Abstract
Our goals were to understand the brain magnetic resonance imaging (MRI) findings in children with retinopathy-negative cerebral malaria (CM) and investigate whether any findings on acute MRI were associated with adverse outcomes. We performed MRI scans on children admitted to the hospital in Blantyre, Malawi with clinically defined CM. Two hundred and seventeen children were imaged during the study period; 44 patients were malarial retinopathy-negative; and 173 patients were retinopathy-positive. We compared MRI findings in children with retinopathy-negative and retinopathy-positive CM. In children who were retinopathy-negative, we identified MRI variables that were associated with death and adverse neurologic outcomes. On multivariate analysis, cortical diffusion weighted imaging (DWI) abnormality and increased brain volume were strongly associated with neurologic morbidity in survivors. Investigations to explore the underlying pathophysiologic processes responsible for these MRI changes are warranted.
Introduction
The World Health Organization (WHO) defines cerebral malaria (CM) as an otherwise unexplained coma in a patient with asexual forms of malaria parasites on peripheral blood smear.1 Annually, the condition affects approximately 600,000 people, most of them children living in sub-Saharan Africa.2 This vulnerable population lives mostly in low- and middle-income countries (LMICs), where advanced neurodiagnostic techniques and therapeutic interventions are often unavailable. In this medical context, comprehensive testing (including magnetic resonance imaging [MRI]) for other causes of coma commonly encountered in high-income countries is usually unavailable.
A clinical–autopsy correlation study of children who fulfilled the standard WHO case definition for CM revealed that 23% of those who died had no evidence of the histological hallmark of this condition (sequestered parasitized erythrocytes in cerebral vasculature).3 Alternative causes of death, including severe non-malarial infections and non-infectious conditions (i.e., trauma), were identified in this subset.3 Among the group with sequestration in cerebral vasculature, no other causes of death were found. Differentiating the group without parasite sequestration from those with parasite sequestration during life was impossible until the recognition of malarial retinopathy.4,5 Three changes in the optic fundus are found in children who, on autopsy, have sequestration of parasitized erythrocytes in cerebral vasculature: retinal hemorrhages, retinal whitening, and/or orange-white vessels. The presence of malaria retinopathy is 95% sensitive and 100% specific for the pre-morbid identification of children with central nervous system (CNS) parasite sequestration at autopsy.6–8 Approximately one-third of children with clinically defined CM are retinopathy-negative.
There are at least two pathophysiologic explanations for the retinopathy-negative CM syndrome. We previously hypothesized that at least some of these children may have an asymptomatic malaria parasitemia (reflecting residence in an area of high malaria transmission) along with a second non-malarial cause of their acute illness.9 Epidemiologically, compared with children with CM who are retinopathy-positive, those who are retinopathy-negative are more likely to be neurologically or developmentally abnormal before admission or have a first-degree relative with epilepsy.10 These associations raise the possibility that, because of a genetic pre-disposition or pre-existing neurodevelopmental abnormality, at least some children diagnosed with retinopathy-negative CM, when challenged with an infectious pathogen (such as malaria), might be more likely to lapse into coma or have complicated seizures compared with children with retinopathy-positive CM.9,10
The Blantyre Malaria Project, located in the Pediatric Research Ward of Queen Elizabeth Central Hospital in Blantyre, Malawi, is a long-standing study of CM pathogenesis. MRI became available at the hospital in 2008. We evaluated admission brain MRI scans in children with retinopathy-negative CM enrolled in the Project between 2009 and 2011. We previously reported the MRI findings found in 120 children with retinopathy-positive CM scanned during this time interval.11 In our previous study, scans from children with retinopathy-positive CM were compared with images from 32 children with retinopathy-negative CM.
Since our previous analysis, interpretations of MRI images from an additional 12 retinopathy-negative and 53 retinopathy-positive children became available. Using this expanded database, our goals in this study were to understand the MRI findings found in retinopathy-negative children, compare MRI findings in children with retinopathy-negative and -positive CM to determine if there were any neuroimaging findings that were associated with retinopathy status, and investigate whether any findings on acute MRI were associated with the adverse outcomes of death or neurologic morbidity in retinopathy-negative CM survivors.
Methods
We performed brain MRI scans on children admitted with WHO clinically defined CM who lacked malaria retinopathy. These children were admitted between January 1, 2009 and June 30, 2011. Enrollment occurred only during the January to June rainy seasons, when the incidence of malaria is highest in Malawi.
Enrollment in our study required written informed consent from the child's caregiver. Our study was approved by the Michigan State University Biomedical Institutional Review Board and the University of Malawi College of Medicine Research Ethics Committee. All children fulfilled WHO diagnostic criteria for CM, having an otherwise unexplained coma (Blantyre coma score ≤ 2), Plasmodium falciparum parasitemia, and no evidence of malarial retinopathy, which was determined by direct and indirect ophthalmoscopy performed by an ophthalmologist with expertise in the diagnosis of malarial retinopathy. All children received intravenous quinine and intensive supportive care.12
At admission, we asked the parent or guardian whether the child had any clinical seizures before admission. We observed for clinical seizures during the admission process and hospitalization. Children were cared for in the Pediatric Research Ward, with nurse-to-patient ratios (usually 1:2) much higher than are typically seen in other LMICs.
Neuroimaging was performed when the child was clinically stable and able to leave the research unit with monitoring by nursing. Scanning was performed with a General Electric Signa Ovation Excite 0.35T Magnet (GE Healthcare, Milwaukee, WI). Details of the scanning protocols used for our patients are found elsewhere.11
Two MRI-trained radiologists interpreted all studies but were blinded to the patient's retinopathy status, because children with malarial retinopathy were intermixed with our patient group. (At this magnet strength, the abnormalities characteristic of malarial retinopathy are not visualized on MRI.) Independent readings were performed using NeuroInterp,13 a searchable database founded on a scoring system of brain MRI interpretation. Using systems that require categorical or dichotomous assessments, radiologists grade T2 signal or diffusion weighted imaging (DWI) changes in various cranial structures, including (but not limited to) supratentorial white and gray matter, the posterior fossa, corpus callosum, and basal ganglia. Criteria have been established for the assessment of overall brain volume.11 Discrepant interpretations between the radiologists were settled by consensus.
At the time of hospital discharge, we performed a physician-level neurologic examination. Caregivers were asked whether their child had functionally returned to baseline. Children whose parents reported new neurologic or behavioral abnormalities or who were found to have new motor, movement, or developmental disorders on neurologic examination were defined as having neurologic sequelae.
Statistical Analysis
We calculated odds ratios (ORs) and 95% confidence intervals (95% CIs) for the presence of individual MRI parameters in children with retinopathy-negative versus -positive CM. For retinopathy-negative patients, the proportion of children in each of the three outcome groups of interest (dead, alive with sequelae, and alive without sequelae) was determined. We determined the proportion of retinopathy-negative CM children in each of the outcome groups who had each of 35 MRI markers of interest. The two highest scores of the NeuroInterp variable edema (+4 and +5) were combined into a single variable of severe edema. (Edema grades of +4 and +5 are assigned to images with severe effacement of sulci and other signs of increased cerebral volume; to receive a grade of +5, the image must also have evidence of herniation, usually transtentorial.) Univariate analyses were performed with Fisher exact test to ascertain whether any of 35 MRI markers from NeuroInterp were associated with the outcomes of mortality or neurologic morbidity in retinopathy-negative CM patients. For variables found to be statistically significantly different between groups, we used χ2 to calculate ORs and 95% CIs. We compared the odds of having a particular MRI finding in a child who died with the odds of having the particular MRI finding in a child who did not die. In survivors, we determined the ratio of the odds of having a particular MRI finding in a child with neurologic disability (alive with sequelae) compared with a child with a normal outcome (alive without sequelae). A P value < 0.05 was considered a statistically significant difference between groups. Because of the exploratory nature of our study, we did not correct for multiple comparisons.
To perform our multivariate analysis of MRI factors associated with mortality or neurologic morbidity in children with retinopathy-negative CM, we combined the variables of caudate DWI abnormality and putamen DWI abnormality into a single variable, because the two took on the same values in all patients. Three other variables (posterior fossa DWI abnormality, pre-existing hemorrhage, and globus pallidus DWI abnormality) were eliminated, because they were not present on the MRI scans from any of the study subjects. Five other variables (proportion of periventricular volume involved, proportion of subcortical volume involved, DWI/T2 match in areas of abnormality, patchy versus confluent abnormalities, and splenial predominance) were excluded, because they were variables conditional on the presence of another finding, and the values were not applicable in > 50% of patients. The variable edema, scored on a scale from −2 (severe atrophy) to +5 (severe edema with herniation), was coded as a quadratic function with the two highest values (+4 and +5) combined into a single variable of severe edema2, because scores on the high end of the edema scale were likely to be more meaningful than those in the center, and biologically, it is unlikely that this variable would behave linearly. Both severe edema and severe edema2 were included in multivariate models.
Assuming on anatomical grounds, that some variables were likely to be collinear, we determined Pearson correlation coefficients between these variables (for example, putamen T2 signal abnormality and globus pallidus T2 signal abnormality). Because several variable pairs were highly collinear (Pearson correlation coefficient > 0.80), we selected Elastic Net, a regularized regression method, to select significant predictors of outcomes in the logistic regression model. Elastic Net is a model selection and estimation method known for handling highly collinear data. It encourages a grouping effect, where strongly correlated predictors tend to be in or out of the model together.14 Elastic Net is not designed to calculate CIs for the non-zero effects of the selected predictors of outcome.
A preliminary review of the dataset for the univariate analysis revealed statistical separation, with some variables having boxes in 2 × 2 contingency tables containing the numeral zero. Statistical separation implies infinite or zero maximum likelihood estimates of ORs, which are usually considered unrealistic. To calculate CIs for significant variables identified by Elastic Net, we used a penalized likelihood approach developed by Firth15 to fit logistic regression models with separated data, hoping to obtain finite ORs and CIs. We used the opt2D function from the R package pensim to implement Elastic Net, where the tuning parameters were selected by leave-one-out cross-validation.
Results
During the 18-month data collection period (January through June of 2009 to 2011), 87 children with retinopathy-negative CM were admitted to the Pediatric Research Ward. Of these children, 44 children had brain MRI scanning performed when they were clinically stable enough to be taken to the scanning suite. The mean (±SD) duration of time between coma onset and scanning was 20.8 ± 13.4 hours, and the mean (±SD) duration between Pediatric Research Ward admission and scanning was 13.7 ± 12.4 hours.
During this same time interval, 173 children with retinopathy-positive CM were scanned. Comparison of the MRI variables from children with retinopathy-negative versus -positive CM reveals several neuroradiologic findings associated with retinopathy status (Table 1). A pre-existing abnormality on MRI (e.g., cortical atrophy from a pre-admission stroke) was more likely to be found on imaging from retinopathy-negative CM patients. The presence of cortical T2 abnormalities and basal ganglia DWI abnormalities dramatically increased the odds that a patient was retinopathy-positive.
Table 1.
Brain MRI findings in retinopathy-negative versus retinopathy-positive CM
| Characteristic | Retinopathy-negative (N = 44) | Retinopathy-positive (N = 173) | Fisher P value | OR (95% CI) |
|---|---|---|---|---|
| Age (mean in months) | 57.0 | 51.6 | 0.259 | |
| Sex (% male) | 43.2 | 49.1 | 0.504 | 0.788 (0.380–1.613) |
| Increased cerebral volume ≥ 4 | 8/44 (18.2%) | 61/172 (35.5%) | 0.03 | 0.406 (0.153–0.961) |
| White matter T2 abnormalities | 22/44 (50.0%) | 136/173 (78.6%) | < 0.0005 | 0.274 (0.129–0.580) |
| White matter DWI abnormalities | 10/43 (23.3%) | 71/170 (41.8%) | 0.034 | 0.424 (0.175–0.952) |
| Cortical T2 abnormalities | 21/44 (47.8%) | 144/173 (83.2%) | < 0.000005 | 0.186 (0.085–0.401) |
| Cortical DWI abnormalities | 11/43 (25.6%) | 22/170 (12.9%) | 0.057 | 2.302 (0.913–5.563) |
| Pontine T2 abnormalities | 16/44 (36.4%) | 89/173 (51.4%) | 0.091 | 0.541 (0.254–1.120) |
| Basal ganglia T2 abnormalities | 16/44 (36.4%) | 140/173 (80.9%) | < 0.00000001 | 0.136 (0.061–0.294) |
| Basal ganglia DWI abnormalities | 0/43 (0%) | 51/170 (30.0%) | < 0.000005 | 0 (0–0.221) |
| Thalamic abnormalities | 11/44 (25.0%) | 83/173 (48.0%) | 0.006 | 0.363 (0.155–0.794) |
| Corpus callosum T2 abnormalities | 7/44 (15.9%) | 75/173 (43.3%) | < 0.001 | 0.249 (0.089–0.606) |
| Corpus callosum DWI abnormalities | 6/43 (14.0%) | 57/169 (33.7%) | 0.014 | 0.320 (0.104–0.826) |
| Posterior fossa T2 abnormalities | 14/44 (31.8%) | 111/173 (64.1%) | < 0.005 | 0.262 (0.119–0.555) |
| Posterior fossa DWI abnormalities | 0/43 (0.0%) | 10/170 (5.9%) | 0.218 | 0 (0–1.740) |
| Pre-existing abnormality | 10/44 (22.7%) | 15/ 173 (8.6%) | 0.016 | 3.078 (1.134–8.074) |
P value for age is based on z test. Denominators of ratios vary because of missing values. White matter T2 abnormality is defined as abnormality in either periventricular or subcortical supratentorial white matter.
Of the patients who were retinopathy-negative, 3 (6.8%) of 44 imaged children died, and 8 of 41 survivors (19.5%) had neurologic sequelae at discharge. During the same period, the overall mortality and morbidity rates for children admitted to the Pediatric Research Ward with retinopathy-negative CM were 9.2% (8 of 87) and 15.2% (12 of 79 survivors), respectively. There was no statistically significant difference in mortality (P = 0.75) or morbidity (P = 0.61) rates between children with retinopathy-negative CM who were scanned and those who were not (Fisher exact test). There was no significant difference in sex or mean age when comparing the children who were and were not scanned.
For children who were retinopathy-negative, we determined the proportion of children in each of the three outcome groups (dead, alive with sequelae, and alive without sequelae) with each of the MRI findings of interest (Table 2). Univariate analysis revealed that only cortical T2 signal abnormalities were positively associated with mortality (Table 3). There were statistically significant positive associations between the following MRI variables and morbidity: mass effect in the parenchyma, cortical T2 signal abnormalities (Figure 1A), cortical DWI abnormalities, focal cortical abnormality (Figure 1B), and severe edema (Figure 2). All of the children who died or were neurologically abnormal at discharge had either cortical DWI or T2 signal abnormalities. Cortical T2 signal abnormalities were more common (9 of 33 or 27.2%) than cortical DWI abnormalities (1 of 32 or 3.0%; one value missing) in children who survived without sequelae (P value for difference = 0.013, Wilcoxon signed rank test). White matter abnormalities were not associated with an increased odds of mortality or neurologic morbidity.
Table 2.
NeuroInterp data for 44 children with retinopathy-negative CM
| MRI characteristic | Dead (N = 3) | Alive with sequelae (N = 8) | Alive without sequelae (N = 33) | P value for difference (Fisher exact test)* |
|---|---|---|---|---|
| Severe edema | 1 (33.3%) | 5 (62.5%) | 2 (6.1%) | < 0.001 |
| Mass effect in parenchyma | 0 (0%) | 4 (50%) | 0 (0%) | < 0.001 |
| Periventricular white matter T2 abnormality | 1 (33.3%) | 3 (37.5%) | 9 (27.3%) | 0.85 |
| Subcortical white matter T2 abnormality | 2 (66.7%) | 2 (25%) | 8 (24.2%) | 0.33 |
| Periventricular white matter DWI abnormality | 1 (33.3%) | 2 (25%) | 7 (21.2%) | 0.84 |
| Cortical T2 abnormality | 3 (100%) | 8 (100%) | 9 (27.3%) | < 0.001 |
| Cortical DWI abnormality | 2 (66.7%) | 8 (100%) | 1 (3.0%) | < 0.001 |
| Focal cortical abnormality | 0 (0%) | 5 (62.5%) | 1 (3.0%) | < 0.001 |
| Pontine T2 abnormality | 2 (66.7%) | 5 (62.5%) | 9 (27.3%) | 0.09 |
| Other brainstem T2 abnormality | 2 (66.7%) | 5 (62.5%) | 8 (24.2%) | 0.058 |
| Globus pallidus T2 abnormality | 2 (66.7%) | 3 (37.5%) | 10 (30.3%) | 0.42 |
| Globus pallidus DWI abnormality | 0 (0%) | 0 (0%) | 0 (0%) | 1.0 |
| Putamen T2 abnormality | 2 (66.7%) | 4 (50%) | 10 (30.3%) | 0.26 |
| Putamen DWI abnormality | 0 (0%) | 0 (0%) | 0 (0%) | 1.0 |
| Caudate T2 abnormality | 2 (66.7%) | 3 (37.5%) | 10 (30.3%) | 0.42 |
| Caudate DWI abnormality | 0 (0%) | 0 (0%) | 0 (0%) | 1.0 |
| Thalamus T2 abnormality | 0 (0%) | 3 (37.5%) | 8 (24.2%) | 0.59 |
| Corpus callosum T2 abnormality | 0 (0%) | 0 (0%) | 6 (18.2%) | 0.57 |
| Corpus callosum DWI abnormality | 0 (0%) | 0 (0%) | 5 (15.2%) | 0.70 |
| Posterior fossa T2 abnormality | 1 (33.3%) | 3 (37.5%) | 10 (30.3%) | 0.86 |
| Posterior fossa DWI abnormality | 0 (0%) | 0 (0%) | 0 (0%) | 1.0 |
| Pre-existing abnormality not otherwise specified | 0 (0%) | 2 (25%) | 8 (24.2%) | 1.0 |
| Focal cortical atrophy | 0 (0%) | 0 (0%) | 1 (3.0%) | 1.0 |
| Widespread cortical atrophy | 0 (0%) | 2 (25%) | 1 (3.0%) | 0.15 |
| White matter gliosis | 3 (100%) | 4 (50%) | 15 (45.5%) | 0.33 |
| Pre-existing hemorrhage | 0 (0%) | 0 (0%) | 0 (0%) | 1.0 |
| Extra-axial fluid collection | 0 (0%) | 1 (12.5%) | 0 (0%) | 0.25 |
The following Neurointerp variables not shown: front_post (in those with supratentorial abnormalities, does the severity of abnormalities vary between frontal and occipital regions, or is it equal in both), pfossa_supra (in those with abnormalities in either the posterior or anterior fossae, does the severity of abnormalities vary between the anterior and posterior fossae, or is it equal in both), p_vent_volume (in those with periventricular white matter involvement, what is the percentage of volume of the periventricular white matter involved), scort_wm (in those with subcortical white matter involvement, what is the percentage of the volume of the subcortical white matter involved), splenial_predom (if the corpus callosum is involved, is the splenium the primary area affected), DWI_v_T2 (in those with both T2 and DWI abnormalities in white matter, do areas of T2 and DWI abnormality match), DWI_cort_match (in those with both T2 and DWI abnormalities in cortical gray matter, do areas of T2 and DWI abnormality match), and patchy_confluent (in those with cortical involvement, is it patchy or confluent). Values are numbers (proportions) with finding.
Comparison of MRI characteristics among the three groups was made by Fisher exact text for a 2 × 3 table.
Table 3.
Association of MRI markers, mortality, or morbidity in survivors (N = 44)
| Variable | OR (95% CI) for mortality (died) in subjects with this MRI finding (N = 3) | OR (95% CI*) for neurologic morbidity (in survivors; alive with sequelae) with this MRI finding (N = 41) |
|---|---|---|
| Severe edema | 2.4 (0.04–51.7) | 22.2 (2.5–333.7) |
| Mass effect in parenchyma† | Undefined‡ | Undefined |
| Cortical T2 abnormality§ | Undefined | Undefined |
| Cortical DWI abnormality¶ | 6.5 (0.31–417.8)‖ | Undefined |
| Focal cortical abnormality** | Undefined | 43.5 (3.5–2539.1) |
Exact CIs were calculated when any expected cell count in the 2 × 2 contingency table was < 5; otherwise, CIs based on the normal approximation are reported.
Mass effect in parenchyma was not present in any child who died or was alive without sequelae.
Undefined ORs are caused by statistical separation, with either the numerator or denominator of the OR calculation equaling zero.
Cortical T2 abnormality was present in all children who died or were alive with sequelae, and it was present in 9 of 33 children who were alive without sequelae.
Cortical DWI abnormality was present in all children who were alive with sequelae and 1 of 33 children who was alive without sequelae.
Calculation based on 43 children with complete data.
Focal cortical abnormality was not present in any of the children who died.
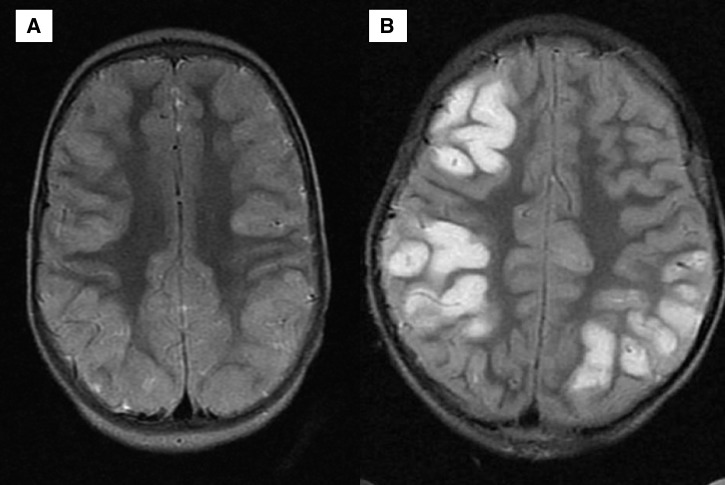
Figure 1.
Brain MRIs of two children alive with neurologic sequelae at discharge. (A) Cortical abnormalities were generally diffuse, and an axial T2-weighted MRI shows pronounced diffuse cortical abnormalities exemplified by bright signal and marked thickening, which is made more pronounced by the sparing of the underlying white matter. (B) Focal abnormalities on the backdrop of more subtle diffuse involvement.
Figure 2.
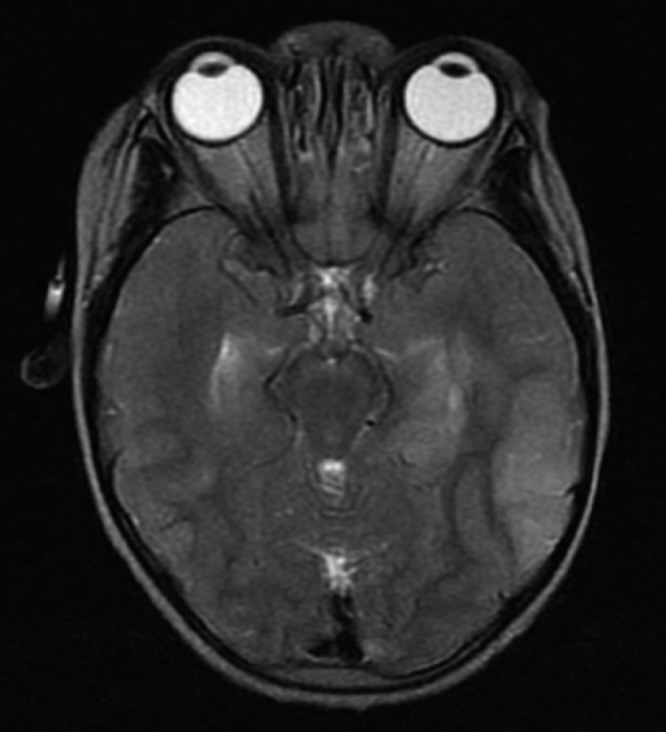
Increased brain volume in a child who survived with neurologic sequelae. Severe edema is illustrated on this axial T2-weighted image by the effacement of the ambient cisterns as well as the loss of the sulcal marking. Note the bilateral uncal herniation.
Seizures were present before, during, or after admission in 97.7% (43 of 44) of children. To investigate whether cortical MRI findings might be a consequence of seizures, we determined the proportions of children with or without clinical seizures at any time (before, during, or after admission) who had cortical DWI changes or cortical T2 abnormalities; 20 of 44 children had cortical T2 abnormalities, all of whom (20 of 20 or 100%) had seizures. Of 24 children who did not have cortical T2 abnormalities, 23 of 24 (95.8%) had seizures. These proportions were not significantly different (P = 1.00, two-sided Fisher exact test). In total, 11 of 44 children had cortical DWI changes, all of whom (11 of 11 or 100%) had seizures. Of 33 children who did not have cortical DWI changes, 32 or 33 (96.9%) had seizures. These proportions were not significantly different (P = 1.00, two-sided Fisher exact test).
On multivariate analysis, there were no variables significantly associated with mortality. Eight variables were selected by Elastic Net as having ORs associated with morbidity that were different than one (Table 4). Cortical DWI abnormality was most strongly associated with neurologic morbidity in survivors. Children with this MRI abnormality had an adjusted OR of 7.4 of having an adverse neurologic outcome compared with children who lacked this finding on neuroimaging. One variable, focal cortical atrophy, was negatively correlated with the presence of neurologic sequelae in survivors. Likely because of the statistical separation of our data, for variables that remained in the final model, the estimated 95% CIs (calculated by Firth logistic regression) encompassed 1.0 (Table 3).
Table 4.
Multivariate analysis of MRI markers associated with neurologic morbidity in retinopathy-negative CM survivors
| Comparison | OR estimated from Elastic Net | 95% CI for OR estimated by Firth logistic regression |
|---|---|---|
| 1-Unit increase in severe edema2 | 1.46 | 0.58–1.53 |
| Mass effect in parenchyma (yes/no) | 1.44 | 0.0004–1,410 |
| Cortical T2 abnormality (yes/no) | 1.53 | 0.0012–1,080 |
| Cortical DWI abnormality (yes/no) | 7.4 | 0.361–269,000 |
| Focal cortical change | 1.24 | 0.0003–1,730 |
| Diffuse cortical atrophy (yes/no) | 1.003 | 0.0004–1,312 |
| Focal cortical atrophy (yes/no) | 1.12 | 0.0002–915 |
| Corpus callosum T2 abnormality | 0.96 | 0.0042–1,490 |
Discussion
MRI findings in children with retinopathy-negative CM are heterogeneous, likely reflecting the pathophysiologic heterogeneity of this patient group. Some children with retinopathy-negative CM may have a non-malarial etiology of coma coupled with an asymptomatic malarial parasitemia, whereas others may be genetically vulnerable or have a pre-existing neurodevelopmental abnormality increasing their likelihood of lapsing into coma during acute malaria infection. We found that 22.2% (10 of 45) of the children scanned had some pre-existing neuroradiologic abnormality. This finding supports the hypothesis that at least some children with retinopathy-negative CM have a pre-existing neurologic abnormality that may increase their risk of coma when challenged with an infectious (or non-infectious) process.
In children with CM, there are multiple MRI findings statistically associated with malarial retinopathy (absent versus present) (Table 1). The clinical use of these findings remains limited. The only variables completely predictive of retinopathy status are posterior fossa and basal ganglia DWI abnormalities. When present, these MRI findings were associated with a subject being retinopathy-positive. Children who were retinopathy-negative had a lower odds of T2 signal abnormalities of white matter, cerebral cortex, thalamus, corpus callosum, and the posterior fossa, and they were less likely to have DWI abnormalities of basal ganglia and corpus callosum compared with children who were retinopathy-positive.
Our exploratory analysis of the MRI findings in children with retinopathy-negative CM reveals at least two MRI findings associated with morbidity in survivors: increased cerebral volume and cortical DWI and T2 abnormalities. Radiographic evidence of increased brain volume (coded as edema) and mass effect in the parenchyma are positively associated with adverse neurologic outcomes in survivors. If the increased cerebral volume seen on MRI is indicative of cerebral edema, antiedema medications may be a potentially useful adjunctive therapy to decrease morbidity rates in children with retinopathy-negative CM.
Cortical T2 and DWI abnormalities are common in brain MRI imaging of children with retinopathy-negative CM, and their presence is associated with neurologic morbidity in survivors. The differential diagnosis of cortical gray matter abnormalities seen on T2 and/or DWI neuroimaging includes infection, infarction, and uncontrolled seizures. These data suggest that the MRI findings seen in our patient group are not a consequence of seizures. Because of resource limitations, our diagnosis of seizure was made with clinical observation and by report from the child's caregiver. These measures are insensitive to the presence of subclinical ictal phenomena. We did not perform continuous electroencephalogram (EEG) monitoring in our patients to confirm if they had electrographic-only seizures, which may have led to some of the findings seen on MRI. This investigative modality is not routinely available in Malawi. Electrographic-only seizures are common in CM.16 Although we found no statistically significant difference in the rate of clinical seizures before or at the time of hospitalization between children with or without cortical T2 or DWI abnormalities, it is possible that rates of subclinical seizures differed between these groups.
Other conditions that may produce cortical gray matter abnormalities include CNS infection and infarction. Possible infectious pathogens include the malaria parasite itself or a bacterial or viral coinfection. Acute CNS viral infections may produce a pattern of cortical DWI and T2 signal abnormalities with relative sparing of white matter similar to that seen in our patients.17 Viral coinfection in patients with CM has only rarely been studied. A case series of 49 Kenyan children with clinically defined CM revealed that 9% of the studied patients had CNS herpesvirus or enterovirus coinfection.18 Patients were not stratified by malarial retinopathy status, and testing for other types of viruses was not performed. CNS coinfection with a wide variety of viral pathogens has recently been documented in Malawian children with retinopathy-negative CM.19
The MRI findings seen in our patients are not typical of the classic neuroimaging findings in patients with acute herpesvirus encephalitis. Although neuroimaging may be normal or show multifocal cortical involvement in this condition,20 the classical temporal lobe involvement of CNS herpesvirus infection was not seen in our study children. Enterovirus encephalitis commonly produces MRI abnormalities in cerebellar dentate nuclei and the dorsal brainstem,21 both of which were seen in some of our study subjects. The MRI findings of neurotropic viruses show considerable overlap,22 and it is possible that some of the findings seen in our patients represent acute CNS viral infection.
Other MRI findings in our study group support a possible role of coinfection in children with retinopathy-negative CM. In five (83%) of six of the children with T2 signal abnormalities and in all (five of five or 100%) of the children with DWI abnormalities in the corpus callosum, the splenium was the primary area affected. The splenium is often preferentially involved in children with viral encephalitis17,23,24 and Salmonella enteritidis-associated encephalopathy,25,26 although other studies postulate that these findings are non-specific and may be caused by seizures or metabolic abnormalities in addition to CNS infections.27 The high proportion of splenial predominance in children with callosal abnormalities in our study group further supports the possibility that at least some children with retinopathy-negative CM may have an acute systemic or CNS viral coinfection. If a substantial proportion of children with retinopathy-negative CM have a viral coinfection producing these MRI abnormalities, clinical trials targeting these pathogens may be warranted.
Focal cortical atrophy seems to be protective against the development of morbidity in retinopathy-negative CM survivors. A biological explanation for this association is unclear. Because of our small sample size, it is possible that this association was spurious. Studies to explore potential epidemiological confounders between focal cortical atrophy and normal neurologic outcome in survivors of retinopathy-negative CM are warranted.
Our study has many limitations, foremost of which is the small sample size. Although we found associations between MRI variables and the presence of neurologic sequelae in retinopathy-negative CM survivors, the small number of patients studied (N = 44) compared with high number of MRI variables (N = 35) may have led to type II error, which is most clearly shown in the determination of MRI factors associated with mortality. Because only three of the children died, even when a variable was strongly associated with mortality on univariate analysis, no MRI abnormalities were associated with death in regression models. Increasing the number of patients with MRI studies would likely improve study power, clarifying the association between MRI findings and mortality. Because of the statistical separation of our data, the 95% CIs (as calculated using Firth logistic regression) were very wide and all overlapped 1.0. Increasing the number of patients with interpreted MRI studies would allow use of regular logistic regression in future analyses, hopefully resulting in narrower CIs for ORs.
Although the majority of the potential retinopathy CM patient population was scanned, only children who were clinically stable could be taken to the scanning suite, which may have led to selection bias. An additional limitation of our study is our relatively weak magnet strength (0.35T) and absence of diffusion gradients in all three axes during axial scanning; they may have led to underrecognition of MRI abnormalities and possibly, type II error. The unavailability of diffusion gradients on all three axes during axial echoplanar DWI precludes correction of anisotropic effects. We have recently validated a three-plane echoplanar DWI method of obtaining axial, coronal, and sagittal series with superior–inferior, anterior–posterior, and right–left b = 900 diffusion gradients, respectively, that will allow for the correction of anisotropic effects, but these techniques were not available when images were acquired. Also, serial MRI acquisitions were not analyzed. Serial scans might allow additional elucidation of factors associated with outcomes in children with retinopathy-negative CM but were not included in this initial study.
Our assessment for neurologic sequelae did not entail a comprehensive developmental test, which may have led to some non-differential misclassification in survivors. Long-term follow-up of these children after hospital discharge was not done. Because CM sequelae may develop weeks to months after hospital discharge,28 this may have also led to misclassification of outcomes in survivors. This misclassification may have been more common in younger children, whose discharge neurologic and developmental examinations may have been more challenging.
Additional studies of neuroimaging in retinopathy-negative CM are warranted. MRI abnormalities associated with mortality or morbidity may provide clues to inform additional studies of the underlying pathophysiologic processes leading to adverse outcomes. Our study provides preliminary evidence of possible therapeutic targets for clinical trials with goals of decreasing rates of morbidity in retinopathy-negative CM survivors.
ACKNOWLEDGMENTS
The authors thank the children hospitalized in the Paediatric Research Ward at Queen Elizabeth Central Hospital in Blantyre, Malawi for allowing us to care for them. The authors also thank their parents and guardians for agreeing to participate in our study. These data were collected on the Pediatric Research Ward as part of a collaboration between Blantyre Malaria Project and the Malawi-Liverpool-Wellcome Trust Programme which is supported by a Core grant award from the Wellcome Trust.
Footnotes
Financial support: This study was supported by National Institutes of Health Grant 5R01AI034969.
Authors' addresses: Douglas G. Postels, International Neurologic and Psychiatric Epidemiology Program, Michigan State University, East Lansing, MI, E-mail: douglas.postels@ht.msu.edu. Chenxi Li, Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, MI, E-mail: cli@epi.msu.edu. Gretchen L. Birbeck, Department of Neurology, University of Rochester, Rochester, NY, E-mail: Gretchen_Birbeck@URMC.Rochester.edu. Terrie E. Taylor, Department of Osteopathic Medical Specialties, Michigan State University, East Lansing, MI, and Blantyre Malaria Project, University of Malawi College of Medicine, Blantyre, Malawi, E-mail: ttmalawi@msu.edu. Karl B. Seydel, Department of Osteopathic Medical Specialties, Michigan State University, East Lansing, MI, E-mail: seydel@msu.edu. Sam D. Kampondeni, Department of Radiology, University of Malawi College of Medicine, Blantyre, Malawi, E-mail: s.kampo154@gmail.com. Simon J. Glover, Anatomy Department, School of Medicine, University of St. Andrews, St. Andrews, Scotland, E-mail: simontheeyeman@hotmail.com. Michael J. Potchen, Department of Imaging Sciences, University of Rochester, Rochester, NY, E-mail: Michael_Potchen@URMC.Rochester.edu.
References
- 1.World Health Organization Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94((Suppl 1)):S1–S90. [PubMed] [Google Scholar]
- 2.World Health Organization . World Malaria Report. Geneva: World Health Organization; 2012. [Google Scholar]
- 3.Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 4.Lewallen S, Taylor TE, Molyneux ME, Wills BA, Courtright P. Ocular fundus findings in Malawian children with cerebral malaria. Ophthalmology. 1993;100:857–861. doi: 10.1016/s0161-6420(93)31563-0. [DOI] [PubMed] [Google Scholar]
- 5.Lewallen S, Wills BA. Retinal haemorrhage in children with malaria. Lancet. 1993;341:442. doi: 10.1016/0140-6736(93)93043-z. [DOI] [PubMed] [Google Scholar]
- 6.Beare NA, Glover SJ, Molyneux M. Malarial retinopathy in cerebral malaria. Am J Trop Med Hyg. 2009;80:171. [PubMed] [Google Scholar]
- 7.Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–797. [PMC free article] [PubMed] [Google Scholar]
- 8.Lewallen S, Bronzan RN, Beare NA, Harding SP, Molyneux ME, Taylor TE. Using malarial retinopathy to improve the classification of children with cerebral malaria. Trans R Soc Trop Med Hyg. 2008;102:1089–1094. doi: 10.1016/j.trstmh.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postels DG, Birbeck GL. Children with retinopathy-negative cerebral malaria: a pathophysiological puzzle. Pediatr Infect Dis J. 2011;30:953–956. doi: 10.1097/INF.0b013e3182271c69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birbeck GL, Beare N, Lewallen S, Glover SJ, Molyneux M, Kaplan P, Taylor TE. Identification of malaria retinopathy improves the specificity of the clinical diagnosis of cerebral malaria: findings from a prospective cohort study. Am J Trop Med Hyg. 2010;82:231–234. doi: 10.4269/ajtmh.2010.09-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potchen MJ, Kampondeni SD, Seydel KB, Birbeck GL, Hammond CA, Bradley WG, DeMarco JK, Glover SJ, Ugorji JO, Latourette M, Siebert J, Molyneux M, Taylor TE. Acute brain MRI findings in 120 Malawian children with cerebral malaria: new insights into an ancient disease. AJNR Am J Neuroradiol. 2012;33:1740–1746. doi: 10.3174/ajnr.A3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor TE. Caring for children with cerebral malaria: insights gleaned from 20 years on a research ward in Malawi. Trans R Soc Trop Med Hyg. 2009;103((Suppl 1)):S6–S10. doi: 10.1016/j.trstmh.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 13.Potchen MJKS, Ibrahim K, Bonner J, Seydel KB, Taylor TE, Birbeck GL. NeuroInterp: a method for facilitating neuroimaging research on cerebral malaria. Neurology. 2013;81:585–588. doi: 10.1212/WNL.0b013e31829e6ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou HHT. Regularization and variable selection via the Elastic Net. J R Stat Soc Ser A Stat Soc. 2005;67:301–320. [Google Scholar]
- 15.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 16.Birbeck GL, Molyneux ME, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, Taylor TE. Blantyre Malaria Project EpilepsyStudy (BMPES) of neurological outcomes in retinopathy positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9:1173–1181. doi: 10.1016/S1474-4422(10)70270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta RK, Soni N, Kumar S, Khandelwal N. Imaging of central nervous system viral diseases. J Magn Reson Imaging. 2012;35:477–491. doi: 10.1002/jmri.22830. [DOI] [PubMed] [Google Scholar]
- 18.Schubart CD, Mturi N, Beld MG, Wertheim PM, Newton CR. Role of viruses in Kenyan children presenting with acute encephalopathy in a malaria-endemic area. Am J Trop Med Hyg. 2006;75:1148–1150. [PubMed] [Google Scholar]
- 19.Mallewa M, Vallely P, Faragher B, Banda D, Klapper P, Mukaka M, Khofi H, Pensulo P, Taylor T, Molyneux M, Solomon T. Viral CNS infections in children from a malaria-endemic area of Malawi: a prospective cohort study. Lancet Glob Health. 2013;1:e153–e160. doi: 10.1016/S2214-109X(13)70060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sili U, Kaya A, Mert A. Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol. 2014;60:112–118. doi: 10.1016/j.jcv.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Lee KY, Lee YJ, Kim TH, Cheon DS, Nam SO. Clinico-radiological spectrum in enterovirus 71 infection involving the central nervous system in children. J Clin Neurosci. 2014;21:416–420. doi: 10.1016/j.jocn.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 22.Crawford JR. Advances in pediatric neurovirology. Curr Neurol Neurosci Rep. 2010;10:147–154. doi: 10.1007/s11910-010-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takanashi J, Barkovich AJ, Yamaguchi K, Kohno Y. Influenza-associated encephalitis/encephalopathy with a reversible lesion in the splenium of the corpus callosum: a case report and literature review. AJNR Am J Neuroradiol. 2004;25:798–802. [PMC free article] [PubMed] [Google Scholar]
- 24.Beattie GC, Glaser CA, Sheriff H, Messenger S, Preas CP, Shahkarami M, Venkatesan A. Encephalitis with thalamic and basal ganglia abnormalities: etiologies, neuroimaging, and potential role of respiratory viruses. Clin Infect Dis. 2013;56:825–832. doi: 10.1093/cid/cis990. [DOI] [PubMed] [Google Scholar]
- 25.Kobuchi N, Tsukahara H, Kawamura Y, Ishimori Y, Ohshima Y, Hiraoka M, Hiraizumi Y, Ueno M, Mavumi M. Reversible diffusion-weighted MR findings of Salmonella enteritidis-associated encephalopathy. Eur Neurol. 2003;49:182–184. doi: 10.1159/000069074. [DOI] [PubMed] [Google Scholar]
- 26.Ogura H, Takaoka M, Kishi M, Kimoto M, Shimazu T, Yoshioka T, Sugimoto H. Reversible MR findings of hemolytic uremic syndrome with mild encephalopathy. AJNR Am J Neuroradiol. 1998;19:1144–1145. [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YW, Yu CY. Reversible focal splenium lesion–MRS study of a different etiology. Acta Neurol Taiwan. 2009;18:203–206. [PubMed] [Google Scholar]
- 28.Postels DG, Taylor TE, Molyneux M, Mannor K, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, Birbeck GL. Neurologic outcomes in retinopathy-negative cerebral malaria survivors. Neurology. 2012;79:1268–1272. doi: 10.1212/WNL.0b013e31826aacd4. [DOI] [PMC free article] [PubMed] [Google Scholar]