Abstract
Leptospira spp. isolated from patients during a multiyear outbreak in Thailand were genotyped using multilocus sequence typing and a majority were identified as ST34, especially in earlier years. We tested whether ST34 isolates were better adapted to survive in various pH levels, temperatures, and water sources. Motility and growth were monitored over a 12-week period. Early year ST34 isolates did not appear to have a significant fitness advantage over non-ST34, however, this may have been because a majority of the isolates survived to the termination of the study, with the exception being at high temperature (37°C) and/or basic pH (8.65). Failure to detect a significant fitness advantage of ST34 may be a result of the length of the study or the small sample size. Lengthening the study and looking at virulence and maintenance in the host could yield additional information about this outbreak.
Leptospirosis has a broad range of symptoms, from mild flu-like illness to acute renal failure and pulmonary hemorrhage.1,2 The disease is found worldwide, but is more common in countries with a rainy season and warmer climate that promotes the survival of leptospires in the environment.1,2 A sustained outbreak of leptospirosis occurred in northeast Thailand between 1999 and 2005, with the peak occurring in 2000.3,4 Blood culture isolates of Leptospira spp. from 2000 to 2005 were typed using multilocus sequence typing (MLST).3 There were 12 sequence types (STs) identified by MLST; 76% of the isolates were identified as ST34 (Leptospira interrogans serovar Autumnalis), but the dominance of ST34 decreased between 2000 and 2005.3 The outbreak could not be explained by climate or regional flooding; the authors hypothesized that the increased incidence may have been caused by the presence of a biologically successful strain of pathogenic Leptospira.3 In this study, we tested whether early year ST34 isolates (2001 and 2003) were better adapted than later year ST34 and/or non-ST34 isolates (2005 and 2006) from this outbreak for survival in water from different sources and at variable pH and temperature.
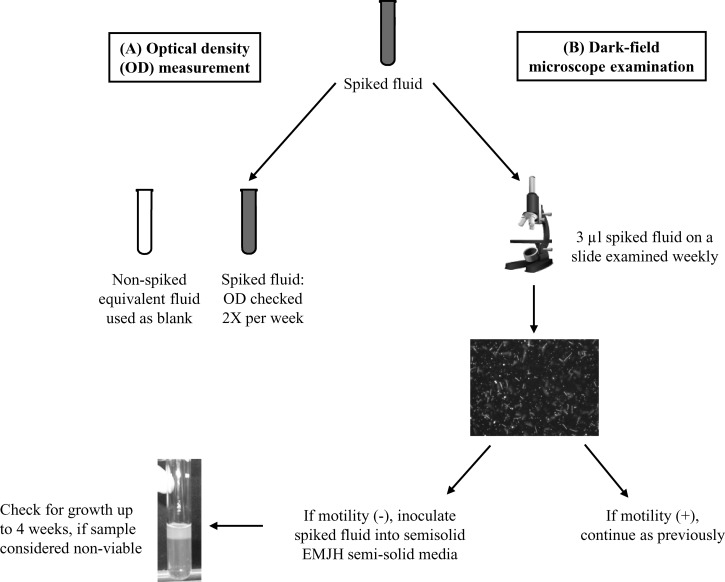
Isolates representing four of the 11 STs from various years of the outbreak were selected (Table 1). Isolates had been maintained at 25–30°C and had gone through 18–23 passages in Ellinghausen, McCullough, Johnson, and Harris (EMJH) semisoild media. Isolates were inoculated into liquid EMJH, grown at 30°C to the logarithmic phase, and adjusted to a McFarland standard of 0.5.5–9 Spiking was performed by centrifuging 12 mL of the adjusted culture, discarding the supernatant, and then resuspending with 12 mL of suspending fluid. Rice field water and pond water from the outbreak location in Thailand were autoclaved before use. For each suspending fluid, conditions were tested in duplicate with various temperatures (25°C, 30°C, or 37°C) and pH (unadjusted, 5.65 or 8.65) combinations. The unadjusted pH was found to be pH 6.95 in rice field water and pH 7.79 in pond water. Once the Leptospira were resuspended, tubes were placed in a dark incubator at the appropriate temperature for 12 weeks. For each test combination, growth and viability of the Leptospira isolates were determined by measuring optical density (OD), leptospiral motility, and growth in semisolid EMJH (Figure 1 ).
Table 1.
Species, serovar, strain, sequence type (ST), collection date, and week that Leptospira isolates from Thailand outbreak were found to be non-viable in rice field (R) and pond (P) water at various pH levels*
| Species | Serovar | Strain (BEI number†) | ST | Collection date | pH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5.65 | Unadjusted‡ | 8.65 | |||||||||||
| 25°C | 30°C | 37°C | 25°C | 30°C | 37°C (R,P) | 25°C | 30°C | 37°C (R, P) | |||||
| L. interrogans | Autumnalis | L0382 | 34 | 1-Aug-01 | V All isolates survived to Week 12 at 25°C, 30°C, and 37°C | V All isolates survived to week 12 at 25°C and 30°C | V,11 | V All isolates survived to Week 12 at 25°C and 30°C | V,V | ||||
| L. interrogans | Autumnalis | L0388 | 34 | 6-Aug-01 | V,V | V,V | |||||||
| L. interrogans | Autumnalis | UT105 | 34 | 23-Sep-03 | 10,V | V,8 | |||||||
| L. interrogans | Autumnalis | UT108 | 34 | 25-Sep-03 | 10,11 | V,8 | |||||||
| L. interrogans | Autumnalis | UT342 | 34 | 24-Jul-05 | 10,11 | V,8 | |||||||
| L. interrogans | Autumnalis | UT567 | 34 | 26-Jul-06 | 10,10 | V,V | |||||||
| L. interrogans | Autumnalis | UT670 | 34 | 24-Oct-06 | 10,11 | V,V | |||||||
| L. interrogans | Medanensis | L0448 (NR-20184) | 46 | 23-Aug-01 | 10,12 | V,V | |||||||
| L. interrogans | Medanensis | L0996 (NR-20158) | 46 | 1-Sep-02 | 10,11 | V,8 | |||||||
| L. interrogans | Pyrogenes | L0374 (NR-20157) | 49 | 1-Aug-01 | 10,11 | V,V | |||||||
| L. kirschneri | Grippotyphosa | UT130 (NR-20327) | 68 | 15-May-03 | 10,12 | V,V | |||||||
V = viable: Leptospira isolate was found to be viable at the termination of the study (12 weeks).
Select strains have been deposited at BEI Resources (Manassas, VA).
Unadjusted pH: R = 6.95 and P = 7.79.
Figure 1.
Schematic of how leptospiral isolates were determined to be viable after spiking into suspending fluids at various pHs (unadjusted, 5.65 or 8.65) and incubated at 25°C, 30°C, or 37°C. (A) Optical density (OD) was measured at 400 nm. (B) Leptospiral motility and culturing.
The OD at 400 nm was measured twice each week as a method of monitoring viable Leptospira (Figure 1A).10,11 Repeated measures analysis of variance and Wilks test were performed to compare the mean OD over time across sequence types under differing conditions12; controlling for temperature, pH, and media, there was no difference over time for early year ST34 versus later year ST34 and/or non-ST34 isolates (P = 0.3). The OD measurements did not correlate with viability. All statistical tests were performed using SAS 9.2 (SAS Institute, Cary, NC). P values < 0.05 were considered statistically significant; all tests were two-sided.
Spiked suspending fluids were checked weekly under dark field microscopy to determine if leptospiral motility could be observed. If the leptospires appeared immotile, 500 μL was inoculated into EMJH semisolid weekly and incubated at 30°C for 4 weeks (Figure 1B). A spiked fluid was considered non-viable if three consecutive weeks of inoculated EMJH did not yield growth after the 4-week incubation period. Replicates yielded identical results. The only conditions in which all isolates did not survive to the termination of the study (Week 12) was when incubated at either unadjusted or pH 8.65 at 37°C (Table 1). At the unadjusted pH for both rice field and pond water, only three early year ST34 isolates survived to 12 weeks, all other isolates were found to be non-viable between Weeks 10 and 12. All isolates were viable at Week 12 in rice field water and seven of the isolates were viable in pond water in adjusted pH 8.65 and at 37°C (Table 1).
Survival time data were analyzed using Efron's tail correction to the Product-Limit estimator for mean survival time, pairwise differences were tested with the Sidak multiple comparison adjustment, and Cox proportional hazards model was used for comparing survival rates.13,14 Results are expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). Most of the observations survived to the longest measured point, which led to low or no variability, therefore CIs are not presented. The mean survival time among all isolates was 11.5 weeks; there was no difference in mean survival time among the isolates (P = 0.9). There was no significant difference in survival between ST34 compared with other STs (P = 0.16), early ST34 versus late ST34 (P = 0.7), early ST34 versus early other STs (P = 0.3), or early ST34 versus late ST34 and other STs (P = 0.7), when controlling for media, temperature, and pH. The survival rate was higher at 25°C (HR 0.1, 95% CI 0.04–0.27; P < 0.001) and 30°C (HR 0.1, 95% CI 0.03–0.23; P < 0.001) compared with 37°C. There was a higher survival rate at unadjusted pH compared with pH 8.65 (HR 0.5, 95% CI 0.25–0.91; P = 0.02) and no difference at pH 5.65 compared with pH 8.65 (P = 0.98).
We determined that early year ST34 isolates did not appear to have a fitness advantage over later year ST34 and non-ST34 isolates in the conditions that were tested; no statistically significant differences were found when looking at mean survival or survival rates. Although we did not find statistically significant results indicating a survival advantage of early year ST34s, ST34s from 2001 and 2003 were the only isolates that survived in unadjusted pH rice field and pond water at 37°C to Week 12. One potential issue when determining the mean survival and survival rate was that the study was only conducted for 12 weeks or the limited sample size. The study was conducted for 12 weeks because results from previous studies showed Leptospira isolates did not survive longer than 3 months in various fluids and conditions, with some only surviving for 7 days or less.15–18 The adaptation of leptospires for survival in artificial media could have an effect on their ability to survive in the environment. It has previously been shown that Leptospira maintained for many years in culture media survived better at some pH levels compared with strains that were recently isolated from animals, although this could also be attributed to the fact that they were different serovars.18 Other studies have mentioned if isolates are high or low passage but there has been no comparison of passages versus survival.7,15,17–19 Although the isolates in this study went through a similar number of passages in media, it is not known if these passages would have the same effect for each of them.
The conditions that had an effect on survival of isolates were incubation at high temperature (37°C) and/or basic pH (8.65), similar to previously published studies that showed pH levels outside the neutral zone and higher temperatures (< 35°C) are not favorable for survival of leptospires.15–19 Variations in survival between Leptospira serovars has been shown, with one study finding differences in survival between the four different serovars incubated in distilled water.18 It is difficult to compare the survival of isolates between the different published studies because of the use of different strains and serovars and differences in study design.
There are many challenges when designing a study to mimic the environmental conditions of a microorganism. We did not monitor the pH levels of the liquids throughout the course of the study to see if they remained stable; however, one study found that pH of tap and river water with and without microbiota present did not change at all or changed very slightly (0.1 to 0.4)15 and another study found that pH levels changed toward neutral during an experiment to a greater degree if the initial pH was < 6.0.18 This study did not address the influence of UV light and other microorganisms.
This study did not find a statistically significant difference between survival of early ST34 versus later year ST34 and other STs. If future studies were to be conducted on fitness advantages of the Leptospira from this outbreak, it would be recommended to conduct the study for longer than a 12-week period and attempt to add additional variables such as light and other microorganisms. In addition, it would be beneficial to determine if early year ST34 isolates are better able to infect and cause disease in the host or if they are better adapted to persist in the maintenance host.
ACKNOWLEDGMENTS
We thank Renee Galloway and Sharon Peacock, University of Cambridge, UK for assistance with study design and manuscript review.
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. Names of vendors or manufacturers are provided as examples of available product sources; inclusion does not imply endorsement of the vendors, manufacturers, or products by the Centers for Disease Control and Prevention, the U.S. Department of Health and Human Services.
Footnotes
Authors' addresses: Robyn A. Stoddard, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: RAStoddard@cdc.gov. Duy Bui, CDC, Zoonotic and Select Agent Laboratory, Atlanta, GA, E-mail: dbui85@gmail.com. Dana L. Haberling, Centers for Disease Control and Prevention, Division of High-Consequence Pathogens and Pathology, Atlanta, GA, E-mail: fnj2@cdc.gov. Vanaporn Wuthiekanun, Mahidol University, Wellcome Unit, Faculty of Tropical Medicine, Bangkok, Thailand, E-mail: lek@tropmedres.ac. Janjira Thaipadungpanit, Mahidol University, Mahidol-Oxford Tropical Medicine Research Unit, Bangkok, Thailand, E-mail: janjira@tropmedres.ac. Alex R. Hoffmaster, Centers for Disease Control and Prevention, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, Atlanta, GA, E-mail: ahoffmaster@cdc.gov.
References
- 1.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 3.Thaipadungpanit J, Wuthiekanun V, Chierakul W, Smythe LD, Petkanchanapong W, Limpaiboon R, Apiwatanaporn A, Slack AT, Suputtamongkol Y, White NJ, Feil EJ, Day NP, Peacock SJ. A dominant clone of Leptospira interrogans associated with an outbreak of human leptospirosis in Thailand. PLoS Negl Trop Dis. 2007;1:e56. doi: 10.1371/journal.pntd.0000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tangkanakul W, Smits HL, Jatanasen S, Ashford DA. Leptospirosis: an emerging health problem in Thailand. Southeast Asian J Trop Med Public Health. 2005;36:281–288. [PubMed] [Google Scholar]
- 5.Johnson RC, Harris VG. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J Bacteriol. 1967;94:27–31. doi: 10.1128/jb.94.1.27-31.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner LH. Leptospirosis. 3. Maintenance, isolation and demonstration of leptospires. Trans R Soc Trop Med Hyg. 1970;64:623–646. doi: 10.1016/0035-9203(70)90087-8. [DOI] [PubMed] [Google Scholar]
- 7.Trueba G, Zapata S, Madrid K, Cullen P, Haake D. Cell aggregation: a mechanism of pathogenic Leptospira to survive in fresh water. Int Microbiol. 2004;7:35–40. [PubMed] [Google Scholar]
- 8.Goris MG, Hartskeerl RA. Leptospirosis serodiagnosis by the microscopic agglutination test. Curr Protoc Microbiol. 2014;32:12E 5 1–12E 5 18. doi: 10.1002/9780471729259.mc12e05s32. [DOI] [PubMed] [Google Scholar]
- 9.Cole JR, Jr, Sulzer CR, Pursell AR. Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol. 1973;25:976–980. doi: 10.1128/am.25.6.976-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellinghausen HC., Jr Growth temperatures, virulence, survival, and nutrition of leptospires. J Med Microbiol. 1973;6:487–497. doi: 10.1099/00222615-6-4-487. [DOI] [PubMed] [Google Scholar]
- 11.Schreier S, Triampo W, Doungchawee G, Triampo D, Chadsuthi S. Leptospirosis research: fast, easy and reliable enumeration of mobile leptospires. Biol Res. 2009;42:5–12. [PubMed] [Google Scholar]
- 12.Stevens JP. Applied Multivariate Statistics for the Social Sciences. Fifth edition. Mahwah, NJ: Lawrence Erlbaum Associates; 2009. [Google Scholar]
- 13.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. Second edition. New York: Springer; 2003. [Google Scholar]
- 14.Westfall PH, Tobias RD, Rom D, Wolfinger RD, Hochberg Y. Multiple Comparisons and Multiple Tests Using the SAS System. Cary, NC: SAS Institute; 1999. [Google Scholar]
- 15.Chang SL, Buckingham M, Taylor MP. Studies on Leptospira icterohaemorrhagiae; survival in water and sewage; destruction in water by halogen compounds, synthetic detergents, and heat. J Infect Dis. 1948;82:256–266. doi: 10.1093/infdis/82.3.256. [DOI] [PubMed] [Google Scholar]
- 16.Kirschner L, Maguire T. Survival of Leptospira outside their hosts. N Z Med J. 1957;56:385–391. [PubMed] [Google Scholar]
- 17.Okazaki W, Ringen LM. Some effects of various environmental conditions on the survival of Leptospira pomona. Am J Vet Res. 1957;18:219–223. [PubMed] [Google Scholar]
- 18.Smith CE, Turner LH. The effect of pH on the survival of leptospires in water. Bull World Health Organ. 1961;24:35–43. [PMC free article] [PubMed] [Google Scholar]
- 19.Parker J, Walker M. Survival of a pathogenic Leptospira serovar in response to combined in vitro pH and temperature stresses. Vet Microbiol. 2011;152:146–150. doi: 10.1016/j.vetmic.2011.04.028. [DOI] [PubMed] [Google Scholar]