Abstract
A modified imprint method, Press-Imprint-Smear, was compared with histopathology for the diagnosis of cutaneous leishmaniasis. Amastigotes were seen in 69 (92%) of 75 individuals in one or both assays. The Press-Imprint-Smear was positive in 85.3%, and histopathology was positive in 44%. Press-Imprint-Smear is a rapid and relatively sensitive method for the diagnosis of cutaneous leishmaniasis.
Introduction
Leishmaniasis has been reported from 98 countries.1 Brazil is highly endemic for both cutaneous and visceral leishmaniasis, which pose important public health problems.2,3 Brazil is among 10 countries that account for 75% of cutaneous disease worldwide. An average of 26,000 cases are reported annually, but it is estimated that more than 72,000 cases occur each year.1 Autochthonous cases have been reported from all Brazilian states.4 Most cases of cutaneous leishmaniasis occur in areas without good health infrastructure where diagnostic laboratory facilities are limited. The diagnostic method of choice for cutaneous leishmaniasis historically has been microscopic demonstration of the parasite. Isolation of Leishmania in culture and detection of parasite DNA by molecular diagnostic methods are limited to reference or research laboratories. A rapid, sensitive, and inexpensive test for the diagnosis of cutaneous leishmaniasis is needed for use in rural, resource-limited, endemic settings.
The diagnosis of cutaneous leishmaniasis often takes days, even in clinics or hospitals with good laboratories. The time between collection of specimens and results of tests is much longer in rural endemic areas, where facilities are frequently not available. As a result, the diagnosis and treatment are often based on clinical and epidemiological grounds. A sensitive, simple, and inexpensive test is needed. We report such a test, a modified imprint test called Press-Imprint-Smear.
Study
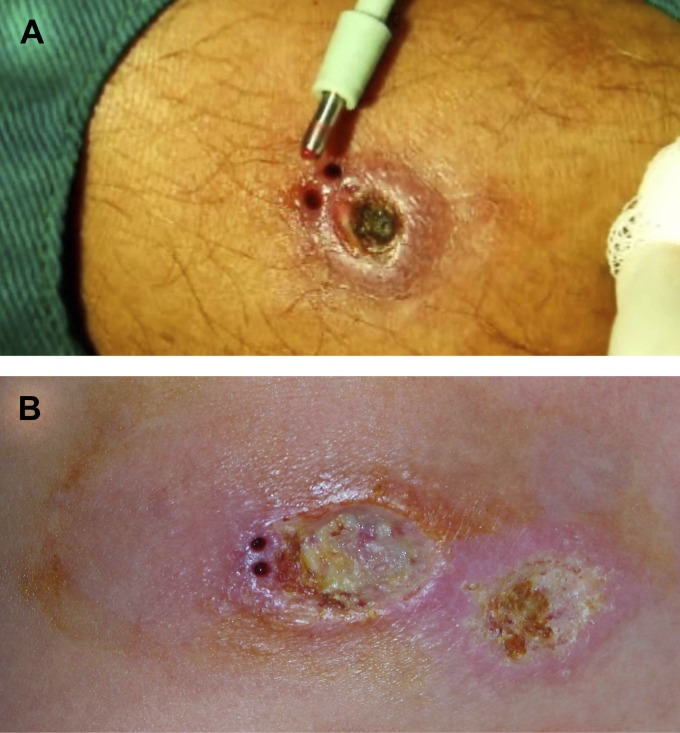
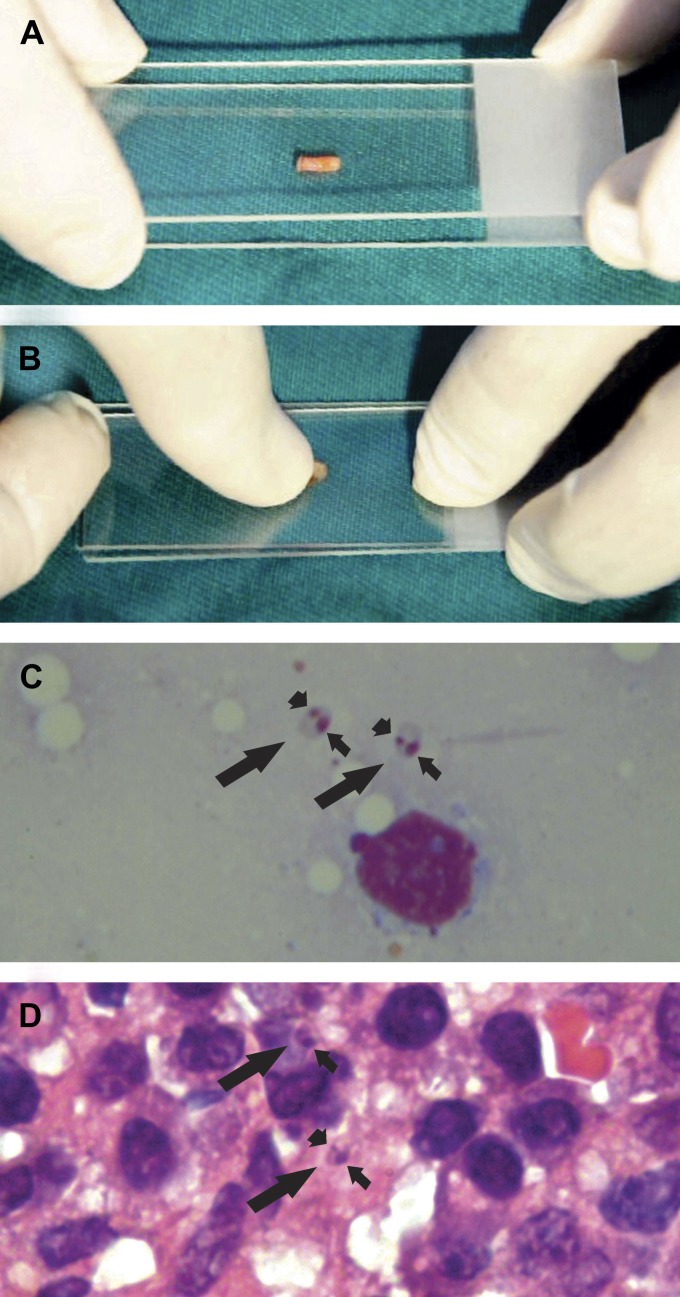
Patients with a clinical diagnosis of cutaneous leishmaniasis seen at São José Hospital for Infectious Diseases in Fortaleza, Ceará, Brazil, from September of 2011 to August 2013 who agreed to skin biopsy were included in the study. Lesions were cleaned with chlorhexidine and anesthetized with 2% xylocaine. Two biopsy samples were taken from the borders of ulcers (lesions) using a 3-mm disposable punch (Figure 1 and Supplemental Videos 1 and 2).5 The samples were taken less than 3 mm from the borders of the ulcers (lesions). One biopsy specimen was fixed in 10% formalin and processed for histopathology. Another specimen was used for Press-Imprint-Smear. For the imprint, the biopsy sample was put on a glass slide, and another glass slide was used to cover the tissue fragment in a sandwich way. On a firm surface, the tissue fragment between both glass slides was squeezed (squashed).6,7 Pressure on the middle of the slides was made, and therefore, the juice and tissue cells were spread on both slides' surfaces that were in contact with the sample (Figure 2A and B). To avoid smears being too thick, slight movements were made (Supplemental Videos 1 and 2). Both smears were air dried, fixed in methanol, stained with Giemsa, and examined microscopically using a 100× oil immersion lens. A Press-Imprint-Smear or histopathology slide was reported to be positive when amastigotes were seen (Figure 2C and D), and a negative Press-Imprint-Smear result was reported when all fields of both slides were analyzed and no amastigotes were found. All Press-Imprint-Smear slides were analyzed by one examiner, and all histopathology slides were examined by one pathologist. The examiners were not part of the research team, and the examiners who analyzed the smear slides did not analyze the histopathology slides and vice versa.
Figure 1.
Typical cutaneous leishmaniasis skin lesions after biopsies were done. A shows a 3-mm punch with the tissue fragment still in it. B shows two biopsy sites after skin fragments were removed. Biopsies were taken less than 3-mm from the border of the lesion.
Figure 2.
A shows a 3-mm skin biopsy fragment on a glass slide and another glass slide almost covering it. B shows the skin fragment being squashed, and it also illustrates the moment that slight movements are made to improve smear quality. C shows two amastigotes (long arrows) with nuclei (short arrows) and kinetoplasts (arrowheads) near a blood cell in a Press-Imprint-Smear stained with Giemsa under an oil immersion lens (100×). D shows a histopathological section stained with hematoxylin/eosin. Two amastigotes are seen with nuclei, and in one of them, the kinetoplast can be visualized (100×).
The Research Ethics Committee at Hospital São José for Infectious Diseases approved the study, and all patients gave informed consent for the skin biopsy.
In total, 75 patients were studied. In 69 (92%) patients, the diagnosis of cutaneous leishmaniasis was confirmed by the identification of amastigotes by one or both methods. In 64 (85.3%) patients, amastigotes were detected by Press-Imprint-Smear. In 33 (44%) patients, amastigotes were detected by histopathology, and in 28 (37.3%) patients, amastigotes were detected by both methods. In 36 (48%) patients, parasites were seen only by Press-Imprint-Smear, and in 5 (6.7%) patients, parasites were seen only by histopathology (Table 1).
Table 1.
Identification of amastigotes by histopathology and Press-Imprint-Smear in the same lesion of 75 patients with a clinical diagnosis of cutaneous leishmaniasis
| Histopathology | Press-Imprint-Smear | Total | P* | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 28 (37.3%) | 5 (6.7%) | 33 (44%) | 0.001 |
| Negative | 36 (48.0%) | 6 (8.0%) | 42 (56%) | 0.001 |
| Total | 64 (85.3%) | 11 (14.7%) | 75 | |
McNemar's test.
Discussion
The results show a much higher sensitivity of Press-Imprint-Smear than histopathology (85.3% versus 44%, P < 0.001). One explanation for the higher sensitivity of Press-Imprint-Smear over histopathology is that, when the tissue is squashed between the glass slides, the result is a monolayer of flatten cells, and if amastigotes are present in the sample, they will be seen in the smear. In a histopathological section, as a rule, there is more than one layer of cells, and other tissue elements are also present, making amastigotes more difficult to be identified. However, in histopathological preparation, cells are not flattened, but they are in a tridimensional position, making it possible for an infected cell to be cut in a non-parasitized area and allowing a false-negative report.
The results showed samples from five patients where amastigotes were seen by histopathology and not Press-Imprint-Smear. Reasons for this discrepancy might be related to sample representation, quality of the smear, and time spent on microscopic examination.
Some studies on histopathology of cutaneous leishmaniasis done in other endemic areas of Brazil as well as other Latin American countries, where the predominant agent is L. (Viannia) braziliensis, have shown amastigotes in 17.8–40% of cases.8–10 These results are in agreement with our results (44%). It is important to emphasize that all patients in our study are from areas where the only parasite ever identified in cutaneous leishmaniasis has been L. (V.) braziliensis,11,12 which makes the sensitivity of our method very good for this species of leishmania.
Previous studies have shown that skin scrapings are also more sensitive than histopathology in diagnosing cutaneous leishmaniasis.10,13–15 The explanation for the difference is probably the same as for Press-Imprint-Smear. Skin scraping has limitations compared with Press-Imprint-Smear. Scrapings are typically done on the borders of ulcerated lesions, but lesions are not always ulcerated, and some lesions are too small to scrape. A scalpel or a curette is required for the procedure. With Press-Imprint-Smear, only a punch is needed, and the procedure is simpler and less expensive. Finally, scrapings are done in ulcerated areas, and there is a theoretical risk of secondary bacterial infection.
Press-Imprint-Smear can be done in less than 1 hour. Histopathology, as a rule, takes several days. All that is needed to perform Press-Imprint-Smear is a punch, staining material, a microscope, and a microscopist. A technician can learn to identify amastigotes with a short training course. Histopathology requires a pathologist and supporting laboratory, which are often available only at referral hospitals.
In addition to cutaneous leishmaniasis, Press-Imprint-Smear has been used to diagnose cutaneous tuberculosis, histoplasmosis, and cryptococcosis with proper stains. In cases of multibacillary leprosy, Press-Imprint-Smear has shown to be more sensitive than earlobe slit smears in identifying Mycobacterium leprae. Press-Imprint-Smear has also been used to evaluate lymph node biopsies in acute Chagas disease and diagnose tuberculosis, disseminated histoplasmosis, and visceral leishmaniasis lymphadenitis. In these situations, the lymph node fragment for the smear should be 2–3 mm in size, and this test has shown to be more sensitive in identifying microorganisms than histopathology (Sousa AQ, unpublished data).
Considering its sensitivity, low cost, and simplicity, Press-Imprint-Smear is a valuable tool for diagnosing cutaneous leishmaniasis and potentially, other infectious diseases at the point of care in rural or resource-limited endemic regions.
Supplementary Material
Footnotes
Financial support: This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq Grant 485964/2012-0 and Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico-FUNCAP Grant 12535705-2.
Authors' addresses: Anastácio Q. Sousa, Margarida M. L. Pompeu, and Juliana M. B. M. Tinel, School of Medicine, Federal University of Ceará, Fortaleza, Ceará, Brazil, E-mails: aqsousa@gmail.com, mpompeu@ufc.br, and julianazuma@gmail.com. Mércia S. Frutuoso, School of Medicine, Federal University of Ceará and Unichristus School of Medicine, Fortaleza, Ceará, Brazil, E-mail: merciasindeaux@gmail.com. José W. O. Lima, State University of Ceará School of Medicine, Fortaleza, Ceará, Brazil, E-mail: jwolima@yahoo.com.br. Richard D. Pearson, University of Virginia School of Medicine, Charlottesville, VA, E-mail: rdp9g@virginia.edu.
References
- 1.Alvar J, Velez ID, Bern C, Herero M, Desjeux P, Cano J, Jannin J, den Boer M. WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Report of the Fifth Consultative Meeting on Leishmania/HIV Coinfection. 2007. http://www.who.int/leishmaniasis/resources/Leishmaniasis_hiv_coinfection5.pdf Available at. Accessed November 1, 2013.
- 4.Ministério da Saúde do Brasil Casos de Leishmaniose Tegumentar Americana. Brasil. Grandes Regiões e Unidades Federadas. 1990–2011. 2013. http://portal.saude.gov.br/portal/arquivos/pdf/2012_11_casos_de_lta_entre_1990_e_2011.pdf Available at. Accessed November 1, 2013.
- 5.Levitt J, Bernardo S, Talley Whang T. How to perform a punch biopsy of the skin—videos in clinical medicine. N Engl J Med. 2013;369:e13. doi: 10.1056/NEJMvcm1105849. [DOI] [PubMed] [Google Scholar]
- 6.De Lorenzi D, Mandara MT, Tranquillo M, Baroni M, Gasparinetti N, Gandini G, Masserdotti C, Bonfanti U, Bertolini G, Vian P, Bernardini M. Squash–prep cytology in the diagnosis of canine and feline nervous system lesions: a study of 42 cases. Vet Clin Pathol. 2006;35:208–214. doi: 10.1111/j.1939-165x.2006.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 7.Mitra S, Kumar M, Sharma V, Mukhopadhyay D. Squash preparation: a reliable diagnostic tool in the intraoperative diagnosis of central nervous system tumors. J Cytol. 2010;27:81–85. doi: 10.4103/0970-9371.71870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alves CF, Alves CF, Figueiredo MM, Souza CC, Machado-Coelho GL, Melo MN, Tafuri WL, Raso P, Soares RP, Tafuri WL. American tegumentary leishmaniasis: effectiveness of an immunohistochemical protocol for the detection of Leishmania in skin. PLoS ONE. 2013;8:e63343. doi: 10.1371/journal.pone.0063343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berman JD. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 10.Weigle KA, de Dávalos M, Heredia P, Molineros R, Saravia NG, D'Alessandro A. Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: a comparison of seven methods. Am J Trop Med Hyg. 1987;36:489–496. doi: 10.4269/ajtmh.1987.36.489. [DOI] [PubMed] [Google Scholar]
- 11.Sousa AQ, Parise ME, Pompeu MM, Coelho Filho JM, Vasconcelos IA, Lima JW, Oliveira EG, Vasconcelos AW, David JR, Maguire JH. Bubonic leishmaniasis: a common manifestation of Leishmania (Viannia) braziliensis infection in Ceara, Brazil. Am J Trop Med Hyg. 1995;53:380–385. doi: 10.4269/ajtmh.1995.53.380. [DOI] [PubMed] [Google Scholar]
- 12.Sousa AQ, Pompeu MML, Frutuoso MS, Moraes EA, Pearson RD. Reply to Drs. Torres and Suarez. Clin Infect Dis. 2012;55:309–310. [Google Scholar]
- 13.Aviles H, Belli A, Armijos R, Monroy FP, Harris E. PCR detection and identification of Leishmania parasites in clinical specimens in Ecuador: a comparison with classical diagnostic methods. J Parasitol. 1999;85:181–187. [PubMed] [Google Scholar]
- 14.Ramirez JR, Agudelo S, Muskus C. Diagnosis of cutaneous leishmaniasis in Colombia: the sampling site within lesions influences the sensitivity of parasitological diagnosis. J Clin Microbiol. 2000;38:3768–3773. doi: 10.1128/jcm.38.10.3768-3773.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharquie KE, Hassen AS, Hassan SA, Al-Hamami IA. Evaluation of diagnosis of cutaneous leishmaniasis by direct smear, culture and histopathology. Saudi Med J. 2002;23:925–928. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.