Abstract
Antivenoms manufactured by bioCSL Limited (Australia) and Instituto Clodomiro Picado (Costa Rica) against the venom of the taipan snakes (Oxyuranus scutellatus) from Australia and Papua New Guinea (PNG), respectively, were compared using antivenomics, an analytical approach that combines proteomics with immunoaffinity chromatography. Both antivenoms recognized all venom proteins present in venom from PNG O. scutellatus, although a pattern of partial recognition was observed for some components. In the case of the Australian O. scutellatus venom, both antivenoms immunorecognized the majority of the components, but the CSL antivenom showed a stronger pattern of immunoreactivity, which was revealed by the percentage of retained proteins in the immunoaffinity column. Antivenoms interacted with taipoxin in surface plasmon resonance. These observations on antivenomics agree with previous neutralization studies.
Introduction
Snakebite envenoming is a significant burden on the public health system in Papua New Guinea (PNG).1–3 Three species are responsible for the majority of cases: Acanthophis laevis (smooth-scaled death adder), Micropechis ikaheka (New Guinea small-eyed snake), and Oxyuranus scutellatus (Papuan taipan). Lower numbers of cases are induced by other Acanthophis species, Pseudechis papuanus (Papuan blacksnake), and Pseudonaja textilis (New Guinea brownsnake).3 In southern PNG and southern Papua, the vast majority of snakebites are inflicted by O. scutellatus.3 Envenomings by this large elapid snake are characterized by minor local effects and severe systemic manifestations, including irreversible flaccid paralysis, coagulopathy associated with systemic spontaneous bleeding, myotoxicity, acute kidney injury, and cardiac damage.2–6
The therapy for envenomings by O. scutellatus in PNG is based on the intravenous administration of either CSL Polyvalent Antivenom or CSL Taipan Antivenom (both manufactured by bioCSL Limited in Melbourne, Victoria, Australia; CSL). They are F(ab')2 antivenoms generated by pepsin digestion and ammonium sulphate precipitation of plasma of hyperimmunized horses.3 Both of these antivenoms, when administered early, have been shown to be effective in halting coagulopathy and bleeding and reduce the incidence of respiratory paralysis. CSL Polyvalent Antivenom is a polyspecific mixture of immunoglobulins (Igs) raised against the venom of Australian elapid species from five genera (Acanthophis, Notechis, Oxyuranus, Pseudechis, and Pseudonaja). Although CSL Taipan Antivenom is therapeutically indicated only for treating envenoming by snakes of the genus Oxyuranus, this antivenom is also the result of hyperimmunizing horses with venom from the same five genera of Australian elapid snakes used in production of CSL Polyvalent Antivenom.7 Recently, a new, truly monospecific taipan antivenom has been prepared at Instituto Clodomiro Picado (ICP) at the University of Costa Rica (San José, Costa Rica) against the venom of O. scutellatus from PNG. It is a whole-IgG preparation generated by caprylic acid fractionation of the plasma of horses immunized with this venom.8 A comparative pre-clinical assessment of the ability of ICP and bioCSL antivenoms to neutralize the venom of PNG taipan revealed a similar potency for the neutralization of lethality and myotoxicity in mouse tests and phospholipase A2 (PLA2) activity, although the ICP whole-IgG antivenom showed a higher efficacy in the neutralization of in vitro coagulant activity.8 These antivenoms are currently being tested in a randomized trial to assess their safety and efficacy in the clinical setting.
In addition to tests designed to evaluate the ability of antivenoms to neutralize toxic effects by venoms, the arena of pre-clinical antivenom testing has been enriched in the last few years with the development of antivenomics (i.e., the application of proteomic tools to the analysis of the immunoreactivity of antivenoms).9–13 Antivenomics bring information on which venom components are recognized by antivenom antibodies and which ones are not bound by antibodies, thus allowing a fine characterization of the reactivity profile of antivenoms. A prerequisite to perform antivenomics is the characterization of the proteomes of the venoms to be analyzed. The proteomes of the venoms of populations of O. scutellatus from PNG and Australia have been recently characterized.14 The most abundant components are PLA2s, including the potent pre-synaptic neurotoxic complex taipoxin15 and other monomeric PLA2s.16,17 In addition, these venoms contain Kunitz-type inhibitors, neurotoxins of the three-finger family, serine proteinases, metalloproteinases, cysteine-rich secretory proteins (CRISPs), and the prothrombin activator Oscutarin-C.14,18 C-type lectin-like proteins and venom natriuretic peptide were identified only in the venom from PNG.14 This proteomic characterization paves the way for investigating the antivenomics of the two antivenoms prepared against O. scutellatus venoms. This study presents an antivenomic analysis of the taipan antivenoms manufactured by bioCSL and ICP and correlates these findings with the previous pre-clinical study of the neutralizing profile of these antivenoms.
Materials and Methods
Venoms and taipoxin.
The venom of O. scutellatus from PNG was a pool obtained from 12 healthy adult specimens collected in the Milne Bay and Central Provinces in PNG. These snakes were maintained in a purpose-built serpentarium at the University of PNG, and venom was collected at 21-day intervals. Venom was obtained using Parafilm-covered Eppendorf tubes and snap-frozen to −80°C before being freeze-dried and stored in the dark at −20°C. The venom of Australian O. scutellatus as well as the venoms of Pseudechis colletti and Notechis scutatus were obtained from Venom Supplies Pty Limited (Tanunda, South Australia, Australia). In some experiments, a preparation of taipoxin provided by Ivan Kaiser was used.
Antivenoms.
Two antivenoms were used in this study. (1) Polyspecific taipan antivenom manufactured by bioCSL Limited (CSL), Melbourne, Victoria, Australia (batch B0548-06301; expiration date March of 2012). CSL taipan antivenom contains a mixture of Igs with activity against venoms from Acanthophis, Notechis, Oxyuranus, Pseudechis, and Pseudonaja but with a minimum of 12,000 neutralizing units of activity to Oxyuranus venom.7 (2) Monospecific taipan antivenom manufactured by ICP (batch 4511209; expiration date November of 2012). The physicochemical characteristics and neutralizing potency of these antivenoms were described by Vargas and others.8 CSL antivenom is made of F(ab')2 antibody fragments prepared by pepsin digestion and ammonium sulphate precipitation. ICP antivenom is a whole-IgG preparation obtained by caprylic acid precipitation of non-IgG plasma proteins.8
Antivenomics: immunoaffinity chromatography.
A modification of the second generation antivenomics protocol described by Pla and others12 was followed. Immunoaffinity columns of antivenoms were prepared by incubating 3 g N-hydroxysuccinimide (NHS)-activated Sepharose with 100 mg antivenom protein overnight. Non-reacting groups were blocked for 2 hours with 0.2 M glycine, and the gel was packed in a column and washed alternately at high and low pH values with coupling buffer (0.1 M NaHCO3 and 0.5 M NaCl, pH 8.3) and acetate buffer (0.1 M sodium acetate and 0.5 M NaCl, pH 4.0). Finally, the columns were equilibrated with 0.14 M NaCl and 0.04 M phosphate (pH 7.2) solution (phosphate-buffered saline [PBS]). A column coupled with equine normal IgG purified by caprylic acid fractionation of normal horse plasma was used as control. For immunoaffinity assays, 5 mg O. scutellatus venom from either Australia or PNG was dissolved in 2 mL PBS and passed through each column. The non-retained fraction of proteins was eluted with PBS, and the retained proteins were then eluted with 0.1 M glycine (pH 3). The pH of the fractions was then neutralized with 1 M NaOH. In addition and for comparative purposes, the immunoreactivity of both antivenoms against the venoms of P. colletti and N. scutatus was also studied using an identical antivenomics protocol.
Non-retained and retained immunoaffinity fractions of the venoms of O. scutellatus were analyzed by reverse-phase high-pressure liquid chromatography (HPLC) using a C18 column (250 × 4.6 mm, 5-μm particle size; Agilent) and an Agilent 1100 system. For the elution of proteins, a flow of 1 mL buffer/minute was used with the following conditions. The column was equilibrated with buffer A (0.1% trifluoroacetic acid [TFA] and 5% acetonitrile). Then, the following gradient was developed: 5% buffer B of 95% acetonitrile and 0.1% TFA for 10 minutes followed by 5–15% buffer B over 20 minutes, 15–45% buffer B over 120 minutes, and 45–70% buffer B over 20 minutes. Proteins were detected at 215 nm. Proteins were identified on the basis of the previous characterization of the proteomes of these venoms.14 To have a quantitative estimation of the extent of protein binding to the affinity column, the areas of the peaks of retained and non-retained fractions in the chromatogram were quantified, and the percentage of retained fraction was estimated by the equation: % retained = 100 – [(non-retained/(retained + non-retained)) × 100].12
Quantification of specific antitaipan venom antibodies in the antivenoms.
An enzyme-linked immunosorbent assay (ELISA) was developed for the quantification of antitaipan venom antibodies in the antivenom. A standard of antitaipan antibodies was obtained by affinity chromatography. Briefly, a volume of either ICP or CSL antivenom was passed through a column in which O. scutellatus venom from PNG had been coupled as a ligand. After elution of the non-retained fraction, the retained fraction (i.e., the antibodies that bound to venom components in the column) was eluted with 0.1 M glycine (pH 3.0), and the pH of the eluate was neutralized with 1 M NaOH. The concentration of specific antitaipan antibodies in antivenoms was determined in an ELISA using as a standard the affinity-purified antitaipan venom antibodies. Plates were coated with 3 μg PNG O. scutellatus venom dissolved in 100 μL PBS per well. The plates were then incubated at room temperature for 1 hour with various dilutions of each antivenom in 2% milk/PBS. In other wells, known amounts of the affinity-purified antivenom antibody standards [either IgG from ICP antivenom or F(ab')2 from CSL antivenom] were added. Plates were then washed five times with distilled water. An anti-horse IgG peroxidase conjugate, diluted 1:6,000 with 2% milk/PBS, was later added, the plates were incubated for 1 hour at room temperature, which was followed by another washing step, and then, o-phenylenediamine and H2O2 were added. Absorbance values at 492 nm were recorded using a microplate reader. A calibration curve was prepared by plotting the absorbance of the affinity-purified antibodies against taipan venom as a function of their corresponding antibody concentration to calculate the concentration of specific antivenom IgGs (in ICP antivenom) or F(ab')2 fragments (in CSL antivenom) in the whole antivenoms. Specific antibody concentrations were expressed in milligrams per milliliter, and the percentage of antivenom antibodies in the whole antivenom was then estimated.
Surface plasmon resonance assays.
Interactions between taipoxin and antivenoms were assessed by surface plasmon resonance (SPR) with a BIAcore T100 system. After sensor chip docking, the instrument was primed three times with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 150 mM NaCl, 3.4 mM (ethylenedinitrilo)tetraacetic acid (EDTA), and 0.005% Tween 20 (pH 7.4; HBS-P) as running buffer, and the biosensor detector response was normalized (automated procedure). Lyophilized taipoxin was suspended and adjusted to 2 μM in 10 mM sodium acetate (pH 4.5) and then covalently immobilized on a BIAcore CM-5 sensor chip (carboxymethylated dextran matrix) according to the manufacturer´s instructions. Briefly, the CM-5 chip was activated with a 1:1 mixture of 0.4 M 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and 0.1 M N-hydroxysuccinimide for 7 minutes. Taipoxin in 10 mM sodium acetate (pH 4.5) was injected over the activated CM-5 chip at 25°C. Remaining active groups on the matrix were blocked with 1 M ethanolamine/HCl (pH 8.5). Immobilization on the CM-5 sensor chip resulted in an average surface taipoxin concentration of 4.1 ng/mm2. A flow chamber subjected to the immobilization protocol but without any addition of protein was used as a reference surface (blank cell) for each experiment. Samples of ICP or CSL antivenoms at a concentration of 20 μg/μL in HBS-P were injected over the immobilized and control surfaces at a flow rate of 20 μL/minute for 2 minutes at 25°C followed by a dissociation time of 300 seconds. Surface regeneration was performed with a single injection of 10 mM glycine-HCl (pH 2.0) for 30 seconds at a flow rate of 100 μL/minute followed by a wash step using running buffer at the same flow rate for 750 seconds. Between experiments, an additional cycle was performed using only running buffer instead of antivenom, because this extra cycle provides a more effective surface regeneration, improving reproducibility. All results were analyzed using Biaevaluation software (version 1.1.1), and sensorgrams were processed by subtracting data from the reference surface and adjusting the response on the y axis (baseline). Individual experiments were performed three times for each antivenom.
Results
Antivenomics: immunoaffinity chromatography.
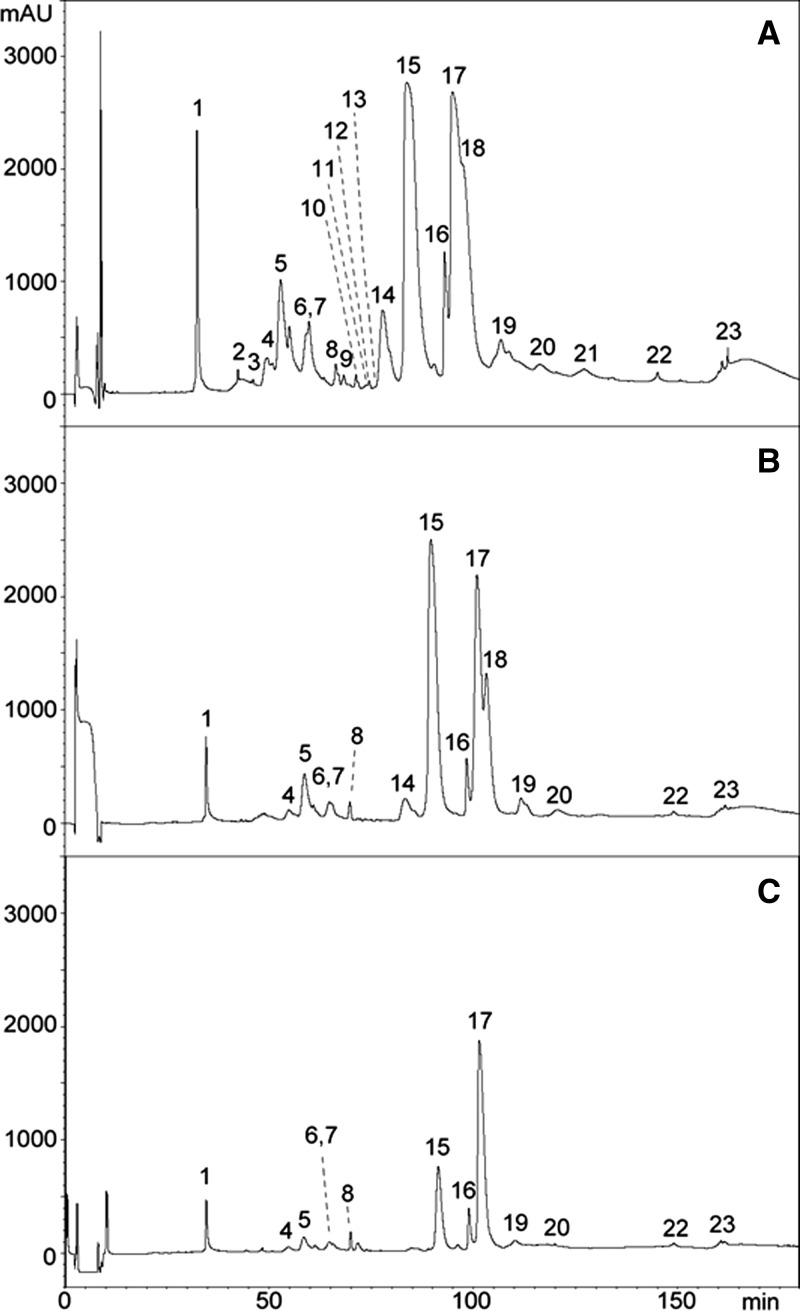
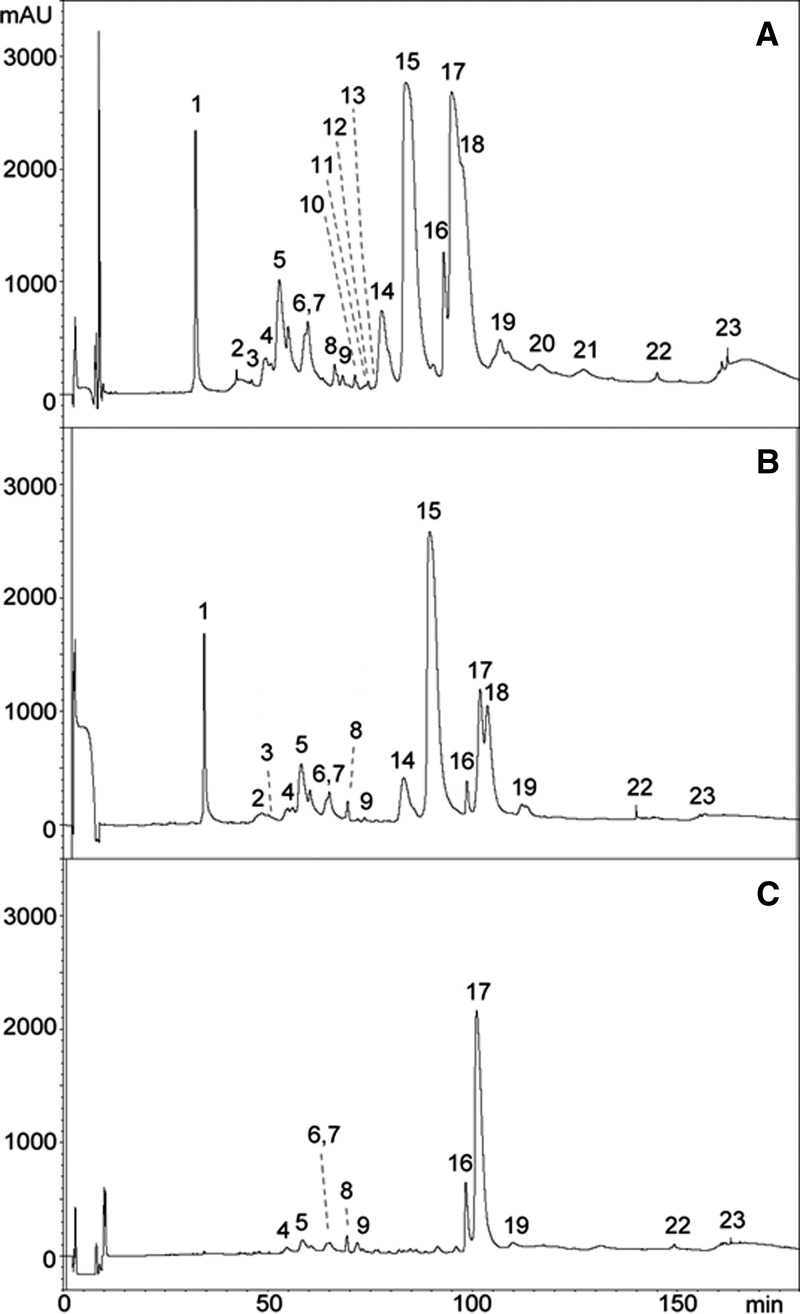
Reverse-phase HPLC separation of O. scutellatus venoms from PNG and Australia was very similar to the separation previously described in the proteomic characterization of these venoms14; therefore, the numbering of fractions used in this previous publication will be followed here (Figures 1A , 2A , 3A , and 4A ). Antivenomics analysis showed that both antivenoms were able to immunocapture the proteins present in all the chromatographic peaks of the venom of taipan from PNG (Figures 1B and 2B). Some fractions were completely immunocaptured, but various fractions were only partially immunocaptured by the antivenoms (i.e., fractions containing the PLA2 OS1 and taipoxin α- and γ-chains [peak 17 in Figures 1C and 2C] and a PLA2 and taipoxin α-chain [peak 16 in Figures 1C and 2C]). However, a fraction containing taipoxin β-chain (peak 15 in Figures 1C and 2C) and a neurotoxin of the three-finger family (peak 1 in Figures 1C and 2C), which were completely immunocaptured by CSL antivenom, were partially recognized by the ICP antivenom (Figure 1C). The estimation of the percentage of retained proteins of the most abundant peaks in the HPLC separation is shown in Table 1.
Figure 1.
Antivenomics analysis of monospecific ICP taipan antivenom with the venom of O. scutellatus from PNG. Chromatograms represent (A) reverse-phase HPLC separations of venom proteins, (B) venom proteins immunocaptured by the affinity column, and (C) venom proteins not immunocaptured in the column (details in Materials and Methods). The identification of proteins in each peak is depicted in Table 1 based on the proteomic characterization described in a previous study.14
Figure 2.
Antivenomics analysis of polyspecific CSL taipan antivenom with the venom of O. scutellatus from PNG. Chromatograms represent (A) reverse-phase HPLC separations of venom proteins, (B) venom proteins immunocaptured by the affinity column, and (C) venom proteins not immunocaptured in the column (details in Materials and Methods). The identification of proteins in each peak is depicted in Table 1 based on the proteomic characterization described in a previous study.14
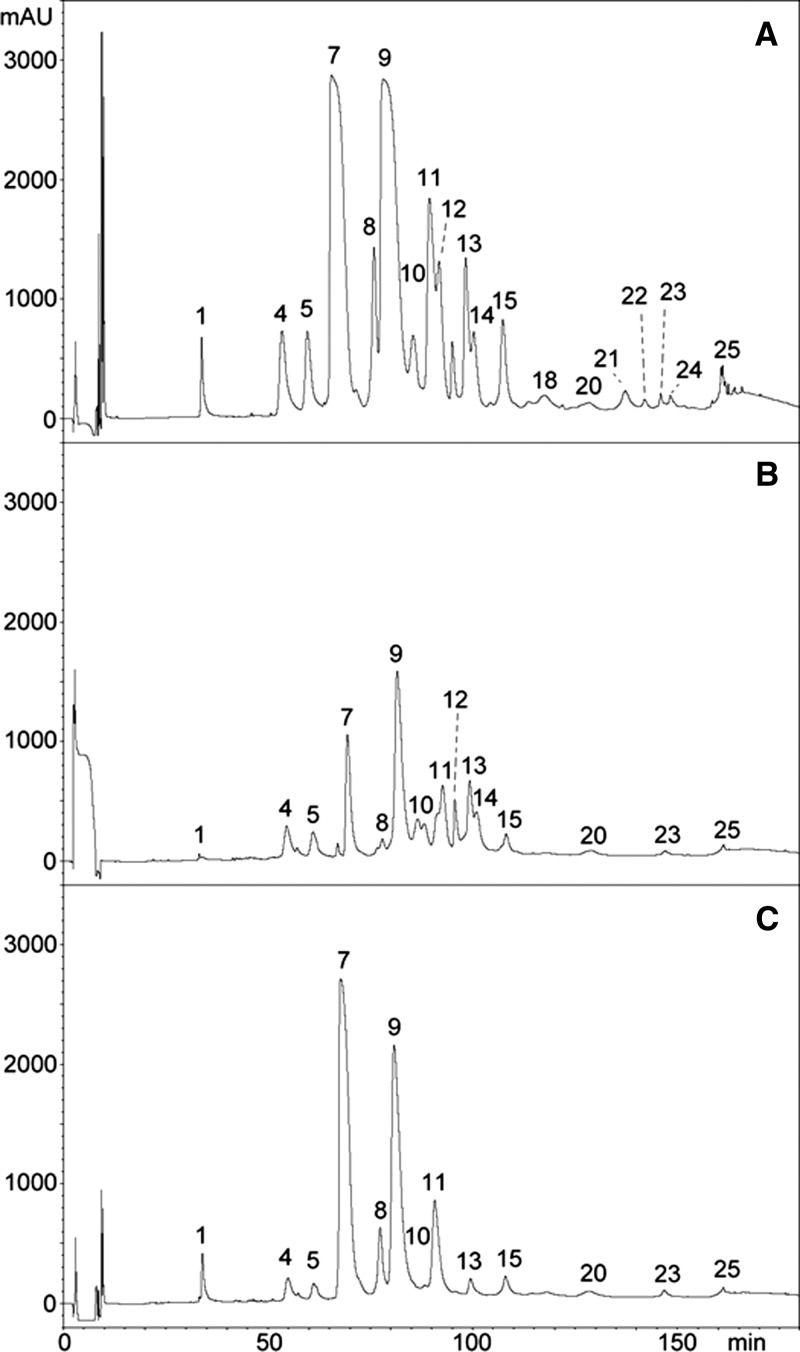
Figure 3.
Antivenomics analysis of monospecific ICP taipan antivenom with the venom of O. scutellatus from Australia. Chromatograms represent (A) reverse-phase HPLC separations of venom proteins, (B) venom proteins immunocaptured by the affinity column, and (C) venom proteins not immunocaptured in the column (details in Materials and Methods). The identification of proteins in each peak is depicted in Table 2 based on the proteomic characterization described in a previous study.14
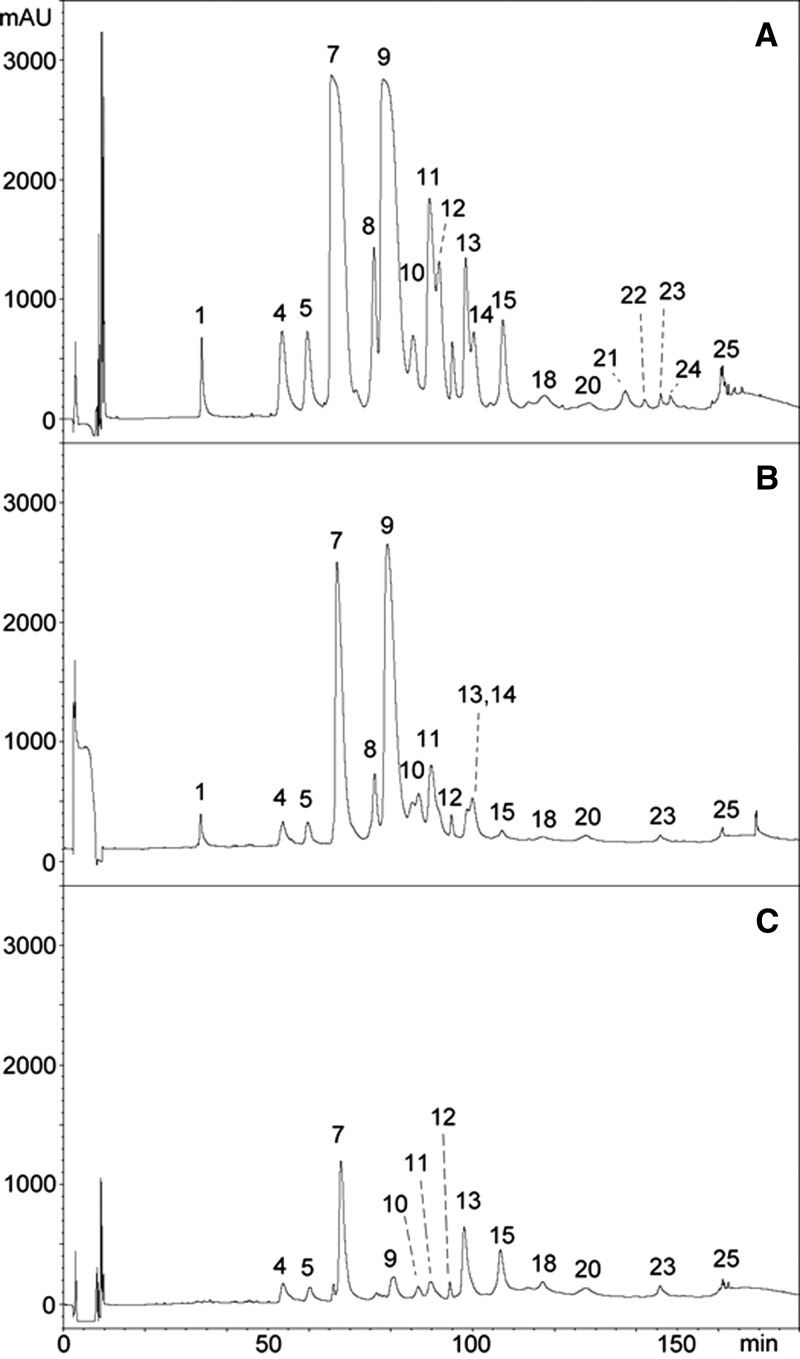
Figure 4.
Antivenomics analysis of polyspecific CSL taipan antivenom with the venom of O. scutellatus from Australia. Chromatograms represent (A) reverse-phase HPLC separations of venom proteins, (B) venom proteins immunocaptured by the affinity column, and (C) venom proteins not immunocaptured in the column (details in Materials and Methods). The identification of proteins in each peak is depicted in Table 2 based on the proteomic characterization described in a previous study.14
Table 1.
Percentages of retained proteins in the most abundant HPLC fractions of O. scutellatus venom from PNG after immunoaffinity with ICP and CSL taipan antivenoms (details in Materials and Methods)
| HPLC peak number* | Types of proteins1 | ICP (% retained fraction) | CSL (% retained fraction) |
|---|---|---|---|
| 1 | Three-finger toxin | 61 | 100 |
| 5 | Kunitz-type proteinase inhibitor (subunit of taicatoxin) | 81 | 82 |
| 14 | PLA2 (OS7 precursor), natriuretic protein | 88 | 91 |
| 15 | PLA2 (taipoxin β-chain) | 81 | 100 |
| 16 | PLA2 (taipoxin α-chain) | 54 | 45 |
| 17 | PLA2 (taipoxin γ-chain, OS1) | 55 | 34 |
| 18 | PLA2 (taipoxin γ-chain) | 100 | 100 |
The peak number and the identification of the proteins present in each peak correspond to those described in a previous study.14
Antivenomic analysis of the venom of O. scutellatus from Australia revealed that both antivenoms recognized all protein peaks, with the exception of a fraction corresponding to a three-finger–type neurotoxin that was not immunocaptured by ICP antivenom (peak 1 in Figure 3B and C). Analysis of the chromatographic patterns of proteins that were not completely immunocaptured revealed differences between antivenoms. Fractions corresponding to serine proteinase inhibitor subunit of taicatoxin (peaks 4 and 5 in Figure 3B and C), PLA2s OS2 and OS7 (peak 7 in Figure 3B and C), other PLA2s (peaks 8 and 9 in Figure 3B and C), and taipoxin α-chain (peak 11 in Figure 3B and C) were only partially immunocaptured by ICP antivenom. In the case of CSL antivenom, fractions that were partially immunocaptured were serine proteinase inhibitor subunit of taicatoxin (peaks 4 and 5 in Figure 3B and C), PLA2s OS2 and OS7 (peak 7 in Figure 3B and C), PLA2 OS6 (peak 13 in Figure 3B and C), and taipoxin β- and γ-subunits (peak 15 in Figure 3B and C). A stronger pattern of immunorecognition of the venom of O. scutellatus from Australia was observed for the CSL antivenom, because it showed a higher percentage of retained proteins than ICP antivenom for the majority of the peaks (Figures 3 and 4 and Table 2). When a control immunoaffinity column, containing IgGs from horses non-immunized with venoms, was used, the percentage of retained proteins was negligible for the majority of the peaks. However, these control antibodies bound peaks 15 (20% retention) and 17 (22% retention) in the venom from PNG O. scutellatus and peak 9 (20% retention) in the venom of Australian O. scutellatus (not shown), evidencing the presence of antibodies in this control equine serum with the ability to bind these venom components, all of which correspond to PLA2s, including some taipoxin chains.
Table 2.
Percentages of retained proteins in the most abundant HPLC fractions of O. scutellatus venom from Australia after immunoaffinity with ICP and CSL taipan antivenoms (details in Materials and Methods)
| HPLC peak number* | Types of proteins | ICP (% retained fraction) | CSL (% retained fraction) |
|---|---|---|---|
| 1 | Three-finger toxin | 3 | 100 |
| 4 | Kunitz-type proteinase inhibitor (subunit of taicatoxin) | 62 | 58 |
| 5 | Kunitz-type proteinase inhibitor (subunit of taicatoxin) | 58 | 58 |
| 7 | PLA2 (OS1 and OS7 precursors) | 19 | 72 |
| 8 | PLA2 | 22 | 92 |
| 9 | PLA2 | 39 | 94 |
| 11 | PLA2 (taipoxin α-chain) | 45 | 84 |
| 13 | PLA2 (OS6 precursor) | 76 | 34 |
| 15 | PLA2 (taipoxin β- and γ-chains) | 53 | 24 |
The peak number and the identification of the proteins present in each peak correspond to those described in a previous study.14
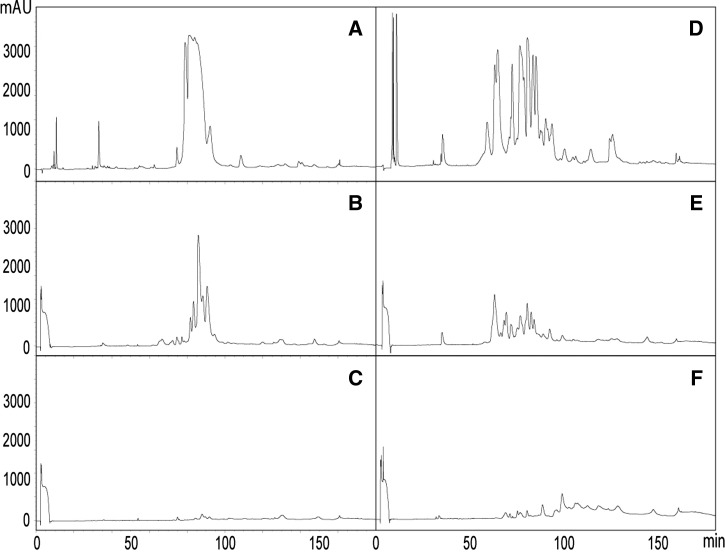
Because ICP antivenom is a truly monospecific taipan antivenom, whereas CSL taipan antivenom is produced from the plasma of horses immunized with venoms from snakes of various genera, we assessed whether these antivenoms would immunoreact differently against venoms of species different from O. scutellatus. To this end, the immunoaffinity columns containing antibodies from taipan antivenoms were used to analyze their immunoreactivity against the venoms of P. colletti and N. scutatus. As shown in Figure 5, CSL taipan antivenom has a much stronger pattern of immunorecognition of these venoms than ICP antivenom, which is in agreement with the polyspecific nature of the former.
Figure 5.
Immunoaffinity chromatography analysis of polyspecific CSL and monospecific ICP taipan antivenoms with the venoms of P. colletti and N. scutatus from Australia. Chromatograms represent reverse-phase HPLC separations of venom proteins of (A) P. colletti and (D) N. scutatus and venom proteins immunocaptured by the affinity column of (B and E) CSL taipan antivenom and (C and F) ICP taipan antivenom (details in Materials and Methods).
Quantification of specific antitaipan venom antibodies in antivenoms.
An ELISA was developed to estimate the concentration of specific anti-PNG taipan venom antibodies in antivenoms. The concentrations of specific antibodies were 13.0 ± 1.8 and 23.2 ± 3.8 mg/mL for ICP and CSL antivenoms, respectively. Taking into account that the total protein concentrations in these antivenoms are 45.9 ± 0.9 and 144.6 ± 0.4 mg/mL, respectively, the percentages of total protein in each antivenom corresponding to specific antitaipan venom antibodies are 28.3% and 16.0% for ICP and CSL antivenoms, respectively.
Interactions studied by SPR.
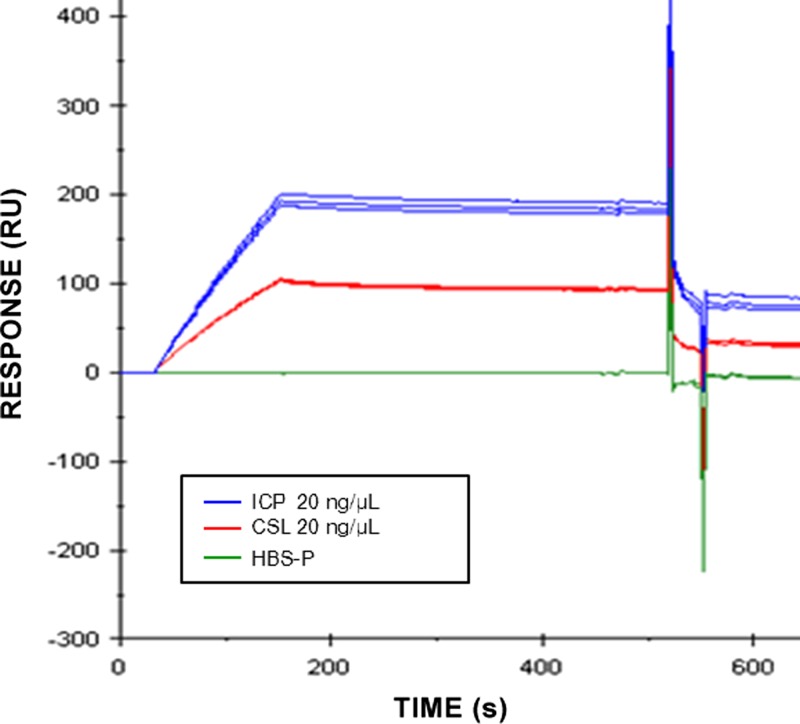
We examined the interaction of antivenoms with taipoxin by the SPR technology using a sensor chip on which the toxin had been immobilized. Figure 6 shows that antivenoms ICP and CSL (at 20 ng/μL) bound to the immobilized toxin; however, ICP showed higher affinity for taipoxin, indicating that it may contain either a higher concentration of antitaipoxin antibodies or antibodies of higher affinity for this toxin.
Figure 6.
Analysis of the interaction of taipoxin with ICP and CSL antivenoms by SPR using the BIAcore T100 system. The antivenoms at 20 μg/mL in HBS-P of 10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA, and 0.005% Tween 20 (pH 7.4) were injected over immobilized taipoxin at a flow rate of 20 μL/minute. The value of zero response corresponds to the baseline.
Discussion
Antivenomics has become a useful tool to identify components in venoms that are either recognized or not recognized by antibodies or antibody fragments in antivenoms.10,11,13 First generation antivenomics were based in the immunoprecipitation of antigen–antibody complexes, with additional identification of non-immunoreacting proteins in the supernatant.9,19 Second generation antivenomics use affinity chromatography to separate immunoreacting and non-immunoreacting venom proteins.12 The latter technique has several advantages over first generation antivenomics, such as the possibility of analyzing not only IgG antivenoms but also antivenoms made of antibody fragments and the fact that both immunocaptured and non-immunocaptured venom components can be analyzed.12 Antivenomics provide additional information to the pre-clinical neutralization tests and have become a tool to gain a deeper understanding of antivenom reactivity by also allowing a knowledge-based selection of venoms to be included in immunizing mixtures for the manufacture of antivenoms.10
Our antivenomics results show a qualitatively similar profile of these two antivenoms when tested with the venom of O. scutellatus from PNG, although quantitative differences in the percentages of proteins were noticed in several peaks. All venom fractions were recognized by the antivenoms, but there were several components, mostly the PLA2 OS1 and some taipoxin subunits, that were partially recognized. In addition, a peak containing a three-finger neurotoxin was partially immunorecognized by ICP antivenom but totally immunocaptured by the CSL antivenom. In the case of O. scutellatus venom from Australia, CSL antivenom showed a stronger pattern of immunorecognition than ICP antivenom. Although all fractions were retained by the two antivenoms, with the exception of a neurotoxin of the three-finger family that was not recognized by ICP antivenom, the HPLC profile of non-bound protein peaks revealed higher peaks of non-retained fractions in the case of ICP antivenom, which was quantitatively reflected in lower percentages of retained proteins. These fractions correspond to PLA2s, including OS2 and OS7, and two chains of taipoxin. One possibility is that these antivenomics data might be revealing immunological differences between the venoms of populations of O. scutellatus from PNG and Australia. Alternatively, the observed differences may, instead, depend on the fact that CSL antivenoms are generated from groups of horses immunized with different types of venoms at different times (i.e., they are, indeed, polyspecific antivenoms). In contrast, ICP antivenom is monospecific, because horses are immunized only with PNG O. scutellatus venom. This result was corroborated in this study, because CSL taipan antivenom reacted strongly with the venoms of P. colletti and N. scutatus, whereas ICP antivenom showed a much lower pattern of recognition of these venoms. Therefore, the presence of antibodies against some fractions of the Australian O. scutellatus venom that are recognized by CSL antivenom but not ICP antivenom might be the consequence of immunization with other elapid venoms containing high concentrations of toxins that cross-react with O. scutellatus venom components, such as neurotoxins of the three-finger family.
It is relevant to correlate these antivenomics findings with the previously described neutralizing ability of these antivenoms.8 In general terms, the widespread immunorecognition revealed by antivenomics correlates with the capacity of these two antivenoms to neutralize lethal, coagulant, myotoxic, and PLA2 activities of PNG and Australian O. scutellatus venoms.8,14 Moreover, these antivenomic patterns correlate with the lethality neutralization tests previously described, because these antivenoms present similar potencies (in terms of milligrams of venom neutralized per milliliter antivenom) for the venom of PNG O. scutellatus, whereas the CSL antivenom has a higher potency than ICP antivenom when pre-incubated with O. scutellatus venom from Australia.14 The ability of antivenoms to neutralize the lethal effect of O. scutellatus venom depends, to a large extent, on their capacity to neutralize taipoxin, the most potent neurotoxin in the venoms of both populations of taipan.15,20 In this regard, the two antivenoms bound taipoxin α-, β-, and γ-subunits to varying degrees in venoms from both PNG and Australian O. scutellatus in the affinity column. In addition, SPR observations corroborated the ability of both antivenoms to bind taipoxin, with a higher affinity in the ICP antivenom. Neutralization of short-chain α-neurotoxins is also important to ameliorating the lethality of O. scutellatus venoms, and ICP antivenom is currently less efficient at achieving immunocapturing of these toxins than CSL antivenom; strategies for improvement are currently being considered.
Our previous studies indicated that, in terms of protein concentration, ICP antivenom shows a higher neutralizing capacity than CSL antivenom, because although they have similar potencies against PNG O. scutellatus venom in terms of milligrams of venom neutralized per milliliter of antivenom, ICP antivenom has higher potency in terms of milligrams of venom neutralized per milligram of antivenom protein owing to differences in total protein concentration (45.9 and 144.6 mg/mL for ICP and CSL antivenoms, respectively).8 Our estimation of the concentration of specific anti-O. scutellatus antibodies present in antivenoms provides a partial explanation of this observation, because only 16% of total proteins were specific anti-O. scutellatus venom antibodies in the case of CSL antivenom, whereas it was 28% in the case of ICP antivenom. This result might have to do with the fact that ICP O. scutellatus antivenom is a truly monospecific antivenom, whereas CSL antivenom has been shown to be polyspecific, because horses have been also used for immunization with other Australian snake venoms. However, although ICP antivenom shows a higher potency than CSL antivenom in terms of milligrams of venom neutralized per milligram of antivenom protein,8,14 our antivenomics analyses highlighted a stronger pattern of immunoreactivity of CSL antivenom against Australian O. scutellatus venom when the same amount of antivenom protein is linked to the affinity column. This apparent discrepancy might be caused by the different conditions in which these two types of tests are performed. In the neutralization tests, antivenom and venom are incubated for 30 minutes before testing, whereas in antivenomics, the venom is passed through the affinity column. Our findings stress the need to study the pre-clinical efficacy of antivenoms by combining neutralization assays and antivenomics. Such studies may be useful in predicting the performance of candidate antivenoms at clinical trials, and certainly, it is imperative that antivenoms be subjected to well-designed clinical trials before they are marketed.
In addition to neurotoxicity leading to respiratory paralysis, envenomings by O. scutellatus are characterized by coagulopathy and associated spontaneous systemic bleeding.4 This effect is caused by the action of a group C prothrombin activator, Oscutarin C, an oligomeric protein comprising factor Xa-like and factor Va-like proteins.18 Despite its pathophysiological relevance in envenomings, this component is present in relatively low concentration in the venom and therefore, difficult to identify in the overall proteome,14 which complicates the antivenomic analysis of these procoagulants. Nevertheless, our results show that peaks containing Oscutarin C, albeit minor, were largely bound to the affinity column. The reason why ICP antivenom shows a higher neutralizing ability than CSL antivenom against procoagulant activity of taipan venoms from both populations8,14 was not answered by this antivenomics study and thus, remains to be further investigated.
Kunitz-type protease inhibitors were identified in the proteomes of taipan venoms.14 A Kunitz-type inhibitor constitutes one of the subunits of taicatoxin, a protein that exerts blocking effects in the membranes of cardiac muscle cells,21 although the role of this complex in the overall toxicity of taipan venom is uncertain. Our observations indicate that the HPLC fractions containing Kunitz-type inhibitors are recognized by both antivenoms, although the corresponding peaks were also observed in the unbound fractions in the case of Australian taipan venom, thus reflecting a pattern of partial immunological recognition.
The interpretation of these antivenomic results vis-à-vis the previous observations of effective neutralization of the lethal, coagulant, myotoxic, and PLA2 activities of both venoms by these antivenoms8,14 suggests that the patterns of partial immunorecognition observed might imply effectiveness in the neutralization of the venom components being recognized in the affinity columns. In these cases, the conditions of the neutralization experiments (i.e., the incubation of venom and antivenom before testing in the corresponding pharmacological or enzymatic systems) are likely to ensure that antibodies against toxins that show a partial recognition pattern in antivenomics bind and neutralize the corresponding toxins. Therefore, the most critical finding in antivenomic studies would be the presence of toxins that are not immunorecognized at all, because neutralization of these toxins in in vivo experiments is highly unlikely. There was only one such case observed in this study (i.e., a neurotoxin of the three-finger family in the venom of Australian taipan was not recognized at all by ICP antivenom). The role of this neurotoxin in the overall toxicity of the venom is unknown, although it is likely to be secondary in light of the preponderance of taipoxin in toxicity. Nevertheless, future improvement of this antivenom should consider enhancing the antibody titer against this neurotoxin.
In conclusion, the antivenomics results presented complement the pre-clinical assessment of these two taipan antivenoms.8,14 Results indicate that antivenoms recognize all the proteins present in the venoms of O. scutellatus of PNG, which is in agreement with previous pre-clinical results on the neutralization of lethal, myotoxic, coagulant, and PLA2 activities. However, a pattern of stronger immunorecognition was observed for the CSL antivenom when tested against O. scutellatus venom from Australia. The combination of antivenomics and toxicity neutralization tests provides complementary information for a proper understanding of the pre-clinical efficacy of antivenoms and is a useful tool for the prediction of in vivo activity and the design of clinical studies of these products.
ACKNOWLEDGMENTS
The authors thank the staff of Instituto Clodomiro Picado for their collaboration. This work was carried out in partial fulfillment of the requirements for the PhD degree for M.H. at the University of Costa Rica.
Disclaimer: M.H., Á.S., M. Vargas, M. Villalta, G.L., and J.M.G. work at Instituto Clodomiro Picado, where one of the antivenoms used in this study was manufactured.
Footnotes
Financial support: This study was supported by Fondos del Sistema-Consejo Nacional de Rectores (FEES-CONARE), Vicerrectoría de Investigación, University of Costa Rica Projects 741-B2-652 and 741-B3-017, and a project grant from the Australian National Health and Medical Research Council (Australia). Additional financial support was also provided through an ad hoc grant from the Papua New Guinea Office of Higher Education.
Authors' addresses: María Herrera, Álvaro Segura, Mariángela Vargas, Mauren Villalta, Guillermo León, and José María Gutiérrez, Instituto Clodomiro Picado, Facultad de Microbiología, Universidad de Costa Rica, San José, Costa Rica, E-mails: maria.herrera_v@ucr.ac.cr, alvaro.seguraruiz@ucr.ac.cr, mariangela.vargasarroyo@ucr.ac.cr, mauren.villaltaarrieta@ucr.ac.cr, guillermo.leon@ucr.ac.cr, and jose.gutierrez@ucr.ac.cr. Owen K. Paiva, Charles Campbell Toxinology Centre, School of Medicine and Health Sciences, University of Papua New Guinea, Port Moresby, Papua New Guinea, E-mail: owen.paiva@gmail.com. Ana Helena Pagotto and Solange M. T. Serrano, Special Laboratory of Applied Toxinology, Center of Toxins, Immune-Response and Cell Signaling (CeTICS), Instituto Butantan, Sao Paulo, Brazil, E-mails: ana.pagotto@butantan.gov.br and solange.serrano@butantan.gov.br. Simon D. Jensen and David J. Williams, Charles Campbell Toxinology Centre, School of Medicine and Health Sciences, University of Papua New Guinea, Port Moresby, Papua New Guinea, and Australian Venom Research Unit, Department of Pharmacology and Therapeutics, University of Melbourne, Parkville, VIC, Australia, E-mails: simondjensen@hotmail.com and david.williams@unimelb.edu.au.
References
- 1.Currie BJ, Sutherland SK, Hudson BJ, Smith AM. An epidemiological study of snakebite envenomation in Papua New Guinea. Med J Aust. 1991;154:266–268. doi: 10.5694/j.1326-5377.1991.tb121088.x. [DOI] [PubMed] [Google Scholar]
- 2.Lalloo DG, Trevett AJ, Korinhona A, Nwokolo N, Laurenson IF, Paul M, Black J, Naraqi S, Mavo B, Saweri A, Hutton RA, Theakston RDG, Warrell DA. Snake bites by the Papuan taipan (Oxyuranus scutellatus canni): paralysis, hemostatic and electrocardiographic abnormalities, and effects of antivenom. Am J Trop Med Hyg. 1995;52:525–531. doi: 10.4269/ajtmh.1995.52.525. [DOI] [PubMed] [Google Scholar]
- 3.Williams D. Snakebite in Papua New Guinea. In: Williams DJ, Jensen SD, Nimorakiotakis B, Winkel KD, editors. Venomous Bites and Stings in Papua New Guinea: A Treatment Guide for Health Workers and Doctors. Melbourne, Australia: University of Melbourne; 2005. pp. 5–32. [Google Scholar]
- 4.Lalloo DG, Trevett AJ, Owens D, Minei J, Naraqi S, Saweri A, Hutton RA, Theakston RDG, Warrell DA. Coagulopathy following bites by the Papuan taipan (Oxyuranus scutellatus canni) Blood Coagul Fibrinolysis. 1995;6:65–72. doi: 10.1097/00001721-199502000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Lalloo DG, Trevett AJ, Nwokolo N, Laurenson IF, Naraqi S, Kevau I, Kemp MW, James R, Hooper L, Theakston RDG, Warrell DA. Electrocardiographic abnormalities in patients bitten by taipans (Oxyuranus scutellatus canni) and other elapid snakes in Papua New Guinea. Trans R Soc Trop Med Hyg. 1997;91:53–56. doi: 10.1016/s0035-9203(97)90394-1. [DOI] [PubMed] [Google Scholar]
- 6.Trevett AJ, Lalloo DG, Nwokolo NC, Naraqi S, Kevau IH, Theakston RDG, Warrell DA. Electrophysiological findings in patients envenomed following the bite of a Papuan taipan (Oxyuranus scutellatus canni) Trans R Soc Trop Med Hyg. 1995;89:415–417. doi: 10.1016/0035-9203(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 7.O'Leary MA, Isbister GK. Commercial monovalent antivenoms in Australia are polyvalent. Toxicon. 2009;54:192–195. doi: 10.1016/j.toxicon.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Vargas M, Segura A, Herrera M, Villalta M, Estrada R, Cerdas M, Paiva O, Matainaho T, Jensen SD, Winkel KD, León G, Gutiérrez JM, Williams DJ. Preclinical evaluation of caprylic acid-fractionated IgG antivenom for the treatment of taipan (Oxyuranus scutellatus) envenoming in Papua New Guinea. PLoS Negl Trop Dis. 2011;5:e1144. doi: 10.1371/journal.pntd.0001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lomonte B, Escolano J, Fernández J, Sanz L, Angulo Y, Gutiérrez JM, Calvete JJ. Snake venomics and antivenomics of the arboreal neotropical pitvipers Bothriechis lateralis and Bothriechis schlegelii. J Proteome Res. 2008;7:2445–2457. doi: 10.1021/pr8000139. [DOI] [PubMed] [Google Scholar]
- 10.Gutiérrez JM, Lomonte B, León G, Alape-Girón A, Flores-Díaz M, Sanz L, Angulo Y, Calvete JJ. Snake venomics and antivenomics: proteomic tools in the design and control of antivenoms for the treatment of snakebite envenoming. J Proteomics. 2009;72:165–182. doi: 10.1016/j.jprot.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Calvete JJ. Proteomic tools against the neglected pathology of snake bite envenoming. Expert Rev Proteomics. 2011;8:739–758. doi: 10.1586/epr.11.61. [DOI] [PubMed] [Google Scholar]
- 12.Pla D, Gutiérrez JM, Calvete JJ. Second generation snake antivenomics: comparing immunoaffinity and immunodepletion protocols. Toxicon. 2012;60:688–699. doi: 10.1016/j.toxicon.2012.04.342. [DOI] [PubMed] [Google Scholar]
- 13.Williams DJ, Gutiérrez JM, Calvete JJ, Wüster W, Ratanabanangkoon K, Paiva O, Brown NI, Casewell NR, Harrison RA, Rowley PD, O'Shea M, Jensen SD, Winkel KD, Warrell DA. Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J Proteomics. 2011;74:1735–1767. doi: 10.1016/j.jprot.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Herrera M, Fernández J, Vargas M, Villalta M, Segura Á, León G, Angulo Y, Paiva O, Matainaho T, Jensen SD, Winkel KD, Calvete JJ, Williams DJ, Gutiérrez JM. Comparative proteomic analysis of the venom of the taipan snake, Oxyuranus scutellatus, from Papua New Guinea and Australia: role of neurotoxic and procoagulant effects in venom toxicity. J Proteomics. 2012;75:2128–2140. doi: 10.1016/j.jprot.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Fohlman J, Eaker D, Karlsson E, Thesleff S. Taipoxin, an extremely potent presynaptic neurotoxin from the venom of the Australian snake taipan (Oxyuranus s. scutellatus). Isolation, characterization, quaternary structure and pharmacological properties. Eur J Biochem. 1976;68:457–469. doi: 10.1111/j.1432-1033.1976.tb10833.x. [DOI] [PubMed] [Google Scholar]
- 16.Lambeau G, Barhanin J, Schweitz H, Qar J, Lazdunski M. Identification and properties of very high affinity brain membrane-binding sites for a neurotoxic phospholipase from the taipan venom. J Biol Chem. 1989;264:11503–11510. [PubMed] [Google Scholar]
- 17.Lambeau G, Cupillard L, Lazdunski M. Membrane receptors for venom phospholipases A2. In: Kini RM, editor. Venom Phospholipase A2 Enzymes. Structure, Function and Mechanism. Chichester, United Kingdom: Wiley; 1997. pp. 389–412. [Google Scholar]
- 18.Speiger H, Govers-Riemslag JWP, Swaal RFA, Rosing J. Prothrombin activation by an activator from the venom of Oxyuranus scutellatus (taipan snake) J Biol Chem. 1986;261:13258–13267. [PubMed] [Google Scholar]
- 19.Calvete JJ, Cid P, Sanz L, Segura A, Villalta M, Herrera M, León G, Harrison R, Durfa N, Nasidi A, Theakston RDG, Warrell DA, Gutiérrez JM. Antivenomic assessment of the immunological reactivity of EchiTAb-Plus-ICP, an antivenom for the treatment of snakebite envenoming in sub-Saharan Africa. Am J Trop Med Hyg. 2010;82:1194–1201. doi: 10.4269/ajtmh.2010.09-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuruppu S, Reeve S, Banerjee Y, Kini RM, Smith I, Hodgson WC. Isolation and pharmacological characterization of cannitoxin, a presynaptic neurotoxin from the venom of the Papuan taipan (Oxyuranus scutellatus canni) J Pharmacol Exp Ther. 2005;315:1196–1202. doi: 10.1124/jpet.105.093641. [DOI] [PubMed] [Google Scholar]
- 21.Possani LD, Martin BM, Yatani A, Mochca-Morales J, Zamudio FZ, Gurrola GB, Brown AM. Isolation and physiological characterization of taicatoxin, a complex toxin with specific effects on calcium channels. Toxicon. 1992;30:1343–1364. doi: 10.1016/0041-0101(92)90511-3. [DOI] [PubMed] [Google Scholar]