Abstract
Background
Health-related quality of life has been shown to impact prognosis in chronic heart failure, however with limited long-term follow-up. We analysed data spanning 8–12 years to assess the impact of health-related quality of life using the Nottingham Health Profile on first hospitalisation and mortality, for cardiovascular and all causes.
Methods
We included 208 patients aged ≥60 years with New York Heart Association class II–IV and left ventricular systolic dysfunction hospitalised in Stockholm during 1996–99. Data on hospital admissions, discharge diagnoses and date and cause of death were collected from administrative databases and medical records until 2007. Cox proportional hazard models were employed to analyse the time to event for mortality and hospitalisations.
Results
Mean age was 76 years, 58% were male and mean ejection fraction was 34%. Median survival was 4.6 years (range 6 days–11.9 years); 148 patients died. All-cause and cardiovascular mortality were determined by physical mobility (by Nottingham Health Profile), age, gender, diuretic dose and haemoglobin level. Glomerular filtration rate was significant for all-cause mortality, while atrioventricular plane displacement was predictive of cardiovascular mortality. Median time to first all-cause and cardiovascular hospitalisation was 5.7 and 11.2 months, respectively. Time to first all-cause hospitalisation was determined by physical mobility, emotional reactions, age, gender and haemoglobin level, while only physical mobility and diuretic dose predicted time to first cardiovascular hospitalisation.
Conclusions
In conclusion, in patients with systolic chronic heart failure, physical mobility as part of health-related quality of life is an independent prognostic marker for cardiovascular and all-cause readmissions and mortality over 12 years.
Keywords: Factor analysis, heart failure, hospitalisation, mortality, Nottingham Health Profile, prognosis, quality of life, survival analysis
Background
Prognosis of chronic heart failure (CHF) is poor,1 and its economic burden is high.2 Low health-related quality of life (HRQoL) has been shown to impact on hospitalisations and mortality in CHF, independently of other factors.3–12 However, the follow-up period in previous studies has generally been limited to between 6 months and 5 years. Only few studies with a follow-up of 10 years have investigated HRQoL and mortality,7,12 and there is a lack of long-term results concerning HRQoL and readmissions. In earlier analyses over a mean follow-up of 37 months, poor HRQoL was predictive of readmissions.9,10 Thus, it is of interest to expand on existing findings using long-term follow-up data with detailed information on clinical and functional variables, taking both morbidity and mortality into account.
The main objective of our study was therefore to assess the long-term (8–12 years) impact of HRQoL on morbidity (first cardiovascular and all-cause hospitalisation) and on cardiovascular and all-cause mortality in patients hospitalised with CHF.
Methods
Study population and procedures
The optimising congestive heart failure outpatient clinic project (OPTIMAL; ClinicalTrials.gov identifier: NCT01671995) prospectively followed 208 consecutive patients hospitalised with CHF at Danderyd University Hospital in Stockholm during 1996–1999. Patients were eligible if aged 60 years and above and with left ventricular systolic dysfunction (left ventricular ejection fraction (LVEF) ≤45%, by echocardiography) and New York Heart Association (NYHA) class II–IV.13 The primary aim of OPTIMAL was to analyse the impact of a nurse-monitored management programme on HRQoL. The study was approved by the institutional review board and patients gave their informed consent to participate in the study (ethics references: original study 95–207; long-term follow-up 2010/23–32; complementary causes of death information 4.2.1-17990/2010).
The OPTIMAL study methods and echocardiographic procedures have been described in detail previously.13,14 In brief, collected data covered patient characteristics (clinical function, functional capacity, neurohormonal activation indices and echocardiographic variables) at baseline, as well as investigations, medications and health outcomes (hospitalisations, outpatient visits and death) during the scheduled follow-up period (minimum 18 months, mean 37 months). The Nottingham Health Profile (NHP) was used to measure HRQoL prior to or soon after discharge (i.e. not in the acute phase) and at 6, 12 and 18 months of follow-up. The NHP is a generic HRQoL instrument that covers physical mobility, pain, sleep, emotional reactions, social isolation and energy. Responses within each section are weighted and added up, with a maximum score of 100 per dimension indicating worst HRQoL.15 Reliability and validity of the Swedish version have been confirmed.16
For the long-term follow-up, data from medical records at Danderyd University Hospital and the Stockholm County Council administrative care database were used for health care consumption, including hospital admissions, up to 31 December 2007. For date and cause of death, data from the Swedish Cause of Death Registry were used over the same time period.
Statistical methods
Results are reported as mean values and standard deviations, and hazard ratios with 95% confidence intervals. For group comparisons, we used Student’s t-test for continuous, normally distributed variables, the Chi square test for categorical variables and the Wilcoxon rank-sum test for ordinal and non-normally distributed variables. A probability (p) <0.05 was considered statistically significant. Analyses were performed in Stata/SE 10.0.
As no single summary index has been defined for the NHP by the instrument developers,17,18 the six dimensions needed to be taken into account separately. As some of them can be expected to be strongly correlated, we used exploratory factor analysis to identify the most important NHP dimensions within correlated groups of dimensions. Factor analysis is commonly used in the development and validation of HRQoL scales and has also been applied in the context of e.g. biomarkers and other risk factors. The identified factors were then included as covariates in the survival models. More details on the factor analysis methods can be found in the Supplementary materials.
Kaplan–Meier survival curves were derived to assess the probability of survival and hospitalisation during the follow-up period. Groups were compared using the log-rank test to ascertain the relationship between HRQoL scores and outcomes. Censoring could occur at end of study (31 December 2007), death or loss to follow-up. The outcomes of interest were all-cause and cardiovascular mortality and all-cause and cardiovascular hospitalisations. The cause of death was defined as stated in the death certificates. Cardiovascular hospitalisations were identified using the Diagnosis Related Groups (DRG) system, where hospitalisations with a DRG within the Major Diagnostic Category 5 were classified as being cardiac. For time to hospitalisation, the time to first admission after initially bridged hospitalisations was used to exclude readmissions that were part of consecutive stays in different clinics or hospitals.
For the outcomes of interest, each potential predictor was examined individually using univariate Cox proportional hazard models. Brain natriuretic peptide (BNP), left ventricular end-diastolic volume and LVEF were log-transformed. We checked for correlation between potential explanatory variables and excluded those with strong correlations (Pearson’s r ≥ 0.6). Predictors were selected for testing based on expected clinical relevance and findings from previous studies and included: age, gender, NYHA class, diabetes, ischaemic heart disease, atrial fibrillation, beta-blocker use, achieved target dose of angiotensin converting enzyme inhibitors, furosemide dose, haemoglobin, estimated glomerular filtration rate (eGFR), BNP, systolic blood pressure, left ventricular end-diastolic volume, LVEF, atrioventricular plane displacement and NHP dimensions at baseline selected through factor analysis.
Variables that were statistically significant at the 0.1 level in the univariate analyses were included in the subsequent multivariate survival models; age and gender were retained regardless of statistical significance. Since some studies9,19 have used summary scores for the NHP, we also performed sensitivity analyses using the total score instead of individual dimensions for the final models.
We tested the proportional hazards assumption using Schoenfeld residuals. If results suggested non-proportional hazards for a variable, an interaction term with time was created and the original and expanded models were compared using the likelihood ratio test to assess its significance.
Results
Sample description
Baseline characteristics are presented in Table 1. Detailed echocardiographic results have been reported elsewhere.14 In addition to the medications listed, aldosterone antagonists and angiotensin receptor blockers were used in a small number of patients. Complete NHP at baseline was available for 177 of the 208 patients. When comparing those who completed the NHP questionnaire to those who did not, only eGFR differed between the two groups. Those who completed the NHP had an eGFR of 53.5 ml/min versus 41.7 ml/min for those with missing information (p = 0.003).
Table 1.
Baseline patient characteristics for patients with HRQoL information.
| Variable | n | |
|---|---|---|
| Age (years) | 75.5 ± 7.2 | 177 |
| Male | 56% | 177 |
| New York Heart Association class I/II/III/IV | 0/112/63/2 | 177 |
| Medical history: | ||
| Ischaemic heart disease | 69% | 175 |
| Atrial fibrillation | 49% | 177 |
| Hypertension | 32% | 173 |
| Diabetes | 22% | 175 |
| Medications: | ||
| Beta-blocker | 50% | 177 |
| Angiotensin converting enzyme (ACE) inhibitor | 74% | 177 |
| Furosemide | 94% | 177 |
| Furosemide dose (mg/day) | 109 ± 198 | 177 |
| Clinical parameters: | ||
| Body mass index (kg/m2) | 26 ± 5 | 169 |
| Heart rate (bpm) | 86 ± 21 | 163 |
| Haemoglobin (g/l) | 132 ± 15 | 171 |
| Estimated glomerular filtration rate (ml/min) | 53 ± 20 | 173 |
| Systolic blood pressure (mm Hg) | 135 ± 25 | 176 |
| Diastolic blood pressure (mm Hg) | 80 ± 14 | 176 |
| Brain natriuretic peptide (ng/l) | 220 [96; 436] | 161 |
| Echocardiographic measures of systolic function: | ||
| Left ventricular ejection fraction (%) | 34 ± 11 | 171 |
| Left ventricular end-diastolic volume (ml) | 124 ± 58 | 171 |
| Left ventricular end-systolic volume (ml) | 85 ± 49 | 171 |
| Atrioventricular plane displacement (mm) | 6.6 ± 2.0 | 174 |
| HRQoL: | ||
| Emotional reactions | 18.4 ± 22.6 | 177 |
| Sleep | 32.8 ± 31.9 | 177 |
| Energy | 52.1 ± 39.1 | 177 |
| Pain | 14.0 ± 20.2 | 177 |
| Physical mobility | 28.4 ± 25.9 | 177 |
| Social isolation | 10.1 ± 18.0 | 177 |
HRQoL, health-related quality of life, where a score of 100 denotes the worst situation.
Data for 177 patients presented as mean values ± standard deviation (SD), median (interquartile range) or proportion, as appropriate; n denotes number of available observations.
Factor analysis
We extracted three factors, which were mostly determined by emotional reactions, physical mobility and sleep. There was a strong correlation between emotional reactions and social isolation (r = 0.67), physical mobility and energy (r = 0.63) as well as physical mobility and pain (r = 0.61). As can be seen from the factor loadings in supplementary Table S1, physical mobility and emotional reactions contribute most information to the model.
Mortality
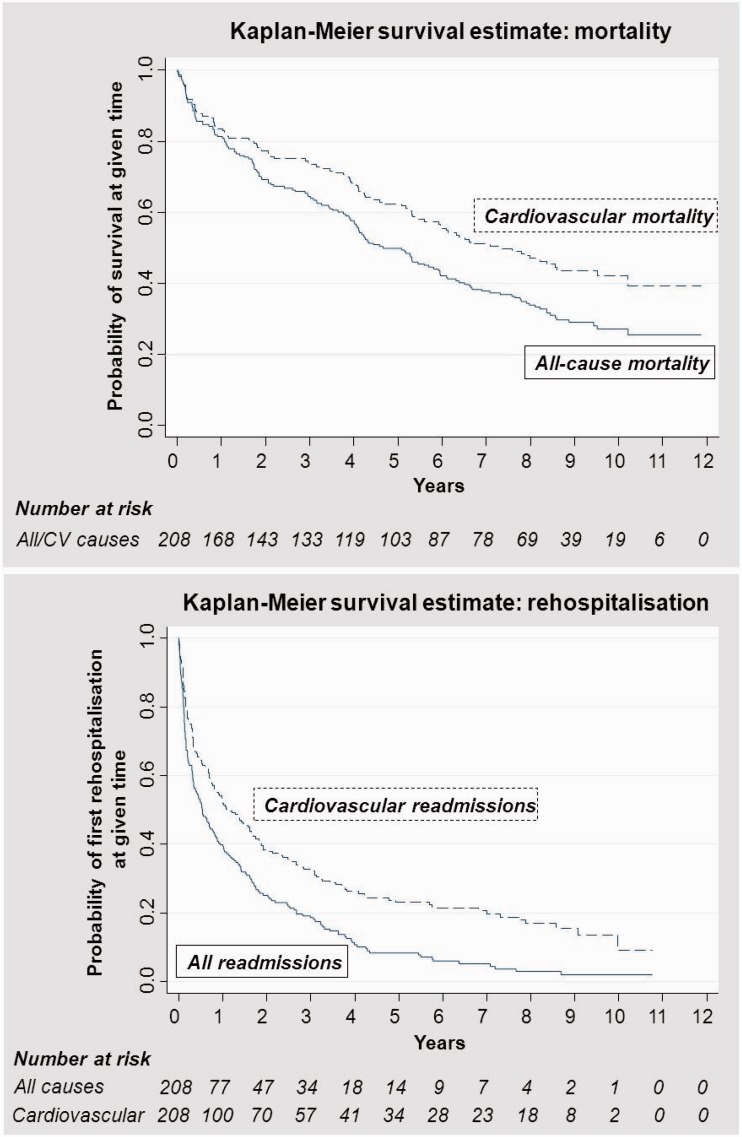
During the 12-year follow-up period, 148 patients (71%) died, of which 101 deaths (68%) were due to cardiovascular causes. Two patients moved from the Stockholm area and were lost to follow-up. Median survival was 4.6 years (range 6 days–11.9 years) and mean survival 5.1 years. Figure 1 shows the Kaplan–Meier curves for all-cause and cardiovascular mortality over time.
Figure 1.
Kaplan–Meier curves for mortality and hospitalisations (all-cause and cardiovascular) over 12-year follow-up.
The results of the multivariate regression models for all-cause and cardiovascular mortality are presented in Table 2. All-cause and cardiovascular mortality were determined by physical mobility, age, gender, diuretic dose and haemoglobin level. The results show that for every 1-point increase on the NHP dimension of physical mobility (corresponding to 1%), the risk of dying increases by 1.8% (p < 0.001) for all causes and 1.2% (p = 0.008) for cardiovascular causes.
Table 2.
Final model specifications for predictors of mortality and rehospitalisation (all-cause and cardiovascular).
| Covariate | Hazard ratio | Lower 95% CI | Upper 95% CI | p |
|---|---|---|---|---|
| All-cause mortality (n = 167) | ||||
| Age (years) | 1.050 | 1.011 | 1.090 | 0.011 |
| Female gender | 0.403 | 0.270 | 0.602 | 0.000 |
| Diuretic dose (mg/day) | 1.001 | 1.000 | 1.001 | 0.077 |
| Haemoglobin (g/l) | 0.984 | 0.971 | 0.997 | 0.020 |
| Estimated glomerular filtration rate (ml/min) | 0.975 | 0.962 | 0.989 | 0.000 |
| Physical mobility | 1.018 | 1.010 | 1.025 | 0.000 |
| Model statistics: Log likelihood = −495.09, Chi squared = 85.62 (p < 0.001) | ||||
| Cardiovascular mortality (n = 168) | ||||
| Age (years) | 1.074 | 1.032 | 1.117 | 0.000 |
| Female gender | 0.476 | 0.296 | 0.766 | 0.002 |
| Diuretic dose (mg/day) | 1.001 | 1.000 | 1.002 | 0.035 |
| Haemoglobin (g/l) | 0.980 | 0.964 | 0.997 | 0.022 |
| Atrioventricular plane displacement (mm) | 0.726 | 0.600 | 0.880 | 0.001 |
| t_AVPDa | 1.047 | 1.004 | 1.091 | 0.033 |
| Physical mobility | 1.012 | 1.003 | 1.021 | 0.008 |
| Model statistics: Log likelihood = −353.44, Chi squared = 48.90 (p < 0.001) | ||||
| All-cause rehospitalisation (n = 171) | ||||
| Age (years) | 1.027 | 1.000 | 1.054 | 0.053 |
| Female gender | 0.655 | 0.457 | 0.940 | 0.022 |
| Haemoglobin (g/l)b | 0.990 | 0.978 | 1.003 | 0.124 |
| Emotional reaction | 1.013 | 1.005 | 1.021 | 0.001 |
| Physical mobility | 1.012 | 1.005 | 1.018 | 0.000 |
| Model statistics: Log likelihood = −639.10, Chi squared = 40.28 (p < 0.001) | ||||
| Cardiovascular rehospitalisation (n = 177) | ||||
| Age (years) | 1.016 | 0.990 | 1.043 | 0.222 |
| Female gender | 0.831 | 0.578 | 1.196 | 0.320 |
| Diuretic dose (mg/day) | 1.001 | 1.000 | 1.002 | 0.046 |
| Physical mobility | 1.007 | 1.000 | 1.013 | 0.041 |
| Model statistics: Log likelihood = −604.10, Chi squared = 9.15 (p < 0.06) | ||||
CI: confidence interval.
t_AVPD denotes the time-varying covariate of atrioventricular plane displacement, which interacts with current values of time.
Haemoglobin was significant in initial multivariate model; retained in final model as it interacts with gender.
In addition, eGFR was significant for all-cause mortality, while atrioventricular plane displacement was predictive of cardiovascular mortality. Since atrioventricular plane displacement displayed a non-proportional hazard, a time-varying covariate was included in the final model, which only had a very small impact on the hazard ratios.
Using the total NHP score instead of physical mobility in the final models provided similar results in terms of significant variables and coefficients. The impact of the total score on the hazard ratio was lower at 0.4% for all-cause mortality (p < 0.001) and 0.3% for cardiovascular mortality (p = 0.003). When interpreting these results, it needs to be kept in mind that the total NHP score can range up to 600 as opposed to 100 for the individual domains.
Hospitalisations
During the follow-up period, 188 patients (90%) were hospitalised at some point, 156 (75%) due to cardiovascular causes and 153 (74%) due to non-cardiovascular causes. The median time to first all-cause hospitalisation was 5.7 months (range 1 day–10.8 years) and to first cardiovascular hospitalisation 11.2 months (range 4 days–10.8 years). Figure 1 shows the Kaplan–Meier curves for first all-cause and cardiovascular hospitalisations over time.
The results of the multivariate regression models for all-cause and cardiovascular hospitalisation are presented in Table 2. Time to first all-cause hospitalisation was determined by physical mobility, emotional reactions, age, gender and haemoglobin level. In contrast, only physical mobility and diuretic dose predicted time to first cardiovascular hospitalisation.
The results show that for every 1-point increase on the NHP dimension of physical mobility, the risk of being hospitalised for any cause increases by 1.2% (p < 0.001), while the risk increases by 1.3% (p = 0.001) for every 1-point increase on the dimension of emotional reactions. For cardiovascular hospitalisations, the risk increase is smaller at 0.7% (p = 0.041) for every 1-point increase on the physical mobility scale.
Using the total NHP score instead of individual domains in the final models, only gender and the total NHP score were significant for all-cause hospitalisations, with a lower impact of the NHP score on the hazard ratio at 0.5% (p < 0.001). For cardiovascular hospitalisations, the significant variables and coefficients were similar using the total NHP score, except for a lower impact of the NHP score on the hazard ratio at 0.1% (p = 0.042).
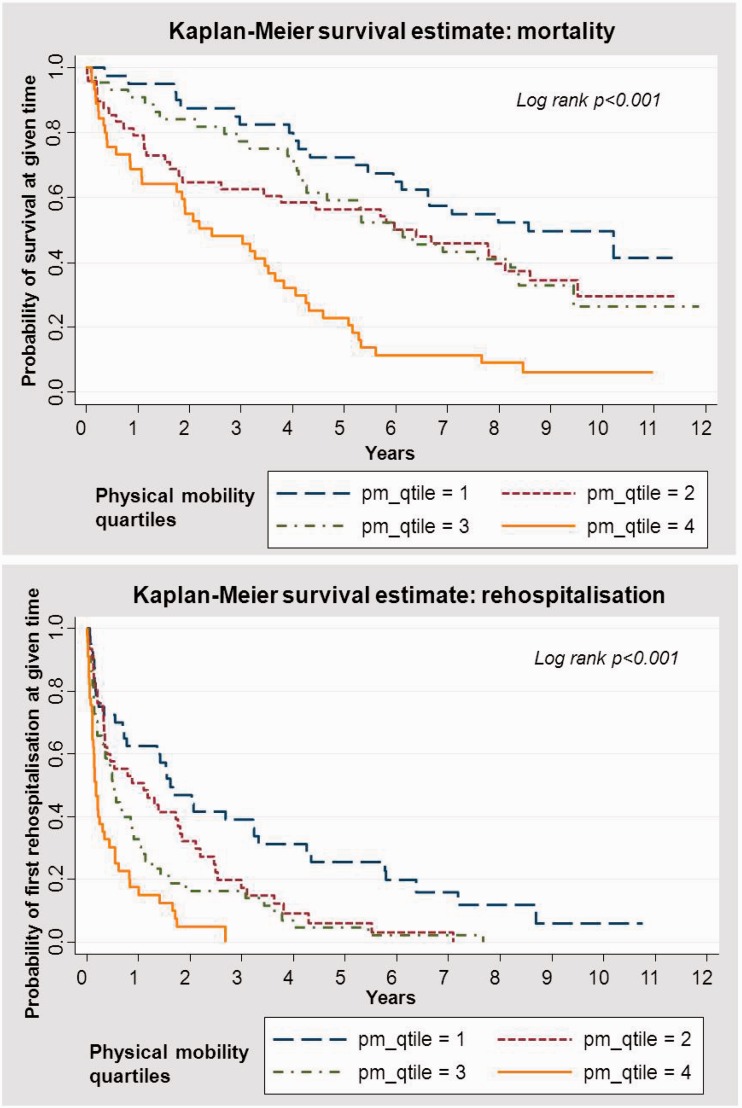
The relationship between impaired HRQoL and increased risk of mortality and morbidity was also clearly seen in the survival curves by different strata of physical mobility scores (Figure 2).
Figure 2.
Kaplan–Meier curves for all-cause mortality and hospitalisations by quartiles of NHP physical mobility score.
Discussion
In this study on systolic CHF in patients aged 60 years or older, we found that HRQoL measured during hospitalisation is an independent predictor of cardiovascular and all-cause morbidity and mortality during a follow-up of 8–2 years. Specifically, physical mobility was the most informative component of the generic HRQoL instrument, with each 1% worsening (increase) on the physical mobility dimension leading to a 1–2% increase in the hazard ratio of being hospitalised or dying. For all-cause hospitalisations, emotional reactions were an additional independent predictor, with a similar impact as physical mobility. The impact of physical mobility was lowest for cardiovascular hospitalisations, albeit still statistically significant.
Factor analysis showed that physical mobility and emotional reactions played the strongest role in determining HRQoL measured with the generic NHP instrument at baseline. This was confirmed when analysing data from the 1-year follow-up visit in outpatient care in OPTIMAL (data not shown). This has important clinical implications and suggests that HRQoL provides similar and useful prognostic information both when assessed in conjunction with heart failure hospitalisations and during outpatient visits.
To our knowledge, this study is the first to analyse the impact of HRQoL on hospitalisations in addition to mortality in patients with CHF over a time frame of up to 12 years. It thus expands on previous findings that HRQoL and patient perception complements demographic and clinical measures in CHF prognosis.3,4,6–8,10–12,20 In a recent analysis focusing on health care consumption and costs in the OPTIMAL study,19 HRQoL was found to be the only independent predictor of the number of cardiac and non-cardiac hospital readmissions.
In addition to HRQoL, we found that age, gender, renal function, haemoglobin, drug treatment and echocardiographic measures of left ventricular systolic function were independent predictors of prognosis. While NYHA class was correlated with mortality (all-cause and cardiovascular causes) in univariate analyses, there was no significant association in the multivariate models. Our results are in agreement with earlier observations.3,6–8,10–12,20 Moreover, they extend our findings in an earlier analysis of OPTIMAL over a 3-year follow-up.9
A recent 6-year follow-up study in 1151 outpatients referred to a heart failure clinic in Spain investigated temporal changes in Minnesota Living With Heart Failure (MLWHF) scores and the impact of HRQoL on mortality, which was of a similar magnitude as in our study (increased hazard of 1.2% for each 1% worsening in MLWHF score).8 This could suggest that the impact on mortality is similar for disease-specific and generic HRQoL instruments.
In earlier heart failure studies, depression has been found to be a prognostic marker for hospitalisations over 1 year21 and for mortality over a period of up to 3 years.21,22 We found the dimension of emotional reactions, which focuses on mental health and is partly related to depression, to be predictive only of time to all-cause hospitalisation.
Our study benefits from a very well characterised study population with detailed information on clinical data, neurohormonal activation and echocardiographic measures. The combination with a very long follow-up provides new insights into long-term prognosis, specifically regarding rehospitalisations. The high quality Swedish national registry system also provided complete information on outcomes in terms of morbidity and mortality. Moreover, we were able to analyse the prognostic impact of specific HRQoL dimensions derived through factor analysis on hospitalisations and mortality, allowing more targeted risk stratification of hospitalised CHF patients.
There are several limitations to our study. Firstly, our sample is relatively small. Secondly, there were missing data for some variables, mostly concerning the NHP, BNP and systolic blood pressure. However, when testing for differences between those with complete information from the NHP and those without, only renal function was found to be different (slightly better) for those with complete information at baseline. Moreover, there was no significant difference in survival or time to rehospitalisation between those with and without complete NHP information. Therefore, this should have no major impact on the conclusions of our study.
Thirdly, our results are intrinsically linked to the patient population studied, and may not be generalised to other populations or settings, e.g. patients with heart failure with preserved left ventricular function, of younger age, or with NYHA class I. Nevertheless, our results complement and confirm findings in e.g. patients with less severe disease treated in primary care.7 It is also inherent to long-term follow-up studies that treatment may no longer correspond to current treatment practice. However, patients in our study received optimised medical therapy during the active part of the study.13
Finally, we used a generic HRQoL instrument that was common at the time of study start; nowadays the generic ShortForm-36 instrument or disease-specific Kansas City Cardiomyopathy Questionnaire are more frequently used. Floor effects have been observed with the NHP; however, these can be expected to pose less of a problem in a relatively severe disease such as CHF.23 Earlier analyses have shown the NHP to be a potentially useful prognostic indicator of survival in patients undergoing heart transplants.18 Overall, our results using the NHP are similar to those obtained with other generic and disease-specific HRQoL instruments.
Our results confirm the independent prognostic value of HRQoL for risk prediction of patients hospitalised for CHF, and for health care consumption (including number of days spent in hospital) and costs.19 Figure 2 suggests that the prognostic information may mostly apply to around the first 5 years of follow-up. We propose that HRQoL should routinely be included in the evaluation of patients hospitalised with CHF to improve risk prediction and to optimise preventive measures and treatment, e.g. through physical activity or rehabilitation. This could occur both at discharge and as part of subsequent follow-up in heart failure clinics to identify patients at high risk of deterioration and rehospitalisation.
Our finding that physical mobility as part of HRQoL predicts prognosis may be explained by a link between oxygen uptake, exercise capacity and mortality in CHF.24 This warrants further research. It would also be of interest to investigate the prognostic impact of interventions to improve HRQoL.
Conclusion
HRQoL measured during hospitalisation of elderly patients with systolic CHF is an independent predictor of cardiovascular and all-cause morbidity and mortality over 12 years. Physical mobility and emotional reactions played the strongest role in determining HRQoL, with physical mobility being the most informative component in terms of prognosis. Our findings support the use of HRQoL to identify hospitalised CHF patients in need of additional treatment and support to reduce future risk and health care consumption.
Acknowledgements
The authors would like to thank Jonas Nilsson, PhD, for advice regarding factor analysis methods and interpretation.
Competing interests
None
Funding
The initial OPTIMAL study was supported by the Vårdal Foundation, the Swedish Heart Lung Foundation, the Swedish Society of Medicine, Karolinska Institutet and the Regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet.
Ethical approval
Original study 95–207; long-term follow-up 2010/23–32; complementary causes of death information 4.2.1-17990/2010.
Guarantor
Jenny Berg is the guarantor for this paper.
Contributorship
JB participated in the study design, performed the statistical analyses and drafted the manuscript. PL participated in the study design, provided statistical input and helped to draft the manuscript. OS obtained the administrative and medical follow-up data. HP and ME conceived the original OPTIMAL study and gave critical input to the manuscript. TK and MM conceived the original OPTIMAL study, participated in the current study design and helped to draft the manuscript.
References
- 1.Jhund PS, Macintyre K, Simpson CR, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: A population study of 5.1 million people. Circulation 2009; 119: 515–523. [DOI] [PubMed] [Google Scholar]
- 2.Liao L, Allen LA, Whellan DJ. Economic burden of heart failure in the elderly. Pharmacoeconomics 2008; 26: 447–462. [DOI] [PubMed] [Google Scholar]
- 3.Faller H, Stork S, Schowalter M, et al. Is health-related quality of life an independent predictor of survival in patients with chronic heart failure? J Psychosom Res 2007; 63: 533–538. [DOI] [PubMed] [Google Scholar]
- 4.Hoekstra T, Jaarsma T, van Veldhuisen DJ, et al. Quality of life and survival in patients with heart failure. Eur J Heart Fail 2013; 15: 94–102. [DOI] [PubMed] [Google Scholar]
- 5.Huynh BC, Rovner A, Rich MW. Long-term survival in elderly patients hospitalized for heart failure: 14-year follow-up from a prospective randomized trial. Arch Intern Med 2006; 166: 1892–1898. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal J, Francis L, Reid J, et al. Quality of life in patients with chronic heart failure and their carers: A 3-year follow-up study assessing hospitalization and mortality. Eur J Heart Fail 2010; 12: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 7.Johansson P, Brostrom A, Dahlstrom U, et al. Global perceived health and ten-year cardiovascular mortality in elderly primary care patients with possible heart failure. Eur J Heart Fail 2008; 10: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 8.Lupon J, Gastelurrutia P, de Antonio M, et al. Quality of life monitoring in ambulatory heart failure patients: Temporal changes and prognostic value. Eur J Heart Fail 2013; 15: 103–109. [DOI] [PubMed] [Google Scholar]
- 9.Mejhert M, Kahan T, Persson H, et al. Predicting readmissions and cardiovascular events in heart failure patients. Int J Cardiol 2006; 109: 108–113. [DOI] [PubMed] [Google Scholar]
- 10.Stull DE, Clough LA, Van DD. Self-report quality of life as a predictor of hospitalization for patients with LV dysfunction: A life course approach. Res Nurs Health 2001; 24: 460–469. [DOI] [PubMed] [Google Scholar]
- 11.Subramanian U, Eckert G, Yeung A, et al. A single health status question had important prognostic value among outpatients with chronic heart failure. J Clin Epidemiol 2007; 60: 803–811. [DOI] [PubMed] [Google Scholar]
- 12.Zuluaga MC, Guallar-Castillon P, Lopez-Garcia E, et al. Generic and disease-specific quality of life as a predictor of long-term mortality in heart failure. Eur J Heart Fail 2010; 12: 1372–1378. [DOI] [PubMed] [Google Scholar]
- 13.Mejhert M, Kahan T, Persson H, et al. Limited long term effects of a management programme for heart failure. Heart 2004; 90: 1010–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mejhert M, Kahan T, Edner M, et al. Sex differences in systolic heart failure in the elderly: The prognostic importance of left ventricular mass in women. J Womens Health (Larchmt) 2008; 17: 373–381. [DOI] [PubMed] [Google Scholar]
- 15.Hunt SM, McKenna SP, McEwen J, et al. The Nottingham Health Profile: Subjective health status and medical consultations. Soc Sci Med A 1981; 15: 221–229. [DOI] [PubMed] [Google Scholar]
- 16.Wiklund I, Romanus B, Hunt SM. Self-assessed disability in patients with arthrosis of the hip joint. Reliability of the Swedish version of the Nottingham Health Profile. Int Disabil Stud 1988; 10: 159–163. [DOI] [PubMed] [Google Scholar]
- 17.Fayers PM, Machin D. Quality of life. The assessment, analysis and interpretation of patient-reported outcomes, Chichester: John Wiley & Sons Ltd., 2007. [Google Scholar]
- 18.O’Brien BJ, Buxton MJ, Ferguson BA. Measuring the effectiveness of heart transplant programmes: Quality of life data and their relationship to survival analysis. J Chronic Dis 1987; 40: 137S–158S. [DOI] [PubMed] [Google Scholar]
- 19.Mejhert M, Lindgren P, Schill O, et al. Long term health care consumption and cost expenditure in systolic heart failure. Eur J Intern Med 2012; 24: 260–265. [DOI] [PubMed] [Google Scholar]
- 20.O’Loughlin C, Murphy NF, Conlon C, et al. Quality of life predicts outcome in a heart failure disease management program. Int J Cardiol 2010; 139: 60–67. [DOI] [PubMed] [Google Scholar]
- 21.Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161: 1849–1856. [DOI] [PubMed] [Google Scholar]
- 22.Junger J, Schellberg D, Muller-Tasch T, et al. Depression increasingly predicts mortality in the course of congestive heart failure. Eur J Heart Fail 2005; 7: 261–267. [DOI] [PubMed] [Google Scholar]
- 23.Coons SJ, Rao S, Keininger DL, et al. A comparative review of generic quality-of-life instruments. Pharmacoeconomics 2000; 17: 13–35. [DOI] [PubMed] [Google Scholar]
- 24.Mejhert M, Linder-Klingsell E, Edner M, et al. Ventilatory variables are strong prognostic markers in elderly patients with heart failure. Heart 2002; 88: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]