Abstract
Histoplasmosis caused by Histoplasma capsulatum var. duboisii is a rare disease outside central and western Africa. In Europe, all cases are imported. We report a case of an African histoplasmosis with isolated pulmonary involvement in a non-immunocompromised patient that occurred 40 years after his stay in a disease-endemic area. The patient was given itraconazole. 18F-fluoro-2-deoxy-d-glucose positron emission tomography–computed tomography was used to assess evolution during treatment. The outcome for the patient was favorable.
African histoplasmosis may occur in persons without identified immunodeficiency, even a long time after exposure. African histoplasmosis caused by Histoplasma capsulatum var. duboiisi, a dimorphic fungus, is an invasive fungal disease endemic to central and western Africa and Madagascar. About thirty cases were reported in Europe and all are imported were cases.1–8 We report an immunocompetent Caucasian patient without identified immunodeficiency who had chronic pulmonary African histoplasmosis diagnosed four decades after a stay in western Africa.
A 60-year-old man from Portugal was admitted to an emergency department in October 2006 for cough with clear sputum and chest pain. His medical history indicated a gastroduodenal ulcer. He still visited his native country each year even though he had lived in France for 40 years. Forty years ago, he lived in Guinea-Bissau for two years during his military service. He is now a factory worker and enjoys gardening.
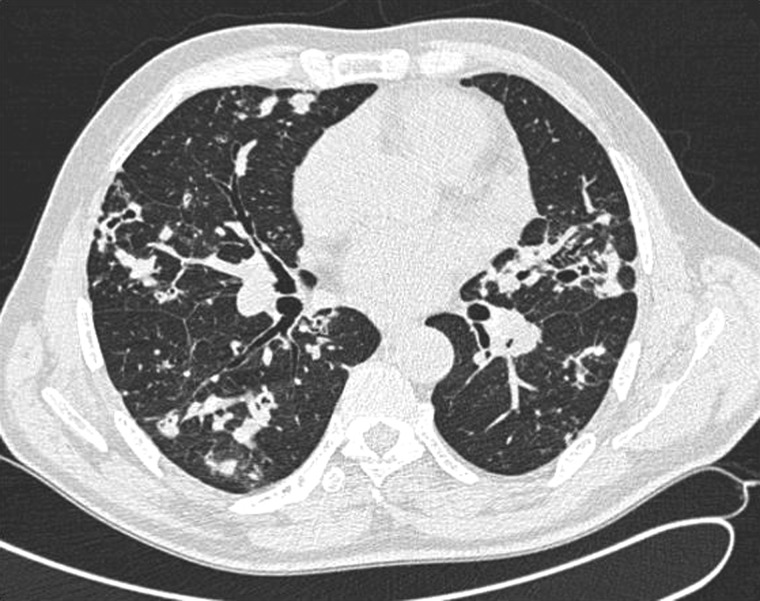
The functional pulmonary symptoms occurred gradually without chills, sweats or fever for several weeks. Results of a clinical examination were normal. Oxygen saturation was 96%, temperature was 37°C, and there was no weight loss. A chest radiograph showed bilateral diffuse opacities with nodular cavitations. Chest computed tomography confirmed the presence of disseminated nodules (3–6 cm in diameter), some with cavitations and one of them calcified, but no adenopathy, pleural effusion, nor underlying parenchymal abnormalities (Figure 1).
Figure 1.
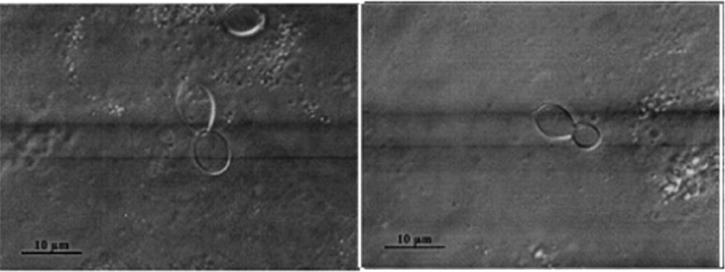
Microscopic examination of sputum and lung biopsy specimens for the patient, showing large oval yeast with narrow budding.
Laboratory tests showed normal blood cell counts: 5,400 leukocytes/mm3 with 3,400 neutrophils/mm3, 1,400 lymphocytes/mm3, and 200 eosinophils/mm3. The C-reactive protein level was 138 mg/L, and serum levels of liver enzymes, bilirubin, and lactate dehydrogenase were within reference ranges. Sputum cultures were negative for bacteria, mycobacteria, and fungi. Serologic test results for aspergillosis and hydatidosis were negative. Abdominal and cardiac ultrasound and bronchoscopic evaluations show normal results. Bronchoalveolar lavage fluid contained 223 × 103 cells/mL, 93% alveolar macrophages, 5% lymphocytes, 2% neutrophils, and no neoplastic cells. Specific staining and/or cultures were negative for Pneumocystis jirovecii, mycobacteria, and bacterial microorganisms. Gomori-Grocott staining showed oval-shaped yeast 8–10 μm in diameter with narrow budding compatible with H. capsulatum var. duboisii (Figure 2). The culture yielded growth of a mycelial phase with tuberculate macroconidia that was visible after lactophenol staining. Moreover, a serologic test result was also positive for histoplasmosis (arc M).
Figure 2.
Chest computed tomography at diagnosis for the patient, showing disseminated parenchymal macronodules, some with excavation, and no evidence for an underlying chronic pulmonary disease.
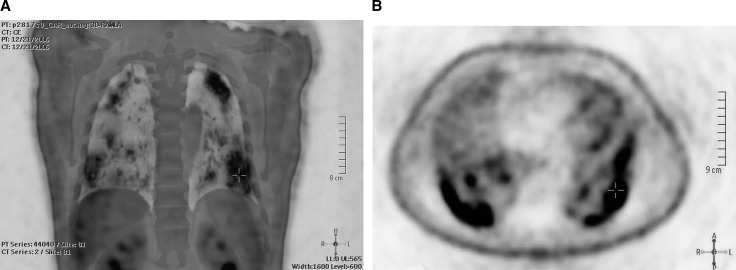
A biopsy specimen of a right pulmonary nodule showed granuloma and suppuration and evidence of the same microorganisms after hematoxylin and Grocott staining. Initial 18F-fluoro-2-deoxy-d-glucose positron emission tomography–computed tomography (PET-CT) showed exclusive uptake by lung lesions and a maximum standardized uptake value (SUVmax) of 8.4 mGy (Figure 3).
Figure 3.
Initial positron emission tomography–computed tomography for the patient, showing exclusive uptake on the lung lesions A, Frontal section. B, Transverse section.
Screening results for an innate or acquired immunodeficiency were negative, except for a slightly reduced CD4 cell count of 480 cells/mm3 (reference range = 500–1,500 cells/ mm3) and a CD8 cell count of 216 cells/mm3 (reference range = 300–900 cells/mm3) compared with a normal CD56 cell count of 216 cells/mm3 (reference range = 100–350 cells/mm3). During treatment, CD4 and CD8 cell counts returned to reference levels. Serum protein electrophoresis and immunoelectrophoresis showed normal results, and IgA, IgG, and IgM levels were normal. Serologic results for infection with human immunodeficiency virus (HIV) were negative. Production of interferon-γ (INF-γ) or interleukin-12 (IL-12)p40 in response to bacillus Calmette Guérin (alone or with INF-γ or IL-12) was normal, excluding a genetic defect in the IL-12/IFN-γ axis or auto-antibodies against IFN-γ. The T cell lymphocyte in vitro proliferative responses to mitogen and antigens were normal. Antinuclear antibodies and cytoplasmic antibodies against neutrophils were absent. No iron overload was detected (transferrin saturation rate = 23%).
The patient was treated orally with itraconazole (600 mg/day for 3 days, then 400 mg/day). Plasma drug levels were carefully monitored: peak levels for hydroxyl-itraconazole and itraconazole ranged from 1,900 to 3,000 μg/mL and from 1,560 to 3,000 μg/mL, respectively (reference values < 2,000 μg/mL for itraconazole and < 6,000 μg/mL for hydroxyl-itraconazole), Residual levels for itraconazole ranged from 1,053 to 2,065 μg/mL (reference value ≤ 250 μg/mL). Initial respiratory symptoms resolved in less than six months.
Although the patient is now healthy and has not showed any respiratory symptoms after seven years of treatment, sequential annual chest CT did not show any improvement. Multiple excavated nodules, some calcified, persisted although bronchoalveolar lavage fluid was sterile four years after initiation of treatment. A PET-CT performed after more than seven years of well-observed treatment, with careful screening for plasma concentrations, showed a decrease, but not an extinction, in uptake by lung nodules (SUVmax = 4.6 mGy). We then decided to stop antifungal treatment but continued clinical and radiologic monitoring. Six months after treatment was stopped, no symptoms were observed.
We describe a clinical case of histoplasmosis with localized pulmonary involvement caused by H. capsulatum var. duboisii that occurred 40 years after exposure. African histoplasmosis is caused by H. capsulatum var. duboisii. The exact pathogenesis remains unclear. The usual routes of acquisition are believed to be airborne contamination from soil and rarely by direct inoculation. However, the most frequent radiologic findings for the lungs are either miliary or calcified parenchymal nodules, which suggest hematogeneous or lymphatic spreading.9,10
Two forms of African histoplasmosis are typically described: a localized chronic form that involves skin, subcutaneous tissue, bones, or lymph nodes; and a disseminated rapidly evolving form, which can also involve the spleen, liver, lungs, and abdominal viscera through ematogeneous invasion.9 For the patient in this study, we suggest a chronic pulmonary form, as described for infection with H. capsulatum var. capsulatum, because he had a persistent productive cough and cavitary disease, and H. capsulatum var duboisii was isolated from this patient. This clinical feature is not a classical picture for infection with H. capsulatum var. duboisii. In contrast to histoplasmosis caused by H. capsulatum, exclusive lung involvement seems to be rare with infection with H. capsulatum var. duboisii, as observed for our patient.1,11–13
Histoplasma capsulatum var. duboisii infections have been reported mainly in patients not infected with HIV; 17 case-patients were reported in Europe during 1980–1994, only 3 of these patients infected with HIV, and no other underlying immunodeficiency was described. Nevertheless, all three patients had disseminated disease in comparison with only one patient not infected with HIV.4 In contrast, H. capsulatum var. capsulatum infections are clearly related to a cellular immunodeficiency, especially for disseminated disease. Thus, in areas to which this organism is highly endemic, it remains the main acquired immunodeficiency syndrome–defining disease.14–16 In the present case, we did not find evidence of any underlying chronic pulmonary disease (including vasculitis associated with cytoplasmic antibodies against neutrophils) or underlying immunodeficiency. One may hypothesize that the natural history of such chronic pulmonary disease may be caused by association of a pulmonary inoculation and a progressive long-lasting local infection in a context of competent immune system that prevents dissemination associated with unusual fungal pathogen characteristics. As for tuberculosis, such chronic pulmonary features highlight the ability of H. capsulatum var. duboisii to produce latent infection in granuloma and local reactivation several years after initial infection.
Because histoplasmosis has only observed in persons from Europe who traveled to Africa or in persons born in Africa, invasive infection is believed to be caused by endogenous reactivation of a latent infection imported from disease-endemic countries. Time between infection and reactivation may be long, which would explain the cases reported several years after persons returned from Africa,17 as found with tuberculosis or cryptococcal disease (serotype B/C). An epidemiologic survey in Europe showed that for at least 25% of patients, symptoms did not occur for at least five years after travel.18 To the best of our knowledge, the present case is the most delayed diagnosis ever recorded for H. capsulatum var. duboisii, other than a case of H. capsulatum var. capsulatum lung reactivation reported 45 years after a person traveled to Africa.19
Amphotericin B, a lipid formulation of amphotericin B, and itraconazole are the drugs of choice for treating H. capsulatum var. capsulatum infections.2,20 These drugs are used to treat H. capsulatum var. duboisii infections because of the absence of specific guidelines for the management of such patients. Itraconazole appeared to be clinically efficient and cost-effective in the patient in this study. However, the persistence of radiologic and PET-CT lesions suggested a residual infection rather than scar lesions. We then decided to maintain antifungal therapy21 until the patient appeared clinically cured and radiologic (chest CT and PET-CT) stability was assessed.
Although rare, a diagnosis of African histoplasmosis should be kept in mind for persons born in Africa or for travelers to Africa,22,23 even many years after their return from the disease-endemic area or if they do not show underlying immunosuppression. Because the exact duration of antifungal treatment is not known, we usually recommend prolonged therapy. However, PET-CT findings may improve monitoring and lead to a more precise therapeutic strategy.
ACKNOWLEDGMENTS
We thank Nicolas Hamilton, Perrine Parize, Blandine Rammaert, Arnaud Hot, and Frédéric Méchai for assistance during the study.
Footnotes
Authors' addresses: Clémence Richaud, Service des Maladies Infectieuses et Tropicales, hôpital Necker, 149 rue de Sèvres, 75015, Paris, France, E-mail: clemence.richaud@gmail.com. Marie-Olivia Chandesris, Service d'Hématologie Adultes, Université Paris Descartes, Sorbonne Paris Cité Hôpital Necker-Enfants-Malades, Assistance Publique–Hôpitaux de Paris, Paris, France, E-mail: olivia.chandesris@nck.aphp.fr. Fanny Lanternier, Service d'Hématologie Adultes, Université Paris Descartes, Sorbonne Paris Cité Hôpital Necker-Enfants-Malades, Assistance Publique–Hôpitaux de Paris, Paris, France, and Institut Pasteur, Unité de Mycologie Moléculaire, Centre National de Référence Mycoses Invasives et Antifongiques, Centre National de la Recherche Scientifique, Unité Recherche Associée 3012, Paris, France, E-mail: fanny.lanternier@nck.aphp.fr. Hélène Benzaquen-Forner, Service de Pneumologie, Hôpital Simone Veil, Eaubonne, France, E-mail: helene.benzaquen@ch-simoneveil.fr. Dea Garcia-Hermoso, Istitut Pasteur, Unité de Mycologie Moléculaire Centre National de Référence Mycoses Invasives et Antifongiques, Centre National de la Recherche Scientifique Unité Recherche Associée 3012, Paris, France, E-mail: dea.garcia-hermoso@pasteur.fr. Capucine Picard, Centre d’Etude des Déficits Immunitaires, Université Paris Descartes, Sorbonne Paris Cité, Hôpital Necker-Enfants-Malades, Assistance Publique–Hôpitaux de Paris, Paris, France, and Laboratoire de Génétique Humaine des Maladies Infectieuses, Université Paris Descartes, Sorbonne Paris Cité, Faculté Necker, Fondation IMAGINE, Institut National de la Santé et de la Recherche Médicale Unité 980, Paris, France, E-mail: capucine.picard@nck.aphp.fr. Emilie Catherinot, Université Paris Descartes, Sorbonne Paris Cité, Hôpital Necker-Enfants-Malades, Assistance Publique–Hôpitaux de Paris, Service des Maladies Infectieuses et Tropicales et Centre d'Infectiologie Necker Pasteur, Paris, France, E-mail: e.catherinot@gmail.com. Marie-Elisabeth Bougnoux, Université Paris Descartes, Sorbonne Paris Cité Hôpital Necker-Enfants-Malades, Assistance Publique–Hôpitaux de Paris, Service de Microbiologie, Paris, France, E-mail: mbougnoux6@gmail.com. Olivier Lortholary, Institut Pasteur, Unité de Mycologie Moléculaire Centre National de Référence Mycoses Invasives et Antifongiques, Centre National de la Recherche Scientifique Unité Recherche Associée 3012, Paris, France, and Université Paris Descartes, Sorbonne Paris Cité, Hôpital Necker-Enfants-Malades, Assistance Publique–Hôpitaux de Paris, Service des Maladies Infectieuses et Tropicales et Centre d'Infectiologie Necker Pasteur, Paris, France, E-mail: olivier.lortholary@nck.aphp.fr.
References
- 1.Kennedy CC, Limper AH. Redefining the clinical spectrum of chronic pulmonary histoplasmosis: a retrospective case series of 46 patients. Medicine (Baltimore) 2007;86:252–258. doi: 10.1097/MD.0b013e318144b1d9. [DOI] [PubMed] [Google Scholar]
- 2.Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, Davies SF, Dismukes WE, Hage CA, Marr KA, Mody CH, Perfect JR, Stevens DA. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 3.Loulergue P, Bastides F, Baudouin V, Chandenier J, Mariani-Kurkdjian P, Dupont B, Viard JP, Dromer F, Lortholary O. Literature review and case histories of Histoplasma capsulatum var. duboisii infections in HIV-infected patients. Emerg Infect Dis. 2007;13:1647–1652. doi: 10.3201/eid1311.070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manfredi R, Mazzoni A, Nanetti A, Chiodo F. Histoplasmosis capsulati and duboisii in Europe: the impact of the HIV pandemic, travel and immigration. Eur J Epidemiol. 1994;10:675–681. doi: 10.1007/BF01719280. [DOI] [PubMed] [Google Scholar]
- 5.Pellaton C, Cavassini M, Jaton-Ogay K, Carron PN, Christen-Zaech S, Calandra T, Bille J, Hauser PM. Histoplasma capsulatum var. duboisii infection in a patient with AIDS: rapid diagnosis using polymerase chain reaction-sequencing. Diagn Microbiol Infect Dis. 2009;64:85–89. doi: 10.1016/j.diagmicrobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Pieron R, Mafart Y, Barranger C, Houdard C, Boury G, Favre M, Lancastre F, Le Fichoux Y. Cold thoracic abscess due to Histoplasma duboisii. Treatment using rifampicin [in French] Sem Hop. 1973;49:3009–3014. [PubMed] [Google Scholar]
- 7.Sanguino JC, Rodrigues B, Baptista A, Quina M. Focal lesion of African histoplasmosis presenting as a malignant gastric ulcer. Hepatogastroenterology. 1996;43:771–775. [PubMed] [Google Scholar]
- 8.Tsiodras S, Drogari-Apiranthitou M, Pilichos K, Leventakos K, Kelesidis T, Buitrago MJ, Petrikkos G, Panayiotides I. An unusual cutaneous tumor: African histoplasmosis following mudbaths: case report and review. Am J Trop Med Hyg. 2012;86:261–263. doi: 10.4269/ajtmh.2012.11-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gugnani HC. Histoplasmosis in Africa: a review. Indian J Chest Dis Allied Sci. 2000;42:271–277. [PubMed] [Google Scholar]
- 10.Williams AO, Lawson EA, Lucas AO. African histoplasmosis due to Histoplasma duboisii. Arch Pathol. 1971;92:306–318. [PubMed] [Google Scholar]
- 11.Dupont B. 36th Interscience Conference on Antimicrobial Agents and Chemotherapy; New Orleans, Louisiana: 1996. Imported histoplasmosis due to H. duboisii in France (1968–1994) [Google Scholar]
- 12.Dupont B, Drouhet E, Lapresle C. Systemic histoplasmosis with Histoplasma duboisii. Miliary pulmonary form with fatal termination [in French] Nouv Presse Med. 1974;3:1005–1007. [PubMed] [Google Scholar]
- 13.Mandell GL, Bennett JE, Dolin R. Principles and Practice of Infectious Diseases. Oxford: United Kingdom; 2009. Histoplasmosis; pp. 3305–3318. [Google Scholar]
- 14.Couppie P, Sobesky M, Aznar C, Bichat S, Clyti E, Bissuel F, El Guedj M, Alvarez F, Demar M, Louvel D, Pradinaud R, Carme B. Histoplasmosis and acquired immunodeficiency syndrome: a study of prognostic factors. Clin Infect Dis. 2004;38:134–138. doi: 10.1086/379770. [DOI] [PubMed] [Google Scholar]
- 15.Nacher M, Adenis A, Adriouch L, Dufour J, Papot E, Hanf M, Vantilcke V, Calvez M, Aznar C, Carme B, Couppie P. What is AIDS in the Amazon and the Guianas? Establishing the burden of disseminated histoplasmosis. Am J Trop Med Hyg. 2011;84:239–240. doi: 10.4269/ajtmh.2011.10-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peigne V, Dromer F, Elie C, Lidove O, Lortholary O. the French Mycosis Study Group Imported acquired immunodeficiency syndrome–related histoplasmosis in metropolitan France: a comparison of pre–highly active anti-retroviral therapy and highly active anti-retroviral therapy eras. Am J Trop Med Hyg. 2011;85:934–941. doi: 10.4269/ajtmh.2011.11-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dallot A, Monsuez JJ, Chanu B, Vittecocq D, Verola O, Badillet G, Rouffy J, Morel P, Puissant A. Cutaneous localizations of disseminated Histoplasma capsulatum histoplasmosis in a case of acquired immunodeficiency [in French] Ann Dermatol Venereol. 1988;115:441–447. [PubMed] [Google Scholar]
- 18.Ashbee HR, Evans EG, Viviani MA, Dupont B, Chryssanthou E, Surmont I, Tomsikova A, Vachkov P, Enero B, Zala J, Tintelnot K. Histoplasmosis in Europe: report on an epidemiological survey from the European Confederation of Medical Mycology Working Group. Med Mycol. 2008;46:57–65. doi: 10.1080/13693780701591481. [DOI] [PubMed] [Google Scholar]
- 19.Torres-Rodriguez JM, Segura-Roca G, Coll J. Histoplasmosis 45 years after infection in an immunocompetent man [in Spanish] Rev Iberoam Micol. 2009;26:244–246. doi: 10.1016/j.riam.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Kauffman CA. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 21.Hot A, Maunoury C, Poiree S, Lanternier F, Viard JP, Loulergue P, Coignard H, Bougnoux ME, Suarez F, Rubio MT, Mahlaoui N, Dupont B, Lecuit M, Faraggi M, Lortholary O. Diagnostic contribution of positron emission tomography with [18F]fluorodeoxyglucose for invasive fungal infections. Clin Microbiol Infect. 2010;17:409–417. doi: 10.1111/j.1469-0691.2010.03301.x. [DOI] [PubMed] [Google Scholar]
- 22.Lortholary O, Charlier C, Lebeaux D, Lecuit M, Consigny PH. Fungal infections in immunocompromised travelers. Clin Infect Dis. 2013;56:861–869. doi: 10.1093/cid/cis935. [DOI] [PubMed] [Google Scholar]
- 23.Richaud C, Lebeaux D, Lortholary O. Fungal infections associated with travel. Curr Fungal Infect Rep. 2013;7:311–319. [Google Scholar]