Abstract
During the process of blood feeding insect vectors are exposed to an array of vertebrate-derived blood factors ranging from byproducts of blood meal digestion to naturally occurring products in the blood including growth hormones, cytokines and factors derived from blood-borne pathogens themselves. In this review, we examine the ability of these ingested vertebrate blood factors to alter the innate pathogen defenses of insect vectors. The ability of these factors to modify the immune responses of insect vectors offers new intriguing targets for blocking or reducing transmission of human disease-causing pathogens.
Keywords: mosquito, sand fly, tsetse fly, Reduviidae, Plasmodium, Leishmania, insulin, insulin-like growth factor 1 (IGF1), transforming growth factor-beta (TGF-β), complement, chitinase
Introduction
Insect vectors of human disease-causing pathogens are exposed to a unique range of vertebrate blood factors that can persist through the process of blood digestion and directly impact their immune system. This review provides a summary of the various effects of vertebrate-derived blood factors on insect immune responses. Blood feeding behavior has evolved independently several times during insect evolution and, as a result, the feeding stage and rate and frequency of feeding vary greatly among hematophagous insect vector species. This review will focus on the best-studied of these insects: mosquitoes, sand flies, and kissing bugs. Only female adult mosquito and sand fly species feed on blood while non-holometabolous kissing bugs require blood at every life stage. For most hematophagous insects a blood meal is necessary for the successful completion of a reproductive or gonotrophic cycle; however, there are species that are capable of autogenous reproduction.
Hemoglobin
A hematophagous insect can ingest up to 10 times its body weight in vertebrate blood, which is primarily composed of hemoglobin (Hb) [1••]. The degradation of Hb during the digestive process releases heme and can yield antimicrobial peptides that are bioactive in both humans and insects [2]. These Hb-derived peptides are an important part of both vertebrate and insect innate immune responses and adversely affect the growth of parasites, fungi, and bacteria. The presence of these antimicrobial peptides in the midguts of hematophagous insects can inhibit the growth of invading organisms. For example, Hb peptides with activity against Trypanosoma cruzi (the causative agent of Chagas disease) have been isolated from the midguts of the kissing bugs Triatoma infestans [3] and Rhodnius prolixus [4]. The fact that these antimicrobial Hb peptides exists in both humans and insects [5], implies that this physiology is both ancient and highly conserved. The release of heme during Hb digestion can also catalyze the synthesis of reactive oxygen species (ROS), which can directly lyse blood stages of Trypanosoma and Plasmodium (the causative agent of malaria) parasites [6, 7]. In mosquitoes, blood digestion generates elevated levels of ROS that are further enhanced in the presence of malaria parasites [8•]. In response to these damaging levels of ROS, hematophagous insects have evolved an array of heme-inactivating mechanisms [1••]. However, these responses are not immediately saturating and ROS are likely to be present throughout the process of blood digestion.
In addition, low concentrations of ROS can regulate the innate immune responses of a variety of organisms. For example, in mosquitoes the control of dengue virus in Wolbachia-infected Aedes aegypti is mediated by ROS-dependent activation of the Toll pathway [9]. In contrast, ROS induced by the insulin/insulin-like growth factor signaling (IIS) pathway in Anopheles stephensi favors malaria parasite development [10]. Given the conserved nature of ROS physiology, other insect vectors are likely have these signaling responses as well.
Pathogen-derived factors
Pathogen-derived factors present in the vertebrate blood meal also have the potential to alter mammalian and insect biology. Examples of such pathogen-derived factors are the glycosylphosphatidylinositols (GPIs) and GPI-anchored proteins. Plasmodium, Leishmania (the causative agent of leishmaniasis), and Trypansoma GPIs anchor proteins to parasite cell surfaces and are also secreted [11••]. The GPIs of all three parasite genera can modulate the production of pro-inflammatory cytokines in infected mammals [11••]. In addition, parasite-derived GPIs can modulate the innate immune responses of insect vectors. For example, Plasmodium falciparum GPIs can induce anti-microbial peptide secretion [12•] and NOS expression [13] in Anopheles mosquitoes. The GPI-anchored cell surface lipophosphoglycans (LPGs) of Leishmania [14•] and Trypansoma [15] parasites are critical for their survival and infectivity in their respective insect vectors.
Complement
An important component of the vertebrate innate immune response is the complement cascade which recognizes and induces the targeted lysis of invading organisms. Elements of both the classical and alternative complement cascades of humans can persist and alter pathogen development in insect hosts [16–18]. In mosquitoes, human complement can reduce malaria parasite development by either binding directly to zygotes and inhibiting their development into ookinetes [17] or by killing the parasites through complement-mediated lysis [18]. To evade complement-mediated killing in the mammalian host, malaria gametocytes have evolved the ability to bind complement regulator factor H. Factor H is a regulatory protein found in circulation that normally protects vertebrate host cells from complement activation and is therefore likely to present in a blood meal as well [19•].
Chitinase
Most blood feeding insects synthesize a peritrophic matrix (PM) composed of proteins and chitin around an ingested blood meal to protect their gut [20]. To establish an infection, and avoid digestion and expulsion by the insect midgut, pathogens must traverse the physical barrier of the PM. Chitinases are highly conserved enzymes that facilitate the breakdown of the PM in insects. The human ortholog chitotriosidase (CHIT) can similarly catalyze the hydrolysis of chitin [21]. During P. falciparum infection, plasma CHIT activity is elevated in humans [22] and mosquitoes fed blood supplemented with human CHIT exhibited a reduction in PM thickness [23•]. Leishmaniasis can also increase CHIT levels in human blood [24], which could similarly alter the PM of sand flies upon ingestion to impact the transmission of Leishmania parasites.
Insulin and insulin-like growth factor-1
The IIS pathway is highly conserved and regulates a variety of physiological functions in insects including immunity [25••]. IIS protein orthologs can be found in a broad range of insect species including the true bug R. prolixus, tsetse flies, sand flies, mosquitoes, and the human body louse Pediculus humanus humanus [26–32]. In addition to conservation of IIS architecture, mammalian insulin and invertebrate insulin-like peptides (ILPs) share a conserved structure that facilitates the binding of mammalian insulin to insect ILP receptors [33]. Indeed, exogenous insulin from vertebrate blood activates IIS in mosquitoes [26, 27] and tsetse flies [34]. In anopheline mosquitoes, physiological levels of insulin (170 pM) can significantly increase P. falciparum oocyst development [26–28], and control of malaria parasite infection requires at least three IIS proteins (ERK [35], Akt/PKB [36••, 37], PTEN [38]). In humans, IIS modifies innate immune responses through the regulation of NF-κB transcription factors [39]. Insects also possess NF-κB transcription factors (reviewed in [40]) and in mosquitoes IIS inhibits NF-κB-dependent immune responses [30•].
Although human insulin and insulin-like growth factor-1 (IGF-1) are structurally similar, they vary considerably in their effects in both humans and blood feeding insects [32]. Unlike insulin, ingested human IGF-1 increases resistance of A. stephensi to P. falciparum through the induction of midgut mitochondrial ROS and nitric oxide (NO) [32, 41•]. In humans, IGF binding proteins (IGFBPs) regulate the bioavailability of IGF-1 and can also independently activate the IGF receptor [42]. In the fruit fly Drosophila melanogaster ILP-2 and ILP-5 signaling is regulated, in part, by interaction with IGFBP-like proteins [43]. IGFBP-like proteins have been described in Ae. aegypti [44] and in the moth species Spodoptera frugiperda [45], raising the possibility that insect vectors may also possess IGFBP-related proteins that could interact with ingested vertebrate growth factors to alter their downstream effects.
TGF-β1
Mammalian transforming growth factor (TGF)-β1 is a cytokine that is often present in peripheral blood during infection and is critical in regulating host immune responses [46]. In addition, TGF-β1 is also induced by infection with Trypanosoma and Leishmania, and these parasites may benefit directly from its subsequent downstream signaling effects [46]. Most mammalian cells produce TGF-β1 in its latent form and it is only after its activation that TGF-β1 exerts is cellular effects.
Mosquitoes ingest human TGF-β1 primarily in a latent form that is rapidly activated by factors such as heme and NO that are released during the digestion of a blood meal [47•]. Levels of circulating latent TGF-β1 in healthy, uninfected humans can reach 5 ng/ml, therefore mosquitoes ingest a biologically relevant level of TGF-β1 [48]. Orthologous proteins from the TGF-β signaling pathway have been identified in a diversity of blood feeding insects [49], raising the possibility that ingested human TGF-β1 activates endogenous TGF-β1 signaling pathways in other insect vectors as well. One of the most potent effects of TGF-β1 is the regulation of NO production, which is used by both mammals and mosquitoes to kill Plasmodium parasites [50]. In mosquitoes, low levels of human TGF-β1 (≤ 200 pg/ml) ingested in an infectious blood meal induce a moderate increase in nitric oxide synthase (NOS) activity that inhibits malaria parasite development. In contrast, high concentrations of TGF-β1 (2,000 pg/ml) do not alter malaria parasite development, but instead induce negative feedback to regulate NO synthesis [35]. The dose dependent effects of TGF-β1 signaling observed in mosquitoes are consistent with findings from mammalian biology that highlight the ability of TGF-β1 to regulate NOS activity on multiple levels [51].
Other cytokines
Both vertebrates and invertebrates use cytokines and cytokine-like factors to regulate immunity and wounding healing. To date, no mammalian cytokines have been identified that signal in insect vectors. However, the strong conservation of signaling pathways between insects and their vertebrate hosts suggests that mammalian cytokines capable of altering the physiology of insect vectors exist. For example, human interferon-γ (IFNγ) signals through the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway. Binding of IFNγ by its membrane receptors leads to the activation of JAK, which phosphorylates the immune-regulatory transcription factor STAT1. JAK/STAT signaling is regulated in part by suppressor of cytokine signaling-1 (SOCS-1) [52]. Orthologs of STAT, JAK, and SOCS proteins exist in Anopheles, Aedes and Culex mosquitoes [53, 54] and activation of JAK/STAT signaling in A. gambiae can inhibit the development Plasmodium parasites [55]. In addition, a cytokine with homology to mammalian interferon has been identified in West Nile virus (WNV)-infected Culex quinquefasciatus cell lines [56•]. This secreted peptide, termed Vago, restricts WNV infection in mosquito cells through the activation of the JAK/STAT signaling pathway [56•]. The use of cytokines by insect vector species to regulate their innate immune responses and the presence of a clear signaling architecture suggests that exogenous human cytokines may signal in insect vectors as well.
Conclusions
In this review we highlighted a variety of vertebrate blood-derived factors that modify the innate immune responses of insect vectors. The conservation of these signaling pathways, and the breadth of cross-talk identified, suggest that other connections remain to be discovered between mammalian hosts and blood feeding insects. Although in this review we discussed blood-derived factors and their impact on insect immunity individually, a single blood meal will most likely contain a multitude of these factors concurrently. Therefore, considerable work is still required to understand how these signaling pathways network with one another to understand their ultimate downstream affects on insect immunity and pathogen transmission.
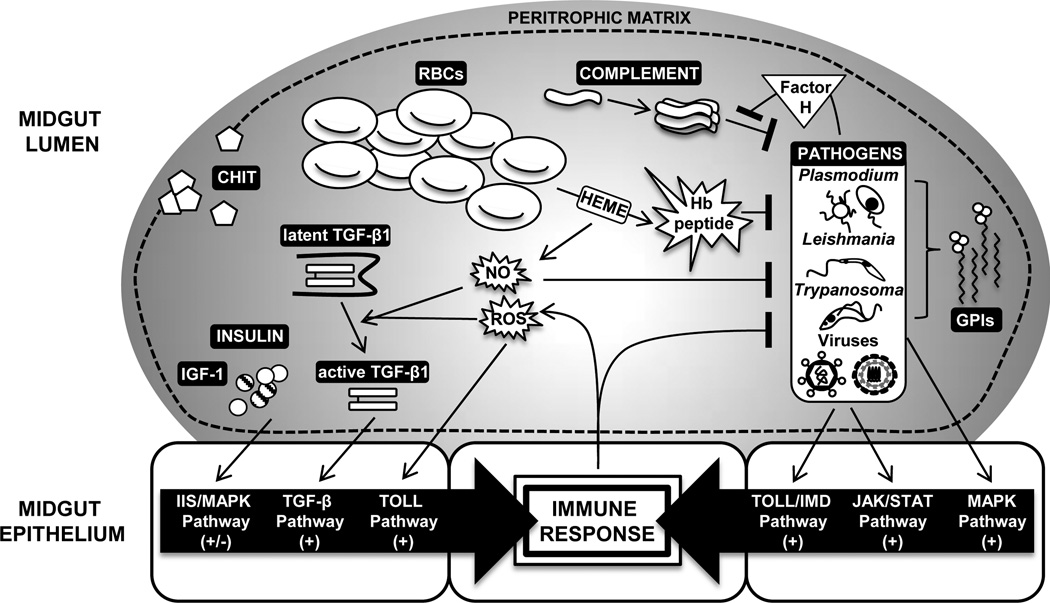
Figure 1.
The effects of ingested blood from an infected vertebrate host (large gray oval, top) on the insect midgut epithelium (large white squares, bottom). Dashed line indicates peritrophic matrix and black boxes indicate ingested factors/pathogens that are directly active or activated after reactions in the midgut lumen. Insect signaling pathways activated by these ingested factors and their downstream effects on insect immunity are indicated.
Highlights.
Ingested blood-derived factors can alter the immune response of insect vectors
Factors released by hemoglobin digestion can limit the growth of pathogens
Pathogen-derived factors can signal in the insect midgut to alter immunity
Human chitotriosidase can alter the peritrophic matrix of insects
Human insulin, IGF-1, and TGF-α1 signal in the insect midgut to alter immunity
Acknowledgements
We apologize to all our colleagues whose studies could not be covered because of space limitations. This work was supported by the National Institute of Health, National Institute of Allergy and Infectious Disease grants AI080799, AI073745, and AI078183.
Abbreviations
- CHIT
Chitotriosidase
- GPIs
glycosylphosphatidylinositols
- Hb
hemoglobin
- IIS
insulin/insulin-like growth factor signaling
- IGF-1
insulin-like growth factor-1
- IGFBPs
IGF binding proteins
- ILPs
insulin-like peptides
- IFNγ
interferon-γ
- JAK/STAT
Janus kinase/signal transducers and activators of transcription
- LPGs
lipophosphoglycans
- NO
nitric oxide
- NOS
nitric oxide synthase
- PM
peritrophic matrix
- ROS
reactive oxygen species
- SOCS-1
suppressor of cytokine signaling-1
- (TGF)-β1
transforming growth factor
- WNV
West Nile Virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. Graca-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, Sorgine MH, Oliveira MF, Oliveira PL. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol. 2006;36:322–335. doi: 10.1016/j.ibmb.2006.01.009. ••This paper provides an overview of the evolution of blood feeding behavior in insects.
- 2.Ivanov VT, Karelin AA, Philippova MM, Nazimov IV, Pletnev VZ. Hemoglobin as a source of endogenous bioactive peptides: the concept of tissue-specific peptide pool. Biopolymers. 1997;43:171–188. doi: 10.1002/(SICI)1097-0282(1997)43:2<171::AID-BIP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 3.Fraidenraich D, Pena C, Isola EL, Lammel EM, Coso O, Anel AD, Pongor S, Baralle F, Torres HN, Flawia MM. Stimulation of Trypanosoma cruzi adenylyl cyclase by an alpha D-globin fragment from Triatoma hindgut: effect on differentiation of epimastigote to trypomastigote forms. Proc Natl Acad Sci U S A. 1993;90:10140–10144. doi: 10.1073/pnas.90.21.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia ES, Gonzalez MS, de Azambuja P, Baralle FE, Fraidenraich D, Torres HN, Flawia MM. Induction of Trypanosoma cruzi metacyclogenesis in the gut of the hematophagous insect vector, Rhodnius prolixus by hemoglobin and peptides carrying alpha D-globin sequences. Exp Parasitol. 1995;81:255–261. doi: 10.1006/expr.1995.1116. [DOI] [PubMed] [Google Scholar]
- 5.Parish CA, Jiang H, Tokiwa Y, Berova N, Nakanishi K, McCabe D, Zuckerman W, Xia MM, Gabay JE. Broad-spectrum antimicrobial activity of hemoglobin. Bioorg Med Chem. 2001;9:377–382. doi: 10.1016/s0968-0896(00)00263-7. [DOI] [PubMed] [Google Scholar]
- 6.Meshnick SR, Chang KP, Cerami A. Heme lysis of the bloodstream forms of Trypanosoma brucei. Biochem Pharmacol. 1977;26:1923–1928. doi: 10.1016/0006-2952(77)90167-8. [DOI] [PubMed] [Google Scholar]
- 7.Orjih AU, Banyal HS, Chevli R, Fitch CD. Hemin lyses malaria parasites. Science. 1981;214:667–669. doi: 10.1126/science.7027441. [DOI] [PubMed] [Google Scholar]
- 8. Peterson TM, Gow AJ, Luckhart S. Nitric oxide metabolites induced in Anopheles stephensi control malaria parasite infection. Free Radic Biol Med. 2007;42:132–142. doi: 10.1016/j.freeradbiomed.2006.10.037. •This paper provides the first evidence of reactive nitrogen oxides generated during blood feeding controlling susceptibility to infection in an insect.
- 9.Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2012;109:E23–E31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baton LA, Ranford-Cartwright LC. Plasmodium falciparum ookinete invasion of the midgut epithelium of Anopheles stephensi is consistent with the Time Bomb model. Parasitology. 2004;129:663–676. doi: 10.1017/s0031182004005979. [DOI] [PubMed] [Google Scholar]
- 11. Ropert C, Gazzinelli RT. Signaling of immune system cells by glycosylphosphatidylinositol (GPI) anchor and related structures derived from parasitic protozoa. Curr Opin Microbiol. 2000;3:395–403. doi: 10.1016/s1369-5274(00)00111-9. ••This paper provides a review of glycosylphosphatidylinositols in parasitic protozoa and their ability to activate immune signaling cascades.
- 12.Arrighi RB, Debierre-Grockiego F, Schwarz RT, Faye I. The immunogenic properties of protozoan glycosylphosphatidylinositols in the mosquito Anopheles gambiae. Dev Comp Immunol. 2009;33:216–223. doi: 10.1016/j.dci.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Lim J, Gowda DC, Krishnegowda G, Luckhart S. Induction of nitric oxide synthase in Anopheles stephensi by Plasmodium falciparum: mechanism of signaling and the role of parasite glycosylphosphatidylinositols. Infect Immun. 2005;73:2778–2789. doi: 10.1128/IAI.73.5.2778-2789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Assis RR, Ibraim IC, Nogueira PM, Soares RP, Turco SJ. Glycoconjugates in New World species of Leishmania: Polymorphisms in lipophosphoglycan and glycoinositolphospholipids and interaction with hosts. Biochim Biophys Acta. 2012;1820:1354–1365. doi: 10.1016/j.bbagen.2011.11.001. •This paper demonstrates how lipophosphoglycans determine insect vector host specificity of Leishmania parasites.
- 15.Guther ML, Lee S, Tetley L, Acosta-Serrano A, Ferguson MA. GPI-anchored proteins and free GPI glycolipids of procyclic form Trypanosoma brucei are nonessential for growth, are required for colonization of the tsetse fly, are not the only components of the surface coat. Mol Biol Cell. 2006;17:5265–5274. doi: 10.1091/mbc.E06-08-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gouagna LC, Bonnet S, Gounoue R, Verhave JP, Eling W, Sauerwein R, Boudin C. Stage-specific effects of host plasma factors on the early sporogony of autologous Plasmodium falciparum isolates within Anopheles gambiae. Trop Med Int Health. 2004;9:937–948. doi: 10.1111/j.1365-3156.2004.01300.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsuboi T, Cao YM, Torii M, Hitsumoto Y, Kanbara H. Murine complement reduces infectivity of Plasmodium yoelii to mosquitoes. Infect Immun. 1995;63:3702–3704. doi: 10.1128/iai.63.9.3702-3704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margos G, Navarette S, Butcher G, Davies A, Willers C, Sinden RE, Lachmann PJ. Interaction between host complement and mosquito-midgut-stage Plasmodium berghei. Infect Immun. 2001;69:5064–5071. doi: 10.1128/IAI.69.8.5064-5071.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simon N, Lasonder E, Scheuermayer M, Kuehn A, Tews S, Fischer R, Zipfel PF, Skerka C, Pradel G. Malaria parasites co-opt human factor H to prevent complement-mediated lysis in the mosquito midgut. Cell Host Microbe. 2013;13:29–41. doi: 10.1016/j.chom.2012.11.013. •This paper provides the first example of a pathogen co-opting human blood-derived factors in an insect midgut to avoid complement-mediated killing.
- 20.Terra WR. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch Insect Biochem Physiol. 2001;47:47–61. doi: 10.1002/arch.1036. [DOI] [PubMed] [Google Scholar]
- 21.Malaguarnera L. Chitotriosidase: the yin and yang. Cell Mol Life Sci. 2006;63:3018–3029. doi: 10.1007/s00018-006-6269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barone R, Simpore J, Malaguarnera L, Pignatelli S, Musumeci S. Plasma chitotriosidase activity in acute Plasmodium falciparum malaria. Clin Chim Acta. 2003;331:79–85. doi: 10.1016/s0009-8981(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 23. Di Luca M, Romi R, Severini F, Toma L, Musumeci M, Fausto AM, Mazzini M, Gambellini G, Musumeci S. High levels of human chitotriosidase hinder the formation of peritrophic membrane in anopheline vectors. Parasitol Res. 2007;100:1033–1039. doi: 10.1007/s00436-006-0372-z. •This paper demonstrates that ingested human chitotriosidase can alter the peritrophic matrix of an insect.
- 24.Hollak CE, van Weely S, van Oers MH, Aerts JM. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93:1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luckhart S, Riehle MA. The insulin signaling cascade from nematodes to mammals: insights into innate immunity of Anopheles mosquitoes to malaria parasite infection. Dev Comp Immunol. 2007;31:647–656. doi: 10.1016/j.dci.2006.10.005. ••This paper reviews the conserved functional biology of insulin signaling between vertebrates and invertebrates.
- 26.Kang MA, Mott TM, Tapley EC, Lewis EE, Luckhart S. Insulin regulates aging and oxidative stress in Anopheles stephensi. J Exp Biol. 2008;211:741–748. doi: 10.1242/jeb.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surachetpong W, Pakpour N, Cheung KW, Luckhart S. Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid Redox Signal. 2011;14:943–955. doi: 10.1089/ars.2010.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horton AA, Wang B, Camp L, Price MS, Arshi A, Nagy M, Nadler SA, Faeder JR, Luckhart S. The mitogen-activated protein kinome from Anopheles gambiae: identification, phylogeny and functional characterization of the ERK, JNK and p38 MAP kinases. BMC Genomics. 2011;12:574. doi: 10.1186/1471-2164-12-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marquez AG, Pietri JE, Smithers HM, Nuss A, Antonova Y, Drexler AL, Riehle MA, Brown MR, Luckhart S. Insulin-like peptides in the mosquito Anopheles stephensi: Identification and expression in response to diet and infection with Plasmodium falciparum. Gen Comp Endocrinol. 2011;173:303–312. doi: 10.1016/j.ygcen.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pakpour N, Corby-Harris V, Green GP, Smithers HM, Cheung KW, Riehle MA, Luckhart S. Ingested human insulin inhibits the mosquito NFkappaB- dependent immune response to Plasmodium falciparum. Infect Immun. 2012;80:2141–2149. doi: 10.1128/IAI.00024-12. •This paper demonstrates that exogenous human insulin activates the endogenous insulin signaling cascade to inhibit the insect immune response.
- 31.Akman-Anderson L, Olivier M, Luckhart S. Induction of nitric oxide synthase and activation of signaling proteins in Anopheles mosquitoes by the malaria pigment, hemozoin. Infect Immun. 2007;75:4012–4019. doi: 10.1128/IAI.00645-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drexler A, Nuss A, Hauck E, Glennon E, Cheung K, Brown M, Luckhart S. Human IGF1 extends lifespan and enhances resistance to Plasmodium falciparum infection in the malaria vector Anopheles stephensi. J Exp Biol. 2013;216:208–217. doi: 10.1242/jeb.078873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sajid W, Kulahin N, Schluckebier G, Ribel U, Henderson HR, Tatar M, Hansen BF, Svendsen AM, Kiselyov VV, Norgaard P, et al. Structural and biological properties of the Drosophila insulin-like peptide 5 show evolutionary conservation. J Biol Chem. 2011;286:661–673. doi: 10.1074/jbc.M110.156018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumann AA, Benoit JB, Michalkova V, Mireji PO, Attardo GM, Moulton JK, Wilson TG, Aksoy S. Juvenile hormone and insulin suppress lipolysis between periods of lactation during tsetse fly pregnancy. Mol Cell Endocrinol. 2013;372:30–41. doi: 10.1016/j.mce.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surachetpong W, Singh N, Cheung KW, Luckhart S. MAPK ERK signaling regulates the TGF-beta1-dependent mosquito response to Plasmodium falciparum. PLoS Pathog. 2009;5:e1000366. doi: 10.1371/journal.ppat.1000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Corby-Harris V, Drexler A, Watkins de Jong L, Antonova Y, Pakpour N, Ziegler R, Ramberg F, Lewis EE, Brown JM, Luckhart S, et al. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 2010;6:e1001003. doi: 10.1371/journal.ppat.1001003. ••This paper the first example of genetically engineered complete resistance to P. falciparum infection in a mosquito vector.
- 37.Luckhart S, Giulivi C, Drexler AL, Antonova-Koch Y, Sakaguchi D, Napoli E, Wong S, Price MS, Eigenheer R, Phinney BS, et al. Sustained activation of Akt elicits mitochondrial dysfunction to block Plasmodium falciparum infection in the mosquito host. PLoS Pathog. 2013;9:e1003180. doi: 10.1371/journal.ppat.1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauck ES, Antonova-Koch Y, Drexler A, Pietri J, Pakpour N, Liu D, Blacutt J, Riehle MA, Luckhart S. Overexpression of phosphatase and tensin homolog improves fitness and decreases Plasmodium falciparum development in Anopheles stephensi. Microbes Infect. 2013;15:775–787. doi: 10.1016/j.micinf.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257–3265. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 40.Cirimotich CM, Dong Y, Garver LS, Sim S, Dimopoulos G. Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol. 2010;34:387–395. doi: 10.1016/j.dci.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drexler AL, Pietri JE, Pakpour N, Hauck E, Wang B, Glennon EKK, Georgis M, Riehle MA, Luckhart S. Human IGF1 regulates midgut oxidative stress and epithelial homeostasis to balance lifespan and Plasmodium falciparum resistance in Anopheles stephensi. PLoS Pathog. 2014 doi: 10.1371/journal.ppat.1004231. in press. •This paper highlights the different downstream effects of ingested human IGF-1 versus ingested human insulin on the mosquito response to infection.
- 42.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 43.Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, Stocker H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol. 2008;7:10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2008;105:5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sloth Andersen A, Hertz Hansen P, Schaffer L, Kristensen C. A new secreted insect protein belonging to the immunoglobulin superfamily binds insulin and related peptides and inhibits their activities. J Biol Chem. 2000;275:16948–16953. doi: 10.1074/jbc.M001578200. [DOI] [PubMed] [Google Scholar]
- 46.Omer FM, Kurtzhals JA, Riley EM. Maintaining the immunological balance in parasitic infections: a role for TGF-beta? Parasitol Today. 2000;16:18–23. doi: 10.1016/s0169-4758(99)01562-8. [DOI] [PubMed] [Google Scholar]
- 47. Luckhart S, Crampton AL, Zamora R, Lieber MJ, Dos Santos PC, Peterson TM, Emmith N, Lim J, Wink DA, Vodovotz Y. Mammalian transforming growth factor beta1 activated after ingestion by Anopheles stephensi modulates mosquito immunity. Infect Immun. 2003;71:3000–3009. doi: 10.1128/IAI.71.6.3000-3009.2003. •This paper demonstrates that latent human TGF-β1 can be activated after ingestion in the midgut of mosquitoes.
- 48.Lieber MJ, Luckhart S. Transforming growth factor-betas and related gene products in mosquito vectors of human malaria parasites: signaling architecture for immunological crosstalk. Mol Immunol. 2004;41:965–977. doi: 10.1016/j.molimm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Kirkness EF, Haas BJ, Sun W, Braig HR, Perotti MA, Clark JM, Lee SH, Robertson HM, Kennedy RC, Elhaik E, et al. Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proc Natl Acad Sci U S A. 2010;107:12168–12173. doi: 10.1073/pnas.1003379107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vodovotz Y, Zamora R, Lieber MJ, Luckhart S. Cross-talk between nitric oxide and transforming growth factor-beta1 in malaria. Curr Mol Med. 2004;4:787–797. doi: 10.2174/1566524043359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vodovotz Y. Control of nitric oxide production by transforming growth factor-beta1: mechanistic insights and potential relevance to human disease. Nitric Oxide. 1997;1:3–17. doi: 10.1006/niox.1996.0105. [DOI] [PubMed] [Google Scholar]
- 52.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 53. Zhou F, Agaisse H. JAK/STAT Signaling and Invertebrate Immune Responses. In: Decker T, Müller M, editors. Jak-Stat Signaling: From Basics to Disease. Vienna, AU: Springer; 2012. pp. 133–151. ••This paper reviews the conserved biology of JAK/STAT signaling between vertebrates and invertebrates.
- 54.Zhou F, Agaisse H. JAK/STAT Signaling and Invertebrate Immune Responses. In: Decker T, Müller M, editors. Jak-Stat Signaling : From Basics to Disease. Springer Vienna; 2012. pp. 133–151. [Google Scholar]
- 55.Gupta L, Molina-Cruz A, Kumar S, Rodrigues J, Dixit R, Zamora RE, Barillas-Mury C. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe. 2009;5:498–507. doi: 10.1016/j.chom.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paradkar PN, Trinidad L, Voysey R, Duchemin JB, Walker PJ. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc Natl Acad Sci U S A. 2012;109:18915–18920. doi: 10.1073/pnas.1205231109. •This paper provides the first evidence of a cytokine an insect vector species.