract Extra, and 0.04–2.7 ppm (24 h) and 0–1.1 ppm (30–60 d) for other commercially available composites [37]. Since the mole-percent fluoride compositions of the commercial products are not known, it is difficult to make a direct comparison to these materials. The results are, however, within the same range.
Figure 5.
Cumulative release of fluoride from the BAG 61 and 81 composites over the 2–444 hour time period for the BAG 61 and 81 composites (n=5).
As can be seen in Figure 2, after an initial burst of fluoride from the BAG 61 and BAG 81 composites, the rate of fluoride release decreased over subsequent time periods. Similar behavior has been observed in all prior studies of fluoride release from dental materials [38]; it has been suggested that surface elution results in the initial rapid release of fluoride and that this is followed by a slower diffusion process [22]. This decline in the rate of release for either process is likely due to a change with time in the concentration gradient. When the disks were first placed in DI water, the concentration gradient was large and fluoride diffused most rapidly from the disks. In addition, fluoride ions at the surface of the fresh composite were directly in contact with the water, so its release was not slowed due to diffusion from the bulk material. Thus, the rate of fluoride release was most rapid within the first two hours of being placed in the DI water. Over the subsequent longer time periods, the rate of fluoride release decreased; this was due to development of a smaller concentration gradient as more fluoride was released into the solution, and due to a slower rate-limiting diffusion process necessary for the fluoride ions to migrate from within the bulk material to the surface of the disk prior to release.
The results plotted in Figure 2 show that the BAG 81 and BAG 61 composites released fluoride ions at essentially the same rate over the 2 and 22 hour time periods. Based upon the much larger surface area of BAG 81 (316 m2·g−1 compared to 129 m2·g−1 for BAG 61), fluoride ion release from BAG 81 might have been expected to be much faster. Additionally, because the fluoride ions in the glass are essentially entirely associated with the calcium ions [39,40], the fluoride ions would be expected to be more mobile in BAG 81 (11% Ca) compared to BAG 61 (31 % Ca); this would also make the release of fluoride ions from the BAG 81 composite more facile. However, since the amount of fluoride ion in the two BAG materials was the same (3 mole %), the concentration gradient was identical for both BAG materials and it is apparent that the identical concentration gradient dominated fluoride ion release at these earlier time points. At 222 h, after which a substantial number of the fluoride ions would have probably diffused out of the glass, the rate of fluoride ion release was greater for BAG 81 than for BAG 61. Thus, at longer times, a larger surface area coupled with the increased mobility of the fluoride ions in the BAG 81 dominated the fluoride ion release.
In the time period immediately after immersion in a 5,000 ppm fluoride solution, both the BAG 61 and 81 composites showed a burst of fluoride release. A burst of fluoride was also seen for the control composite which contained OX-50 in place of BAG, even though the control, like the sample specimens, was rinsed with DI water after immersion in the 5,000 ppm fluoride solution. Since the control lacked any components that are capable of forming a chemical interaction with fluoride ions, this shows that fluoride can be adsorbed directly onto the surface and into the polymer network of the composite. It is apparent that this involved a simple adsorption/absorption process since the release of fluoride from the control returned to the pre-fluoride recharge levels in the two subsequent time periods. Unlike the control, the BAG 61 and 81 composites continued to release the same or even more fluoride at subsequent time periods.
Figure 5 shows that the BAG 61 and 81 composites were recharged beyond the simple surface uptake noted above. There was an increase in the fluoride released over the 22 to 222 hour time period following recharge for both composites. For the BAG 61 composite, the slope over the 22 to 222 hour time periods prior to and following fluoride recharge remains essentially unchanged. For the BAG 81 composite, however, the slope over the 22 to 222 hour time period following fluoride recharge is almost 20% greater than that for the same time period prior to recharge. Without any recharge, the slopes would have been expected to decrease based upon previous studies which showed that the rate of fluoride release in composites is proportional to (time)−1/2 [41]. The substantial increase in the slope for the BAG 81 composite indicates recharging occurred to a greater extent compared to the BAG 61 composite. This can be attributed to the greater surface area of BAG 81 which allows this material to recharge more rapidly than the BAG 61, resulting in a greater recharged-fluoride concentration in the BAG 81 composite, and consequently, more rapid release of fluoride following recharge. This is consistent with studies which showed that the magnitude of recharge is governed by the number of sites available for the incoming fluoride ions [37b]. These results, taken with the above-discussed release of fluoride ions prior to recharge, indicate that both BAG 61 and BAG 81 act as reservoirs for fluoride ions when added to composites, and the resultant composites can be readily recharged upon exposure to topical applications of fluoride. The results also indicate that the composite with BAG 81 offers superior performance with respect to fluoride release and recharge.
As shown in Figure 4, both composites continued to release calcium over the entire 444 hour period. When the disks were first placed in DI water, the concentration gradient was large and calcium diffused most rapidly from the disks within the first two hours. Over the next 20 hours, the rate of calcium release decreased due to the concentration gradient which became smaller as more calcium was released into the solution, and due to diffusion effects as discussed above for fluoride release. This was repeated over the next 200 h time period.
The rate of release of calcium from the BAG 61 composite at 2 hours was substantially greater than that of the BAG 81 composite. Since BAG 61 contains 31 mole-percent calcium compared to 11 mole percent calcium for BAG 81, it is apparent that the concentration gradient provided the dominant driving force for calcium release. The much greater surface area of BAG 81 compared to BAG 61, 316 and 129 m2·g−1, respectively, which would favor the more rapid release of calcium from the BAG 81 composite, played a lesser role in the kinetics of calcium release. In addition, since the fluoride ion (3 mole-percent in both BAG 61 and 81) is essentially entirely associated with calcium in the glass [39,40], there is more “free” calcium in BAG 61; this would also make the diffusion of the calcium ions out of BAG 61 more facile than that in BAG 81. At the 22 and 222 hour time points, with depletion of calcium from the BAG 61 composite, there was apparently no significant difference in the concentration gradient for either BAG material. Consequently, the rates of calcium release at these times were not significantly different. Following fluoride recharge, both BAG composites continued to release calcium ions. The average rates of calcium release were, however, not significantly different for the 2 hour time period following recharge, or at any subsequent time period. These results indicate that either BAG 61 or BAG 81 will be a good reservoir for calcium when added to a composite, but that BAG 61 will have superior performance with respect to calcium release, at least at earlier time periods.
Conclusion
The addition of fluoride-containing bioactive glass, derived by the sol-gel route, to a composite formulation has been shown to act as a single source of both calcium and fluoride ions, providing a reservoir of ions that are readily available to diffuse from the material. Both BAG 61 and BAG 81 composites tested showed sustained release of calcium and fluoride ions over a 444 hour time period, and both materials were recharged by exposure to 5,000 ppm fluoride. The release of calcium ions is controlled far more by concentration gradients than by the different surface areas of BAG 61 and BAG 81, whereas surface area and ion mobility seem to play a larger role in the release of fluoride ions by BAG 81.
Acknowledgments
This study was supported in part by an Oregon Translational Research Institute grant OCTRI0201N12, and NIH/NIDCR grant DE021372.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilder AD, Jr, May KN, Jr, Bayne SC, Taylor DR, Leinfelder KF. Seventeen-year clinical study of ultraviolet-cured posterior class I and II restorations. J Esthetic Dentistry. 1999;11(3):135–142. doi: 10.1111/j.1708-8240.1999.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 2.Collins C, Bryant R, Hodge K. A clinical evaluation of posterior composite restorations: 8-year findings. J Dent. 1998;26(4):311–317. doi: 10.1016/s0300-5712(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 3.Duarte MA, Martins CS, de Oliveira Cardoso Demarchi AC, de Godoy LF, Kuga MC, Yamashita JC. Calcium and hydroxide release from different pulp capping materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:66–9. doi: 10.1016/j.tripleo.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Estrela C, Sydney GB, Bammann BL, Felippe Junior O. Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Brazil Dent J. 1995;6:85–90. [PubMed] [Google Scholar]
- 5.Takita T, Hayashi M, Takeichi O, Ogiso B, Suzuki N, Otsuka K, Ito K. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J. 2006;39:415–22. doi: 10.1111/j.1365-2591.2006.01097.x. [DOI] [PubMed] [Google Scholar]
- 6.a Melo MAS, Weir MD, Rodrigues LKA, Xu HKK. Novel calcium phosphate nonocomposite with caries-inhibition in a human in situ model. Dent Mat. 2013;29:231–40. doi: 10.1016/j.dental.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dickens SH, Flaim GM, Tagagi S. Mechanical properties and biochemical activity or remineralizing resin-based Ca-PO4 based cements. Dent Mat. 2003;19:558–66. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]; c Langhorst SE, O’Donnell JNR, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dent Mat. 2009;25:884–91. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Xu HKK, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mat. 2011;27:762–9. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate in remineralizing resin composites. Dent Mat. 1996;12:295–301. doi: 10.1016/s0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 7.Duggal MS, Chawla HS, Curzon ME. A study of the relationship between trace elements in saliva and dental caries in children. Arch Oral Biol. 1991;36:881–4. doi: 10.1016/0003-9969(91)90118-e. [DOI] [PubMed] [Google Scholar]
- 8.Bruun C, Thylstrup A. Fluoride in whole saliva and dental caries experience in areas with high or low concentrations of fluoride in the drinking water. Caries Res. 1984;18:450–6. doi: 10.1159/000260802. [DOI] [PubMed] [Google Scholar]
- 9.Sjogren K, Birkhed D. Factors related to fluoride retention after toothbrushing and possible connection to caries activity. Caries Res. 1993;27:474–7. doi: 10.1159/000261583. [DOI] [PubMed] [Google Scholar]
- 10.Featherstone JDB, Zero DT. Laboratory and human studies to elucidate the mechanics of action of fluoride-containing dentifrices. In: Embery G, Rølla G, editors. Clinical and biological aspects of dentrifices. New York: Oxford University Press; 1992. pp. 41–50. [Google Scholar]
- 11.Hamilton JR. Biochemical effects of fluoride on oral bacteria. J Dent Res. 1990;69:660–667. doi: 10.1177/00220345900690S128. [DOI] [PubMed] [Google Scholar]
- 12.Seppä L, Korhonen A, Nuutinen A. Inhibitory effect on S. mutans by fluoride treated conventional and resin modified glass ionomer cements. Eur J Oral Sci. 1995;103:182–185. doi: 10.1111/j.1600-0722.1995.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 13.Ten Cate JM. In vitro studies on the effects of fluoride on de and remineralization. J Dent Res. 1990;69:614–619. doi: 10.1177/00220345900690S120. [DOI] [PubMed] [Google Scholar]
- 14.Featherstone JDB, Glena R, Shariati M, Sheilds CP. Dependence of in vitro demineralization of apatite and remineralization of dental enamel on fluoride concentration. J Dent Res. 1990;69(Spec Iss):620–625. doi: 10.1177/00220345900690S121. [DOI] [PubMed] [Google Scholar]
- 15.Brauer DS, Karpukhina N, Law RV, Hill RG. Structure of fluoride-containing bioactive glasses. J Mat Chem. 2009;19(31):5629–36. [Google Scholar]
- 16.Weigand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials-Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mat. 2007;12:343–362. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Forsten L. Short and long-term fluoride release from glass ionomers and other fluoride-containing filling materials in vitro. Scand J Dent Res. 1993;98:179–85. doi: 10.1111/j.1600-0722.1990.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 18.Hsu C-YS, Donly KJ, Drake DR, Wefel JS. Effects of aged fluoride-containing restoriative materials on recurrent root caries. J Dent Res. 1998;77(2):418–25. doi: 10.1177/00220345980770021101. [DOI] [PubMed] [Google Scholar]
- 19.Arends J, Dijkman GE, Dijkman AE. Review of fluoride release and secondary caries reduction by fluoridating composites. Adv Dent Res. 1995;9:367–76. [Google Scholar]
- 20.Dionysopoulus D, Kolinotou-Koumpia E, Helvatzoglou-Antoniades M, Kotansos N. Fluoride release and Recharge abilities of contemporary fluoride-containing materials and dental adhesives. Dent Mater. 2012;32(2):296–304. doi: 10.4012/dmj.2012-144. [DOI] [PubMed] [Google Scholar]
- 21.Naoum S, Eilakwa A, Martin F, Swain M. Fluoride release, recharge, and mechanical property stability of various fluoride-containing resin composites. Oper Dent. 2011;36(4):422–32. doi: 10.2341/10-414-L. [DOI] [PubMed] [Google Scholar]
- 22.Chan CD, Lifang Y, Wankel W, Rizkalla AS. Fluoride release from dental cements and composites: A mechanistic study. Dent Mater. 2006;22:366–71. doi: 10.1016/j.dental.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 23.Lim B-S, Lee S-J, Lim Y-J, Ahn S-J. Effects of periodic fluoride treatment on fluoride ion release from fresh orthodontic adhesives. J Dent. 2011;39:788–94. doi: 10.1016/j.jdent.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Yli-Urpo H, Vallittu PK, Narhi TO, Forskack A-P, Vikiparta M. Release of silica, calcium, phosphorus, and fluoride from glass ionomer cement containing bioactive glass. J Biomat Appl. 2004;19:5–20. doi: 10.1177/0085328204044538. [DOI] [PubMed] [Google Scholar]
- 25.Sepulveda P, Jones JR, Hench LL. Characterization of melt-derived 45S5 and sol-gel-derived S85 bioactive glasses. J Biomed Mat Res. 2001;58:734–740. doi: 10.1002/jbm.10026. [DOI] [PubMed] [Google Scholar]
- 26.Khvostenko D, Mitchell JC, Hilton TJ, Ferracane JL, Kurzic JJ. Mechanical performance of novel bioactive glass containing dental composites. Dent Mater. 2013;39:1139. doi: 10.1016/j.dental.2013.08.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oval O, Lassila LV, Kumbuloglu O, Vallitu PK. Bioactive glass filler composite: effect of coupling of fillers and filler loading on some physical properties. doi: 10.1016/j.dental.2014.02.017. http://dx.doi.org/j.dental2014.02.017. [DOI] [PubMed]
- 28.Yu B, Poologasundarampillai G, Turdean-Ionescu C, Smith ME, Jones JR. A new calcium source for bioactive sol-gel hybrids. Bioceram Devel App. 2011;1:1–3. [Google Scholar]
- 29.Brunauer S, Emmett P, Teller E. J Amer Chem Soc. 1938;60:309. [Google Scholar]
- 30.On loan from the Department of Chemistry, Portland State University, Portland, OR.
- 31.Tung M. Paffenbarger Research Center; Gaithersburg, MD: [Google Scholar]
- 32.Standard Methods for the Examination of Water and Wastewater. 22. American Water Works Assoication/American Public Works Association/Water Environment Federation; 2012. [Google Scholar]
- 33.Forssh H, Nase L, Seppä L. Fluoride concentration, mutans streptococci and lactobacilli in plaque from old glass-ionomer fillings. Caries Res. 1995;29:50–3. doi: 10.1159/000262040. [DOI] [PubMed] [Google Scholar]
- 34.Benelli EM, Serra MC, Rodrigues AL, Jr, Cury JA. In situ anticariogenic potential of glass ionomer cement. Caries Res. 1993;27:280–4. doi: 10.1159/000261551. [DOI] [PubMed] [Google Scholar]
- 35.Duckworth RM, Morgan SN, Murray AM. Fluoride in saliva and plaque following use of fluoride-containing mouthwashes. J Dent Res. 1987;66:1730–4. doi: 10.1177/00220345870660120701. [DOI] [PubMed] [Google Scholar]
- 36.Caldeira1 Érika Machado, Osório Amanda, Oberosler Edna Lúcia Couto, Vaitsman Delmo Santiago, Alviano Daniela Sales, da Cunha Gonçalves Nojima Matilde. Antimicrobial and fluoride release capacity of orthodontic bonding materials. J Appl Oral Sci. 2013;21(4):327–34. doi: 10.1590/1679-775720130010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.a Attar N, Turgut MD. Fluoride uptake and capacities of fluoride releasing restorative materials. Oper Dent. 2003;28:395–402. [PubMed] [Google Scholar]; b Attar N, őnen A. Fluoride release and uptake characteristics of aesthetic restorative materials. J Oral Rehabil. 2002;29:791–8. doi: 10.1046/j.1365-2842.2002.00902.x. [DOI] [PubMed] [Google Scholar]
- 38.Chan WD, Yang L, Wankel W, Rizkalla AS. Fluoride release from dental cements and composites: A mechanistic study. Dent Mat. 2006;22:366–73. doi: 10.1016/j.dental.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 39.Christie JK, Pedone A, Menziani MC, Tilocca A. Fluorine environment in bioactive glasses: ab initio molecular dynamics simulations. J Phys Chem. 2011;115:2038–45. doi: 10.1021/jp110788h. [DOI] [PubMed] [Google Scholar]
- 40.Lusvardi G, Malavasi G, Cortada M, Menabue L, Menziani MC, Pedone A, Segre U. Elucidation of the structural role of fluorine in potentially bioactive glasses by experimental and computational investigation. J Phys Chem B. 2008;112:12730–39. doi: 10.1021/jp803031z. [DOI] [PubMed] [Google Scholar]
- 41.a Verbeek RM, Dijkman GE, de Vries J, Lodding A, Arends J. Long term fluoride release of visible light-activated composites in vitro: a correlation with in situ demineralization data. Caries Res. 1993;27:117–23. doi: 10.1159/000261528. [DOI] [PubMed] [Google Scholar]; b Cooley RS, Sandoval VA, Barnwell SE. Fluoride release and color stability of a fluoride-containing composite resin. Quintessence Int. 1988;19:899–904. [PubMed] [Google Scholar]