Abstract
Objectives
In this study, we have developed an electrocardiogram-based scoring system to predict secondary pulmonary hypertension.
Design
A cross-sectional study.
Setting
Single tertiary-care hospital in Scranton, Pennsylvania, USA.
Participants
Five hundred and fifty-two consecutive patients undergoing right heart catheterization between 2006 and 2009.
Main outcome measures
Surface electrocardiogram was assessed for R-wave in lead V1 ≥ 6mm, R-wave in V6 ≤ 3mm, S-wave in V6 ≥ 3mm, right atrial enlargement, right axis deviation and left atrial enlargement. Pulmonary hypertension was defined as mean pulmonary artery pressure ≥25 mmHg, determined by right heart catheterization.
Results
A total of 297 (54%) patients in the study cohort had pulmonary hypertension. In total, 332 patients from the study cohort formed the development cohort and the remaining 220 patients formed the validation cohort. In the development cohort, based on log odds ratios of association, RAE, LAE, RAD, R-wave in V1 ≥ 6 mm were assigned scores of 5, 2, 2 and 1, respectively, to form a 10-point scoring system “Scranton PHT (SP) score”. SP scores of 5 points and 7 points in DC showed C-statistic of 0.83 and 0.89, respectively, for discriminating pulmonary hypertension. C-statistic for RAE alone was significantly lower compared to an SP score of 7 (0.83 vs. 0.89, P = 0.021). The reliability of SP score in the validation cohort was acceptable.
Conclusion
SP score provides a good point-of-care tool to predict pulmonary hypertension in patients with clinical suspicion of it.
Keywords: Pulmonary hypertension, electrocardiography, scoring method
Introduction
Secondary pulmonary hypertension (PHT) is common in patients with respiratory, cardiac and systemic diseases and predicts poor prognosis and decreased survival.1–3 PHT may be the only presenting feature in diseases, such as systemic sclerosis, obstructive sleep apnea, and mitral stenosis, which when investigated, may lead the clinician to identify the underlying diagnosis.4–6 PHT is a significant risk factor for early and sudden postoperative deaths.7,8 In critically ill patients, PHT is associated with increased risk of right ventricular failure and poor prognosis.9 Hence, timely recognition of PHT is important for primary care providers, subspecialists, intensivists, and surgeons alike. However, PHT is asymptomatic during its initial course making early diagnosis difficult.10 Although investigative tools like echocardiogram, pulmonary function test, and assessment for carbon monoxide diffusion capacity show promise in early diagnosis of PHT,10 these tools are expensive and are often not available at the bedside.
Clinically applicable scoring systems are in common practice for diagnosis and prognostication of cardiovascular diseases.11,12 Alteration in treatment strategies based on the additional information provided by these scoring systems has been shown to improve the outcomes.12 EKG is a common investigative modality, which is cost-effective and has point-of-care applicability. There is no published report describing an EKG-based scoring system to predict PHT. We sought to develop an EKG-based score (Scranton PHT score) that can predict the presence or absence of PHT.
Materials and methods
This cross-sectional study was conducted at a tertiary care hospital in Scranton, Pennsylvania. Institutional review board approval was obtained from the Wright Center for Graduate Medical Education's institutional review board for conducting the study. Written informed consent was obtained from the study participants.
Case records of 602 consecutive adult patients (age ≥18 years) who underwent elective right heart catheterization between the years 2006 and 2009 were reviewed. Patients with known diagnosis of primary PHT, atrial fibrillation, and other baseline rhythm abnormalities on EKG were excluded. Hence, the patients included in the study were those who were referred for elective right heart catheterization due to a clinical suspicion of secondary PHT, with no persistent baseline EKG rhythm abnormalities.
Baseline data collection
Baseline data were collected from case records at the time of admission for elective cardiac catheterization. Demographic data (age, gender, race/ethnicity), comorbidities (smoking, diabetes, hypertension, coronary artery disease, chronic obstructive pulmonary disease (COPD)), clinical parameters (height, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP)), and laboratory parameters (hemoglobin, serum creatinine, estimated glomerular filtration rate (eGFR), total cholesterol and LDL cholesterol) were recorded. Body mass index was calculated using standard measures.13
EKG variables
Standard 12 lead surface EKG was performed, with 10 mm/mv calibration and 25 mm/s paper speed, for all the study participants at the time of admission into the hospital for an elective right heart catheterization. EKG variables, such as rhythm, axis (in degrees), R-wave in lead V1 (mm), R-wave in V6, S-wave in V6, right atrial enlargement (RAE) (defined as >2.5 mm height of P-wave in Lead II), left atrial enlargement (LAE) (defined as width of P-wave in Lead II >2.5 mm and/or >1mm width and height of the negative component of P-wave in lead V1), were recorded for all the study cohort participants. Right axis deviation (RAD) was defined when frontal QRS axis was > + 90°. These variables were selected by virtue of them being the variables used by several common EKG criteria to identify right ventricular hypertrophy associated with PHT.14–17 A single observer who was an internist and had 4 years post-medical school experience abstracted all the EKG variables used in this study. These findings were confirmed by a senior cardiologist (post-cardiology board certification experience >15 years).
Right heart catheterization and measurement of mean pulmonary artery pressure
Three different cardiologists performed right heart catheterization over the 4-year time course of the study. All catheterization procedures were performed in the same catheterization laboratory of the single tertiary care center where the study was conducted. Right heart catheterization was performed using either femoral or brachial vein approach in a standard fashion. The variables measured via the right heart catheterizations that were of interest to our present study included pulmonary artery (PA) systolic pressure, PA diastolic pressure, and PA mean pressure. PHT was defined as a mean PA pressure ≥25 mmHg.18 Patients with pulmonary capillary wedge pressure <15 mmHg were excluded in view of diagnosis of primary PHT (n = 1).
Statistical analysis and EKG-based scoring system development
Continuous and categorical variables were expressed as mean (±SD) and n (%), respectively. The study cohort was categorized into those with PHT and those without PHT and was compared using Student's t-test or chi-squared test as appropriate with regard to baseline demographic, clinical, and laboratory variables. The study cohort was divided into five random equal groups using a random number table. Three of the five groups formed the development cohort (DC) and were used to develop the risk score. The remaining two of the five groups formed the validation cohort (VC) and were used for validation of the score developed in the DC. The DC was compared with the VC using Student's t-test and chi-squared test as appropriate. Using the DC, bivariate logistic regression analysis was performed with PHT (mean PA pressure ≥25 mmHg) as the dependent variable (treated as categorical variable, yes/no) and variables namely: age, gender, RAE (yes/no), LAE (yes/no), RAD (yes/no), R-wave in lead V1 ≥ 6 mm, R-wave in lead V6 ≤ 3 mm, S-wave in lead V6 ≥ 3, and hypertension (yes/no) as independent variables. On the basis of these bivariate analyses, variables that were significantly associated with PHT (P < 0.05), were used in the multivariate logistic regression analysis treating PHT (yes/no) as dependent variable and the chosen variables (as mentioned above) as independent variables and log odds ratios (beta-coefficients) determining the strength of association of these variables with PHT were obtained. Goodness of fit of the model was determined by means of Hosmer and Lemeshow goodness-of-fit test. On the basis of odds ratios from the multivariate logistic regression analysis, a 10-point score was developed. The accuracy of this 10-point score for predicting PHT was assessed for various cut-offs, namely 1–10 points, using sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratio (LR)+, LR−, and area under receiver operator characteristics curve (ROC).19 The ROC curves obtained for each of the cut-offs were compared with one another using the method described by DeLong et al.20 Furthermore, this 10-point score was used in the VC and accuracy of this score in predicting PHT for cut-offs 1–10 points, were assessed using sensitivity, specificity, PPV, NPV, LR+, LR−, area under ROC curve.19 A P-value ≤0.05 was considered statistically significant. All analysis was performed using STATA 8.2.21
Results
From a total of 601 patient records assessed for eligibility, 49 were excluded (30 due to missing data, 11 due to atrial fibrillation, 7 due to baseline EKG abnormalities, and 1 due to primary PHT). The remaining 552 patients formed the study cohort. Of these 552 study cohort patients, 297 (54%) had PHT and 255 (46%) had normal mean pulmonary artery pressures. Of those with PHT, 234 (79%) had mild PHT, 47 (16%) had moderate PHT, and 15 (5%) had severe PHT. The baseline characteristics of the study cohort are mentioned in Table 1. The study cohort was divided into five random equal groups, containing 110 patients each, using a random number table. Three of these five groups formed the DC and contained 332 participants. The remaining 220 participants formed the VC. The baseline characteristics of the DC and VC are mentioned in Table 1.
Table 1.
Baseline characteristics of the study population.
| Variables | Study cohort (n = 552) | PHT cohort (n = 297) | Non-PHT cohort (n = 255) | P-value | DC (n = 332) | VC (n = 220) | P-value |
|---|---|---|---|---|---|---|---|
| Age (years) | 66.4 (12.3) | 65.4 (12.3) | 67.4 (12.2) | 0.057 | 65.3 (11.7) | 67.8 (13.4) | 0.137 |
| Females | 288 (52.2%) | 145 (48.8%) | 142 (55.7%) | 0.108 | 171 (52.0%) | 117 (53.0%) | 0.471 |
| Caucasians | 480 (87%) | 261 (87.8%) | 219 (85.8%) | 0.487 | 286 (86.1%) | 194 (88.2%) | 0.118 |
| Tobacco use | 252 (45.5%) | 130 (43.8%) | 121 (47.5%) | 0.387 | 145 (44.0%) | 107 (49.0%) | 0.077 |
| Diabetes | 175 (31.7%) | 99 (29.8%) | 76 (33.3%) | 0.374 | 101 (30.4%) | 74 (34.0%) | 0.091 |
| Hypertension | 331 (60.0%) | 171 (57.6%) | 160 (62.8%) | 0.217 | 212 (64%) | 119 (54%) | 0.039 |
| CAD | 302 (54.5%) | 157 (52.9%) | 145 (56.9%) | 0.346 | 166 (50%) | 136 (62%) | 0.044 |
| COPD | 79 (14.6%) | 39 (13.5%) | 40 (16%) | 0.464 | 52 (16%) | 27 (12%) | 0.101 |
| Right axis deviation | 19 (3.26%) | 13 (4.38%) | 6 (2.35%) | 0.420 | 12 (3.6%) | 7 (3.2%) | 0.610 |
| BMI (kg/m2) | 26.5 (7.0) | 26.0 (6.9) | 27.1 (7.1) | 0.111 | 27.1 (5.6) | 26.4 (6.3) | 0.331 |
| Hemoglobin (g/dl) | 12.8 (1.9) | 12.9 (1.8) | 12.7 (1.9) | 0.170 | 13.0 (1.2) | 12.6 (1.7) | 0.299 |
| Creatinine (mg/dl) | 1.1 (0.6) | 1.1 (0.6) | 1.1 (0.6) | 0.298 | 0.9 (0.4) | 1.1 (0.5) | 0.772 |
| eGFR | 69.9 (29.1) | 71.1 (29.4) | 68.5 (28.7) | 0.381 | 73.4 (23.7) | 70.1 (26.4) | 0.113 |
| Total cholesterol (mg/dl) | 156.3 (46.2) | 161.0 (56.3) | 152.6 (36.3) | 0.352 | 166 (51.2) | 157 (43.9) | 0.038 |
| LDL (mg/dl) | 86.6 (39.3) | 91.6 (43.9) | 82.7 (35.0) | 0.237 | 90.7 (40.1) | 110.8 (29.1) | 0.029 |
BMI: body mass index; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; DC: development cohort; eGFR: estimated glomerular filtration rate; LDL: low density lipoprotein; PHT: pulmonary hypertension; VC: validation cohort.
In the univariate analysis, RAE, LAE, RAD, R-wave in lead V1 ≥ 6 were significantly associated with presence of PHT (Table 2). Multivariate logistic regression analysis identified these variables to have significant association with PHT, with relative weights (based on their log-odds ratios of association with PHT) as shown in Table 2. Hence, the proposed 10-point “Scranton PHT (SP) score” was calculated assigning 5 points for RAE, 2 points for LAE, 2 points for RAD, and 1 point for R-wave in lead V1 ≥ 6.
Table 2.
Associations of pulmonary hypertension and the relative weights of each variable (DC—n = 332).
| Variables | Univariate logistic regression analysis |
Multivariate logistic regression analysis |
Relative weights | ||
|---|---|---|---|---|---|
| Log-OR (95% CI) | P-value | Log-OR (95% CI) | P-value | ||
| Age | 0.97 (0.77–1.76) | 0.322 | – | – | – |
| Gender | 1.10 (0.71–1.39) | 0.219 | – | – | – |
| R-wave in lead V6 ≤ 3 | 0.90 (0.62–1.41) | 0.371 | – | – | – |
| S-wave in lead V6 ≥ 3 | 0.99 (0.65–1.98) | 0.150 | – | – | – |
| RAE | 6.91 (3.79–8.44) | 0.020 | 4.78 (2.61–5.77) | 0.024 | 5 |
| LAE | 3.10 (1.91–4.66) | 0.029 | 2.11 (1.29–3.01) | 0.031 | 2 |
| RAD | 2.99 (1.56–3.85) | 0.031 | 2.06 (1.13–2.68) | 0.037 | 2 |
| R-wave in lead V1 ≥ 6 | 1.76 (1.21–2.47) | 0.037 | 1.33 (1.09–1.88) | 0.044 | 1 |
CI: confidence interval; DC: development cohort; LAE: left atrial enlargement; OR: odds ratio; RAD: right atrial enlargement; RAE: right atrial enlargement.
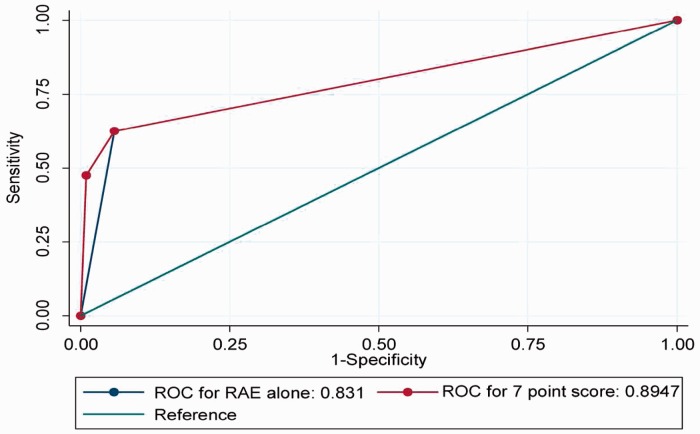
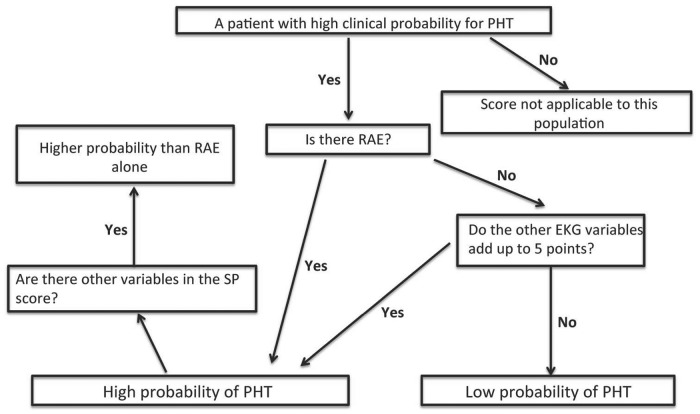
In the DC, 201 (61%),190 (57%), 181 (55%),166 (50%), 141 (42%), 83 (25%), 36 (11%) had scores of 1 point, 2 points, 3 points, 4 points, 5 points, 6 points and 7 points, respectively. In total, 133 (40%) has RAE alone. Area under curve (AUC) for 1 point, 2 points, 3 points, and 4 points were all 0.44, 0.47, 0.46, and 0.59, respectively. The AUC for scores 5, 6, and 7 points in the DC were 0.83, 0.85, and 0.89, respectively (Table 3) for predicting PHT. None in the DC had scores >7 points. Presence of RAE alone had an AUC of 0.83 (Table 3). The AUC for a score of 7 points was significantly better (P = 0.021) than RAE alone in discriminating PHT (Figure 1). In the VC, 124 (56%), 119 (54%), 110 (50%), 96 (44%), 87 (40%), 31 (14%), and 81 (37%) patients had 1 point, 2 points, 3 points, 4 points, 5 points, 6 points, and RAE alone, respectively. AUC for 1 point, 2 points, 3 points, and 4 points were, respectively, 0.33, 0.45, 0.43, and 0.53. The AUC for 5 points, 6 points, and RAE alone were 0.84, 0.91, and 0.81, respectively, in predicting PHT (Table 3). Patients with SP score of 6 had significantly better AUC compared to RAE alone (P = 0.019). There were none in the VC with scores >6. Algorithm that we propose for clinical use of our score is detailed in Figure 2.
Table 3.
Predictive accuracy of the SP score for PHT in the DC and the VC.
| Predictive accuracy | DC |
VC |
|||||
|---|---|---|---|---|---|---|---|
| (n = 332) |
(n = 220) |
||||||
| 5 points | 6 points | 7 points | RAE alone | 5 points | 6 points | RAE alone | |
| Sensitivity | 81.0 | 78.0 | 79.0 | 87.2 | 83.1 | 83.3 | 84.5 |
| Specificity | 92.0 | 94.6 | 97.0 | 91.5 | 90.4 | 96.8 | 86.0 |
| PPV | 90.1 | 84.3 | 73.0 | 87.1 | 83.1 | 80.6 | 75.0 |
| NPV | 84.3 | 92.0 | 97.6 | 91.5 | 92.4 | 97.4 | 92.0 |
| LR+ | 6.9 | 7.5 | 9.1 | 10.2 | 8.68 | 9.8 | 6.0 |
| LR− | 0.21 | 0.23 | 0.22 | 0.14 | 0.19 | 0.17 | 0.18 |
| AUC | 0.83 | 0.85 | 0.89 | 0.83 | 0.84 | 0.91 | 0.81 |
| Number with PHT | 127 | 70 | 26 | 116 | 69 | 25 | 60 |
| Number with this score | 141 | 83 | 36 | 133 | 87 | 31 | 81 |
AUC: area under the curve; LR: likelihood ratio; NPV: negative predictive value; PHT: pulmonary hypertension; PPV: positive predictive value; RAE: right atrial enlargement.
Figure 1.
An SP score of 7 performed significantly better compared to presence of electrocardiogram evidence of right atrial enlargement alone in discriminating pulmonary hypertension.
Figure 2.
Algorithm detailing the step-by-step use of SP score to predict pulmonary hypertension.
Discussion
In this cross-sectional study aimed at developing an EKG-based scoring system to predict PHT, we observed that a 10-point “Scranton PHT score” comprising of RAE, LAE, RAD, and R-wave in V1 ≥ 6 mm with scores 5, 2, 2, and 1 points, respectively, performed well in discriminating patients with secondary PHT. This score showed good accuracy when internally validated within the same dataset.
SP score, comprising of EKG markers of bi-atrial, and right ventricular pathology, parallels the pathophysiology of secondary PHT. In majority of left-sided etiologies, left atrium is known to enlarge, and hence addition of LAE provides EKG representation of this aspect of the pathophysiologic process. Atria are considered the barometers of the ventricles, and hence tend to develop morphologic abnormalities earlier in the natural history of pathologic conditions affecting the cardiac chambers. The right atrium (RA) undergoes significant electrical changes with PHT including a significant reduction in atrial conduction velocity and an increase in activation times that occurs early in the disease process.22 This explains our observed strong association of RAE with PHT and hence its assigned score of 5 in the 10-point scoring system. Although RAE alone had a good predictive accuracy, higher scores performed better in discriminating PHT compared to RAE alone, likely due to additive contribution of markers of right ventricular pathology.
We note that when a scoring system is assessed for its predictive accuracy, the prevalence of the event of interest should be taken into consideration.23 When the prevalence of the event of interest (PHT) is high (54% in our study), values of sensitivity, specificity, positive predictive value, and negative predictive value may be less useful for clinical application, as all of the above-mentioned indicators of test accuracy vary with prevalence. In this context, the indicators of test accuracy, which do not significantly vary with prevalence, are the most clinically relevant. LR + and LR− do not vary with prevalence of the disease.23 An LR + >1 and an LR− < 1 makes a test clinically meaningful, while an LR + >10 and LR− < 0.1 indicates high accuracy.23 AUC > 0.75 also conveys the same meaning.23 In our study, all cut-offs, namely SP score values of 5, 6, and 7 showed LR + >5, LR− < 0.3 and AUC > 0.8 indicating good accuracy of this tool in discriminating PHT.
Generally, EKG-based scores, including those used for right ventricular hypertrophy, tested in general population, are known to have high specificity but low sensitivity.24 Our score had both high sensitivity and high specificity (Table 3) probably due to the high prevalence of PHT in our sample. Doppler echocardiogram, which is considered the standard for non-invasive testing for PHT, has a sensitivity and specificity of 88–89% and 86–89%, respectively, for identifying PHT.24 Our score fares similar with comparable sensitivity and specificity measures as Doppler echocardiography. Predictive accuracy of RAE alone was similar to that of Doppler echocardiography in identifying PHT in our sample; however, higher SP scores had higher predictive accuracy. With the current guidelines not recommending routine echocardiography prior to cardiac catheterization, due to its cost and non-applicability as a point-of-care tool, we believe, our “SP” score serves as a point-of-care tool to predict PHT in patients with systemic illnesses and left heart pathologies in whom PHT may signify poor prognosis as it potentially performs similar to echocardiography in discriminating PHT.
Limitations
Although our study has significant strengths, namely large sample size, PHT confirmed by right heart catheterization, and score having applicability to patients with even mild PHT, study limitations need consideration while interpreting the findings of our study. This is a single-centered study and hence confounding due to study center characteristics is possible. Specific etiologies of PHT patients in our cohort were not ascertained. As our patients were referred for right heart catheterization, they had a high clinical suspicion for PHT and hence selection bias is obvious. This explains the high predictive accuracy observed in our sample. However, the aim of our study was to develop a score to identify PHT in patients with clinical suspicion of PHT, hence this selection bias may not affect the applicability of our score. Our patients predominantly had mild PHT (79%). Hence, applicability of this score in patients with severe PHT needs to be assessed in future studies, although we expect at least similar, and likely a better performance of the score in patients with severe PHT. Also, in our development or the VC there were no patients with scores >7, probably due to less numbers of patients with moderate and severe PHT. Hence, predictive accuracy of scores >7 is not known and needs assessment in future studies. Furthermore, in the setting of elective catheterization of our study patients, it is likely that they were fasting and probably volume depleted. Documentation of left ventricular end-diastolic pressure (LVEDP) after saline loading in patients with normal LVEDP could not be done as patients' case records did not have documentation of saline loading. However, influence of this limitation on our study findings will be miniscule as only one patient had normal LVEDP in our study, and he was excluded from our analysis.
Conclusion
The 10-point “SP score” shows promise as a clinically useful point-of-care tool to predict PHT in patients with clinical suspicion of PHT. Our score needs validation in external samples and in samples from general populations25 before its potential for clinical use can be properly determined.
Acknowledgements
None.
Competing interests
Dr Samir Bipin Pancholy, MD receives research grant from Accumed Radial Systems Inc. and is a speaker for Pfizer and Medtronic and a consultant for Terumo. All other authors declare no competing interests.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
The institutional review board of the Wright Center for Graduate Medical Education approved the study proposal.
Guarantor
SBP is the guarantor for this paper.
Contributorship
SBP and GPSS, were involved in the concept of the study, data cleaning, data analysis, and interpretation, wrote the manuscript, and revised it for scientific rigor. NP, PB, SN, SC, AS, and JS were involved in data collection, data cleaning, revising the manuscript, and literature review. All authors participated in the decision to submit and approved the current version of the manuscript for publication.
References
- 1.Tonelli AR. Pulmonary hypertension survival effects and treatment options in cystic fibrosis. Curr Opin Pulm Med 2013; 19: 652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adir Y, Amir O. Pulmonary hypertension associated with left heart disease. Semin Respir Crit Care Med 2013; 34: 665–680. [DOI] [PubMed] [Google Scholar]
- 3.Lynch JP, Belperio JA, Saggar R, et al. Pulmonary hypertension complicating connective tissue disease. Semin Respir Crit Care Med 2013; 34: 581–599. [DOI] [PubMed] [Google Scholar]
- 4.Pauling JD, Gunawardena H, Coghlan JG, et al. Pulmonary artery hypertension as the presenting feature of systemic sclerosis sine scleroderma. Rheumatology (Oxford) 2008; 47: 1431–1432. [DOI] [PubMed] [Google Scholar]
- 5.Kessler R, Chaouat A, Weitzenblum E, et al. Pulmonary hypertension in the obstructive sleep apnoea syndrome: Prevalence, causes and therapeutic consequences. Eur Respir J 1996; 9: 787–794. [DOI] [PubMed] [Google Scholar]
- 6.Steinberger J, Moller JH, Berry JM, et al. Echocardiographic diagnosis of heart disease in apparently healthy adolescents. Pediatrics 2000; 105: 815–818. [DOI] [PubMed] [Google Scholar]
- 7.Minai OA, Yared JP, Kaw R, et al. Perioperative risk and management in patients with pulmonary hypertension. Chest 2013; 144: 329–340. [DOI] [PubMed] [Google Scholar]
- 8.Ramakrishna G, Sprung J, Ravi BS, et al. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: Predictors of perioperative morbidity and mortality. J Am Coll Cardiol 2005; 45: 1691–1699. [DOI] [PubMed] [Google Scholar]
- 9.Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med 2011; 184: 1114–1124. [DOI] [PubMed] [Google Scholar]
- 10.McCrory DC, Coeytaux RR, Schmit KM, et al. Pulmonary Arterial Hypertension: Screening, Management, and Treatment [Internet], Rockville (MD): Agency for Healthcare Research and Quality (US), 2013. Report No.: 13-EHC087-EF. [PubMed] [Google Scholar]
- 11.Giralt-Steinhauer E, Rodríguez-Campello A, Cuadrado-Godia E, et al. External validation of the DRAGON score in an elderly Spanish population: Prediction of stroke prognosis after IV thrombolysis. Cerebrovasc Dis 2013; 36: 110–114. [DOI] [PubMed] [Google Scholar]
- 12.Chatzikonstantinou A, Wolf ME, Schaefer A, et al. Risk prediction of subsequent early stroke in patients with transient ischemic attacks. Cerebrovasc Dis 2013; 36: 106–109. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey SF, Eleftheria MF. Obesity. In: Kasper E DL, Braunwald AS, Fauci SL, Hauser DL. (eds). Harrison's principles of internal medicine, 21st ed New York: McGraw Hill Inc, 2003, pp. 422–423. [Google Scholar]
- 14.Murphy ML, Thenabadu PN, Blue LR, et al. Descriptive characteristics of the electrocardiogram from autopsied men free of cardiopulmonary disease – a basis for evaluating criteria for ventricular hypertrophy. Am J Cardiol 1983; 52: 1275–1280. [DOI] [PubMed] [Google Scholar]
- 15.Myers GB, Klein HA, Stofer BE. The electrocardiographic diagnosis of right ventricular hypertrophy. Am Heart J 1948; 35: 1–40. [DOI] [PubMed] [Google Scholar]
- 16.Butler PM, Leggett SI, Howe CM, et al. Identification of electrocardiographic criteria for diagnosis of right ventricular hypertrophy due to mitral stenosis. Am J Cardiol 1986; 57: 639–643. [DOI] [PubMed] [Google Scholar]
- 17.Hancock EW, Deal BJ, Mirvis DM, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: Endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119: e251–261. [DOI] [PubMed] [Google Scholar]
- 18.Lafitte S, Pillois X, Reant P, et al. Estimation of pulmonary pressures and diagnosis of pulmonary hypertension by Doppler echocardiography: A retrospective comparison of routine echocardiography and invasive hemodynamics. J Am Soc Echocardiogr 2013; 26: 457–463. [DOI] [PubMed] [Google Scholar]
- 19.Lusted LB. Signal detectability and medical decision-making. Science 1971; 171: 1217–1219. [DOI] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 21.StataCorp. Stata statistical software: Release 8.2, College Station, TX: StataCorp LP, 2004. [Google Scholar]
- 22.Medi C, Kalman JM, Ling LH, et al. Atrial electrical and structural remodeling associated with longstanding pulmonary hypertension and right ventricular hypertrophy in humans. J Cardiovasc Electrophysiol 2012; 23: 614–620. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg RS, Daniels SR, Dana Flanders W, Sanders FH, Robert RB. Diagnostic testing. In: Greenberg RS. (ed.). Medical epidemiology, New York: McGraw-Hill Inc, 2005, pp. 112–117. [Google Scholar]
- 24.Lafitte S, Pillois X, Reant P, et al. Estimation of pulmonary pressures and diagnosis of pulmonary hypertension by Doppler echocardiography: A retrospective comparison of routine echocardiography and invasive hemodynamics. J Am Soc Echocardiogr 2013; 26: 457–463. [DOI] [PubMed] [Google Scholar]
- 25.Whitman I, Patel VV, Soliman EZ, et al. Validity of the surface electrocardiogram criteria for right ventricular hypertrophy: The MESA – right ventricle study. J Am Coll Cardiol 2013; 63: 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]