Abstract
Cutaneous leishmaniasis (CL) is endemic in the Bikaner region situated in the Thar Desert of Rajasthan, India. This study describes clinicoepidemiological data of pediatric CL in pre-school children (0–5 years of age) from this region during 2001–2012. In total, 151 patients with 217 lesions were reported during the study period. The mean age of the study group was 3.29 ± 1.43 years (0.25–5 years), with many (41.7%) cases being in the age group of 2–4 years. Face was the most common site involved, and morphologically, the lesions were either plaque type or papulonodular. Smear for parasitologic examination was positive in 84 (70%) of 120 cases, and histopathologic examination confirmed CL in 10 (55.55%) of 18 cases. Parasite species identification conducted for 13 randomly selected patients by polymerase chain reaction identified Leishmania tropica as the causative species. Intralesional sodium stibogluconate was the most commonly used treatment and found to be well-tolerated. Other therapies that were effective included oral rifampicin, oral dapsone, radiofrequency heat therapy (RFHT), and combinations of the three therapies.
Introduction
Leishmaniasis is a widespread tropical infectious disease caused by a hemoflagellate protozoan parasite of the Leishmania genus. The disease manifests mainly in three forms: visceral (L. donovani and L. infantum), cutaneous (L. tropica, L. major, L. aethiopica, L. mexicana, L. guyanensis, L. peruviana, and L. amazonensis), and mucocutaneous (L. subgenus Vianna braziliensis and L. subgenus Viannia guyanensis).1 Cutaneous leishmaniasis (CL) is the most common type; approximately 1.5 million new cases occur annually, and 90% of these cases are in Asia, the Middle East, north Africa, and South America.2 In India, CL is reported primarily from the Bikaner region situated in the Thar Desert of the Rajasthan State.3 L. tropica has been established as the causative organism in Bikaner in both rural and urban areas.4
CL affects all age groups, and children are as susceptible to the parasite as adults.5 In established endemic areas, the prevalence of CL typically increases with age, up to about 15 years, after which time prevalence levels off, presumably because of the acquisition of immunity.6 Many studies have been published that describe the epidemioclinical profiles, therapeutic modalities, and clinical outcomes of CL in the pediatric age group in various parts of world where CL is endemic.7–12 Most of the these studies have been done in the age group of 0–14 years.
Confirmation of diagnosis in pre-school children is difficult, because invasive procedures, like cutaneous biopsy/slit skin smear, are difficult to perform. A clinicoepidemiological study focused on pre-school children will lead to a better understanding of the clinical presentation of this disease and help in the early diagnosis and proper management of CL in this age group. This study describes the epidemioclinical characteristics of CL in pre-school children. To the best of our knowledge, there has been no such study from India in the past.
Materials and Methods
The study was a retrospective epidemiological study conducted from January of 2001 to December of 2012 at the Department of Dermatology, Sardar Patel Medical College, Bikaner, Rajasthan, India, and it included all patients of CL. Detailed data analysis of pre-school children with CL for the same time period was done separately. CL was diagnosed on the basis of clinical features and demographic profile. Additional confirmation of diagnosis was done by parasitological smear in a majority of patients and histopathological examination in a few of the patients. Demographic parameters, such as age, sex, and geographic location; family history of CL; duration of illness; clinical features, such as number, site, size, and type of the lesions; routine laboratory investigations; results of the slit skin smear and histopathologic finding; treatment administered; and outcome were noted on a printed pro forma.
Depending on the age, numbers of lesions, and sites of lesions, patients were treated with different treatment modalities either alone or in combination. The following treatments were administered on a case-by-case basis.
-
(1)
Intralesional injections of sodium stibogluconate (SSG; Albert David Ltd., Kolkata, India) at a dose of 0.5 mL/cm2 (100 mg/mL solution) one or two times per week for five to seven injections.
-
(2)
For topical radiofrequency heat therapy (RFHT), the controlled heat was applied locally at 50°C for 30 seconds under local anesthesia with a localized current field radio frequency generator (ThermoMed 1.8 device; Thermosurgery Technologies, Inc., Phoenix, AZ).
-
(3)
Oral rifampicin at a dosage of 20 mg/kg body weight per day for 4–6 weeks (Glaxo Smith Kline Pharmaceuticals Ltd., Maharashtra, India).
-
(4)
Oral dapsone at a dose of 20 mg/kg per day for 4–6 weeks (Glaxo Smith Kline Pharmaceuticals Ltd.).
Complete cure was defined as total re-epithelization of the lesion (flattening of the lesion and absence of signs of inflammation) with or without scarring and a negative skin smear. Patients were followed up initially at an interval of 2 weeks and thereafter, on a monthly basis until complete cure. Slit skin smear was taken from completely healed lesions after completion of treatment in cases that were positive at initial diagnosis.
Results
In total, 151 (10.3%) patients in the age group of 0–5 years were clinically diagnosed with CL from January of 2001 to December of 2012 of a total of 1,466 cases reported during the same period. The demographic profile is summarized in Table 1. The mean age was 3.29 ± 1.43 years (0.25–5 years), with the majority (41.7%) of cases being in the age group of 2–4 years. The sex ratio (male/female) was 1.56, with 92 male patients and 59 female patients. Family history of CL was observed in 16 cases (10.9%). The incidence in winter (between November and March) was more than in the summer season (between April and October). The lowest number of cases for a month were reported in October (3 cases), and the maximum number of cases was in March (25 cases).
Table 1.
Demographic profile of CL patients
| Age group (years) | Females | Males | Total |
|---|---|---|---|
| 0–2 | 22 | 19 | 41 (27.1%) |
| 2–4 | 24 | 39 | 63 (41.7%) |
| 4–5 | 13 | 34 | 47 (31.2%) |
| Total | 59 | 92 | 151 |
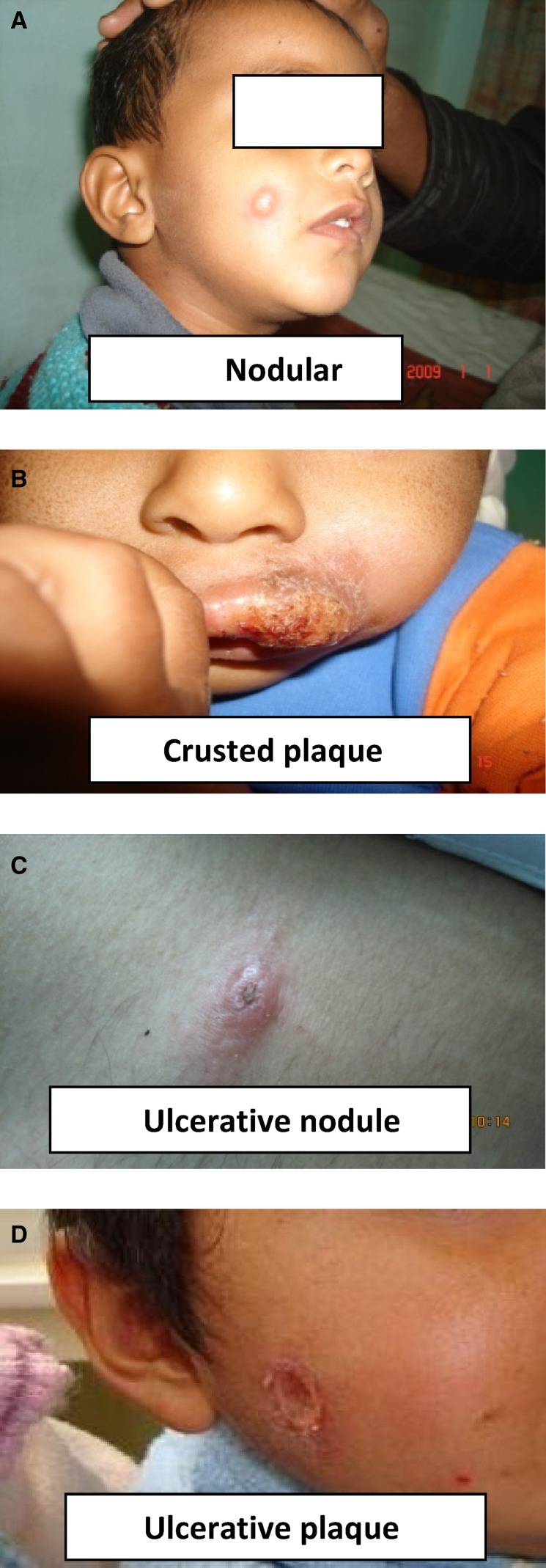
In total, 217 lesions were present in 151 patients, with the mean number of lesions being 1.43 (±0.93, range = 1–8) per patient. Single lesions were most commonly seen (i.e., in 109 patients [72.2%]), whereas double and multiple lesions were recorded in 30 (19.9%) and 12 (7.9%) patients, respectively. Clinically, the lesions were either plaque type (75.5%) or papulonodular (24.5%). The face was the most commonly involved site (72.18%). Upper limb, lower limb, and trunk were affected in 9.93%, 7.94%, and 1.32% cases, respectively, whereas multiple sites were involved in 8.63% of cases (Figure 1). The details of clinical features are provided in Table 2.
Figure 1.
Clinical manifestations of CL in pre-school children at Bikaner. (A) Nodular. (B) Crusted plaque. (C) Ulcerative nodule. (D) Ulcerative plaque.
Table 2.
Clinical features of the cases
| Clinical feature | Frequency |
|---|---|
| Number of lesions | 217 (mean = 1.43 ± 0.93, range = 1–8) |
| Single | 109 (72.2%) |
| Double | 30 (19.9%) |
| Multiple | 12 (7.9%) |
| Clinical form | |
| Plaques (total) | 114 (75.5%) |
| Ulcerated | 54 (35.7%) |
| Non-ulcerated | 60 (39.8%) |
| Papulonodular | 37 (24.5%) |
| Site involved | |
| Face | 109 (72.18%) |
| Lower limb | 12 (7.94%) |
| Upper limb | 15 (9.93%) |
| Trunk | 2 (1.32%) |
| Multiple sites | 13 (8.63%) |
The mean duration of the cutaneous lesions before presentation was 17.88 ± 1.18 weeks (range = 2–48 weeks), whereas the sizes of the lesions varied from 0.50 to 6 cm, with a mean value of 2.42 cm (±1.63 cm). Smears for parasitologic examination were performed in 120 patients, and they were positive in 84 (70%) patients. Skin biopsies were performed in 18 patients, of which 10 (55.5%) patients were positive for Leishmania. Histopathology in all cases showed granulomatous inflammation in the dermis with the presence of parasitized macrophages and epitheloid and plasma cells along with lymphomononuclear infiltrate. Internal transcribed spacer region 1 restriction fragment length polymorphism polymerase chain reaction (PCR) and kinetoplast DNA PCR were performed in 13 randomly selected patients, and all patients were positive for L. tropica.
In total, 148 (98.01%) patients received treatment. Intralesional SSG was most commonly used (58 patients; 38.4%), and it was well-tolerated in all patients. Heat therapy was administered to 14 (9.4%) patients. Fifty (33.1%) patients received oral rifampicin. Three (1.9%) patients were treated with oral dapsone, whereas both dapsone and rifampicin were given orally in 11 (7.3%) patients. Twelve (7.9%) patients received a combination of systemic and local therapies in the form of intralesional injections of SSG and oral rifampicin.
Nine patients were lost to follow-up, whereas response to treatment was recorded in 139 (92.1%) patients. Complete cure of the lesions was obtained within an average period of 9.7 weeks (±3.3 weeks, range = 4–20 weeks). Slit skin smears were performed in 80 (of 84) completely cured cases that were positive at the time of diagnosis, and they were found to be negative in all cases. The mean time for healing varied with the treatment modality used. Complete cure was seen in 125 (82.78%) patients at 20 weeks. Complete cure with local therapies (i.e., intralesional SSG and RFHT) was observed in 84.4% and 91.8% of patients, respectively. Similarly, with oral rifampicin, oral dapsone, and a combination of rifampicin and dapsone, complete healing was recorded in 82%, 66.67%, and 90.1% of patients respectively. The combination of intralesional SSG and oral rifampicin was effective in 100% of cases.
Discussion
Although only a few studies have been published in the literature on the epidemiology of CL in pediatric age groups, none have been published in pre-school children (< 5 years of age). In total, 151 children in the pre-school age group were affected, comprising 10.3% of total CL reported during the same period. Gurel and others13 from Turkey reported 19.7% in the 0–4 year age group of the total CL cases, and Bari6 from Pakistan reported 32.3% of total pediatric cases; both percentages are higher than in our study. The high percentage in this age group may be because of more time indoors or in areas nearby the dwellings, which are the most common breeding and resting grounds of sandfly vectors, placing them at high risk. The high percentage could also be explained by a lack of antileishmanial immunity and an immature immune system characteristic of this age group.
A slight male preponderance was seen in our study, with a male-to-female ratio of 1.56, similar to the studies conducted by Bari6 and Shoaib and others8 from Pakistan. Talari and others10 made similar observations, with a boy-to-girl ratio of 1.2 in Iran. However, a female preponderance was observed in Tunisian and Moroccan studies.9,11 Family history of CL was present in 10.6% of cases in this study, whereas Kharfi and others11 and Qasmi and others9 had reported it to be 5.6% and 15.3% of cases, respectively. Although a single lesion was most commonly observed, multiple lesions (more than one) were not uncommon. Bari,6 in his study, observed multiple lesions in 25% of cases, which was similar to the finding in this study (27.8%). Talari and others10 from Iran reported multiple lesions in 48.7% of cases, which is much higher than other studies, and Zaraa and others7 from Tunisia reported multiple lesions in 8.4% of pediatric cases, which is lower than all other studies.
The average time lag between the appearance of a CL lesion and the first diagnosis was lengthy (4.5 months). Zaraa and others7 and Kharfi and others11 from Tunisia reported an even longer time lags, which were 12.71 and 8 months, respectively. This delay in diagnosis was because of the painless and insidious nature of the disease. Although most CL cases were observed in winter months, it is possible that transmission of infection occurred predominantly in summers because of the increased humidity and temperature, which favor breeding of sandflies. The long incubation period of the disease coupled with the apparent delay in seeking treatment led to more cases in the winter season, although the patients might have acquired the infection during the preceding summer. The most common site affected was the face (72.18%), which is similar to the finding in the study by Kharfi and others11 (76.5%). Bari6 and Zaraa and others7 reported facial lesions in 98.9% and 88.1% of patients, respectively, which are much higher percentages than in this study. Talari and others10 showed facial lesions in only 47.1% of cases, which is much lower than all other studies. The occurrence of the majority of lesions on the face observed in our study can be explained by the fact that pre-school children usually spend a lot of time sleeping, and the face is the most frequently exposed body area. The lesions were predominantly plaques and papulonodular, which has been reported in other studies (27.4–78.44%).6,7,10,11 Some of the uncommon morphological forms of CL that were reported earlier in pediatric age groups, including cheilitis, verruciform, perleche, chancriform,6 sporotrichoid, erysipeloid,10 lupoid, mucoucutaneous, and furunculoid,11 were not observed in this study. Microscopic examination of tissue smears and histopathology were confirmatory in 70% and 55.5% of cases, respectively, in this study, whereas Rodrigues and others14 and Al-Hucheimi and others15 reported 66.7% and 66.2% as well as 66.7% and 59.6%, respectively. Newer DNA-based techniques, like PCR, have a higher sensitivity but are expensive and not widely available.16,17
Many treatment modalities have been used in CL in pediatric age groups, including topical, systemic, and physical treatments. Intralesional SSG was used in 58 cases, with a cure rate of 84.4% in this study, whereas Zaraa and others7 and Kharfi and others11 reported cure rates of 67% and 100%, respectively. Oral rifampicin was administered to 50 patients, and the complete cure rate was 82%. Similar results were reported by the same center earlier by Kochar and others18 and Bumb and Mehta,19 with complete cure in 73.9% and 83.2% of cases, respectively, in all groups. Oral rifampicin was well-tolerated without any serious side effects. It has the advantages of ease of administration, good safety profile, and minimal scarring post-treatment.18,19 Salim and Kandil20 reported complete resolution of lesions in 41 (89.1%) of 46 patients who underwent treatment with oral rifampicin. RFHT is a relatively newer modality for treatment of CL. An Iranian study by Sadeghian and others21 reported a cure rate of > 80% in 117 cases of all age groups with local heat therapy by radiofrequency, whereas Bumb and others22 showed a 94% cure rate. In both studies, RFHT was found to be more effective than intralesional meglumineantimoniate. Similarly, in this study, a cure rate of 92% was observed with RFHT, although it was used in fewer cases and only pre-school children. Dapsone alone or combined with rifampicin was effective in 82.7% cases. A double-blind study assessing the efficacy of oral dapsone by Dogra23 reported a cure rate of 82% with dapsone monotherapy.
In conclusion, L. tropica CL is endemic in Bikaner district of the Thar Desert of Rajasthan in the pre-school age group. The clinical and epidemiology spectra seem to be similar to the pediatric and adult age group. Diagnosis in the pre-school age group is based predominantly on clinical presentation and history of stay in endemic areas because of the difficulty in performing invasive investigations and the low sensitivity of skin smear and biopsy. Intralesional SSG remains the standard treatment, but oral rifampicin was observed to be equally efficacious. RFHT is a good alternative because of its minimal systemic toxicity, and only a single treatment session is required compared with other local modalities of treatment. The preventive measures to control the vector have reduced the incidence in the past few years, but more measures should be adopted to reduce the incidence of this infection that can cause facial disfigurement.
Footnotes
Authors' addresses: Saurabh Agrawal, Kanika Khandelwal, and Ram A. Bumb, Department of Dermatology, S P Medical College, Bikaner, Rajasthan, India, E-mails: sagrawal@yahoo.com, khandelwalk@yahoo.com, and dr_bumb@rediffmail.com. Steve Oghumu and Abhay R. Satoskar, Department of Pathology and Microbiology, Ohio State University, Columbus, OH, E-mails: steve.oghumu@osumc.edu and satoskar.2@osu.edu. Poonam Salotra, Department of Molecular Biology, ICMR, New Delhi, India, E-mail: psalotra@yahoo.com.
References
- 1.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 2.WHO . Geneva, Switzerland: 2010. Control of the leishmaniases. Proceedings of the Meeting of the WHO Expert Committee on the Control of Leishmaniases. WHO Technical Report Series 949, March 22–26, 2010. [Google Scholar]
- 3.Srivastava D, Vyas MCR, Joshi CK. Clinico epidemiological study of cutaneous leishmaniasis in Bikaner (Rajasthan) J Commun Dis. 1987;19:326–331. [PubMed] [Google Scholar]
- 4.Kumar R, Bumb RA, Ansari NA, Mehta RD, Salotra P. Cutaneous leishmaniasis caused by Leishmania tropica in Bikaner, India: parasite identification and characterization using molecular and immunologic tools. Am J Trop Med Hyg. 2007;76:896–901. [PubMed] [Google Scholar]
- 5.Kafetzis DA. An overview of paediatric leishmaniasis. J Postgrad Med. 2003;49:31–38. doi: 10.4103/0022-3859.930. [DOI] [PubMed] [Google Scholar]
- 6.Bari AU. Childhood cutaneous leishmaniasis. J Clin Diagn Res. 2008;2:973–978. [Google Scholar]
- 7.Zaraa I, Ishak F, Kort R, El Euch D, Mokni M, Chaker E, Ben Osman A. Childhood and adult cutaneous leishmaniasis in Tunisia. Int J Dermatol. 2010;49:790–793. doi: 10.1111/j.1365-4632.2010.04467.x. [DOI] [PubMed] [Google Scholar]
- 8.Shoaib S, Tauheed S, Hafeez A. Cutaneous leishmaniasis: an emerging childhood infection. J Ayub Med Coll Abbottabad. 2007;19:40–41. [PubMed] [Google Scholar]
- 9.Qasmi S, Elguelbazouri N, Belgnaoui FZ, Marcil T, Bouhllab J, Senouci K, Aitourhoui M, Hassam B. Childhood cutaneous leishmaniasis: experience of a Moroccan unit of dermatology. Dermatol Online J. 2008;14:18. [PubMed] [Google Scholar]
- 10.Talari SA, Talaei R, Shajari G, Vakili Z, Taghaviardakani A. Childhood cutaneous leishmaniasis: report of 117 cases from Iran. Korean J Parasitol. 2006;44:355–360. doi: 10.3347/kjp.2006.44.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharfi M, Benmously R, El Fekih N, Daoud M, Fitouri Z, Mokhtar I, Ben Becher S, Kamoun MR. Childhood leishmaniasis: report of 106 cases. Dermatol Online J. 2004;10:6. [PubMed] [Google Scholar]
- 12.Fenniche S, Souissi A, Benmously R, Ben Jannet S, Marrak H, Mokhtar I. Childhood cutaneous leishmaniasis in Tunisia: retrospective study of 60 cases. Med Trop (Mars) 2006;66:456–460. [PubMed] [Google Scholar]
- 13.Gurel MS, Ulukanligil M, Ozbilge H. Cutaneous leishmaniasis in Sanliurfa: epidemiologic and clinical features of the last four years (1997–2000) Int J Dermatol. 2002;41:32–37. doi: 10.1046/j.0011-9059.2001.01396.x. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues EH, Felinto de Brito ME, Mendonça MG, Werkhäuser RP, Coutinho EM, Souza WV, Militão de Albuquerque Mde F, Jardim ML, Abath FG. Evaluation of PCR for diagnosis of American cutaneous leishmaniasis in an area of endemicity in northeastern Brazil. J Clin Microbiol. 2002;40:3572–3576. doi: 10.1128/JCM.40.10.3572-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Hucheimi SN, Sultan BA, Al-Dhalimi MA. A comparative study of the diagnosis of Old World cutaneous leishmaniasis in Iraq by polymerase chain reaction and microbiologic and histopathologic methods. Int J Dermatol. 2009;48:404–408. doi: 10.1111/j.1365-4632.2009.03903.x. [DOI] [PubMed] [Google Scholar]
- 16.Vega-López F. Diagnosis of cutaneous leishmaniasis. Curr Opin Infect Dis. 2003;16:97–101. doi: 10.1097/00001432-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Marco JD, Barroso PA, Mimori T, Locatelli FM, Tomatani A, Mora MC, Cajal SP, Nasser JR, Parada LA, Taniguchi T, Korenaga M, Basombrío MA, Hashiguchi Y. Polymorphism-specific PCR enhances the diagnostic performance of American tegumentary leishmaniasis and allows the rapid identification of Leishmania species from Argentina. BMC Infect Dis. 2012;12:191. doi: 10.1186/1471-2334-12-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochar DK, Aseri S, Sharma BV, Bumb RA, Mehta RD, Purohit SK. The role of rifampicin in the management of cutaneous leishmaniasis. QJM. 2000;93:733–737. doi: 10.1093/qjmed/93.11.733. [DOI] [PubMed] [Google Scholar]
- 19.Bumb RA, Mehta RD. Oral rifampicin in cases of cutaneous leishmaniasis with multiple lesions (a pilot study) Indian J Dermatol Venereol Leprol. 2002;68:272. [PubMed] [Google Scholar]
- 20.Salim MM, Kandil E. Rifampicin in the treatment of cutaneous leishmaniasis. J Kuwait Med Assoc. 1972;6:159–166. [Google Scholar]
- 21.Sadeghian G, Nilfroushzadeh MA, Iraji F. Efficacy of local heat therapy by radiofrequency in the treatment of cutaneous leishmaniasis, compared with intralesional injection of meglumineantimoniate. Clin Exp Dermatol. 2007;32:371–374. doi: 10.1111/j.1365-2230.2007.02405.x. [DOI] [PubMed] [Google Scholar]
- 22.Bumb RA, Prasad N, Khandelwal K, Aara N, Mehta RD, Ghiya BC, Salotra P, Wei L, Peters S, Satoskar AR. Long-term efficacy of single-dose radiofrequency-induced heat therapy vs. intralesionalantimonials for cutaneous leishmaniasis in India. Br J Dermatol. 2013;168:1114–1119. doi: 10.1111/bjd.12205. [DOI] [PubMed] [Google Scholar]
- 23.Dogra J. Dapsone in the treatment of cutaneous leishmaniasis. Int J Dermatol. 1986;25:398–401. doi: 10.1111/j.1365-4362.1986.tb03435.x. [DOI] [PubMed] [Google Scholar]