Abstract
Few studies have quantified toxoplasmosis mortality, associated medical conditions, and productivity losses in the United States. We examined national multiple cause of death data and estimated productivity losses caused by toxoplasmosis during 2000–2010. A matched case–control analysis examined associations between comorbid medical conditions and toxoplasmosis deaths. In total, 789 toxoplasmosis deaths were identified during the 11-year study period. Blacks and Hispanics had the highest toxoplasmosis mortality compared with whites. Several medical conditions were associated with toxoplasmosis deaths, including human immunodeficiency virus (HIV), lymphoma, leukemia, and connective tissue disorders. The number of toxoplasmosis deaths with an HIV codiagnosis declined from 2000 to 2010; the numbers without such a codiagnosis remained static. Cumulative disease-related productivity losses for the 11-year period were nearly $815 million. Although toxoplasmosis mortality has declined in the last decade, the infection remains costly and is an important cause of preventable death among non-HIV subgroups.
Introduction
Toxoplasmosis encephalitis is a highly recognized manifestation of Toxoplasma gondii infection associated with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS).1,2 However, this protozoan zoonosis is underappreciated as the second most common cause of foodborne deaths in the United States (US) and a preventable cause of other non-HIV disease.3
With an estimated seroprevalence of about 10–15% in the general US population,4 the clinical presentation of toxoplasmosis varies among several subgroups, ranging from no symptoms in most individuals to cerebral involvement and/or widespread disseminated disease in individuals with severe immune suppression.5,6 A number of contributing factors, including age, sex, underlying medical conditions, and strain of parasite, affect this disease's severity and clinical course.7,8 For example, nearly one-third (30%) of all AIDS patients with seropositivity to this organism who experienced CD4 counts below 100 cells/mm3 have developed reactivated toxoplasmosis, especially in the absence of effective prophylaxis.9 In non-HIV immune suppression (e.g., from cancer chemotherapy, prolonged steroid treatment of connective tissue disorders, post-transplantation therapy, or poorly controlled diabetes), this disease risk is still substantial but generally not as well-characterized in the literature.10–12
To contribute to this latter gap in the literature, we conducted a multipart study using the national multiple cause of death (MCD) dataset from 2000 to 2010: (part 1) quantification and trend analysis of toxoplasmosis mortality in the United States, (part 2) a matched case–control analysis matching associated medical conditions to disease cases and (part 3) a cost analysis to enumerate the cumulative productivity losses attributed to pre-mature death from this zoonosis during the 11-year study period. The analysis complements emerging research showing disparities in the number of toxoplasmosis hospitalizations by HIV status.13
Materials and Methods
Through the National Vital Statistics System monitored by the US Department of Health and Human Services and the Centers for Disease Control and Prevention, the National Center for Health Statistics (NCHS) collects and publishes data on deaths obtained from registration offices of all 50 states in the US, including the District of Columbia. The US Standard Certificate of Death form is completed by a medical certifier (most often the attending physician or coroner) and includes demographic information, such as age, sex, race/ethnicity, and all conditions that led to death (i.e., underlying and associated causes). The underlying cause of death is the disease or injury that initiated the chain of events leading directly to death, whereas the associated causes are conditions other than the underlying cause listed on the death certificate in the sections for both the sequence of events and other contributing conditions. Underlying and associated conditions reported from 2000 to 2010 are listed on the death certificates in accordance with the International Classification of Diseases, 10th Revision (ICD-10).14
Study sample.
We examined toxoplasmosis mortality in the US using MCD data for an 11-year period from 2000 to 2010. Deaths from toxoplasmosis were defined as any observation listed as either the underlying cause or the associated cause of death using the following ICD-10 codes: B58–B58.3, B58.8–B58.9, and P37.1; these codes include the diagnosis of congenital toxoplasmosis. Mortality rates were calculated using bridged-race population approximations from the NCHS.15 Race bridging is a method used to make two different sets of race categories consistent with one another for estimation and comparison purposes. Age, sex, race/ethnicity, and year of death were included in these calculations based on estimates from the US Census Bureau for the 11-year period. Age at death was used to standardize and calculate age-specific rates and ratios as well as categorize decedent cases into the following groups: < 1, 1–4, 5–14, 15–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, and ≥ 85 years. Race/ethnicity was categorized as white (non-Hispanic), Asian/Pacific Islander, black (non-Hispanic), Hispanic, and Native American (American Indian/Alaska Native). Due to changes in ICD coding in 1999 from the 9th version to the 10th version, we restricted our analysis to include only data from 2000 and onwards. Because this study relies on public deidentified data on deceased individuals, it does not constitute research involving human subjects; this is in accordance with Title 45, part 45, of the Code of Federal Regulations.16
Mortality and matched case–control analysis.
Population data for deriving mortality rates were obtained from the US Census Bureau. Toxoplasmosis mortality rates per 100,000 population per year were calculated for the US. Crude rates of toxoplasmosis mortality and 95% confidence intervals (95% CIs) were computed by age, sex, race/ethnicity, year, and state of residence. Using the 2000 US standard population, age-adjusted rates were calculated for sex, race/ethnicity, year, and state.17 Rate ratios (RRs), age-adjusted RRs, and 95% CIs were also calculated. Temporal trends were modeled using Poisson regression methods. Years of potential life lost (YPLL), a measure of the number of years not lived by an individual who dies before reaching a pre-determined age, was calculated using the NCHS ceiling of 75 years of age.18 Redelings and others19 have previously validated the use of MCD data to investigate associations between conditions listed on death certificates. Using these methods, the relationship between comorbid medical conditions and toxoplasmosis mortality was examined using a matched case–control analysis. To select controls, a random sample of non-toxoplasmosis deaths was drawn from the MCD dataset during the same time frame as cases (2000–2010) and matched in a 10:1 ratio (10 controls per 1 case) by age, race/ethnicity, and sex. Co-occurring or comorbid medical conditions were selected and included in the analysis if they were (1) known to be associated with toxoplasmosis, such as HIV/AIDS, and/or (2) known to impair an individual's immune response in the absence of HIV/AIDS (e.g., treatments for autoimmune conditions and cancer chemotherapy). Matched odds ratios (MORs) and 95% CIs were generated for each of the case–control pairs studied.
Because HIV is strongly related to fatal toxoplasmosis reactivation (i.e., more than one half of the death certificates with toxoplasmosis listed as a cause of death also had HIV listed), we conducted a subgroup analysis to compare differences in mortality rates and age-adjusted RRs between toxoplasmosis deaths with HIV involvement versus those without HIV involvement. All analyses were conducted using the SAS 9.2 statistical software package (SAS Institute, Inc., Cary, NC).
Cost analysis: estimating productivity losses.
Productivity losses due to of pre-mature death from toxoplasmosis were estimated using the human capital approach.20,21 The number of deaths during each year of the 11-year period (2000–2010) were multiplied by the present value of lifetime productivity (PVLP) stratified by sex and age. Total costs from these losses were calculated and presented cumulatively for the entire period. The procedure to calculate PVLP has been described elsewhere in the literature.21 PVLP calculations based on 2007 data (latest data available at the time of analysis) accounted for labor force participation, life expectancy, estimates of future values for housekeeping activities, and caregiving. It also accounted for projected estimates of productivity growth. Estimates for years before and after 2007 were adjusted assuming an annual productivity growth of 0.5%. As recommended by the Panel of Cost-Effectiveness in Health and Medicine, a discounted rate of 3% was applied to the analysis.22,23 All estimates were adjusted to 2013 dollars using the Employment Cost Index.24 Similar methods to assess productivity losses using MCD data have been published in the literature.25,26
Results
In total, 789 toxoplasmosis deaths were identified during the study period from 2000 to 2010 (Table 1), of which 271 deaths were caused by toxoplasmosis encephalitis, 112 deaths were caused by other organ involvement, 71 deaths were caused by congenital toxoplasmosis, 22 deaths were caused by pulmonary toxoplasmosis, < 5 deaths were caused by toxoplasma oculopathy, and the remaining deaths were unspecified. Of 71 congenital toxoplasmosis deaths, 44 deaths listed were as the underlying cause of death. The annual percentage change in mortality rates was –11.7% over the 11-year period (P value < 0.0001). Toxoplasmosis was reported as the underlying cause of death for 201 cases (25.5% of total) and as an associated cause of death for 588 cases (74.5% of total). The average age at death was 42.1 years (range = 0–92; median = 41.0). The age-adjusted mortality rates varied during the 11-year time frame (Figure 1), with an overall average age-adjusted mortality rate of 0.025 per 100,000 population (95% CI = 0.023–0.026) and a total of 26,186 YPLLs.
Table 1.
Mortality RRs and YPLLs from toxoplasmosis in the United States, 2000–2010
| Toxoplasmosis deaths n (%)* | Age-adjusted RR (95% CI) | YPLLs | |
|---|---|---|---|
| Sex | |||
| Female | 268 (34.0%) | Referent | 9,027 |
| Male | 521 (66.0%) | 2.01 (1.76–2.30) | 17,159 |
| Race/ethnicity | |||
| White | 253 (32.1%) | Referent | 7,430 |
| Asian/Pacific Islander | 13 (1.6%) | 0.81 (0.67–0.99) | 509 |
| Black | 355 (45.0%) | 8.30 (7.32–9.42) | 11,971 |
| Hispanic | 166 (21.0%) | 3.52 (3.09–4.01) | 6,213 |
| Native American | 2 (0.3%) | 0.64 (0.51–0.80) | 63 |
| Age group (years) | |||
| < 1 | 24 (3.0%) | – | 1,800 |
| 1–4 | 7 (0.9%) | – | 511 |
| 5–14 | 20 (2.5%) | – | 1,300 |
| 15–24 | 27 (3.4%) | – | 1,476 |
| 25–34 | 148 (18.8%) | – | 6,606 |
| 35–44 | 240 (37.0%) | – | 8,548 |
| 45–54 | 171 (21.7%) | – | 4,447 |
| 55–64 | 77 (9.8%) | – | 1,250 |
| 65–74 | 40 (5.1%) | – | 248 |
| 75–84 | 30 (3.8%) | – | 0 |
| > 85 | 5 (0.6%) | – | 0 |
| Total | 789 (100%) | – | 26,186 |
n is the frequency or number of decedents specified in each column.
Figure 1.
Number of toxoplasmosis deaths and age-adjusted mortality rates per 100,000 population in the United States, 2000–2010.
The highest absolute number of deaths and YPLLs attributed to toxoplasmosis occurred within the 35–44 years age group—this represented 37.0% of all toxoplasmosis deaths (0.050 per 100,000 population; 95% CI, 0.044–0.057; N = 240) (Table 1). In general, males were two times as likely to have toxoplasmosis listed as a cause of death than females (age-adjusted RR = 2.01; 95% CI = 1.76–2.30). Black and Hispanic race/ethnicity had the highest toxoplasmosis mortality rates relative to whites (age-adjusted RR = 8.30; 95% CI = 7.32–9.42 and age-adjusted RR = 3.52; 95% CI = 3.09–4.01, respectively). Blacks experienced 11,971 YPLLs; this was considerably more than any other race/ethnic group (Table 1). Florida had the highest frequency of deaths (N = 114; 14.4%), with an age-adjusted mortality rate of 0.060 per 100,000 population (95% CI = 0.049–0.072). Total productivity losses from all toxoplasmosis deaths during 2000–2010 were estimated to be $814.5 million. Cost per death for each year was just over $1 million. Total productivity losses were highest in the 30–34 and 35–39 years female age groups (approximately $84 million combined).
In the matched case–control analysis, HIV was the most often listed codiagnosis on the death certificates (N = 440; 56% of total), and it was strongly associated with toxoplasmosis deaths (MOR = 27.75; 95% CI = 21.78–35.37). Other comorbid medical conditions strongly associated with toxoplasmosis deaths included Hodgkin/non-Hodgkin lymphoma, leukemia, and connective tissue disorders (Table 2).
Table 2.
Comorbid conditions associated with toxoplasmosis deaths with MORs and 95% CIs in the United States, 2000–2010
| Medical conditions | ICD-10 Codes | Toxoplasmosis cases (N = 789) n (%)* | Matched control† (N = 7,882) n (%)* | MOR‡ (95% CI) |
|---|---|---|---|---|
| Connetive tissue disorders | M30–M36 | 23 (2.9) | 53 (0.7) | 4.40 (2.68–7.24) |
| Diabetes | E10–E14 | 18 (2.3) | 452 (5.7) | 0.39 (0.24–0.62) |
| Heart disease | I00–I99 | 171 (21.7) | 2,877 (36.5) | 0.46 (0.39–0.56) |
| HIV | B20–B24 | 440 (55.8) | 414 (5.3) | 27.75 (21.78–35.37) |
| Hodgkin, non-Hodgkin lymphoma§ | C81, C82, C83, C84, C85, C86 | 42 (5.3) | 76 (1.0) | 5.91 (4.03–8.69) |
| Leukemia | C91, C92, C93, C94, C95 | 25 (3.2) | 82 (1.0) | 3.07 (1.95–4.85) |
n is the frequency or number of decedents specified in each column.
During the matching process, one case was only able to find two matched controls because of the characteristics of that case (i.e., in the 5–14 years age range, female, and Asian).
Cases were matched to controls by age, sex, and race/ethnicity.
Among toxoplasmosis deaths with non-Hodgkin lymphoma, there were 11 cases with HIV involvement; among toxoplasmosis deaths with Hodgkin lymphoma, there were no cases with HIV involvement.
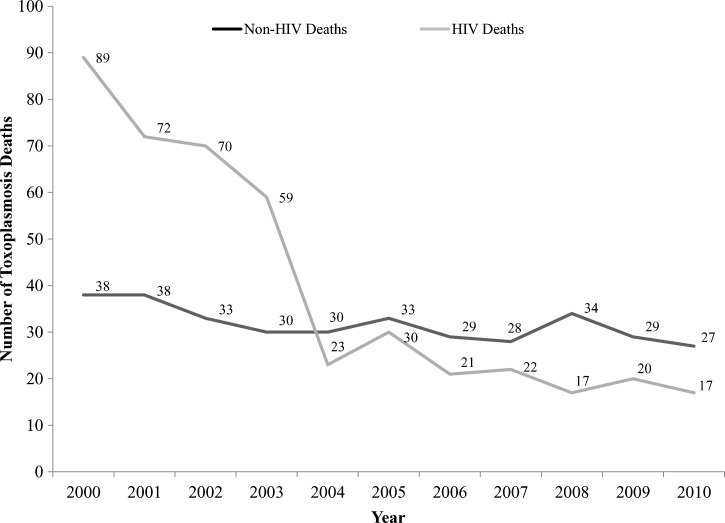
In total, there were 349 toxoplasmosis deaths without HIV (non-HIV) as a codiagnosis compared with 440 toxoplasmosis deaths with HIV (HIV) as a codiagnosis on the death certificates (Table 3). The average age at death for these subgroups (with and without HIV codiagnosis) was similar to the overall sample (caseload)—42.1, 41.9, and 42.1 years, respectively. The average age-adjusted mortality rate for toxoplasmosis deaths with no HIV codiagnosis was 0.011 (95% CI = 0.010–0.012) compared with 0.013 (95% CI = 0.012–0.015) for deaths with HIV codiagnosis. Poisson trend analysis showed that the annual percentage change in mortality rates among toxoplasmosis deaths without HIV involvement was −4.0% (95% CI, −6.6%, −0.18%; P value = 0.04), whereas for deaths with HIV involvement, it was −18.4%. The annual percentage change for the overall sample was −11.7% (Figure 2). The 35–44 years age group comprising toxoplasmosis deaths without an HIV codiagnosis had the highest absolute number of deaths. For both HIV and non-HIV toxoplasmosis deaths, Black and Hispanic race/ethnicity had the highest age-adjusted mortality rates compared with whites (HIV: age-adjusted RR = 14.90; 95% CI = 12.10–18.34 and age-adjusted RR = 6.21; 95% CI = 5.04–7.66, respectively; non-HIV: age-adjusted RR = 4.45; 95% CI = 3.79–5.22 and age-adjusted RR = 1.90; 95% CI = 1.59–2.27, respectively).
Table 3.
Decedent characteristics: toxoplasmosis cases with and without HIV codiagnosis on the death certificates in the United States, 2000–2010
| Characteristic(s) | Toxoplasmosis cases with HIV as a codiagnosis (N = 440) n (%)* | Toxoplasmosis cases without HIV as a codiagnosis (N = 349) n (%)* | χ2† | P value |
|---|---|---|---|---|
| Sex | 16 | < 0.001 | ||
| Female | 123 (28.0) | 145 (41.5) | ||
| Male | 317 (72.0) | 204 (58.5) | ||
| Race/ethnicity‡ | 70 | < 0.001 | ||
| White | 91 (20.9) | 162 (46.4) | ||
| Asian/Pacific Islander | < 5 | 10 (2.9) | ||
| Black | 237 (54.5) | 118 (33.8) | ||
| Hispanic | 107 (24.6) | 59 (16.9) | ||
| Age group (years)§ | 154 | < 0.001 | ||
| < 1 | < 5 | 23 (6.6) | ||
| 1–4 | – | 7 (2.0) | ||
| 5–14 | – | 20 (5.7) | ||
| 15–24 | 10 (2.3) | 17 (4.9) | ||
| 25–34 | 96 (22.0) | 52 (14.9) | ||
| 35–44 | 160 (36.7) | 80 (22.9) | ||
| 45–54 | 123 (28.2) | 48 (13.8) | ||
| 55–64 | 40 (9.2) | 37 (10.6) | ||
| 65–74 | 7 (1.6) | 33 (9.5) | ||
| 75–84 | < 5 | 27 (7.7) | ||
| > 85 | – | 5 (1.4) |
n is the frequency or number of decedents specified in each column.
χ2 statistic for equality of distribution of characteristic over groups and P value.
Native American race was excluded because of small sample size (< 5).
Percentage calculated based on available information from cases and controls; excludes those with missing or incomplete other category information.
Figure 2.
Number of toxoplasmosis cases with and without HIV codiagnosis on the death certificates by year in the United States, 2000–2010.
Discussion
T. gondii as a non-HIV disease in the United States.
Although toxoplasmosis is associated with HIV/AIDS, disease reactivation is common and often suspected in non-HIV immunosuppressive states (e.g., transplant recipients and chemotherapy patients). Based on a report released in 2011, T. gondii was ranked 2 among 14 foodborne pathogens in annual disease burden, with Salmonella (nontyphoidal serotypes) ranking first and Campylobacter ranking third.27 According to the report, $3 billion in illness costs and 11,000 quality-of-adjusted-life-years (QALYs) were attributed to toxoplasmosis; the disease's high mortality rate contributed to its high ranking. In this analysis, toxoplasmosis mortality averaged 71 deaths per year during the 11-year study period. This was considerably higher than for Salmonella (about 53 deaths per year), the number one cause of foodborne illness in the US. The Centers for Disease Control and Prevention currently estimates that approximately 50% of toxoplasmosis cases are foodborne.3
Productivity losses associated with toxoplasmosis pre-mature deaths.
The cumulative productivity losses associated with toxoplasmosis mortality during 2000–2010 were nearly $815 million, representing a significant financial burden to society. Although the highest costs were found in the 35–39 years age group, the 0–4 age subgroup contributed disproportionately to the amount. Although the caseload for congenital toxoplasmosis was relatively small in the MCD dataset (< 4% of total), the early losses of life summed up to a very large number of years lost from pre-mature death. Congenital toxoplasmosis is a condition that can lead to miscarriage, stillbirth, or lifelong complications (e.g., neurological disorders, permanent vision loss, learning disabilities, and paralysis).4 It is also a condition that can be prevented. For example, among pregnant women, increased patient education should stress the importance of reducing risk from food (e.g., eating raw ground beef, undercooked pork, rare lamb or locally produced cured, dried, or smoked meat).27,28 The Centers for Disease Control and Prevention also recommends reducing risk from the environment (e.g., avoid drinking untreated drinking water, wear gloves when gardening to avoid contact with soil that may be contaminated with oocysts excreted by infected cats, avoid changing cat litter, and keep cats indoors).28
The 11-year decline.
In this study, toxoplasmosis deaths declined substantively over an 11-year period from 2000 to 2010. This overall decline is consistent with a similar decline in the prevalence of toxoplasmosis infections observed in another study of US-born individuals.29 Parallel to this mortality trend (with some overlap), toxoplasmosis hospitalizations have also declined during the past 10–15 years (i.e., 10,583 in 1995 versus 3,000 in 2008).13 The decrease in toxoplasmosis hospitalizations has been attributed, partially, to a number of environmental and healthcare factors. For example, the overall prevalence of viable T. gondii in beef, chicken, and pork from retail meat stores in the US was found to be very low in a 2005 study30 and has been declining in humans and pigs4; this lowering of T. gondii contamination of the meat supply may be the result of enhanced efforts by the meat production industry to reduce T. gondii tissue cysts in meat.29 Another perhaps even stronger contributing factor was the introduction and subsequent widespread use of antiretroviral therapy (e.g., HAART) to control HIV infection. The relationship between HAART therapy and a decline in toxoplasmosis-related deaths has been shown in the literature.31 Coupled to other efforts, such as enhanced outreach and education of expectant mothers (by physicians) and the public (by public health authorities), this medical breakthrough likely helped reduce the absolute number of fatal opportunistic infections among individuals with HIV/AIDS. This is confirmed in our subgroup analysis, where a lower annual percentage change in mortality rates was observed for toxoplasmosis deaths that did not contain an HIV codiagnosis compared with deaths that did (−4.0% versus −18.4%; overall sample = −11.7%). However, another contributing factor may have been the advancement in anti-Toxoplasma therapy32–34 and better use of chemoprophylaxis to prevent reactivation or disease progression.35
Limitations.
Although MCD data have several strengths (e.g., population based and large sample size), there are a number of notable limitations and constraints attributed to abstracting information from NCHS death records.19 First, the ordering of the underlying and associated causes of death on death certificates is dependent on the opinions of the reporting physician or coroner, rarely providing insights into how these conditions interacted and contributed to death. Second, death certificates often contain errors and/or missing information that are confounded by a variety of factors, including quality of reporting. Accuracy of HIV reporting on death certificates (or misclassification/failure to identify HIV involvement on a death record), however, is inadequate. For example, Hessol and others36 compared the reported cases through national surveillance registry with the National Death Index and found that approximately 9% of AIDS cases did not have HIV infection or AIDS noted on their death certificates. Third, MCD data are typically not linked to clinical information, which often is required to provide context to the diagnosis and see how disease risk, comorbidities, and social factors influenced the chain of events leading to death. Fourth, using MCD data as a primary data source to inform economic analyses may limit the breadth and comprehensiveness of the cost estimations. For instance, this analysis does not capture productivity losses before death, costs attributed to treatment, or business costs related to replacement of a deceased employee.
Conclusions.
Despite the observed decline in toxoplasmosis mortality in the United States, the absolute number of deaths and productivity losses attributable to this protozoan zoonosis remained substantial during 2000–2010. Based on trend analyses, the notable improvements to disease burden were primarily related to the decline in cases associated with HIV infection. The mortality trends for cases without an HIV codiagnosis on the death certificate, for example, remained static, suggesting that non-HIV–related toxoplasmosis, especially from foodborne sources, continues to be an important cause of preventable death in vulnerable subgroups. Public health surveillance and timely clinical management of this condition remain critical for disease control and prevention of the lethal consequences associated with this infectious disease.
ACKNOWLEDGMENTS
The authors thank Mirna Ponce and Ranjana N. Wickramasekaran for their technical contributions to the analysis.
Footnotes
Authors' addresses: Patricia L. Cummings and Tony Kuo, Division of Chronic Disease and Injury Prevention, Los Angeles County Department of Public Health, Los Angeles, CA, E-mails: pcummings@ph.lacounty.gov and tkuo@ph.lacounty.gov. Marjan Javanbakht and Frank Sorvillo, Department of Epidemiology, Jonathan and Karin Fielding School of Public Health, University of California, Los Angeles, Los Angeles, CA, E-mails: javan@ucla.edu and fsorvill@ucla.edu.
References
- 1.Antinori A, Larussa D, Cingolani A, Lorenzini P, Bossolasco S, Finazzi MG, Bongiovanni M, Guaraldi G, Grisetti S, Vigo B, Gigli B, Mariano A, Dalle Nogare ER, De Marco M, Moretti F, Corsi P, Abrescia N, Rellecati P, Castagna A, Mussini C, Ammassari A, Cinque P, d'Arminio Monforte A. Prevalence, associated factors, and prognostic determinants of AIDS-related toxoplasmic encephalitis in the era of advanced highly active antiretroviral therapy. Clin Infect Dis. 2004;39:1681–1691. doi: 10.1086/424877. [DOI] [PubMed] [Google Scholar]
- 2.Jones JL, Hanson DL, Chu SY, Ciesielski CA, Kaplan JE, Ward JW, Navin TR. Toxoplasmic encephalitis in HIV-infected persons: risk factors and trends. The Adult/Adolescent Spectrum of Disease Group. AIDS. 1996;10:1393–1399. doi: 10.1097/00002030-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States – major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Ho YC, Sun HY, Chen MY, Hsieh SM, Sheng WH, Chang SC. Clinical presentation and outcome of toxoplasmic encephalitis in patients with human immunodeficiency virus type 1 infection. J Microbiol Immunol Infect. 2008;41:386–392. [PubMed] [Google Scholar]
- 6.Montoya JG, Liesenfeld D. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 7.Fuentes I, Rubio JM, Ramírez C, Alvar J. Genotypic characterization of Toxoplasma gondii strains associated with human toxoplasmosis in Spain: direct analysis from clinical samples. J Clin Microbiol. 2001;39:1566–1570. doi: 10.1128/JCM.39.4.1566-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falusi O, French AL, Seaberg EC, Tien PC, Watts DH, Minkoff H, Piessens E, Kovacs A, Anastos K, Cohen MH. Prevalence and predictors of Toxoplasma seropositivity in women with and at risk for human immunodeficiency virus infection. Clin Infect Dis. 2002;35:1414–1417. doi: 10.1086/344462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartelink ML, Hoek L, Freriks JP, Rutten GE. Infections in patients with type 2 diabetes in general practice. Diabetes Res Clin Pract. 1998;40:15–19. doi: 10.1016/s0168-8227(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 11.Bretagne S, Costa JM, Foulet F, Jabot-Lestang L, Baud-Camus F, Cordonnier C. Prospective study of Toxoplasma reactivation by polymerase chain reaction in allogeneic stem-cell transplant recipients. Transpl Infect Dis. 2000;2:127–132. doi: 10.1034/j.1399-3062.2000.020305.x. [DOI] [PubMed] [Google Scholar]
- 12.Voegele L, Cheerva AC, Bertolone S. Cerebral toxoplasmosis after tandem high-dose chemotherapy and autologous hematopoietic cell transplant for neuroblastoma. J Pediatr Hematol Oncol. 2013;35:e50–e52. doi: 10.1097/MPH.0b013e3182677e84. [DOI] [PubMed] [Google Scholar]
- 13.Jones JL, Roberts JM. Toxoplasmosis hospitalizations in the United States, 2008, and trends, 1993–2008. Clin Infect Dis. 2012;54:e58–e61. doi: 10.1093/cid/cir990. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization International Classification of Diseases, 10th Revision. 1992. www.who.int/classifications/icd/en/ Available at. Accessed July 10, 2012.
- 15.National Center for Health Statistics; Centers for Disease Control and Prevention (CDC); US Census Bureau United States Bridged-Race Population Estimates 2010 - NCHS. 2011. http://www.cdc.gov/nchs/nvss/bridged_race.htm Available at. Accessed October 10, 2013.
- 16.United States Department of Health and Human Services Protection of Human Subjects. Title 45 Code of Federal Regulations, Pt 46. 2005. http://www.hhs.gov/ohrp/documents/OHRPRegulations.pdf Available at. Accessed April 29, 2014.
- 17.National Center for Health Statistics Bridged-Race Intercensal Estimates of the July 1, 2000–July 1, 2009. United States Resident Population from the Vintage 2006 Postcensal Series by Year, County, Age, Sex, Race, and Hispanic Origin, Prepared Under Collaborative Arrangement with the U.S. Census Bureau. 2007. www.cdc.gov/nchs/nvss/bridged_race.htm Available at. Accessed April 29, 2014.
- 18.National Center for Health Statistics Life Expectancies for 2000–2010 Were Calculated Using a Revised Methodology and May Differ from Those Previously Published. National Vital Statistics Reports (NVSR), Deaths: Preliminary Data for 2008. 2010. http://www.census.gov/compendia/statab/2012/tables/12s0104.pdf Available at. Accessed July 11, 2012.
- 19.Redelings MD, Wise M, Sorvillo F. Using multiple cause-of-death data to investigate associations and causality between conditions listed on the death certificate. Am J Epidemiol. 2007;166:104–108. doi: 10.1093/aje/kwm037. [DOI] [PubMed] [Google Scholar]
- 20.Bradley CJ, Yabroff KR, Dahman B, Feuer EJ, Mariotto A, Brown ML. Productivity costs of cancer mortality in the United States: 2000–2020. J Natl Cancer Inst. 2008;100:1763–1770. doi: 10.1093/jnci/djn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosse SD, Krueger KV, Mvundura M. Economic productivity by age and sex: 2007 estimates for the United States. Med Care. 2009;47:S94–S103. doi: 10.1097/MLR.0b013e31819c9571. [DOI] [PubMed] [Google Scholar]
- 22.Lipscomb J, Weinstein MC, Torrance GW. Time preference. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. pp. 214–246. [Google Scholar]
- 23.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 24.Halfhill TR. Tom's Inflation Calculator, v7.5.3. Copyright 1997–2012. 2012. http://www.halfhill.com/inflation.html Available at. Accessed October 1, 2012.
- 25.Bristow BN, Casil J, Sorvillo F, Basurto-Dávila R, Kuo T. Melanoma-related mortality and productivity losses in the USA, 1990–2008. Melanoma Res. 2013;23:331–335. doi: 10.1097/CMR.0b013e328361926c. [DOI] [PubMed] [Google Scholar]
- 26.Moschetti K, Barragan N, Basurto-Dávila R, Cummings PL, Sorvillo F, Kuo T. Mortality and productivity losses from Alzheimer disease among U.S. adults aged 40 to 64 years, 1999 to 2010. Alzheimer Dis Assoc Disord. 2014 doi: 10.1097/WAD.0000000000000017. in press. [DOI] [PubMed] [Google Scholar]
- 27.Batz MB, Hoffman S, Morris JG., Jr . Ranking the Risks: The 10 Pathogen-Food Combinations with the Greatest Burden on Public Health. Gainesville, FL: Emerging Pathogens Institute, University of Florida; 2011. [Google Scholar]
- 28.Centers for Disease Control and Prevention; Global Health–Division of Parasitic Diseases and Malaria Parasites–Toxoplasmosis (Toxoplasma infection) 2013. www.cdc.gov/parasites/toxoplasmosis/prevent.html Available at. Accessed June 22, 2014.
- 29.Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999–2004, decline from the prior decade. Am J Trop Med Hyg. 2007;77:405–410. [PubMed] [Google Scholar]
- 30.Dubey JP, Hill DE, Jones JL, Hightower AW, Kirckland E, Roberts JM, Marcet PL, Lehmann T, Vianna MC, Miska K, Sreekumar C, Kwok OC, Shen SK, Gamble HR. Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J Parasitol. 2005;91:1082–1093. doi: 10.1645/GE-683.1. [DOI] [PubMed] [Google Scholar]
- 31.Jones JL, Sehgal M, Maguire JH. Toxoplasmosis-associated deaths among human immunodeficiency virus-infected persons in the United States, 1992–1998. Clin Infect Dis. 2002;15:1161. doi: 10.1086/339752. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H. Centers for Disease Control and Prevention (CDC); National Institutes of Health; HIV Medicine Association of the Infectious Disease Society of America Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 33.Luft BJ, Hafner R, Korzun AH, Leport C, Antoniskis D, Bosler EM, Bourland DD, 3rd, Uttamchandani R, Fuhrer J, Jacobson J, Morlat P, Vilde JL, Remington JS. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. Members of the ACTG 077p/ANRS 009 Study Team. N Engl J Med. 1993;329:995–1000. doi: 10.1056/NEJM199309303291403. [DOI] [PubMed] [Google Scholar]
- 34.Dannemann B, McCutchan JA, Israelski D, Antoniskis D, Leport C, Luft B, Nussbaum J, Clumeck N, Morlat P, Chiu J, Vilde JL, Orellana M, Feigal D, Bartok A, Heseltine P, Leedom J, Remington J. Treatment of toxoplasmic encephalitis in patients with AIDS: randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann Intern Med. 1992;116:33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- 35.Nissapatorn V. Toxoplasmosis in HIV/AIDS: a living legacy. Southeast Asian J Trop Med Public Health. 2009;40:1158–1178. [PubMed] [Google Scholar]
- 36.Hessol NA, Buchbinder SP, Colbert D, Scheer S, Underwood R, Barnhart JL, O'Malley PM, Doll LS, Lifson AR. Impact of HIV infection on mortality and accuracy of AIDS reporting on death certificates. Am J Public Health. 1992;82:561–564. doi: 10.2105/ajph.82.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]