Abstract
Humoral immune response against dengue virus (DENV) is an important component in dengue-endemic transmission. We conducted a cross-sectional nested cohort study to determine the seroprevalence and frequency of neutralizing antibodies against DENV serotypes in two endemic localities in the state of Morelos, Mexico. The cohort participants (N = 1,196) were screened to determine previous exposure to DENV. Overall seroprevalence was 76.6% (95% confidence interval [95% CI] = 73.6–79.2), and prevalence of neutralizing antibodies in the 5- to 9-year-old group was 82.5% (95% CI = 67.2–92.7), 45% (95% CI = 29.3–61.5), and 65% (95% CI = 48.3–79.4) for DENV-1, DENV-2, and DENV-3, respectively. For participants older than 10 years, the observed seroprevalence was above 60% for each serotype, except DENV-4 in the 10- to 25-year-old group (42.9%); 81% of humoral responses were multitypic. The outcomes of our study contribute to understanding the immune component of dengue transmission and provide focal information for the evaluation of vaccine candidates under development.
Introduction
Dengue is a viral disease transmitted by mosquitoes of the Aedes genus, and it is considered a major public health problem.1–3 During the last 50 years, the worldwide dengue incidence has increased.4 Approximately 3 billion people living in tropical and subtropical regions are at risk of infection every year.1,5
In Mexico, according to the Ministry of Health, the states with the highest incidence over the past 7 years are Campeche, Quintana Roo, Yucatan, Colima, and Morelos. The incidence rates in the localities of the state of Morelos were above the national average; for example, the localities of Axochiapan and Tepalcingo in 2010 recorded an incidence of 528.0 per 100,000 habitants, whereas the state and national averages were 105.01 and 39.95 per 100,000 habitants, respectively.6
Considering the lack of treatment and the absence of an effective licensed vaccine, dengue control measures have been focused on reducing the vector density; however, the reduction of the incidence of the disease has not been achieved. Therefore, it is important to consider other factors, such as the immunological human response of short-term cross-protection, that could explain the fluctuating pattern of dengue virus (DENV) transmission.7–10
The human immune response to DENV infection depends on whether it is a primary or secondary infection. For a primary infection, the host's immune system generates neutralizing antibodies against the infecting serotype that offer lifelong protection. In addition, there is a short-term (up to 6 months) heterotypic neutralizing immune response against the other serotypes.11,12 Immune response to a secondary heterotypic DENV infection is characterized by a rapid increase on immunoglobulin G (IgG) antibodies; these antibodies are mainly cross-reactive and predominantly non-neutralizing, which in turn, increase the risk to develop severe dengue by antibody-dependent enhancement. Nevertheless, recent evidence shows that, in endemic communities, heterotypic secondary immune response is associated with low risk of clinical infection depending on the time that separates the first and the second infections.13–16
Few studies of immunity against DENV have been carried out in Mexico. In Veracruz, the reported seroprevalence was 79.6%, similar to the seroprevalence reported in Matamoros.17,18 In Tabasco, the prevalence of IgG antibodies against DENV was 9.1%, although this percentage may be underestimated; the type of diagnostic test used was not optimal, because the dengue IgG capture test used to detect recent infections does not reflect the total seroprevalence.2 However, this study is the only one that reports on the neutralizing antibody titers per serotype, showing the heterogeneity of the immune response of a group exposed to DENV.
Additionally, the seroepidemiological studies can support the decision-making process for selecting the age group to be vaccinated in endemic communities.19 There are many studies in southeast Asia (SEA) that provide the necessary information to set up a vaccination program.20–22 However, there are substantial differences in dengue transmission patterns between SEA and the Americas that can influence the vaccination program.23–25 Consequently, the objective of this study was to determine the seroprevalence of DENV per serotype in two endemic localities in the state of Morelos.
Materials and Methods
Design and study population.
A cross-sectional nested cohort study was performed.26 The cohort included subjects ages 5 years and older who were residents of the Axochiapan and Tepalcingo localities in the state of Morelos, Mexico. Axochiapan is located at an altitude of 1,030 m and has a population of 17,508, and Tepalcingo is located at an altitude of 1,160 m and has a population of 12,053.27
The cohort had two groups for the purpose of determining the risk of infection by an index case (IC). The exposed group was composed of subjects who lived with the IC and others who agreed to participate and lived inside a 50-m radius around the house of the IC (in practice, people who lived in the next four houses around the house of the IC). No blood sample was taken from the IC, because DENV was already diagnosed by the state of Morelos passive epidemiological surveillance system. The non-exposed (NE) group was composed of subjects who lived in a 100-m radius in the same community as the IC with no reported ICs in the 2 months before enrollment. In practice, after a review of the state epidemiological surveillance database, field personnel went to the area and invited people to participate in the study; when the first subject agreed to participate, her/his house was the center of the sampling area (50-m radius). An NE group was enrolled 1–3 weeks after each expose group. In total, 1,196 subjects were included in the cohort. For the purpose of this study, only the baseline diagnostics and interview were analyzed.
We excluded the subjects for whom we did not have blood samples collected (n = 32) and who stated that they had not lived all their life in the state of Morelos (n = 235). These criteria were selected, because we wanted the collected and analyzed information to reflect immunity acquired within only our tested region. The subjects were classified according to three age groups in accordance to exposure to the reported serotypes that have been circulating in Morelos since 1984. The 5- to 9-year-old group may have been exposed to DENV-1, DENV-2, or DENV-3 serotype; the 10- to 25-year-old group may have been exposed at least in one occasion to four serotypes, whereas the over 25-year-old group may have been exposed on two occasions to three serotypes (DENV-1, DENV-2, and DENV-3) and one occasion to DENV-4.6
Sample size.
Selection was based on 15% expected prevalence for the group of subjects between 5 and 9 years old (the group with the least exposure) and 30% prevalence for the other age groups (groups with higher exposure) with a power of 80% and a confidence level of 95%. The calculated sample size was 133 subjects for each group, but only 122 participants between 5 and 9 years old were enrolled from the cohort study; therefore, we decided to determine the seroprevalence for all cohort subjects who met the inclusion and exclusion criteria to the cross-sectional study (n = 929).
A post-hoc analysis was performed on 137 seropositive DENV subjects to describe the frequency of neutralizing antibodies per DENV serotype; it included all the children ages 5–9 years old (n = 40) and the first 97 enrolled subjects over 9 years of age.
Study variables.
The dependent variables were the detection of IgG antibodies against DENV and the detection of neutralizing antibodies against the different DENV serotypes. The main independent variable was the age of the subjects, which served as an indirect measure of exposure to the different serotypes over the course of their lives. Additionally, variables, such as sex, educational level, occupation, and recent infection, were considered.
Laboratory tests.
For each participant, serum from the first visit of the cohort (baseline sample) was stored at −70°C for antibody determination. The seroprevalence was determined with an indirect enzyme-linked immunosorbent assay (ELISA) for anti-DENV IgG (E-DEN-01G; Panbio®) following the instructions of the manufacturer (Inverness Medical Innovations Australia Pty Ldt [IMAI], Sinnamon Park, QLD Australia), the ELISA has a specificity of 98% and a sensitivity of 100%.28 The samples were classified as positive, negative, or indeterminate in accordance with the cutoff point for each kit. Recent infection by dengue was defined as an IgM- or IgG-positive test for DENV using the Dengue IgM Capture ELISA (E-DEN01M; Panbio®) and Dengue IgG Capture ELISA (E-DEN02G; Panbio®).
Sera from the seropositive subjects were assayed to determine the specific neutralizing antibody titers per serotype using the plaque reduction neutralization test (PRNT).29 The PRNT was performed in 24-well Vero microplate cell cultures using a fixed virus inoculum (approximately 1,500 focus forming units [FFUs]) against varying serum dilutions (1:20–1:1,280). The PRNT titers were scored as reciprocal of the highest dilution of serum that inhibited 60% of foci (PRNT60). Samples scored with PRNT60 < 20 were considered negative. The reference strains used were DENV-1 Hawaii, DENV-2 16681, DENV-3 FSP032, and DENV-4 H241.
Statistical analysis.
The characteristics of the sample were described according to age group using the Mann–Whitney test for continuous variables and the χ2 test for categorical variables.
Then, the seroprevalence of IgG antibodies was determined according to age group, and a bivariate analysis was performed. Variables with P value < 0.2 in the bivariate analysis were tested with a binomial regression model. The final model included all those with an adjusted P value < 0.05 or those that modified the prevalence ratios (PRs) of the main independent variable by more than 20%.
The PRNT60 values were transformed log base 10 to obtain normal distribution. The serotype-specific antibodies frequency and titers among the groups were compared using the χ2 and Mann–Whitney tests according to the type of variable. Also, the frequencies of homotypic and multitypic reactions were compared. The Stata SE® 12 software was used for the statistical analysis.
Ethical considerations.
The studies were conducted at the Centro de Investigacion sobre Enfermedades Infecciosas (Research Center for Infectious Diseases), Instituto Nacional de Salud Pública (INSP; National Institute of Public Health), Cuernavaca, Morelos, México. Human sera were obtained from the cohort study “Peridomestic infection as a determinant of the transmission of dengue,” which was approved by the Ethics Board of the INSP (CI986-L62). Also, the cross-sectional study was reviewed by the Ethics Board of the INSP (CI494). Written consent to participate in the cohort study was obtained from each subject. All data were handled anonymously and confidentially.
Results
Demographic characteristics of the population.
In total, 929 of 1,196 serum samples from subjects in the cohort were analyzed. The samples were obtained between June and November of 2011 in the localities of Axochiapan and Tepalcingo, Morelos.
According to the Mexican Institute for Statistics and Geographic Information (INEGI), in 2005, the 5- to 9-year-old group accounted for 11.9% of the population in these localities, the 10- to 25-year-old group accounted for 35.3% of the population in these localities, and the over 25-year-old group accounted for 53.1% of the population in these localities.27 Because the subjects in each age group, resulting from the cohort baseline enrollment, accounted for 12.1% of 5- to 9-year-old group, 33.6% of 10- to 25-year-old group, and 54.4% of the over 25-year-old age group, we considered it as representative of those localities. The main occupation for the first and second groups was student, which is consistent with their ages, whereas the third group, which is in productive age, was employed either in their homes or elsewhere. In total, 85.8% of the subjects stated that they had some type of healthcare coverage, of which 74.7% of the subjects had public healthcare insurance (Seguro Popular) at the time of the survey. For 17.1% (159 of 929) of the subjects, a recent DENV infection before their enlistment was detected (positive capture ELISA IgM or IgG test from the baseline sample) (Table 1).
Table 1.
Demographic data of the participants in the 2011 study of the localities of Axochiapan and Tepalcingo, Morelos, Mexico
| Variable | Total (n = 929) | 5–9 years (n = 112; 12.1%; INEGI: 11.6%) | 10–25 years (n = 312; 33.6%; INEGI: 35.3%) | > 25 years (n = 505; 54.4%; INEGI: 53.12%) | P |
|---|---|---|---|---|---|
| Median age, years (range) | 28 (5–86) | 7 | 17 | 47 | |
| Male (%) | 381 (40.1) | 65 (58.0) | 129 (41.3) | 187 (37.0) | < 0.001 |
| Exposed* | 539 (58) | 69 (61.6) | 183 (58.7) | 287 (56.8) | 0.626 |
| Locality: Tepalcingo | 325 (35) | 38 (33.9) | 89 (28.5) | 198 (39.2) | 0.008 |
| Attends school | 278 (30) | 108 (96.4) | 164 (52.6) | 6 (1.2) | < 0.001 |
| Educational level | |||||
| Illiterate | 111 (12) | 21 (18.8) | 6 (1.9) | 84 (16.6) | < 0.001 |
| Reads or writes | 260 (28) | 91 (81.3) | 59 (18.9) | 110 (21.8) | |
| Basic | 188 (20.2) | 0 (0) | 81 (26) | 107 (21.2) | |
| Secondary | 240 (25.8) | 0 (0) | 110 (35.3) | 130 (25.7) | |
| High school | 70 (7.5) | 0 (0) | 44 (14.1) | 26 (5.1) | |
| Bachelor's degree | 25 (2.7) | 0 (0) | 7 (2.2) | 18 (3.6) | |
| Postgraduate | 35 (3.8) | 0 (0) | 5 (1.6) | 30 (5.9) | |
| Occupation | |||||
| Student | 246 (26.5) | 106 (94.6) | 139 (44.6) | 1 (0.2) | < 0.001 |
| Studies and works | 28 (3) | 2 (1.8) | 24 (7.7) | 2 (0.4) | |
| Employee | 170 (18.3) | 0 (0) | 50 (16) | 120 (23.8) | |
| Independent | 140 (15.1) | 0 (0) | 17 (5.5) | 123 (24.4) | |
| Housewife | 277 (29.8) | 0 (0) | 63 (20.2) | 214 (42.4) | |
| Other† | 68 (7.3) | 4 (3.6) | 19 (6.1) | 45 (8.9) | |
| Health insurance coverage | 797 (85.8) | 104 (92.9) | 262 (83.9) | 431 (85.4) | 0.211 |
| Infection pre-recruitment‡ | 159 (17.1) | 19 (17) | 78 (25) | 62 (12.3) | < 0.001 |
Exposed group of cohort subjects.
Unemployed, retirees, senior citizens without salary, and disabled.
Recently infected cohort group.
Seroprevalence of IgG antibodies against DENV.
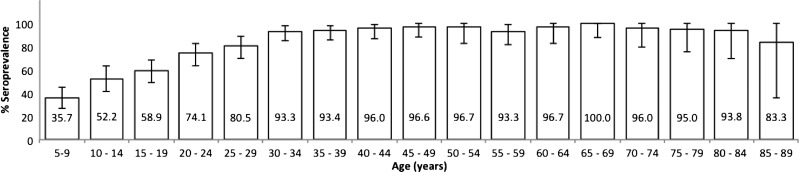
The global seroprevalence for DENV was 76.6% (713 of 929; 95% confidence interval [95% CI] = 73.6–79.2), indicating that most of the population had already been exposed to DENV infection. The seroprevalence was higher than 90% for ages 30 years and over, except in the group of 85–89 years old, for which the prevalence was 83.3% (95% CI = 35.8–99.6); this finding may be because of the size of the sample for this group (Figure 1). There are reports of circulation of West Nile virus (WNV) and other flaviviruses in wild and domestic animals in Mexico, specifically on the US–Mexico border and in some southern Mexican states.6 Nevertheless, only a couple of WNV human cases have been reported by the federal sentinel surveillance system. Therefore, although we cannot rule out silent transmission of other flaviviruses in the studied area, we believe that neutralizing antibodies detected in our study are generated only by DENV infection.30
Figure 1.
Global seroprevalence of IgG against DENV by quinquennium in the localities of Axochiapan and Tepalcingo, Morelos, Mexico (2011). The graph shows the percentage of seroprevalence by quinquennium with 95% CI (error bars).
A 72.8% (95% CI = 67.6–77.6) seroprevalence was detected in Tepalcingo, and a 78.4% (95% CI = 74.9–81.6, P = 0.112) seroprevalence was detected in Axochiapan. An increase in seroprevalence with age was observed in both localities.
The demographic characteristics associated with seroprevalence against DENV were analyzed in 928 of 929 subjects; one of the subjects was reported in the IgG ELISA test as indeterminate and thus, was not included in this analysis (Table 2).
Table 2.
Characteristics associated with the seroprevalence of DENV in the localities of Axochiapan and Tepalcingo, Morelos, Mexico
| Variable | Total (N = 928) | Seronegative (N = 215) | Seropositive (N = 713) | PR (95% CI) | PRa*(95% CI) |
|---|---|---|---|---|---|
| Age group (years) n (%) | |||||
| 5–9 | 112 (12.1) | 72 (33.5) | 40 (5.6) | 1.0 | |
| 10–25 | 311 (33.5) | 117 (54.4) | 194 (27.2) | 1.75 (1.34–2.27) | 1.49 (1.15–1.94) |
| > 25 | 505 (54.4) | 26 (12.1) | 479 (67.2) | 2.66 (2.07–3.41) | 2 (1.5–2.66) |
| Sex | |||||
| Female | 547 (58.9) | 115 (53.5) | 432 (60.6) | 0.93 (0.87–1.01) | 1.06 (1.01–1.11) |
| Male | 381 (41.6) | 100 (46.5) | 281 (39.4) | ||
| Locality | |||||
| Tepalcingo | 325 (35.0) | 88 (40.9) | 237 (33.2) | 1.08 (1–1.17) | 1.11 (1.05–1.17) |
| Axochiapan | 603 (65) | 127 (59.1) | 476 (66.7) | ||
| Occupation | |||||
| Student | 246 (26.5) | 135 (62.8) | 111(15.6) | 1.0 | |
| Student and employee | 28 (3) | 12 (5.6) | 16 (2.2) | 1.27 (0.89–1.8) | 1.04 (0.72–1.51) |
| Employee | 170 (18.3) | 23 (10.7) | 147 (20.6) | 1.92 (1.65–2.23) | 1.29 (1.06–1.57) |
| Independent | 140 (15.1) | 10 (4.7) | 130 (18.2) | 2.06 (1.78–2.38) | 1.37 (1.12–1.66) |
| Housewife | 276 (29.7) | 29 (13.5) | 247 (34.6) | 1.98 (1.72–2.29) | 1.37 (1.12–1.66) |
| Other† | 68 (7.3) | 6 (2.8) | 62 (8.7) | 2.02 (1.73–2.36) | 1.31 (1.08–1.6) |
PRa = adjusted PR.
Multivariate regress binomial model. Standard errors adjusted for sampling house cluster (N = 342).
Unemployed, retirees, senior citizens without salary, and disabled.
The final multivariate analysis model for global seroprevalence was adjusted in accordance with the variables age, sex, location, and occupation. The analysis showed that the prevalence ratio of being seropositive in the over 25-year-old age group was 49% higher than in the 5- to 9-year-old age group. We found an association between sex and seropositivity, for which males displayed a higher seroprevalence than females (PR = 1.06, 95% CI = 1.01–1.11). In regard to occupation, the highest probability of being seropositive corresponded to those subjects who spend more time at home compared with the group of students, because according to the baseline interview (question: how long, on average, do you stay at home on working and non-working days?), housewife and others (definition in Table 2) spend more time at home during working days (median of 22 and 23 hours, respectively) than students (median of 17 hours, P < 0.0001); however, employees spent more time outside the home (15 hours, P < 0.0001). A similar behavior was observed with regard to sex, because females older than 10 years of age spent more time at home than males; for the 10- to 25-year-old group, for females, the median time spent in their home was 19 hours (interquartile range [IQR] = 16–22) compared with 16 hours (IQR = 14–17) for males (P < 0.0001). For the group older than 25 years, the median time was 22 hours for females (IQR = 19–23) compared with 16 hours (IQR = 13–20) for males (P < 0.0001). Overall, these findings strongly suggest that occupation and sex could be associated with exposure to dengue transmission around a clinical case.
Seroprevalence of neutralizing antibodies against specific DENV serotypes per age group.
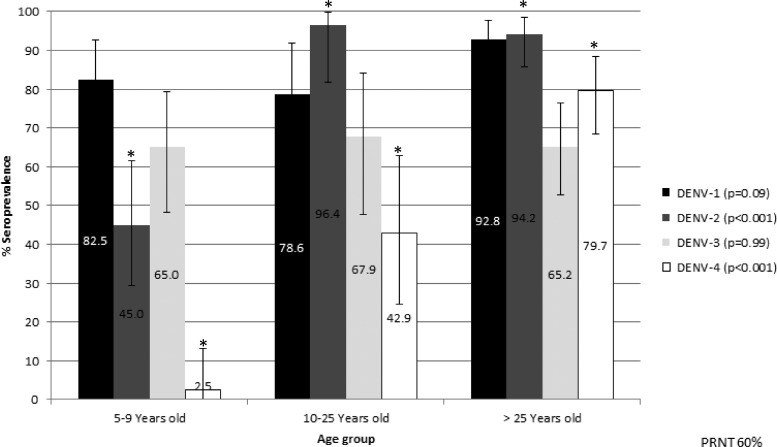
The seroprevalence of neutralizing antibodies for the first age group (n = 40) was 82.5% (95% CI = 67.2–92.7), 45% (95% CI = 29.3–61.5), and 65% (95% CI = 48.3–79.4) for DENV-1, DENV-2, and DENV-3, respectively. It is important to point out that 2.5% (95% CI = 0.06–13.2) of the seroprevalence against DENV-4 was associated with a short-term multitypic neutralizing immune response, because the subjects were individuals recently infected by DENV who may not have been exposed to DENV-4 according to the viral surveillance of the region6 (Figure 2). However, viral surveillance in the studied area by local health authorities, although passive, is particularly robust compared with other areas within Mexico, but we cannot exclude the undetected circulation of any other serotype, which Montoya and others14 reported previously.
Figure 2.
Seroprevalence per serotype against DENV per age group. Distribution of the seroprevalence by serotype per age group. The error bars are 95% CI. *Statistically significant differences.
In the second age group (n = 27), a seroprevalence higher than 60% was observed, except for DENV-4 (42.9%, 95% CI = 24.5–62.8), whereas for the third age group (n = 70), the seroprevalence was higher than 65% for all four serotypes. Moreover, a statistically significant difference was observed between DENV-2 and DENV-4 seroprevalence for the three age groups; this result was not observed with DENV-1 and DENV-3 (Figure 2).
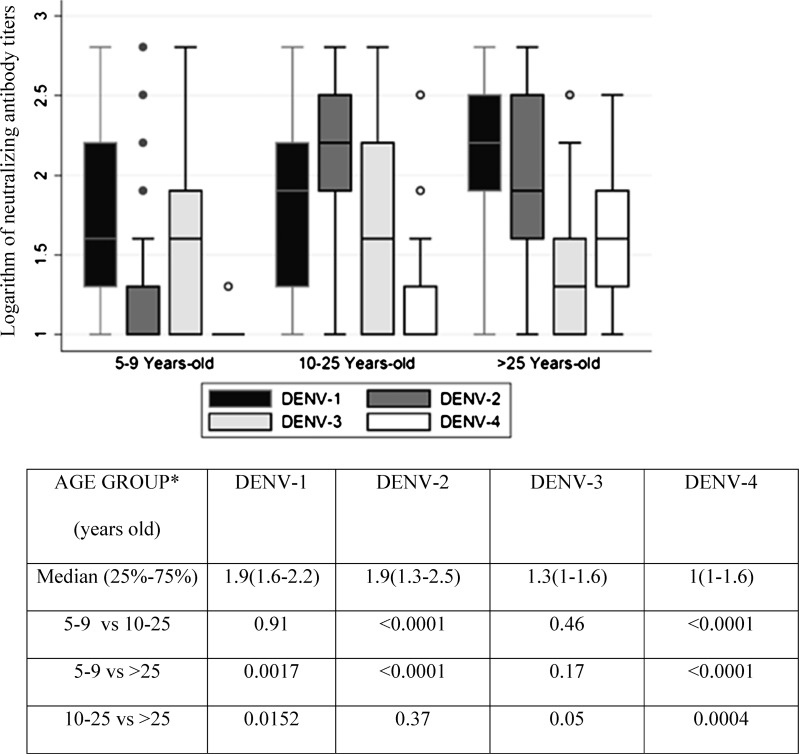
With respect to the neutralizing antibody titers, they reflected the pattern of exposure to the serotypes that have circulated in this region. The 5- to 9-year-old age group may have been exposed to at least one of the three serotypes (DENV-1, DENV-2, and DENV-3), and the second and third age groups may have been exposed in more than one occasion to one of four DENV serotypes (Figure 3) according to epidemiological surveillance reports by the local health services.
Figure 3.
Logarithm of neutralizing antibody titers against the four DENV serotypes (PRNT60). Mann–Whitney test. The error bars are 95% CI.
DENV-3 has circulated over the last decade more often than DENV-4, which was only reported in the region of Morelos in 1996 and 1997. Nonetheless, the neutralizing antibody titers against DENV-3 in the over 25-year-old age group were lower than those against DENV-4 (Figure 3). Moreover, DENV-3 neutralizing antibody titers were not significantly different among the three age groups, unlike the neutralizing antibodies from the other serotypes (Figure 3).
For DENV-1 neutralizing antibodies, the titers observed are higher in the group older than 25 years compared with the other groups (Figure 3). Because this serotype has circulated more often over the past 5 years, the described antibody pattern suggests a booster effect.
In accordance with the Mexican virological surveillance system, exposure to DENV-2 by the 5- to 9-year-old age group occurred one time (2007–2008), and a significant difference between neutralizing antibody titers was observed compared with the other groups (P < 0.0001). Another interesting observation is that DENV-3 neutralizing antibodies were observed less frequently compared with those for DENV-4, although DENV-3 had circulated on more occasions than DENV-4 (Figure 3).
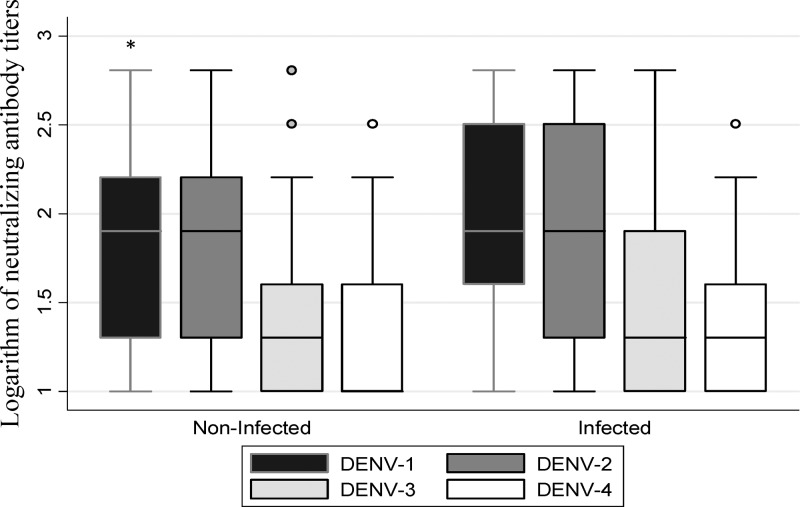
The relationship between the neutralizing antibody titers and recent infection was studied.31,32 The neutralizing antibody titer against DENV-1 was higher in recently infected subjects (Figure 4). According to the “original antigenic sin” concept, this observation could be the result of a prolonged and sustained circulation of DENV-1 in the studied communities; however, it should be taken in account that, at the time of the study, DENV-1 was the only serotype circulating in these populations.16
Figure 4.
Logarithm of neutralizing antibody titers against the four DENV serotypes in recently infected and non-infected subjects (PRNT60). Mann–Whitney test. Comparison the logarithm of neutralizing antibody titers between recently infected and non-infected subjects. The error bars are 95% CI. *P = 0.0005 for DENV-1 infected (N = 38) versus DENV-1 (N = 99) not recently infected.
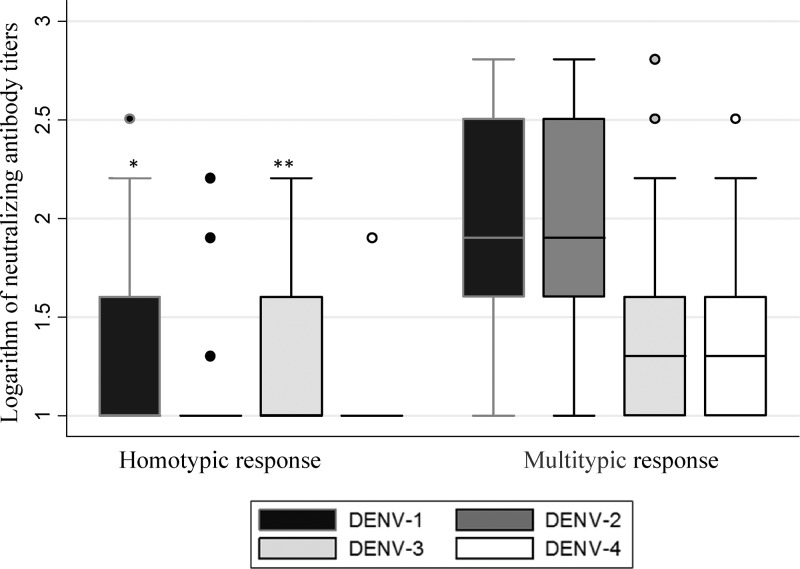
A total of 81.6% (n = 111) of the immune response was multitypic, and its frequency increased with age. This behavior is typical of endemic communities, because age is a reflection of the time that the individuals may have been exposed to the different DENV serotypes in circulation (Figure 5).
Figure 5.
Neutralizing antibody titers per DENV serotype with respect to the type of response of the individuals. Mann–Whitney test. Comparison the logarithm of neutralizing antibody titers between homotypic and multitypic subjects. The error bars are 95% CI. *P < 0.0001 for homotypic response (N = 25) versus multitypic response (N = 111). **P < 0.005 for homotypic response for DENV-3 (N = 25) versus multitypic response for DENV-3 (N = 111).
Discussion
We found a global seroprevalence of 76.6% in the studied population; this proportion is typical of endemic communities in Mexico and other Latin America countries.17,18 However, for the 5- to 9-year-old group, the seroprevalence found in this study is lower compared with SEA countries.33,34 This difference can probably be explained by the higher intensity of dengue transmission in SEA compared with that in Latin America-endemic countries.16
In general, studies have shown an increase in seroprevalence with age as a result of a longer period of exposure to the DENV circulating in endemic regions.17,18,35–38 In the localities of Axochiapan and Tepalcingo, the magnitude of seroprevalence detected in this study as well as the presence of dengue cases over the last 5 years situate them in the category of endemic areas in Mexico. Although the incidence is higher in Axochiapan than Tepalcingo, the difference in global seroprevalence between the localities was not statistically significant, but in the adjusted analysis by age and other variables, we observed a small difference that was statistically significant (11%, 95% CI = 5–17) and may be explained by differences in population density (Axochiapan = 0.193 habitants/km2; Tepalcingo = 0.072 habitants/km2).27 In fact, population density is considered one of the decisive factors in the transmission of dengue.39
An association between seroprevalence and male sex was found with the binomial regression model, suggesting a possible association with human movement. Because males seem to spend more time away from their houses, the possibility of being infected by dengue in the places that they frequently visit increases.40 It is intriguing that other studies did not find an association with sex; cultural determinants in these communities could explain this finding.37,41–43
In regard to occupation, when adjusting by age, locality, and sex, independent workers, senior citizens without salary, disabled individuals, retirees, and housewives were associated with a higher seroprevalence compared with students. In addition, the relationship between seroprevalence and housewife status has previously been reported in Singapore, and these observations may have two explanations.36 First, the attendance of students in schools for long periods of time results in less exposure to the vector. Unlike at home, there is an absence of potential mosquito breeding grounds at schools as a result of the Healthy School program, which has been implemented in schools in Mexico. Second, the other explanation is based on the assumption that infected mosquitoes live near homes and that the children are at school during the time that the infected mosquitoes are more active near their homes. For subjects whose stated occupation was employee, given the socioeconomic characteristics of this particular population, we considered the working environment to be very similar to the home environment in regards of exposure to mosquitoes.36,44 These observations imply that subjects move between relatively static localities with infected vectors, and therefore, the mobility of the individual is a factor involved in the transmission. This result is suggested by mathematical models that consider humans as both DENV hosts and vectors for the mosquitoes.45,46
In regards to the frequency of neutralizing antibodies per serotype, differences were observed among the age groups for DENV-2 and DENV-4 but not DENV-1 and DENV-3. The explanation for the latter may be that the circulation of DENV-1 has been predominant in the area over the past 5 years, and this fact tends to homogenize the frequency between groups. Additionally, in the case of DENV-3, a phylogenetic relationship between serotypes 1 and 3 has been reported, which is reflected in an antigenic relationship in such a way that the frequency of antibodies against DENV-3 is proportional to that of DENV-1.1,47–49 These observations are in agreement with previous studies, which showed neutralizing activity against DENV-1 and DENV-3.50 Likewise, other studies that detected neutralizing antibodies in primary and secondary infections observed that, in the presence of an infection caused by serotype 1, the serotypes with the highest antibody titers corresponded to serotypes 1 and 3.13,51,52
The multitypic response was positively associated with age because of the diversity of antibodies generated as a result of ongoing exposure in addition to triggering an increase in the titer against the others as a result of a cross-reaction response. Higher neutralizing antibody titers observed in the study for the four serotypes corresponded to a multitypic response caused by the booster effect (Figure 3).13,53,54
This study was not able to establish the identity of the serotype of the participant's first infection or the sequence of infection using the neutralizing response, because doing so would require comparing antibody titers between blood samples pre- and post-infection. In accordance with the concept of “original antigenic sin,” during a secondary DENV infection, the neutralizing antibody titers from the serotype that caused the first infection will be higher than those resulting from the serotype responsible for the second infection.16 Thus, it is difficult to identify with certainty the serotype that caused the first infection if there was no viral isolation at that moment.51
One limitation of this study is that the results are only generalized to subjects older than 5 years of age. Younger children were not included in the sample, because they are less affected; also, there are ethical restrictions of the cohort project.
In addition, the circulation dynamics of the serotypes must influence the immune response at least in terms of magnitude; there is no cocirculation of DENV in the study area, and therefore, in principle, this study would only be applicable to endemic populations where one serotype circulates predominantly. Nevertheless, both the global seroprevalence and the frequency of neutralizing antibody suggest that, in endemic populations, the cocirculation of serotypes does not significantly contribute to the frequency and diversity of the immune response against DENV.
This study is the first to report global seroprevalence according to DENV serotype in an endemic Mexican population with a wide age range. Because the results are similar to other endemic populations in the world, we consider this study to provide valid information for the entire Americas region. This work is important, because results from phase II clinical trials to evaluate the effectiveness of a vaccine have shown that neutralizing immunity against DENV needs to be evaluated to understand the relationship between immunity and protection.20 Finally, based on this work, it is possible to design studies with the objective of establishing the neutralizing antibody titers required to develop protection against the disease, thereby contributing to the design of effective immunization schemes against dengue.
ACKNOWLEDGMENTS
The authors thank the personnel of Sanitary Jurisdiction III in Morelos State, Health Services Morelos and Robert Tesh and Hilda Guzman of the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) for providing reference dengue virus strains and antisera. This work was presented in part at the 60th Annual Meeting of the American Society for Tropical Medicine and Hygiene in Atlanta, GA in December of 2012.
Footnotes
Financial support: This project was supported, in part, by a grant from Instituto Cientifico Pfizer (2010 Epidemiology Award) and FOSISS Grant SSA-IMSS-ISSSTE CONACYT-1385 (Fondo Sectorial de Investigación en Salud y Seguridad Social). I.Y.A.-L. and R.A.M.-V. were CONACYT (Consejo Nacional de Ciencia y Tecnologia) fellows. S.V.M. is supported by the James W. McLaughlin Fellowship Fund; N.V. is supported by startup funds provided by the Department of Pathology, a grant from the Institute for Human Infection and Immunity, University of Texas Medical Branch, and National Institutes of Health Contract HHSN272201000040I/HHSN27200004/D04.
Authors' addresses: Irma Y. Amaya-Larios, Marisol Galeana-Hernández, Andreu Comas-García, Karla J. Sepúlveda-Salinas, and Jorge A. Falcón-Lezama, Instituto Nacional de Salud Pública, Cuernavaca, Morelos, México, E-mails: irma.amaya@insp.mx, mgaleana@insp.mx, andreu.comas@espm.insp.mx, karla.sepulveda@insp.mx, and jorge.falcon@insp.mx. Ruth Aralí Martínez-Vega, Instituto Nacional de Salud Pública, Cuernavaca, Morelos, México, and Organización Latinoamericana para el Fomento de la Investigación en Salud, Bucaramanga, Stder, Colombia, E-mail: rutharam@yahoo.com. Sandra V. Mayer and Nikos Vasilakis, Department of Pathology and Center for Biodefense and Emerging Infectious Diseases, Center for Tropical Diseases, and Institute of Human Infections and Immunity, University of Texas Medical Branch, Galveston, TX, E-mails: samayer@utmb.edu and nivasila@utmb.edu. José Ramos-Castañeda, Instituto Nacional de Salud Pública, Cuernavaca, Morelos, México, Organización Latinoamericana para el Fomento de la Investigación en Salud, Bucaramanga, Stder, Colombia, and Center for Tropical Diseases, University of Texas Medical Branch, Galveston, TX, E-mail: jramos@insp.mx.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sánchez-Burgos GG, López-Alvarado MA, Castañeda-Desales D, Ruiz-Gómez J, Ramos-Castañeda J. Prevalence of neutralizing antibodies to dengue virus serotypes in university students from Tabasco, Mexico. Salud Publica Mex. 2008;50:362–366. doi: 10.1590/s0036-36342008000500008. [DOI] [PubMed] [Google Scholar]
- 3.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martínez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8((Suppl)):S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21(st) century. Trop Med Health. 2011;39((Suppl)):3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Dengue Guidelines, 2010. 2010. http://www.health.gov.bz/www/index.php/publications/environmental-health/160-environmental-health/322-guidelines-for-dengue-diagnosis-treatment-prevention-and-control Available at. Accessed July 7, 2011.
- 6.Direccion General de Epidemiologia Panorama epidemiologico de dengue. 2012. http://www.dgepi.salud.gob.mx/anuario/html/anuarios.html Available at. Accessed May 15, 2012.
- 7.Adams B, Holmes EC, Zhang C, Mammen MP, Jr, Nimmannitya S, Kalayanarooj S, Boots M. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proc Natl Acad Sci USA. 2006;103:14234–14239. doi: 10.1073/pnas.0602768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 9.Teoh BT, Sam SS, Tan KK, Johari J, Shu MH, Danlami MB, Abd-Jamil J, MatRahim N, Mahadi NM, AbuBakar S. Dengue virus type 1 clade replacement in recurring homotypic outbreak. BMC Evol Biol. 2013;13:213. doi: 10.1186/1471-2148-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen R, Vasilakis N. Dengue–quo tu et quo vadis? Viruses. 2011;3:1562–1608. doi: 10.3390/v3091562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 12.Wahala WM, Silva AM. The human antibody response to dengue virus infection. Viruses. 2011;3:2374–2395. doi: 10.3390/v3122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 14.Montoya M, Gresh L, Mercado JC, Williams KL, Vargas MJ, Gutierrez G, Kuan G, Gordon A, Balmaseda A, Harris E. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl Trop Dis. 2013;7:e2357. doi: 10.1371/journal.pntd.0002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olkowski S, Forshey BM, Morrison AC, Rocha C, Vilcarromero S, Halsey ES, Kochel TJ, Scott TW, Stoddard ST. Reduced risk of disease during postsecondary dengue virus infections. J Infect Dis. 2013;208:1023–1033. doi: 10.1093/infdis/jit273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halstead SB, Rojanasuphot S, Sangkawibha N. Original antigenic sin in dengue. Am J Trop Med Hyg. 1983;32:154–156. doi: 10.4269/ajtmh.1983.32.154. [DOI] [PubMed] [Google Scholar]
- 17.Brunkard JM, Robles López JL, Ramirez J, Cifuentes E, Rothenberg SJ, Hunsperger EA, Moore CG, Brussolo RM, Villarreal NA, Haddad BM. Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg Infect Dis. 2007;13:1477–1483. doi: 10.3201/eid1310.061586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarrete-Espinosa J, Acevedo-Vales JA, Huerta-Hernández E, Torres-Barranca J, Gavaldón-Rosas DG. Prevalence of dengue and leptospira antibodies in the state of Veracruz, Mexico. Salud Publica Mex. 2006;48:220–228. doi: 10.1590/s0036-36342006000300006. [DOI] [PubMed] [Google Scholar]
- 19.Ndeffo Mbah ML, Durham DP, Medlock J, Galvani AP. Country- and age-specific optimal allocation of dengue vaccines. J Theor Biol. 2014;342:15–22. doi: 10.1016/j.jtbi.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 21.Chao DL, Halstead SB, Halloran ME, Longini IM., Jr Controlling dengue with vaccines in Thailand. PLoS Negl Trop Dis. 2012;6:e1876. doi: 10.1371/journal.pntd.0001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coudeville L, Garnett GP. Transmission dynamics of the four dengue serotypes in southern Vietnam and the potential impact of vaccination. PLoS ONE. 2012;7:e51244. doi: 10.1371/journal.pone.0051244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halstead SB. Dengue in the Americas and Southeast Asia: do they differ? Rev Panam Salud Publica. 2006;20:407–415. doi: 10.1590/s1020-49892006001100007. [DOI] [PubMed] [Google Scholar]
- 24.Tapia-Conyer R, Betancourt-Cravioto M, Méndez-Galván J. Dengue: an escalating public health problem in Latin America. Paediatr Int Child Health. 2012;32((Suppl 1)):14–17. doi: 10.1179/2046904712Z.00000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douglas DL, DeRoeck DA, Mahoney RT, Wichmann O. Will dengue vaccines be used in the public sector and if so, how? Findings from an 8-country survey of policymakers and opinion leaders. PLoS Negl Trop Dis. 2013;7:e2127. doi: 10.1371/journal.pntd.0002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez-Vega RA, Danis-Lozano R, Velasco-Hernández J, Díaz-Quijano FA, González-Fernández M, Santos R, Román S, Argáez-Sosa J, Nakamura M, Ramos-Castañeda J. A prospective cohort study to evaluate peridomestic infection as a determinant of dengue transmission: protocol. BMC Public Health. 2012;12:262. doi: 10.1186/1471-2458-12-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Instituto Nacional de Estadistica y Geografia (INEGI) Censo General de Población y vivienda 2005. 2005. http://www.inegi.org.mx Available at. Accesses July 8, 2011.
- 28.Groen J, Koraka P, Velzing J, Copra C, Osterhaus AD. Evaluation of six immunoassays for detection of dengue virus-specific immunoglobulin M and G antibodies. Clin Diagn Lab Immunol. 2000;7:867–871. doi: 10.1128/cdli.7.6.867-871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasilakis N, Durbin AP, da Rosa AP, Munoz-Jordan JL, Tesh RB, Weaver SC. Antigenic relationships between sylvatic and endemic dengue viruses. Am J Trop Med Hyg. 2008;79:128–132. [PubMed] [Google Scholar]
- 30.Elizondo-Quiroga D, Elizondo-Quiroga A. West Nile Virus and its theories, a big puzzle in Mexico and Latin America. J Glob Infect Dis. 2013;5:168–175. doi: 10.4103/0974-777X.122014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 32.van Panhuis WG, Gibbons RV, Endy TP, Rothman AL, Srikiatkhachorn A, Nisalak A, Burke DS, Cummings DA. Inferring the serotype associated with dengue virus infections on the basis of pre- and postinfection neutralizing antibody titers. J Infect Dis. 2010;202:1002–1010. doi: 10.1086/656141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashiro T, Disla M, Petit A, Taveras D, Castro-Bello M, Lora-Orste M, Vardez S, Cesin AJ, Garcia B, Nishizono A. Seroprevalence of IgG specific for dengue virus among adults and children in Santo Domingo, Dominican Republic. Am J Trop Med Hyg. 2004;71:138–143. [PubMed] [Google Scholar]
- 34.Thai KT, Binh TQ, Giao PT, Phuong HL, Hung le Q, Van Nam N, Nga TT, Groen J, Nagelkerke N, de Vries PJ. Seroprevalence of dengue antibodies, annual incidence and risk factors among children in southern Vietnam. Trop Med Int Health. 2005;10:379–386. doi: 10.1111/j.1365-3156.2005.01388.x. [DOI] [PubMed] [Google Scholar]
- 35.Espinoza-Gómez F, Hernández-Suárez CM, Rendón-Ramírez R, Carrillo-Alvarez ML, Flores-González JC. Interepidemic transmission of dengue in the city of Colima, Mexico. Salud Publica Mex. 2003;45:365–370. [PubMed] [Google Scholar]
- 36.Yew YW, Ye T, Ang LW, Ng LC, Yap G, James L, Chew SK, Goh KT. Seroepidemiology of dengue virus infection among adults in Singapore. Ann Acad Med Singapore. 2009;38:667–675. [PubMed] [Google Scholar]
- 37.Braga C, Luna CF, Martelli CM, de Souza WV, Cordeiro MT, Alexander N, de Albuquerque Mde F, Júnior JC, Marques ET. Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop. 2010;113:234–240. doi: 10.1016/j.actatropica.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sissoko D, Ezzedine K, Giry C, Moendandzé A, Lernout T, D'Ortenzio E, Pettinelli F, Malvy D. Seroepidemiology of dengue virus in Mayotte, Indian Ocean, 2006. PLoS ONE. 2010;5:e14141. doi: 10.1371/journal.pone.0014141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, Reiner RC, Jr, Vilcarromero S, Elder JP, Halsey ES, Kochel TJ, Kitron U, Scott TW. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci USA. 2013;110:994–999. doi: 10.1073/pnas.1213349110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raghwani J, Rambaut A, Holmes EC, Hang VT, Hien TT, Farrar J, Wills B, Lennon NJ, Birren BW, Henn MR, Simmons CP. Endemic dengue associated with the co-circulation of multiple viral lineages and localized density-dependent transmission. PLoS Pathog. 2011;7:e1002064. doi: 10.1371/journal.ppat.1002064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teixeira Mda G, Barreto ML, Costa Mda C, Ferreira LD, Vasconcelos PF, Cairncross S. Dynamics of dengue virus circulation: a silent epidemic in a complex urban area. Trop Med Int Health. 2002;7:757–762. doi: 10.1046/j.1365-3156.2002.00930.x. [DOI] [PubMed] [Google Scholar]
- 42.Van Benthem BH, Vanwambeke SO, Khantikul N, Burghoorn-Maas C, Panart K, Oskam L, Lambin EF, Somboon P. Spatial patterns of and risk factors for seropositivity for dengue infection. Am J Trop Med Hyg. 2005;72:201–208. [PubMed] [Google Scholar]
- 43.Trravassos da Rosa AP, Vasconcelos PF, Travassos Da Rosa ES, Rodrigues SG, Mondet B, Cruz AC, Sousa MR, Travassos Da Rosa JF. Dengue epidemic in Belem, Para, Brazil, 1996–97. Emerg Infect Dis. 2000;6:298–301. doi: 10.3201/eid0603.000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iturrino-Monge R, Avila-Agüero ML, Avila-Agüero CR, Moya-Moya T, Cañas-Coto A, Camacho-Badilla K, Zambrano-Mora B. Seroprevalence of dengue virus antibodies in asymptomatic Costa Rican children, 2002–2003: a pilot study. Rev Panam Salud Publica. 2006;20:39–43. doi: 10.1590/s1020-49892006000700005. [DOI] [PubMed] [Google Scholar]
- 45.Adams B, Kapan DD. Man bites mosquito: understanding the contribution of human movement to vector-borne disease dynamics. PLoS ONE. 2009;4:e6763. doi: 10.1371/journal.pone.0006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoon IK, Getis A, Aldstadt J, Rothman AL, Tannitisupawong D, Koenraadt CJM, Fansiri T, Jones JW, Morrison AC, Jarman RG, Nisalak A, Mammen MP, Jr, Thammapalo S, Srikiatkhachorn A, Green S, Libraty DH, Gibbons RV, Endy T, Pimgate C, Scott TW. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis. 2012;6:e1730. doi: 10.1371/journal.pntd.0001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Twiddy SS, Holmes EC, Rambaut A. Inferring the rate and time-scale of dengue virus evolution. Mol Biol Evol. 2003;20:122–129. doi: 10.1093/molbev/msg010. [DOI] [PubMed] [Google Scholar]
- 48.Zanotto PM, Gould EA, Gao GF, Harvey PH, Holmes EC. Population dynamics of flaviviruses revealed by molecular phylogenies. Proc Natl Acad Sci USA. 1996;93:548–553. doi: 10.1073/pnas.93.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis JA, Chang GJ, Lanciotti RS, Kinney RM, Mayer LW, Trent DW. Phylogenetic relationships of dengue-2 viruses. Virology. 1993;197:216–224. doi: 10.1006/viro.1993.1582. [DOI] [PubMed] [Google Scholar]
- 50.Schieffelin JS, Costin JM, Nicholson CO, Orgeron NM, Fontaine KA, Isern S, Michael SF, Robinson JE. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. J Virol. 2010;7:28. doi: 10.1186/1743-422X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graham RR, Juffrie M, Tan R, Hayes CG, Laksono I, Ma'roef C, Erlin, Sutaryo, Porter KR, Halstead SB. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I. studies in 1995–1996. Am J Trop Med Hyg. 1999;61:412–419. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 52.Kuno G, Gubler DJ, Oliver A. Use of ‘original antigenic sin’ theory to determine the serotypes of previous dengue infections. Trans R Soc Trop Med Hyg. 1993;87:103–105. doi: 10.1016/0035-9203(93)90444-u. [DOI] [PubMed] [Google Scholar]
- 53.Midgley CM, Bajwa-Joseph M, Vasanawathana S, Limpitikul W, Wills B, Flanagan A, Waiyaiya E, Tran HB, Cowper AE, Chotiyarnwong P, Grimes JM, Yoksan S, Malasit P, Simmons CP, Mongkolsapaya J, Screaton GR. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol. 2011;85:410–421. doi: 10.1128/JVI.01826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons M, Burgess T, Lynch J, Putnak R. Protection against dengue virus by non-replicating and live attenuated vaccines used together in a prime boost vaccination strategy. Virology. 2010;396:280–288. doi: 10.1016/j.virol.2009.10.023. [DOI] [PubMed] [Google Scholar]