Abstract
Dengue transmission in Venezuela has become perennial and a major public health problem. The increase in frequency and magnitude of recent epidemics prompted a comprehensive community-based cross-sectional study of 2,014 individuals in high-incidence neighborhoods of Maracay, Venezuela. We found a high seroprevalence (77.4%), with 10% of people experiencing recent infections. Multivariate logistic regression analysis showed that poverty-related socioeconomic factors (place and duration of residence, crowding, household size, and living in a shack) and factors/constraints related to intradomiciliary potential mosquito breeding sites (storing water and used tires) were linked with a greater risk of acquiring a dengue infection. Our results also suggest that transmission occurs mainly at home. The combination of increasingly crowded living conditions, growing population density, precarious homes, and water storage issues caused by enduring problems in public services in Maracay are the most likely factors that determine the permanent dengue transmission and the failure of vector control programs.
Introduction
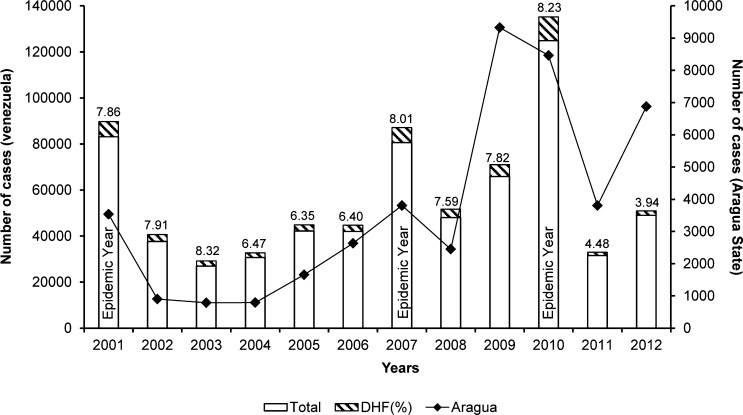
Despite control measures, dengue has become a major public health problem in Venezuela. Epidemics of increasing magnitude regularly occur against a background of an established endemic situation. The most recent and largest outbreak took place in 2010 with 124,931 reported cases; 8.2% of them represented severe cases (Figure 1).1 Concomitantly, the number of severe dengue cases has risen with time. Venezuela reported the highest proportion of severe cases (35.1%) in the Americas during 1980–2007,2 and together with Colombia, Mexico, and Brazil, it is predicted to bear the highest burden of disease in the region.3 Although previous studies have pointed out certain risk factors for dengue transmission,4–6 a detailed evaluation is warranted to identify possible control targets that can inform health authorities and ameliorate future dengue epidemics.7
Figure 1.
Number of reported dengue cases in Venezuela and Aragua from 2001 to 2012. DHF shown as a proportion (hatched bars) of the total number of cases (white bars), and values are shown on top of the bars. Source: Ministerio del Poder Popular para la Salud (MPPS) Boletines Epidemiológicos 2002–2012.1
Dengue virus (DENV) belongs to the Flavivirus genus of the family Flaviviridae.8 DENV is transmitted in urban and periurban settings by female infected day-biting Aedes mosquitoes, predominantly Ae. aegypti and Ae. albopictus.9 There are four closely related serotypes (DENV-1 to -4), and each is capable of causing the entire range of dengue-related disease symptoms. Infected individuals can be asymptomatic or present with clinical manifestations varying from mild febrile illness and dengue fever (DF) to severe illness, such as dengue hemorrhagic fever (DHF) and dengue shock syndrome.10 According to recent estimates, 390 million dengue infections occur annually worldwide, with approximately 96 million apparent infections.3 The World Health Organization (WHO) estimates that 500,000 people develop severe disease, and of these people, 2.5% die.9 Between 2000 and 2006, the Americas experienced 68% of worldwide dengue infections,11 and over the last three decades, the number of dengue cases increased 4.6 times, with a concomitant 8.3 times increase of severe cases.2 Several factors are related to the increase of dengue incidence in the Americas. Among the most important ones are uncontrolled urbanization and absence of public services, such as water supply, sewers, and waste disposal.12–14 Deterioration of mosquito eradication programs, decay in public health infrastructure, and changes in public health policy contribute to the increase of vector density.15 Globalization, international travel, and climate change have also influenced the recent rapid spread of dengue.10
Epidemic outbreaks of DF were first recorded in Venezuela in 196416 and partly attributed to the reintroduction of previously non-circulating DENV serotypes and strains coinciding with increased spread and densities of Ae. aegypti.16–20 In South America, infection with the Asian DENV-2 genotype has been related with higher risk for DHF.17,20,21 The first documented DHF case in Venezuela occurred in 1989 (specifically in Maracay, Aragua) and coincided with the introduction of this strain.22–24 Since that time, Maracay has become one of the most important endemic urban areas of the country with the cocirculation of all four dengue serotypes.5 Recently, the presence of Ae. albopictus has been reported for the first time in Venezuela.25 The introduction of this new vector may affect transmission patterns.26
The persistence and increase of dengue transmission and severe disease in Venezuela (Figure 1) merit an assessment of the epidemiological dynamics of dengue infection. This study was designed to estimate dengue seroprevalence and identify current risk factors for dengue transmission in high-incidence areas of Maracay, Venezuela.
Materials and Methods
Study area.
Maracay is the fourth largest city of Venezuela and has become highly endemic for dengue transmission and DHF epidemics.6,27 It is the capital of Aragua state in the northcentral region of Venezuela (10°15′ N, 67°36′ W) with an estimated 1,300,000 inhabitants.28 The climate is tropical with two defined seasons: a dry (November to April) season and a rainy (May to October) season. The temperature ranges between 25°C and 35°C, with a mean total annual precipitation of 834 mm.
This study was conducted in two municipalities of Maracay with high dengue incidence. Within them, three neighborhoods or barrios (Candelaria, Cooperativa, and Caña de Azúcar) were chosen for their proximity and access to a local (governmental) health center (HC), where dengue cases can be identified. Cooperativa neighborhood is located in the northeast area of Maracay, whereas Caña de Azúcar and Candelaria are close to each other and located in the northwest. To place this study into epidemiological context, national and regional dengue incidence data between the years 2001 and 2012 were compiled from the Epidemiological Bulletins reported by the Venezuelan Ministry of Health (Figure 1).1
Study design.
A cross-sectional study was carried out during the recruitment process of a prospective community-based cohort study to estimate dengue seroprevalence and identify risk factors for dengue infection. The study was set up with the intention of recruiting 2,000 individuals between 5 and 30 years old living in the neighborhoods of Caña de Azúcar (sectors 1 and 2), Cooperativa, and Candelaria in Maracay. Participants were recruited from August of 2010 to January of 2011 through house-to-house visits. The aims and scope of the project were clearly explained to all members of the household. Individuals invited to participate in the study were asked to sign a written informed consent, and a copy was left with the participant. The inclusion criteria were (1) age between 5 and 30 years old, (2) living in the study area with no intention to move in the next 3 years, (3) consenting to attend the designated HC in case of any symptoms, and (4) absence of immune-compromising conditions, such as current immunosuppressive therapy or human immunodeficiency virus infection.
A structured questionnaire was given to consenting individuals, and demographic (age, sex, and place of residence), socioeconomic (occupation), and epidemiological data plus clinical history were collected. Given that informal work is a frequent source of income in developing countries, apart from asking to define their occupation, we asked our interviewees to indicate if they performed any kind of job, were engaged in any kind of study, or did not do any of these types of work at the time of interview. All participants were physically examined by medically qualified study personnel. A 10-mL blood sample was collected to perform baseline dengue serology and a full blood count. A unique identification number was assigned to each participant to maintain anonymity. Socioeconomic and environmental data were gathered through a household questionnaire that included questions on type of housing; household ownership; number of people and rooms in a household (all rooms except bathrooms); the presence of indoor/outdoor water containers (see below), litter, used tires, and other possible mosquito breeding sites; the quality of public services (particularly piped water supply); and the use of mosquito-protective measures (windows/door screening, indoor insecticide sprays/coils, mosquito repellent, mosquito nets, and frequent washing of water containers). In Venezuela, the supply of piped water is not always regular, and some households may store water in tanks and/or containers to have constant access to water. In this study, tanks were defined as large plastic or concrete water storage repositories of at least 1,000-L capacity that supply water to the entire dwelling (mostly through the intradomiciliary piping system), whereas containers were any type of water storage devices of different sizes, including 200-L metal drums. Each house was identified with a unique code, and its geoposition was recorded with a handheld Global Positioning System (GPS; Garmin Ltd.).
The study was approved by the Ethics Review Committee of the Biomedical Research Institute, Carabobo University (Aval Bioetico CBIIB[UC]-014), Maracay, Venezuela; the Ethics, Bioethics and Biodiversity Committee (CEBioBio) of the National Foundation for Science, Technology and Innovation (FONACIT) of the Ministry of Science, Technology and Innovation, Caracas, Venezuela; and the Regional Health Authorities of Aragua State (CORPOSALUD Aragua). The study was conducted according to the principles expressed in the Declaration of Helsinki.29 All adult subjects provided written informed consent, and a parent or guardian of any child participant provided written informed consent on their behalf. Children between 8 and 17 years old provided written informed assent.
Hemaglutination inhibition assay.
Previous DENV infection at baseline for cohort study participants was determined using the hemaglutination inhibition (HI) test.30 Dengue antigens, obtained from mouse brain, were provided by the Laboratory of Viral Immunoserology of the Venezuelan National Institute of Hygiene. The treated samples were screened in serial twofold dilutions from 1:40 to 1:2,560 using DENV-1 as a broadly reactive antigen.31 Those samples with titers below 40 were retested in serial twofold dilutions from 1:10 to 1:40 with a pool of all four DENV antigens using 4–8 hemaglutinin units per antigen. In accordance with the distribution of our data, a titer > 20 was taken as the cutoff point for seropositivity.
Statistical analysis.
Two outcome variables for dengue infection were defined based on serology testing: (1) past dengue infection: HI titers > 20 and (2) recent dengue infection: HI titers ≥ 1,280.31–33 Univariate and multivariate analyses of potential risk factors for dengue infection were performed using SPSS (version 20.0; SPSS Inc., Chicago, IL) and STATA (release 10; Stata Statistical Software, College Station, TX) softwares. Data were analyzed anonymously. Variables considered as confounders were age, sex, and place of residence. Continuous variables were converted into ordered categorical variables when suitable. Proportions were compared using χ2 tests, and means were compared using Z and Student's t test. Fisher's exact test was used when applicable. The Mantel–Haenszel score test examined trends in ordered categorical variables. Logistic regression was used to compare crude and adjusted odds ratios (ORs). Significance was determined at the 5% level (P value ≤ 0.05). Variables with a P value ≤ 0.2 after adjusting by age group were fitted into multivariate logistic regression models and adjusted for additional confounders. Effect modification was analyzed, and resulting models were compared by likelihood ratio test. Two separate final models contained variables independently associated with dengue infection for each of the outcome variables defined above.
Results
General description of the study population.
Between August of 2010 and January of 2011, 2,014 individuals living in 840 households were enrolled. Table 1 shows the general characteristics of the study population. The mean age was 17.2 years and differed between males (15.9 years) and females (18.2 years; P < 0.001) but not between neighborhoods. There was a higher proportion of females recruited (57.1%; P = 0.023), mainly in Cooperativa neighborhood (Table 1). The (geometric) mean number of persons per household was similar between neighborhoods. However, a lower mean number of rooms per household was found in Caña de Azúcar compared with the other neighborhoods (5.45 versus 5.74; P = 0.017) as well as a higher proportion of people living under more crowded conditions (P < 0.001) (Table 1).
Table 1.
General characteristics of the study population (N = 2,014)
| Place of residence | ||||||||
|---|---|---|---|---|---|---|---|---|
| Candelaria | Cooperativa | Caña de Azúcar | Total | |||||
| n | Percent | n | Percent | n | Percent | n | Percent | |
| Age (years) | ||||||||
| 5–10 | 119 | 24.8 | 144 | 24.0 | 195 | 20.9 | 458 | 22.8 |
| 11–15 | 96 | 20.0 | 110 | 18.3 | 204 | 21.9 | 411 | 20.4 |
| 16–20 | 85 | 17.7 | 117 | 19.5 | 188 | 20.2 | 390 | 19.4 |
| 21–25 | 89 | 18.5 | 105 | 17.5 | 148 | 15.9 | 342 | 17.0 |
| 26–30 | 91 | 19.0 | 125 | 20.8 | 197 | 21.1 | 413 | 20.5 |
| Sex | ||||||||
| Male | 226 | 47.1 | 233 | 38.8 | 404 | 43.4 | 864 | 42.9 |
| Female | 254 | 52.9 | 368 | 61.2 | 528 | 56.7 | 1,150 | 57.1 |
| Households | 210 | 25.0 | 267 | 31.7 | 363 | 43.2 | 840 | 100 |
| Number of persons per household* | 5.68 | 1–17 | 6.01 | 1–21 | 6.07 | 1–15 | 5.96 | 1–21 |
| Number of people living in houses with ≤ 4 rooms | 117 | 25.6 | 153 | 25.5 | 266 | 28.9 | 536 | 27.1 |
| Crowding† (people/room ≥ 1.5) | 117 | 26.0 | 152 | 26.5 | 311 | 35.3 | 580 | 30.5 |
Geometric mean (range).
Crowding is defined as the number of people in a household divided by the number of rooms of the household. This variable reports the number of people (and the proportion) who live in houses with high crowding defined as ≥ 1.5 persons per room.
Seroprevalence and previous clinical dengue disease.
The recruitment of participants for our cohort study and therefore, the cross-sectional survey coincided with the largest dengue epidemic experienced by the country and Aragua (Figure 1). Dengue seroprevalence was determined by HI assay in 2,002 participants whose samples were available for testing. Most individuals (1,550; 77.4%) had been previously infected by DENV. Recent dengue infections (HI titers ≥ 1,280) were detected in 10% (201) of the individuals.31,32
Overall, 20.5% (410 of 2,005) of the individuals reported having had dengue, but 55 (13%) of them had HI levels below the cutoff point. Of those individuals who had antibodies showing past or recent dengue infection, 77% (1,190 of 1,544) and 71.3% (142 of 199), respectively, reported not having had dengue previously.
Demographic and socioeconomic risk factors.
Table 2 shows adjacent univariate analyses of risk factors for each of the two outcome variables.
Table 2.
Univariate analysis of demographic risk factors and dengue infection
| Total number of subjects* | Past dengue infection | Recent dengue infection | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Crude OR | 95% Confidence interval | P value (PT)† | n (%) | Crude OR | 95% Confidence interval | P value | ||
| Age (years; N = 2,002) | (< 0.001)† | ||||||||
| 5–10 | 450 | 220 (48.9) | 1 | − | − | 29 (6.4) | 1 | − | − |
| 11–15 | 408 | 296 (72.5) | 2.76 | 2.07–3.67 | < 0.001 | 45 (11.0) | 1.80 | 1.11–2.93 | 0.018 |
| 16–20 | 389 | 338 (86.9) | 6.93 | 4.89–9.81 | < 0.001 | 57 (14.7) | 2.49 | 1.56–3.99 | < 0.001 |
| 21–25 | 342 | 319 (93.3) | 14.50 | 9.14–23.01 | < 0.001 | 37 (10.8) | 1.76 | 1.06–2.93 | 0.029 |
| 26–30 | 413 | 377 (91.3) | 10.95 | 7.42–16.15 | < 0.001 | 33 (8.0) | 1.26 | 0.75–2.12 | 0.381 |
| Sex (N = 2,002) | |||||||||
| Male | 856 | 626 (73.1) | 1 | − | − | 81 (9.5) | 1 | − | − |
| Female | 1,146 | 924 (80.6) | 1.53 | 1.24–1.89 | < 0.001 | 120 (10.5) | 1.12 | 0.83–1.51 | 0.458 |
| Place of residence (N = 2,001) | |||||||||
| Candelaria | 476 | 355 (74.6) | 1 | − | − | 34 (7.1) | 1 | − | − |
| Cooperativa | 595 | 470 (79.0) | 1.28 | 0.96–1.70 | 0.088 | 48 (8.1) | 1.14 | 0.72–1.80 | 0.572 |
| Caña de Azúcar | 930 | 724 (77.8) | 1.19 | 0.93–1.55 | 0.170 | 119 (12.8) | 1.91 | 1.28–2.84 | 0.001‡ |
| Occupation (N = 2,001) | |||||||||
| Unemployed | 123 | 109 (88.6) | 1 | − | − | 12 (9.8) | 1 | − | − |
| Student | 1,369 | 971 (70.9) | 0.31 | 0.18–0.55 | < 0.001 | 142 (10.4) | 1.07 | 0.58–1.99 | 0.830 |
| Housewife/domestic worker | 183 | 168 (91.8) | 1.44 | 0.67–3.10 | 0.353 | 27 (14.8) | 1.60 | 0.78–3.30 | 0.202 |
| Manual worker | 182 | 170 (93.4) | 1.82 | 0.81–4.08 | 0.146 | 11 (6.0) | 0.59 | 0.25–1.40 | 0.233 |
| Merchant/employee/office worker | 119 | 109 (91.6) | 1.40 | 0.60–3.29 | 0.440 | 9 (7.6) | 0.76 | 0.31–1.87 | 0.546 |
| Professional/university staff | 25 | 22 (88.0) | 0.94 | 0.25–3.56 | 0.930 | 0 | 0 | 0 | 0.998 |
Total number of subjects is the denominator for each variable category.
P value of the Mantel–Haenszel score test for trend.
P values that remained or became significant after adjusting by age group (in the text).
Demographic factors.
Age, sex, and occupation were significantly associated with past dengue infection, whereas age and place of residence were related with recent dengue infection (Table 2). As expected, past dengue infection was associated with increasing age (score test for trend P < 0.001) and peaked at ages 21–25 years old. This age group was 14.5 times more likely to have been infected than children ages 5–10 years old (P < 0.001). However, subjects ages 16–20 years old were more likely to be recently infected by DENV (OR = 2.49; P < 0.001). Females were 1.53 times more infected by DENV in the past (P < 0.001), but there was no sex difference with regard to recent DENV infection. The three neighborhoods chosen in this study have a history of high dengue incidence. However, people living in Cooperativa were 1.28 times more likely to be infected in the past than those living in the other two neighborhoods. On the other hand, subjects living in Caña de Azúcar were almost two times more likely to be recently infected by DENV (P = 0.001).
The majority of the individuals recruited into the study reported to be students (68.5%), and of these individuals, 61% were younger than 15 years old. Only 123 (6%) people reported unemployment status. The only significant association within the variable occupation was found for students, who were 69% less likely to have been exposed to DENV in the past (P < 0.001). However, when controlled by age group, the association disappeared (OR = 0.76, P = 0.362). In relation with recent dengue infection, housewives/domestic workers were at a higher risk of acquiring a recent dengue infection. This association became more evident when controlling for age (OR = 1.92, P = 0.086). Individuals who work outside homes were less likely of having a recent infection (Table 2).
Socioeconomic risk factors.
The type of housing (P = 0.061), residing more than 16 years in a household, having a job, or being unemployed without any other activity at the time of the interview increased the likelihood of a past dengue infection, whereas the number of people living in a household and studying were protective (Table 3). The risk of having acquired a recent dengue infection was negatively associated with a higher number of rooms in the house and having a job at the time of interview, whereas a higher number of years living in a household and crowding, defined as the number of people per room in a house, were positively associated (Table 3).
Table 3.
Univariate analysis of socioeconomic risk factors and dengue infection
| Total number of subjects* | Past dengue infection | Recent dengue infection | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Crude OR | 95% Confidence interval | P value | n (%) | Crude OR | 95% Confidence interval | P value | ||
| Type of housing (N = 2,001) | |||||||||
| House/apartment/other | 1,977 | 1,526 (77.2) | 1 | − | − | 199 (10.1) | 1 | − | − |
| Rancho† (shack) | 24 | 23 (95.8) | 6.80 | 0.92–50.47 | 0.061‡ | 2 (8.3) | 0.81 | 0.19–3.48 | 0.779 |
| Number of household rooms (N = 1,967) | |||||||||
| 1–4 | 533 | 408 (76.6) | 1 | − | − | 66 (12.4) | 1 | − | − |
| ≥ 5 | 1,434 | 1,118 (77.9) | 1.08 | 0.86–1.37 | 0.503 | 132 (9.2) | 0.72 | 0.52–0.98 | 0.038‡ |
| Number of persons per household (N = 1,906) | |||||||||
| 1–6 | 1,121 | 890 (79.4) | 1 | − | − | 118 (10.5) | 1 | − | − |
| ≥ 7 | 785 | 592 (75.4) | 0.80 | 0.64–0.99 | 0.040 | 75 (9.6) | 0.90 | 0.66–1.22 | 0.489 |
| Crowding (people/room; N = 1,893) | |||||||||
| 0.17–1.49 | 1,319 | 1,027 (77.9) | 1 | 121 (9.2) | 1 | − | − | ||
| 1.5–8.0 | 574 | 446 (77.7) | 0.99 | 0.78–1.25 | 0.938 | 70 (12.2) | 1.38 | 1.01–1.88 | 0.046‡ |
| Duration of residence (years; N = 1,948) | |||||||||
| 0–16 | 1,475 | 1,067 (72.3) | 1 | − | − | 136 (9.2) | 1 | − | − |
| ≥ 17 | 473 | 444 (93.9) | 1.56 | 1.41–1.72 | < 0.001 | 60 (12.7) | 1.09 | 1.01–1.19 | 0.030 |
| People who did not study or work§ (N = 2,002) | |||||||||
| No | 1,708 | 1,283 (75.1) | 1 | − | − | 164 (9.6) | 1 | − | − |
| Yes | 294 | 267 (90.8) | 3.28 | 2.17–4.94 | < 0.001 | 37 (12.6) | 1.36 | 0.93–1.98 | 0.117 |
| People following any type of study§ (N = 2,000) | |||||||||
| No | 620 | 569 (91.8) | 1 | − | − | 59 (9.5) | 1 | − | − |
| Yes | 1,380 | 979 (70.9) | 0.22 | 0.16–0.30 | < 0.001‡ | 142 (10.3) | 1.09 | 0.79–1.50 | 0.595 |
| People who had a job§ (N = 2,000) | |||||||||
| No | 1,525 | 1,115 (73.1) | 1 | − | − | 167 (11.0) | 1 | − | − |
| Yes | 475 | 433 (91.2) | 3.79 | 2.71–5.31 | < 0.001 | 34 (7.2) | 0.63 | 0.43–0.92 | 0.017‡ |
Total number of subjects is the denominator for each variable category. The univariate analysis for the two outcome variables is presented as in Table 2.
In Venezuela, the word rancho is used to define a shack or informal housing typical of slum areas.
P values that remained or became significant after adjusting by age group (in the text).
At the time of interview.
Rancho is the vernacular name used in Venezuela to define a shack or an informal substandard type of housing typical of slum areas.34 In our area of study, ranchos were found clustered in the outskirts of the neighborhoods. Most of them lacked piped water supply. Despite the small sample size (N = 24 individuals in 13 dwellings), people who lived in ranchos were nearly seven times more likely to have a previous dengue infection than those living in a house with a better structure (P = 0.061) (Table 3). This association was not confounded by age, sex, number of people living in the house, or crowding.
The number of rooms and persons per household as well as crowding are surrogate markers of socioeconomic status. Individuals who inhabited households with more than four rooms were less likely to have had a recent DENV infection (P = 0.038), whereas those sharing a crowded household with more than 1.5 persons per room were at higher risk (OR = 1.4, P = 0.046) (Table 3). The strength of both associations increased when controlled by age. A larger number of household dwellers decreased the risk of past dengue infection (Table 3), but this association disappeared when controlled by age (P = 0.207).
As expected, the longer that a person lives in an endemic area, the higher the likelihood of infection by DENV. Both recent and past dengue infection were positively associated with the number of years that people have lived in the same residence (Table 3). The risk of past dengue infection increased by 6.3% for every 1 year lived in the household, and those who inhabited the same place for more than 16 years were at a 1.56 times higher risk. This effect was not completely explained by age, because the association remained significant, although its strength diminished after adjustment (OR = 1.18, P = 0.004). The association of this variable with recent dengue infection is somewhat weaker and loses significance after adjusting (P = 0.088).
Performing any kind of job, following any kind of study, or not doing either at the time of interview were all associated with past dengue infection, whereas recent dengue infection was only significantly associated with having a job (Table 3, last three variables). After adjusting for age, only following any kind of study remained significantly associated and was protective against acquiring dengue in the past, whereas having a job provided almost 40% protection against being recently infected by dengue and remained significant after adjustment (P = 0.005).
Household and environmental risk factors.
Univariate analysis showed that storing water at home in general and storing water in containers (whether stored in tanks or not) increased the risk of having been infected with DENV in the past by 1.4 (Table 4). People who stored water in containers were 1.34 times more likely to have acquired a recent DENV infection (Table 4). The number of water storage devices (tanks, containers, and other) ranged from 1 to 20 per house, with a mean of 1.8; however, there was no relation between the number of storage devices and dengue seropositivity. Approximately 94% (1,897 of 2,014) of the individuals reported that their homes were provided with all public services, such as water, electricity, sewer, and urban garbage collection, whereas 72% reported daily supply of piped water or regular water supply to their dwellings. There was no association between the availability of all public services and regular water supply with dengue infection. The different ways of final trash disposal were not associated with DENV infection (Table 4). However, the presence of used car tires in people's gardens/patios increased the risk of having had a recent dengue infection by 1.58 (P = 0.035). Other known potential mosquito breeding sites, such as litter and bottles in people's gardens (outdoors) or flower/plant pots indoors, did not show a statistically significant association with dengue infection (Table 4). Similar results were found after adjusting by age group.
Table 4.
Univariate analysis of household and environmental risk factors and dengue infection
| Total number subjects* | Past dengue infection | Recent dengue infection | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Crude OR | 95% Confidence interval | P value | n (%) | Crude OR | 95% Confidence interval | P value | ||
| Water storage at home (N = 1,993) | |||||||||
| No | 175 | 125 (71.4) | 1 | − | − | 11 (6.3) | 1 | − | − |
| Yes | 1,818 | 1,420 (78.1) | 1.42 | 1.00–2.00 | 0.044 | 190 (10.5) | 1.74 | 0.93–3.26 | 0.084 |
| Water storage in tanks (N = 1,993) | |||||||||
| No | 428 | 334 (78.0) | 1 | − | − | 36 (8.4) | 1 | − | − |
| Yes | 1,565 | 1,211 (77.4) | 0.96 | 0.74–1.25 | 0.773 | 165 (10.5) | 1.28 | 0.88–1.87 | 0.195 |
| Water storage in containers (N = 1,993) | |||||||||
| No | 871 | 647 (74.3) | 1 | − | − | 75 (8.6) | 1 | − | − |
| Yes | 1,122 | 898 (80.0) | 1.39 | 1.12–1.71 | 0.002† | 126 (11.2) | 1.34 | 0.99–1.81 | 0.055 |
| Trash disposal | |||||||||
| Collected by public services (N = 1,993) | |||||||||
| No | 435 | 333 (76.6) | 1 | − | − | 45 (10.3) | 1 | − | − |
| Yes | 1,558 | 1,212 (77.8) | 1.07 | 0.83–1.38 | 0.584 | 156 (10.0) | 0.96 | 0.68–1.37 | 0.839 |
| On grounds of the house (N = 1,993) | |||||||||
| No | 1,961 | 1,519 (77.5) | 1 | − | − | 201 (10.2) | − | − | − |
| Yes | 32 | 26 (81.2) | 1.26 | 0.52–3.08 | 0.611 | 0 | − | − | 0.068‡ |
| Potential mosquito breeding sites | |||||||||
| Litter outdoors (N = 1,993) | |||||||||
| No | 1,194 | 939 (78.6) | 1 | − | − | 119 (10.0) | 1 | − | − |
| Yes | 799 | 606 (75.8) | 0.85 | 0.69–1.06 | 0.143 | 82 (10.3) | 1.03 | 0.83–1.39 | 0.830 |
| Car tires outdoors (N = 1,993) | |||||||||
| No | 1,791 | 1,386 (77.4) | 1 | − | − | 172 (9.6) | 1 | − | − |
| Yes | 202 | 159 (78.7) | 1.08 | 0.76–1.54 | 0.669 | 29 (14.4) | 1.58 | 1.03–2.41 | 0.035† |
| Bottles outdoor (N = 1,993) | |||||||||
| No | 1,296 | 1,004 (77.5) | 1 | − | − | 131 (10.1) | 1 | − | − |
| Yes | 697 | 541 (77.6) | 1.01 | 0.81–1.26 | 0.939 | 70 (10.0) | 0.99 | 0.73–1.35 | 0.963 |
| Indoor flower vases (N = 1,994) | |||||||||
| No | 873 | 667 (76.4) | 1 | − | − | 79 (9.0) | 1 | − | − |
| Yes | 1,120 | 878 (78.4) | 1.12 | 0.91–1.38 | 0.291 | 122 (10.9) | 1.23 | 0.91–1.66 | 0.176 |
Total number of subjects is the denominator for each variable category. The univariate analysis for the two outcome variables is presented as in Table 2.
P values that remained or became significant after adjusting by age group (in the text).
Fisher's exact test.
Mosquito preventive measures.
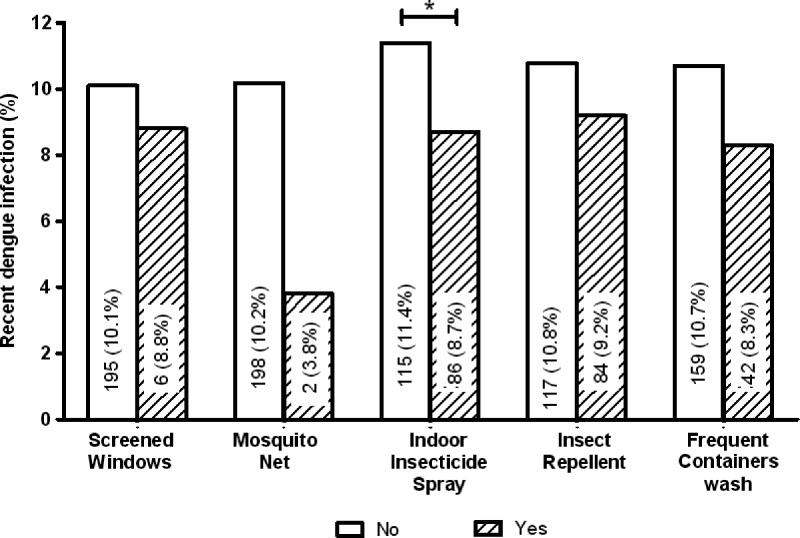
All mosquito prevention measures showed a degree of protection against past (non-significant associations; data not shown) and particularly, recent dengue infection (Figure 2). In Venezuela, the use of indoor insecticide spraying is one of the most frequently used personal control measures against mosquito bites. This measure reduced the risk of recent dengue infection by 30% (P = 0.046). The strongest protective effect against recent infection was experienced by those reporting the use of mosquito nets (OR = 0.34), although the association was not significant (P = 0.14), probably because of the small sample size (N = 53).
Figure 2.
Mosquito prevention measures and recent dengue infection. Association of the use of measures against mosquitoes or mosquito breeding sites and the risk of recent dengue infection. Values within bars indicate the number and proportion (in parentheses) of seropositive individuals using/performing each measure (yes; hatched bars) compared with seropositive people not using/carrying out those measures (no; white bars). The total sample size for each mosquito prevention measure variable was N = 1,993, except for the variable mosquito net (N = 1,985). Screened windows indicates household's where windows/doors houses were/were not screened. Mosquito net indicates people reporting/not reporting the use of nets. Indoor insecticide spray indicates the proportion of people reporting/not reporting the use of insecticide spray to kill indoor adult mosquitoes. Insect repellent indicates the proportion of people reporting/not reporting the use of insect repellent body lotions/spray to repel mosquitoes. Frequent containers wash indicates the proportion of people reporting/not reporting the washing of containers used for storing water at least one time per week. *OR = 0.74 (0.55–0.99); P = 0.046.
Multivariate analyses.
The final models of factors independently associated with past and recent dengue infection are shown in Table 5. Increasing age, storing water in containers at home, increasing length of time living in the same residence, and living in a shack (rancho) were all positively associated with past dengue infection (Table 5, final model of risk factors for past dengue infection). Those factors that increased the risk of recent dengue infection were age, living in Caña de Azúcar (place of residence), and having used car tires outdoors of households. People who reported having a job or living in a house with more than four rooms were less likely to have acquired a recent dengue infection. Individuals ages 16–20 years old showed a higher risk of having been infected recently with DENV. Participants living in Caña de Azúcar were 1.71 times more at risk of exposure to a recent dengue infection than participants living in Cooperativa and Candelaria, whereas those individuals who kept used tires in their household's gardens or patios were 1.56 times more likely to have been infected recently with DENV. People who reported performing a job of any kind had a 44% reduction in the risk of being infected by DENV, whereas a 26% reduction was observed for those living in better housing conditions (represented by households with more rooms).
Table 5.
Multivariate logistic regression models of risk factors for dengue infection
| OR | 95% Confidence interval | P value | |
|---|---|---|---|
| Final model of risk factors for past dengue infection (N = 1,939) | |||
| Age (years) | |||
| 5–10 | 1 | − | − |
| 11–15 | 2.65 | 1.98–3.54 | < 0.001 |
| 16–20 | 5.72 | 3.95–8.29 | < 0.001 |
| 21–30 | 9.31 | 6.44–13.46 | < 0.001 |
| Water storage in containers | |||
| No | 1 | − | − |
| Yes | 1.35 | 1.07–1.71 | 0.013 |
| Number of years living in household | |||
| 0–16 | 1 | − | − |
| ≥ 17 | 1.18 | 1.06–1.32 | 0.004 |
| Type of housing | |||
| House/apartment/other | 1 | − | − |
| Rancho* | 7.65 | 0.99–59.15 | 0.051 |
| Final model of risk factors for recent dengue infection (N = 1,965) | |||
| Age (years) | |||
| 5–10 | 1 | − | − |
| 11–15 | 1.92 | 1.16–3.17 | 0.011 |
| 16–20 | 2.77 | 1.70–4.52 | < 0.001 |
| 21–30 | 2.07 | 1.26–3.39 | 0.004 |
| Place of residence | |||
| Candelaria/Cooperativa | 1 | − | − |
| Caña de Azúcar | 1.71 | 1.26–2.31 | < 0.001 |
| Used car tires outdoors | |||
| No | 1 | − | − |
| Yes | 1.56 | 1.01–2.40 | 0.046 |
| Having a job | |||
| No | 1 | − | − |
| Yes | 0.56 | 0.36–0.86 | 0.008 |
| Number of rooms in the household | |||
| 1–4 | 1 | − | − |
| ≥ 5 | 0.73 | 0.53–1.00 | 0.053 |
The word rancho in Venezuela is used to define a shack or informal housing typical of slum areas.
Discussion
During the recruitment phase of a dengue community-based cohort study in Maracay, Aragua, Venezuela, a baseline cross-sectional study involving all 2,014 recruited individuals was carried out. The study aimed to estimate dengue seroprevalence and assess risk factors for dengue transmission that could partially explain the increase in the frequency and magnitude of recent epidemics in Venezuela, despite control measures. We found that 77.4% of the individuals had been exposed to dengue infection. However, only 23% recalled a disease episode; 10% of the sampled population showed serological markers of a recent dengue infection. Multivariate analysis showed that socioeconomic factors (place of residence, number of rooms in the household, number of years living in this household, and living in a shack) and environmental factors related to mosquito breeding sites (storing water at home and having used car tires in gardens/patios) determine the risk of acquiring a dengue infection in this population. Our data also suggest that people become infected mostly at home.
Maracay is one of the highly dengue endemic cities in Venezuela. The gradual introduction or re-emergence of DENV serotypes has been associated with substantial epidemics, which mostly began in the central region of the country.6,17,18,20,35 In the last decade, Venezuela has witnessed three dengue epidemic years (Figure 1).1 Dengue incidence in Aragua has mirrored these national epidemics. However, the last epidemic was exceptional in magnitude and duration, lasting for 2 years (Figure 1). Our cross-sectional survey took place during the second half of 2010, when the epidemic was at its peak. We found a high overall seroprevalence of 77.4% among the individuals recruited into the study. A previous study in schoolchildren 5–13 years old in Maracay carried out during the 2001 epidemic found a lower prevalence (51%)27 compared with our study, where 59% of children from the same age group were seropositive. This finding denotes an increase in transmission intensity in time, although the increase is smaller than in areas where DENV was recently introduced.36 Similar high population-based seroprevalences have been reported from other Latin American countries, such as Peru,37 Mexico,38 Brazil,39,40 and Nicaragua, which reported higher figures.41 Transmission intensity in Maracay is lower compared with the current figures for Asia42,43 but similar to those reported 15–20 years ago.44,45
Poverty-related socioeconomic factors found in our study increased the risk for both recent and past infection. We have also found indirect evidence suggesting that DENV transmission occurs mainly at home, which is in agreement with other studies.39,46 People who spent more time within homes, such as domestic workers and housewives, were at a higher risk of acquiring a recent dengue infection (Table 2), whereas individuals who worked away from home were 44% less likely to have been recently infected (Table 5). The place of residence, living in households with fewer rooms, duration of residence in the same household, and living in a shack all increased the likelihood of dengue infection. Individuals who lived in more spacious dwellings were 27% less likely to have had a recent dengue infection, whereas those sharing a crowded household were at higher risk. Braga and others39 reported a three times increase in seropositivity in those living in crowded conditions in Brazil, whereas others have reported that low income was the main risk factor for past and recent dengue infection.47 In our study neighborhoods, population growth and the need for extra income resulted in the subdivision of one-family dwellings into smaller apartments to house two or more families. The consequences are more crammed living conditions and deprivation.48 Furthermore, people who live in ranchos or shacks are subjected to the poorest living standards34 and higher dengue risk.33,39,40 Despite the small sample size, living in ranchos was strongly associated with the risk of past dengue exposure (OR = 7.65, P = 0.051). This result suggests that the low socioeconomic situation of these individuals had not substantially changed over time,48 keeping them at a higher risk of dengue infection. Between 0.4% and 21% of the dwellings in Aragua are classified as ranchos.49 Unplanned urbanization and population growth are some of the major determinants that have shaped the spread and persistence of dengue in the last decades.2,50,51
Although most people in Maracay are aware of dengue as a disease, preventive measures to avoid mosquito breeding sites are not always taken.52 People who kept discarded car tires on their household premises had a 50% higher risk of a recent dengue infection, consistent with another study.53 Vehicle tires have played a major role in the international spread of dengue.54 They seem to be a preferred breeding site for Ae. aegypti55 as well as Ae. albopictus,56 a newly introduced species in Venezuela.25 Other intradomiciliary potential mosquito breeding sites, such as indoor flower vases, discarded bottles, animal pans and other type of litter kept in the open, were not associated with dengue seropositivity in our study, contrary to other studies.6,53 Individuals who lived in dwellings where water was stored had, on average, a 1.4 times higher seroprevalence indicative of recent or past DENV infection (Table 4), which is in agreement with some57 but not other47 studies. In our multivariate analysis, storing water in containers remained independently associated with dengue infection in the past, corroborating that this practice is not new and may account for sustained dengue transmission at our study sites.6,14 People in all neighborhoods reported long-lasting deficits in public services, such as frequent and prolonged interruptions in water supply and electricity and irregular and insufficient garbage collection. Such shortcomings in public services have been associated with higher DF and DHF incidence and persistence in a 6-year analysis (1993–1998) in Maracay that included our study areas.6 Although present for many years, these inadequacies have become more pronounced in recent years, because people now report daily interruptions of water supply for up to 12 hours or several days a week. Shortage of piped water supply has obliged residents to store water intradomiciliary, maintaining adequate breeding conditions for dengue vectors during the dry season and throughout the year.6,14,50,58
The three neighborhoods selected for our study are areas of high incidence and persistence of dengue.6 Although there were no significant differences between areas for past dengue seroprevalence, people living in Caña de Azúcar were 71% more at risk of acquiring a recent infection (Tables 2 and 5, final model of risk factors for recent dengue infection). This neighborhood is one of the most densely populated areas of Maracay.49 Caña de Azúcar had a higher proportion of water storage in general (98%; P < 0.001) and in containers (67%; P < 0.001) than the other neighborhoods combined with living in smaller houses and more crowded conditions (Table 1), increasing the chance for dengue transmission.7,40 The characteristics of this neighborhood seem to encompass the major risk factors found in this study. However, place of residence remained independently associated with dengue infection in the multivariate model, implying that hidden confounders not measured in this study may determine the higher dengue transmission in this neighborhood.5,36,59 Finally, we evaluated the effect of personal mosquito prevention measures on the risk of dengue infection and found that all measures afforded some degree of protection. However, only the indoor use of insecticide spray was significantly associated with a lower prevalence of recent infection (Figure 2), which is in agreement with other studies.60 Mosquito nets showed the biggest effect against recent infection, but random error could not be excluded because of the small sample size (N = 53).
Limitations.
In Cooperativa neighborhood, 5–10% of households with a probable higher socioeconomic status refused to participate. Socioeconomic variables were similar between Cooperativa and Candelaria, where refusal was minimal. Therefore, we believe that selection bias in Cooperativa was small. A degree of recall bias is present when interviewing people about past experiences, such as having had dengue. However, people's awareness about dengue is high in Maracay; thus, we do not expect a significant bias.
Conclusions and implications for dengue control.
To our knowledge, this study is the first comprehensive, systematic, community-based study of the impact of dengue and risk factors for transmission in Venezuela. High dengue seroprevalences were found in the studied neighborhoods of Maracay. An important proportion of the population with pre-existing immunity is a risk factor for the development of DHF in Venezuela.27,61,62 A high proportion of individuals with dengue antibodies denied having had dengue previously. Dengue surveillance in Aragua is based on a proactive system, whereby laboratory confirmation of suspected cases identified in health centers is made.20 This system may recognize increases in symptomatic cases before epidemics but might not detect increases in silent transmission of unapparent infections. We suggest to complement this method with serological surveillance at the community level during interepidemic years in sentinel areas already known for their high and perennial dengue incidence.5,6,63
In an endemic situation where all four DENV serotypes circulate, factors other than solely reintroduction of different DENV genotypes may influence the maintenance of dengue transmission and rise in epidemic peaks.2 Our findings show that socioeconomic status and environmental factors/constraints linked to intradomiciliary potential mosquito breeding sites determined the risk of recent and past dengue infection. Moreover, transmission seems to occur mainly at home; thus, control strategies should be aimed at reducing vector–host contact in residential areas. Increasingly crowded living conditions, higher population density,51 precarious homes (shacks), and a high proportion of households storing water because of lasting problems in public services in Maracay4,6,14 are the most likely factors that determine the permanent dengue transmission and the failure of vector control programs.
Dengue control in Venezuela is restricted to actions around the notification of suspected dengue cases, such as fogging around blocks of houses of reported cases, and campaigns of larval/pupae control plus information on dengue preventive measures during high-transmission/epidemic periods.20,64 Unfortunately, these focal activities have proven unsuccessful.51,52,65 We suggest to improve the current focal control activities by (1) establishing community sentinel site surveillance alongside the already existing health center-based surveillance, (2) establishing a permanent and updated vector control program,52,64,66 and (3) continuing public education on dengue prevention measures (e.g., elimination of discarded used tires). Additionally, political commitment is essential to direct enough resources to improve public services and socioeconomic conditions of the less privileged if a change in epidemic patterns is to be expected.
ACKNOWLEDGMENTS
The authors thank the inhabitants, community leaders, and community councils of our study neighborhoods Candelaria, la Cooperativa, and Caña de Azúcar. We express our gratitude to the late Prof. Francisco Triana (ex-Director of Instituto de Investigaciones Biomédicas de la Universidad de Carabobo [BIOMED-UC]) and all members of this institute for their support, especially the members of the laboratory for dengue and other viral diseases. Our thanks go to Dr. Matilde Jimenez, Lic. Maritza Cabello de Quintana, and other staff at the Laboratorio Regional de Diagnóstico e Investigación del Dengue y otras Enfermedades Virales (LARDIDEV) for epidemiological information on dengue in Aragua. We also thank Dr. Angel Melchor Director of the Regional Ministry of Health (CORPOSALUD-ARAGUA); Lic. Zulay Ramirez and Lic. Milagros Viloria (heads of the laboratory of Ambulatorio del Norte, where part of the participants' baseline blood counts were done); Dr. María F. Correa, Dr. Alexis Rodriguez (Director and Subdirector of the National Institute of Health “Rafael Rangel,” respectively), and staff members of the Viral Sero-Immunological Section of this institute for support and assistance with the hemaglutination inhibition assay; Dr. José Pauletti for his invaluable technical and logistical support; and Prof. Hajo Grundmann for critical review of the manuscript. We finally thank all field workers, technicians, and nurses who participated in the study.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This study received financial support from Shell de Venezuela, Industrias Venezolanas de Iluminación S.A. (IVISA), Ferretería Hermanos Fridegotto, PC Actual Valencia according to the Organic Law of Science, Technology and Innovation (LOCTI; Certification Number DGCAFIDCTI/204-214-10) and was approved by the Coordinación de Aplicación de Fondos e Incentivos para el Desarrollo de Planes de Ciencia, Tecnología e Innovación. Financial support was also through the Fondo Nacional de Ciencia y Tecnología e Innovación (FONACIT; Project 2011000303, Contract Number 201100129), Venezuelan Ministry of Science, Technology and Innovation, Venezuela. The project was also partly financed by the Vollmer Foundation of Venezuela and the Department of Medical Microbiology, Molecular Virology Section, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Authors' addresses: Zoraida I. Velasco-Salas, Jan C. Wilschut, and Adriana Tami, Department of Medical Microbiology, University Medical Center Groningen, Groningen, The Netherlands, E-mails: z.velasco@umcg.nl, zvelasco@uc.edu.ve, J.C.Wilschut@umcg.nl, and a.tami@umcg.nl. Gloria M. Sierra, Diamelis M. Guzmán, and Guillermo Comach, Laboratorio Regional de Diagnóstico e Investigación del Dengue y otras Enfermedades Virales (LARDIDEV), Instituto de Investigaciones Biomédicas de la Universidad de Carabobo (BIOMED-UC), Maracay, Venezuela, E-mails: gmsierrac@yahoo.com, diaguz17@yahoo.es, and gcomach@yahoo.com. Julio Zambrano, Instituto Nacional de Higiene “Rafael Rangel,” Ministerio del Poder Popular para la Salud, Caracas, Venezuela, E-mail: jcz_2000@yahoo.com. Daniel Vivas, Unidad de Proyectos de Aragua, Facultad de Ciencias de la Salud, Universidad de Carabobo, Maracay, Venezuela, E-mail: vivaszdaniel@yahoo.com.
References
- 1.Ministerio del Poder Popular para la Salud (MPPS) Boletines Epidemiologicos (2002–2012) 2013. http://www.mpps.gob.ve/index.php?option=com_phocadownload&view=section&id=4:boletin-epidemiologico&Itemid=915 Available at. Accessed March. 3, 2013.
- 2.San Martin JL, Brathwaite O, Zambrano B, Solorzano JO, Bouckenooghe A, Dayan GH, Guzman MG. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg. 2010;82:128–135. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrera R, Navarro JC, Mora JD, Dominguez D, Gonzalez J. Public service deficiencies and Aedes aegypti breeding sites in Venezuela. Bull Pan Am Health Organ. 1995;29:193–205. [PubMed] [Google Scholar]
- 5.Barrera R, Delgado N, Jimenez M, Villalobos I, Romero I. Stratification of a city with hyperendemic dengue hemorrhagic fever. Rev Panam Salud Publica. 2000;8:225–233. doi: 10.1590/s1020-49892000000900001. [DOI] [PubMed] [Google Scholar]
- 6.Barrera R, Delgado N, Jimenez M, Valero S. Eco-epidemiological factors associated with hyperendemic dengue haemorrhagic fever in Maracay city, Venezuela. Dengue Bull. 2002;26:84–94. [Google Scholar]
- 7.Kuno G. Review of the factors modulating dengue transmission. Epidemiol Rev. 1995;17:321–335. doi: 10.1093/oxfordjournals.epirev.a036196. [DOI] [PubMed] [Google Scholar]
- 8.Westaway E, Blok J. Taxonomy and evolutionary relationships of flaviviruses. In: Gubler DJ, Kuno G, editors. Dengue and Dengue Hemorrhagic Fever. London, UK: CAB International; 1997. pp. 147–173. [Google Scholar]
- 9.World Health Organization Dengue and Severe Dengue. Fact Sheet Number 117. 2012. http://www.who.int/mediacentre/factsheets/fs117/en/ Available at. Accessed July 10, 2013.
- 10.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . Dengue Net. 2009. http://www.who.int/csr/disease/dengue/denguenet/en/index.html Available at. Accessed April 21, 2009. [Google Scholar]
- 12.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 13.Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 14.Barrera R, Avila J, Gonzalez-Tellez S. Unreliable supply of potable water and elevated Aedes aegypti larval indices: a causal relationship? J Am Mosq Control Assoc. 1993;9:189–195. [PubMed] [Google Scholar]
- 15.Guzman MG, Kouri G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol. 2003;27:1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 16.Pan American Health Organization (PAHO) Dengue in the Caribbean, 1977; Proceedings of a workshop held in; Montego Bay, Jamaica. 1977. May 8–11, 1978. PAHO Sci Publ. 1979, 375. [Google Scholar]
- 17.Uzcategui NY, Camacho D, Comach G, Cuello de Uzcategui R, Holmes EC, Gould EA. Molecular epidemiology of dengue type 2 virus in Venezuela: evidence for in situ virus evolution and recombination. J Gen Virol. 2001;82:2945–2953. doi: 10.1099/0022-1317-82-12-2945. [DOI] [PubMed] [Google Scholar]
- 18.Uzcategui NY, Comach G, Camacho D, Salcedo M, Cabello de Quintana M, Jimenez M, Sierra G, Cuello de Uzcategui R, James WS, Turner S, Holmes EC, Gould EA. Molecular epidemiology of dengue virus type 3 in Venezuela. J Gen Virol. 2003;84:1569–1575. doi: 10.1099/vir.0.18807-0. [DOI] [PubMed] [Google Scholar]
- 19.Gubler DJ, Clark GG. Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerg Infect Dis. 1995;1:55–57. doi: 10.3201/eid0102.952004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camacho DE, Alvarez M, Rodriguez-Henriquez F, de Quintana M, Soler M, Chiarello A, Sierra G, Comach G. Laboratory diagnosis of dengue virus infections in Aragua state, Venezuela: October 1997–December 1998. Invest Clin. 2003;44:91–103. [PubMed] [Google Scholar]
- 21.Rico-Hesse R. Molecular evolution and distribution of dengue viruses type 1 and 2 in nature. Virology. 1990;174:479–493. doi: 10.1016/0042-6822(90)90102-w. [DOI] [PubMed] [Google Scholar]
- 22.Pan American Health Organization (PAHO) Dengue hemorrhagic fever in Venezuela. Epidemiol Bull. 1990;11:7–9. [PubMed] [Google Scholar]
- 23.Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, Boshell J, de Mesa MT, Nogueira RM, da Rosa AT. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–251. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]
- 24.Salas RA, Tovar D, Barreto A, de Miller E, Leitmeyer K, Rico-Hesse R. Serotypes and genotypes of dengue virus circulating in Venezuela, 1990–1997. Acta Cient Venez. 1998;49((Suppl 1)):33–37. [PubMed] [Google Scholar]
- 25.Navarro J, Zorrilla A, Moncada N. First record of Aedes albopictus (skuse) in Venezuela. Its importance as dengue vector and actions to address it. Bol Mal Salud Amb. 2009;49:161–166. [Google Scholar]
- 26.Ali M, Wagatsuma Y, Emch M, Breiman RF. Use of a geographic information system for defining spatial risk for dengue transmission in Bangladesh: role for Aedes albopictus in an urban outbreak. Am J Trop Med Hyg. 2003;69:634–640. [PubMed] [Google Scholar]
- 27.Comach G, Blair PJ, Sierra G, Guzman D, Soler M, de Quintana MC, Bracho-Labadie M, Camacho D, Russell KL, Olson JG, Kochel TJ. Dengue virus infections in a cohort of schoolchildren from Maracay, Venezuela: a 2-year prospective study. Vector Borne Zoonotic Dis. 2009;9:87–92. doi: 10.1089/vbz.2007.0213. [DOI] [PubMed] [Google Scholar]
- 28.Instituto Nacional de Meteorologia (INAMEH) http://www.inameh.gob.ve/pestadistico.php Available at. Accessed June 14, 2012.
- 29.World Medical Association (WMA) Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. 2013. http://www.wma.net/en/30publications/10policies/b3/ Available at. Accessed April 12, 2013. [DOI] [PubMed]
- 30.Clarke D, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization . Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd Ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 32.Balmaseda A. Manual de procedimientos de técnicas para el diagnóstico del dengue. Organización Panamericana de la Salud. Oficina regional de la Organización Mundial de la salud. 2002. http://www.paho.org/Spanish/AD/DPC/CD/manual-procedimientos-tecnicos.pdf Available at. Accessed September 18, 2012.
- 33.Waterman SH, Novak RJ, Sather GE, Bailey RE, Rios I, Gubler DJ. Dengue transmission in two Puerto Rican communities in 1982. Am J Trop Med Hyg. 1985;34:625–632. doi: 10.4269/ajtmh.1985.34.625. [DOI] [PubMed] [Google Scholar]
- 34.UN-HABITAT Slums of the World: The Face of Urban Poverty in the New Millennium?; United Nations Human Settlements Programme (UN-HABITAT) Report; 2003. United Nations, Nairobi, Kenya. [Google Scholar]
- 35.Rodriguez-Roche R, Villegas E, Cook S, Poh Kim PA, Hinojosa Y, Rosario D, Villalobos I, Bendezu H, Hibberd ML, Guzman MG. Population structure of the dengue viruses, Aragua, Venezuela, 2006–2007. Insights into dengue evolution under hyperendemic transmission. Infect Genet Evol. 2012;12:332–344. doi: 10.1016/j.meegid.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siqueira-Junior JB, Maciel IJ, Barcellos C, Souza WV, Carvalho MS, Nascimento NE, Oliveira RM, Morais-Neto O, Martelli CM. Spatial point analysis based on dengue surveys at household level in central Brazil. BMC Public Health. 2008;8:361. doi: 10.1186/1471-2458-8-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison AC, Minnick SL, Rocha C, Forshey BM, Stoddard ST, Getis A, Focks DA, Russell KL, Olson JG, Blair PJ, Watts DM, Sihuincha M, Scott TW, Kochel TJ. Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis. 2010;4:e670. doi: 10.1371/journal.pntd.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarrete-Espinosa J, Acevedo-Vales JA, Huerta-Hernandez E, Torres-Barranca J, Gavaldon-Rosas DG. Prevalence of dengue and leptospira antibodies in the state of Veracruz, Mexico. Salud Publica Mex. 2006;48:220–228. doi: 10.1590/s0036-36342006000300006. [DOI] [PubMed] [Google Scholar]
- 39.Braga C, Luna CF, Martelli CM, de Souza WV, Cordeiro MT, Alexander N, de Albuquerque Mde F, Junior JC, Marques ET. Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop. 2010;113:234–240. doi: 10.1016/j.actatropica.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honorio NA, Nogueira RM, Codeco CT, Carvalho MS, Cruz OG, Magalhaes Mde A, de Araujo JM, de Araujo ES, Gomes MQ, Pinheiro LS, da Silva Pinel C, Lourenco-de-Oliveira R. Spatial evaluation and modelling of dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2009;3:e545. doi: 10.1371/journal.pntd.0000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balmaseda A, Hammond SN, Tellez Y, Imhoff L, Rodriguez Y, Saborio SI, Mercado JC, Perez L, Videa E, Almanza E, Kuan G, Reyes M, Saenz L, Amador JJ, Harris E. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop Med Int Health. 2006;11:935–942. doi: 10.1111/j.1365-3156.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 42.Thai KT, Binh TQ, Giao PT, Phuong HL, Hung le Q, Van Nam N, Nga TT, Groen J, Nagelkerke N, de Vries PJ. Seroprevalence of dengue antibodies, annual incidence and risk factors among children in southern Vietnam. Trop Med Int Health. 2005;10:379–386. doi: 10.1111/j.1365-3156.2005.01388.x. [DOI] [PubMed] [Google Scholar]
- 43.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 44.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 45.Graham RR, Juffrie M, Tan R, Hayes CG, Laksono I, Ma'roef C, Erlin, Sutaryo, Porter KR, Halstead SB. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia. I. Studies in 1995–1996. Am J Trop Med Hyg. 1999;61:412–419. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 46.Yew YW, Ye T, Ang LW, Ng LC, Yap G, James L, Chew SK, Goh KT. Seroepidemiology of dengue virus infection among adults in Singapore. Ann Acad Med Singapore. 2009;38:667–675. [PubMed] [Google Scholar]
- 47.Brunkard JM, Robles Lopez JL, Ramirez J, Cifuentes E, Rothenberg SJ, Hunsperger EA, Moore CG, Brussolo RM, Villarreal NA, Haddad BM. Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg Infect Dis. 2007;13:1477–1483. doi: 10.3201/eid1310.061586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization and the World Bank . Dying for Change: Poor People's Experience of Health and Ill Health. The Voices of the Poor Study. Geneva: World Health Organization; 2002. [Google Scholar]
- 49.Instituto Nacional de Estadístitca (INE) http://www.ine.gov.ve/ Available at. Accessed June 2012.
- 50.San Martin JL, Brathwaite-Dick O. Integrated strategy for dengue prevention and control in the region of the Americas. Rev Panam Salud Publica. 2007;21:55–63. doi: 10.1590/s1020-49892007000100011. [DOI] [PubMed] [Google Scholar]
- 51.Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21(st) century. Trop Med Health. 2011;39:3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008;5:e68. doi: 10.1371/journal.pmed.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayes JM, Garcia-Rivera E, Flores-Reyna R, Suarez-Rangel G, Rodriguez-Mata T, Coto-Portillo R, Baltrons-Orellana R, Mendoza-Rodriguez E, De Garay BF, Jubis-Estrada J, Hernandez-Argueta R, Biggerstaff BJ, Rigau-Perez JG. Risk factors for infection during a severe dengue outbreak in El Salvador in 2000. Am J Trop Med Hyg. 2003;69:629–633. [PubMed] [Google Scholar]
- 54.Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB., Jr Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- 55.Moore CG, Cline BL, Ruiz-Tiben E, Lee D, Romney-Joseph H, Rivera-Correa E. Aedes aegypti in Puerto Rico: nevironmental determinants of larval abundance and relation to dengue virus transmission. Am J Trop Med Hyg. 1978;27:1225–1231. doi: 10.4269/ajtmh.1978.27.1225. [DOI] [PubMed] [Google Scholar]
- 56.Yee DA, Allgood D, Kneitel JM, Kuehn KA. Constitutive differences between natural and artificial container mosquito habitats: vector communities, resources, microorganisms, and habitat parameters. J Med Entomol. 2012;49:482–491. doi: 10.1603/me11227. [DOI] [PubMed] [Google Scholar]
- 57.Vanwambeke SO, van Benthem BH, Khantikul N, Burghoorn-Maas C, Panart K, Oskam L, Lambin EF, Somboon P. Multi-level analyses of spatial and temporal determinants for dengue infection. Int J Health Geogr. 2006;5:5. doi: 10.1186/1476-072X-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pham HV, Doan HT, Phan TT, Minh NN. Ecological factors associated with dengue fever in a Central Highlands province, Vietnam. BMC Infect Dis. 2011;11:172. doi: 10.1186/1471-2334-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mammen MP, Pimgate C, Koenraadt CJ, Rothman AL, Aldstadt J, Nisalak A, Jarman RG, Jones JW, Srikiatkhachorn A, Ypil-Butac CA, Getis A, Thammapalo S, Morrison AC, Libraty DH, Green S, Scott TW. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 2008;5:e205. doi: 10.1371/journal.pmed.0050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez-Figueroa L, Rigau-Perez JG, Suarez EL, Reiter P. Risk factors for dengue infection during an outbreak in Yanes, Puerto Rico in 1991. Am J Trop Med Hyg. 1995;52:496–502. doi: 10.4269/ajtmh.1995.52.496. [DOI] [PubMed] [Google Scholar]
- 61.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 62.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 63.McBride WJ, Bielefeldt-Ohmann H. Dengue viral infections; pathogenesis and epidemiology. Microbes Infect. 2000;2:1041–1050. doi: 10.1016/s1286-4579(00)01258-2. [DOI] [PubMed] [Google Scholar]
- 64.Perez-Pinto EE, Molina de Fernandez DE. Focal resistance to organosynthetic insecticides in Aedes aegypti (Linneaus, 1762) (Diptera: Culicidae) from different municipalities in Aragua state, Venezuela. Bol Mal Salud Amb. 2009;49:143–150. [Google Scholar]
- 65.Newton EA, Reiter P. A model of the transmission of dengue fever with an evaluation of the impact of ultra-low volume (ULV) insecticide applications on dengue epidemics. Am J Trop Med Hyg. 1992;47:709–720. doi: 10.4269/ajtmh.1992.47.709. [DOI] [PubMed] [Google Scholar]
- 66.Luz PM, Vanni T, Medlock J, Paltiel AD, Galvani AP. Dengue vector control strategies in an urban setting: an economic modelling assessment. Lancet. 2011;377:1673–1680. doi: 10.1016/S0140-6736(11)60246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]