Abstract
Human amnion/chorion tissue derived from the placenta is rich in cytokines and growth factors known to promote wound healing; however, preservation of the biological activities of therapeutic allografts during processing remains a challenge. In this study, PURION® (MiMedx, Marietta, GA) processed dehydrated human amnion/chorion tissue allografts (dHACM, EpiFix®, MiMedx) were evaluated for the presence of growth factors, interleukins (ILs) and tissue inhibitors of metalloproteinases (TIMPs). Enzyme‐linked immunosorbent assays (ELISA) were performed on samples of dHACM and showed quantifiable levels of the following growth factors: platelet‐derived growth factor‐AA (PDGF‐AA), PDGF‐BB, transforming growth factor α (TGFα), TGFβ1, basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), placental growth factor (PLGF) and granulocyte colony‐stimulating factor (GCSF). The ELISA assays also confirmed the presence of IL‐4, 6, 8 and 10, and TIMP 1, 2 and 4. Moreover, the relative elution of growth factors into saline from the allograft ranged from 4% to 62%, indicating that there are bound and unbound fractions of these compounds within the allograft. dHACM retained biological activities that cause human dermal fibroblast proliferation and migration of human mesenchymal stem cells (MSCs) in vitro. An in vivo mouse model showed that dHACM when tested in a skin flap model caused mesenchymal progenitor cell recruitment to the site of implantation. The results from both the in vitro and in vivo experiments clearly established that dHACM contains one or more soluble factors capable of stimulating MSC migration and recruitment. In summary, PURION® processed dHACM retains its biological activities related to wound healing, including the potential to positively affect four distinct and pivotal physiological processes intimately involved in wound healing: cell proliferation, inflammation, metalloproteinase activity and recruitment of progenitor cells. This suggests a paracrine mechanism of action for dHACM when used for wound healing applications.
Keywords: Amnion, Chorion, Chronic ulcers, Dermal fibroblasts, dHACM, Growth factors, Mesenchymal stem cells, Wound healing
Introduction
The molecular mechanisms responsible for chronic wound formation remain poorly understood, partly because wound healing involves a well‐coordinated sequence of complex and interrelated physiological processes, including inflammation, angiogenesis, cell‐mediated regeneration of the vasculature and extracellular matrix, and epithelialization. The failure to heal of chronic wounds could be the result of deficiencies in one or any combination of these processes. This is exemplified by the pathological conditions in which chronic wounds develop, such as diabetic neuropathic ulcers, chronic venous insufficiencies, pressure ulcers and arterial disease. Targeting therapies for a single process within this sequence of events, such as a single growth factor that affects one aspect of angiogenesis or is limited to promoting extracellular matrix production, is unlikely to achieve full tissue regeneration and wound closure. A multifaceted therapy that addresses multiple defective physiological processes and revitalises the cascade of normal reparative mechanisms is clearly needed.
The therapeutic potential of human amnion/chorion tissue grafts in wound healing has been well established 1, 2, 3, 4. Early use of fresh amniotic membrane containing both amnion and chorion has proved beneficial in treating ulcers, burns and dermal injuries 4. In the last 50 years, amnion/chorion tissue allografts have also been used successfully for chronic neuropathic wounds, corneal surface wounds as well as pterygium and conjuntivochalasis, dental applications, and for orthopaedic, general and neurosurgical applications.
Human amnion is known to be non‐immunogenic, to reduce inflammation, pain and scarring and provide a matrix for cell colonisation as well as a natural biological barrier. Given this functionality, it is not surprising that human amnion allografts containing amnion and chorion (HACM) have also been used therapeutically for cutaneous wounds, and appear effective at accelerating wound healing 5, 6, 7, 8, 9. While the basis for this therapeutic effect has not been fully elucidated, native human amnion/chorion membrane contains an array of growth factors, including epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), keratinocyte growth factor, transforming growth factor α (TGFα) and TGFβ, nerve growth factor and hepatocyte growth factor 10, 11, that are known to play critical roles in the physiological processes leading to normal wound healing and tissue regeneration.
Difficulties inherent to supplying a safe and effective amnion allograft, particularly those related to blood borne pathogens, stability during storage and off the shelf availability, have led to efforts towards preserving amnion allograft tissue while at the same time retaining its biological activities and clinical effectiveness. While many methods for processing human allografts have been developed, most are directed to completely removing the cells and DNA, soluble macromolecules and antigenic and immunogenic macromolecules. These processes remove the biologically active components leaving only an extracellular scaffold. Recently, a gentle cleansing and dehydration process was developed to preserve and maintain the biological activities inherent in the native amnion (PURION® process; 12–14). As shown below, this process retains the natural growth factors and regulatory molecules contained in the amnion and chorion. However, the mere presence of these factors does not mean they are biologically active.
The aim of this study was to determine whether PURION® processed and dehydrated human amnion/chorion allografts (dHACM) preserve biological activities that are therapeutically relevant to wound healing. Growth factors and selected regulatory proteins were identified and measured in this advanced wound care product (EpiFix®, MiMedx). The ability of dHACM to regulate cellular functions was evaluated in cell cultures using human cells known to participate in wound healing. An in vivo mouse model was employed to test whether dHACM can influence regenerative cells to migrate into the wound bed. The overall results establish that dHACM is biologically active and contains factors that are important in regulating wound healing and tissue regeneration.
Methods
Dehydrated human amnion/chorion allografts
dHACM is a dehydrated human allograft comprising amnion and chorion 12, 13, 14. Amnion and chorion from placenta donated following scheduled Caesarean sections are processed with a proprietary PURION® process that involves gentle cleansing of the layers. The amnion and chorion are laminated to form the graft and then the graft is dehydrated under controlled drying conditions 14. EpiFix® (MiMedx) was used as the dHACM in this study. The results of this study apply only to dehydrated human amnion/chorion composite grafts.
ELISA assays
Growth factors, interleukins (ILs) and tissue inhibitors of metalloproteinases (TIMPs) were measured in samples of processed, dehydrated human amnion/chorion grafts (dHACM) with standard enzyme‐linked immunosorbent assays (ELISA plates obtained from RayBiotech, Norcross, GA). Weighed, minced samples were placed in lysis buffer containing protease inhibitors for 24 hours at 4°C. Tissues were then homogenised, centrifuged to remove tissue residue, and the amount of each factor in the lysis buffer was measured in diluted aliquots with standard ELISA assays.
In vitro proliferation studies on human dermal fibroblasts: proliferation assays
Cell culture: adult human dermal fibroblasts (HDFa, Life Technologies Corp., Grand Island, NY) were plated on 96‐well plates for 24 hours in Dulbecco's modified Eagle's medium (DMEM) containing 10% calf serum (GIBCO, Life Technologies Corp.). After 24 hours, the medium was aspirated from the wells and replaced with one of the following: DMEM lacking serum (control), DMEM plus 10% calf serum (positive control) or DMEM containing extracts of dHACM at 20, 10, 2 and 0·2 mg/ml. After 72 hours, the plate was washed to remove unattached cells and a CyQuant assay (Molecular Probes CyQuant, Life Technologies C7026) was performed to quantify the number of cells.
To obtain dHACM extracts, sterilised dHACM grafts containing amnion and chorion with an intact epithelial cell layer were minced and extracted at 4°C in DMEM without 10% calf serum, at a concentration of 20 mg of tissue per millilitre of medium. After 24 hours of extraction at 4°C, the tissue was removed by centrifugation and the extract was sterile filtered.
In vitro trans‐well migration studies with human mesenchymal stem cells
Human bone marrow mesenchymal stem cells (MSCs) were obtained from the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine (Temple, TX) at passage 1. Cells were expanded following recommended protocols, in growth medium containing α‐minimal essential medium (MEM) (Mediatech, Manassas, VA) with 16·3% foetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA), 1% antibiotic/antimycotic (Mediatech), and 2 mM l‐glutamine (Mediatech). Following expansion, cells were frozen at passage 2 in liquid nitrogen until further use.
Migration assays were performed in 24‐well transwell inserts with 8 µm pore membrane filters (Becton Dickinson, Franklin Lakes, NJ). Human MSCs were serum‐starved in serum‐free medium for 24 hours prior to the start of the experiment, and 200 µl of 5 µg/ml human fibronectin (Becton Dickinson) in phosphate‐buffered saline (PBS) were placed into each transwell insert and 700 µl fibronectin into each bottom well of the 24‐well plate to enable adsorption of fibronectin to the transwell surfaces overnight. On the day of the experiment, 700 µl of serum‐free culture medium was loaded into the bottom wells, followed by the addition of differently sized portions of dHACM tissue, including 1·5 and 4·0 mm diameter disks (n = 6 dHACM tissue donors tested). Serum‐free medium and medium containing 10% FBS (n = 6) acted as negative and positive controls, respectively. Next, 4·0 × 104 MSCs (passage 3) were loaded into the transwell inserts in 300 µl serum‐free medium and cultured for 24 hours to permit migration.
After 24 hours, both sides of the inserts were rinsed with PBS, and non‐migrating cells were removed with a cotton‐tipped applicator. Remaining cells were fixed in 10% neutral buffered formalin for 20 minutes, and stained with haematoxylin for 5 minutes prior to imaging in distilled water on an inverted phase‐contrast microscope (Nikon TE2000‐U, 10× objective, Tokyo, Japan; SPOT Software 4·6, Sterling Heights, MI). These migration experiments were repeated thrice using MSCs isolated from three separate human donors. Migrated cells were counted and averaged across four micrographs per insert. To mitigate differences in migration between MSC donors, data were also normalised to the 10% FBS positive control where appropriate.
In vivo mouse model of mesenchymal progenitor cell recruitment
All murine experiments were approved by the Stanford Administrative Panel on Laboratory Animal Care (APLAC), protocol #20627. dHACM products from six donors were used for implantation in normal mice. A 5 × 5 mm square of EpiFix® was surgically placed subcutaneously in 4‐month‐old wild‐type mice. Four mice were implanted per sample per time point. Negative controls were intact skin and sham‐operated sites (surgical incision but no implant). At 3, 7, 14 and 28 days, the implant and overlying skin was harvested for fluorescence‐activated cell sorting (FACS) analysis: Implants and overlying skin were harvested, minced and incubated in a collagenase solution at 37°C for 1 hour. After centrifugation, the samples were incubated with a lineage negative (lin−) antibody cocktail (Ter119/CD4/CD8a/Gr‐1/CD45R/CD11b) conjugated to PE‐Cy5. For mesenchymal progenitor cell analysis, conjugated antibodies were also added against CD45 [phycoerythrin (PE)] and Sca‐1 [fluoroscein isothiocyanate (FITC)]. Following staining, samples were washed by adding five volumes of 2% FBS in PBS. Cells were centrifuged and then resuspended in propidium iodide for 1 minute at 4°C. Samples were analysed using an LSR Flow Cytometer (Becton Dickinson). Using CellQuest software, samples were gated for viability in addition to lin−/Sca‐1 + /CD45− to define mesenchymal progenitor cells.
Statistical analyses
All values were reported as mean ± standard deviation. For the trans‐well migration assay, statistical comparisons were performed by first using a Box‐Cox transformation to normalise data variance 15. Statistical outliers were determined by fitting the normalised data to a general linear model, and removing data points outside of a 95% confidence interval of a normal probability plot. For both the trans‐well migration assay and the FACS analysis, a one‐ or two‐factor analysis of variance (ANOVA) was used to determine statistical significance of groups, and Tukey's post‐hoc multiple comparison test with significance set at P ≤ 0·05 indicated significance between individual samples. All statistical analyses were carried out using Minitab (v15·1, State College, PA).
Results
Contents of biological factors
ELISA assays were performed on samples of dHACM and showed quantifiable levels of the following growth factors: platelet‐derived growth factor‐AA (PDGF‐AA), PDGF‐BB, TGFα, TGFβ1, bFGF, EGF, PLGF and granulocyte colony‐stimulating factor (GCSF) (Table 1). ELISA assays identified the presence of ILs 4, 6, 8 and 10, and TIMPs 1, 2 and 4 also.
Table 1.
The amount of growth factors, interleukins and TIMPs in dHACM
| Average (pg/mg) | SD | n | |
|---|---|---|---|
| Growth factors | |||
| bFGF | 1649·4 | 925·4 | 56 |
| EGF | 5·1 | 3·8 | 56 |
| GCSF | 54·4 | 30·3 | 6 |
| PDGF‐AA | 10327·2 | 7892·9 | 56 |
| PDGF‐BB | 46·5 | 21·6 | 55 |
| PLGF | 101·1 | 92·8 | 57 |
| TGFα | 1·3 | 0·7 | 45 |
| TGFβ1 | 431·6 | 251·6 | 55 |
| Interleukins | |||
| IL4 | 0·7 | 0·5 | 45 |
| IL6 | 22·3 | 17·9 | 6 |
| IL8 | 474·4 | 807·3 | 6 |
| IL10 | 1·7 | 0·8 | 45 |
| Tissue inhibitors of metalloproteinases | |||
| TIMP‐1 | 6356·8 | 3410·1 | 55 |
| TIMP‐2 | 1254·7 | 566·7 | 45 |
| TIMP‐4 | 36·2 | 24·7 | 45 |
bFGF, basic fibroblast growth factor; dHACM, dehydrated human amnion/chorion tissue allografts; EGF, epidermal growth factor; GCSF, granulocyte colony‐stimulating factor; PDGF‐AA, platelet‐derived growth factor‐AA; PLGF, placental growth factor; TGFα, transforming growth factor α; SD, standard deviation.
Elution of growth factors from dHACM
In order for the growth factors in dHACM to be delivered to the wound, they must elute from the tissue either through simple diffusion or tissue remodelling. To determine how much the growth factor elutes from the material, weighed samples of micronised dHACM were incubated in normal saline for 24 hours, the tissue was removed by centrifugation, and the amount of growth factor eluting into the saline was determined by ELISA (Figure 1). Micronised samples were used to ensure that all of the soluble growth factors eluted from the tissue. The content of the growth factors remaining in the tissue was measured with ELISAs as described above. The relative amount of growth factor eluting from the tissue was normalised to the total tissue growth factor content. All growth factors eluted from the tissue into the saline. The relative amount that eluted differed depending on the growth factor and ranged from 4% PDGF‐BB to 62% EGF.
Figure 1.
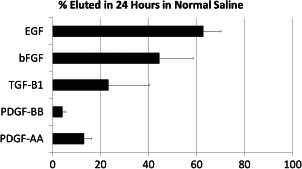
Relative amount of growth factors eluting from dehydrated human amnion/chorion tissue allografts (dHACM) after 24 hours in normal saline. Each bar represents the average ±standard deviation of five samples tested.
Human dermal fibroblast proliferation
The control HDFa cells proliferated to a minor extent, whereas inclusion of calf serum in the positive control wells caused the cells to double in number (Figure 2). Extracts of dHACM caused a dose‐dependent increase in fibroblast proliferation. The largest proliferative effect was observed at 20, 10, 5 and 2 mg/ml concentrations of the extract, where cell number increased three‐ to four‐fold. The effect was comparable at these concentrations. A dose‐dependent proliferative effect was observed at concentrations between 0·2 mg/ml and 0·025 mg/ml. At concentrations below 0·025 mg/ml there was no effect on proliferation. These results establish (i) that growth factors will elute from the tissue graft under physiological conditions and (ii) that dHACM contains biologically active factor/s that cause HDFa to proliferate.
Figure 2.
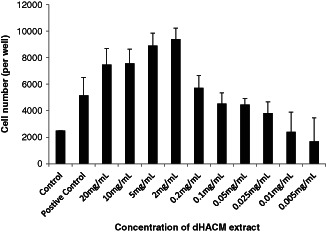
Effects of extracts of dehydrated human amnion/chorion tissue allografts (dHACM) on adult human dermal fibroblast proliferation. The control wells contained Dulbecco's modified Eagle's medium (DMEM) only. The positive control contained DMEM supplemented with calf serum and established the capacity of adult human dermal fibroblast (HDFa) cells to proliferate in response to active factors. dHACM extract concentration in the DMEM is shown on the x‐axis. Each value represents the average ± standard deviation of five wells.
Effects of dHACM on human MSC migration in vitro
MSC migration in the negative controls (serum‐free) and the smallest 1·5 mm samples was not significantly different for any MSC donor; however, samples containing 4·0 mm diameter disks of dHACM tissue demonstrated significantly greater migration compared with negative controls in all MSC donors (Figure 3). Additionally, MSC donors #1 and #2 experienced significantly greater migration in response to 4·0 mm EpiFix® disks than 1·5 mm samples. Donor #3, however, experienced a lesser degree of migration than the other donors in response to 4·0 mm dHACM samples, and there was no significant difference between 4·0 mm samples and 1·5 mm samples in donor #3. No experimental group reached the number of cells comparable to positive controls (10% FBS); however, the 4·0 mm diameter samples demonstrated that dHACM tissue in the culture medium was capable of directing moderate MSC migration in vitro.
Figure 3.
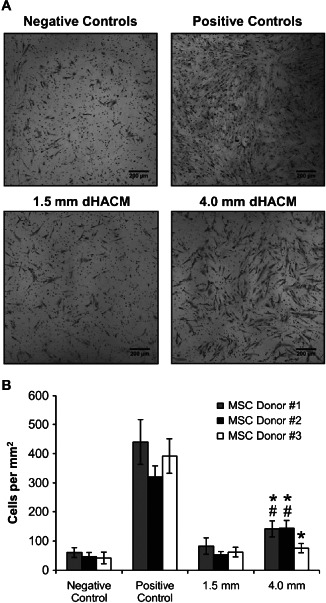
Cell density of migrated mesenchymal stem cells (MSCs) in response to dehydrated human amnion/chorion tissue allografts (dHACM), as well as negative and positive controls, for three MSC donors. (A) Representative micrographs and (B) cell density measurements indicated that greater migration was observed in response to larger samples, relative to their smaller counterparts. MSC migration in positive controls was significantly greater than all other samples (P≤0·05). * Indicates significantly greater migration than negative controls (serum‐free) in same MSC donor (P≤0·05). # Indicates significantly greater migration than 1·5 mm group in same MSC donor (P≤0·05).
Effects of dHACM on mesenchymal progenitor cell recruitment in vivo
FACS analysis was used to quantify mesenchymal progenitor cell recruitment into the implanted tissue graft (Figure 4). The number of progenitor cells associated with the dHACM implant was greater than those in normal skin and the sham implant site at all time points, with these differences becoming statistically significant at day 7. These results indicate that the dHACM implant is associated with a progenitor cell migration to the implant site.
Figure 4.
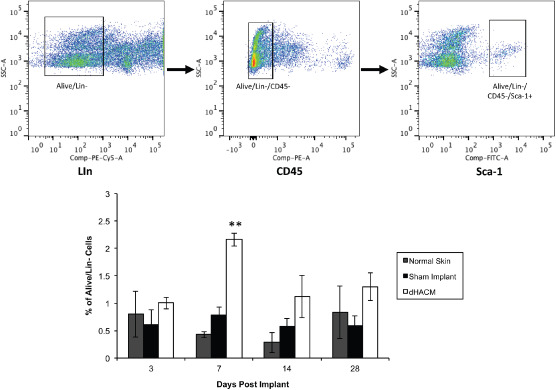
Effects of dehydrated human amnion/chorion tissue allografts (dHACM) on mesenchymal progenitor cell recruitment in an in vivo mouse model. Upper panels demonstrate the fluorescence‐activated cell sorting (FACS) gating scheme and identification of progenitor cells. Bottom chart shows the relative number of mesenchymal stem cells in specimens and dHACM and sham implant site on days 3, 7, 14 and 28 post implant. Values shown are means ± standard deviations, n = 4 specimens. ** Indicates P < 0·05 when comparing dHACM to normal skin and sham implant via one‐way analysis of variance (ANOVA).
Discussion
dHACM contains multiple growth factors, including PDGF‐AA, PDGF‐BB, TGFα, TGFβ1, bFGF, EGF and GCSF. ELISA assays also measured the presence of other biological regulatory proteins, IL‐ 4, 6, 8 and 10, and TIMPs 1, 2 and 4. This list of growth factors and regulatory proteins does not encompass the entire array of physiologically important and biologically active regulatory molecules present in dHACM. However, these particular growth factors are likely to be responsible for some of the clinical benefits of this dHACM allograft, specifically for wound healing or soft tissue repair 16.
dHACM was shown to retain soluble biological molecules that cause human dermal fibroblast proliferation and migration of human MSCs. Additionally, an in vivo mouse model showed that dHACM implantation was associated with a recruitment of mesenchymal progenitor cells. These results indicate that PURION® processed dHACM retains biological activities and, moreover, suggest that these activities are in part responsible for its therapeutic efficacy when used for wound healing 6, 7, 8, 9.
Human amnion has also been shown to reduce inflammation. The mechanism by which it accomplishes this is not completely understood, but ILs are likely involved because they regulate the inflammatory process 17. Accordingly, the present analyses established that dHACM contains IL‐4, 6, 8 and 10, with IL‐4 and IL‐10 being potent inhibitors of pathologic inflammation. Interestingly, IL‐6 can act both as a pro‐inflammatory and anti‐inflammatory agent, suggesting an intricate functional relationship among the factors in dHACM that affect inflammation. Moreover, IL‐8 is a chemokine with wide ranging functions in a variety of cell types, and is even known to be a strong promoter of angiogenesis.
In addition to ILs, the ELISA assays established that dHACM contains at least three TIMPs. TIMPs directly inhibit the activity of matrix metalloproteinases (MMPs), which degrade extracellular matrix components, including collagen, and are known to be persistently and abnormally elevated in many chronic wounds. These inhibitory proteins are critical components for regulating the action of MMPs in normal extracellular matrix homeostasis. The balance between biosynthesis and resorption of extracellular matrix is pivotal for maintaining a healthy and functional tissue. In chronic wounds, the balance can be severely skewed towards matrix degradation, thereby limiting the generation of competent granulation tissue and the ultimate production of normal dermal matrix 16. Of particular concern is the abundance of MMPs 2 and 9, which can be overexpressed in chronic wounds. Nonetheless, preliminary evidence from our laboratory suggests that TIMP containing extracts of dHACM can inhibit exogenous MMPs 2 and 9. The presence of these TIMPs thus suggests that one mechanism underlying dHACM's efficacy in healing chronic wounds is inhibition of active MMPs in the wound bed, resulting in a shift from degradation to synthesis, and ultimately regeneration of a competent extracellular matrix.
Mesenchymal stem cells and progenitor cells have been implicated in normal skin homeostasis and proper wound healing in both animal models and clinical use 18, 19, 20. In fact, excisional wound studies in mice and rats have shown that administration of bone marrow‐mesenchymal stem cells (BM‐MSC) to the wound increased granulation tissue and angiogenesis, accelerated wound closure and increased the strength or competence of the healed wound 21. Animal models utilising green fluorescent protein tagged BM‐MSCs have also shown that these cells are recruited to the site of cutaneous wounds, suggesting that stem‐cell recruitment is essential to wound healing. The progenitor cells recruited to the dHACM implant in this study suggest a similar involvement; however, the source of these cells is unclear, as they may have originated from the local wound environment, the circulatory system or from sources outside the wound, like bone marrow. Further studies will be necessary to determine their origin. Nonetheless, the results from the in vitro and in vivo experiments clearly establish that dHACM contains one or more soluble factors that cause mesenchymal stem and progenitor cell migration and recruitment.
Reported clinical use of dHACM for non‐healing wounds, while limited in scope, has nevertheless supported the clinical efficacy of this particular dehydrated amnion/chorion allograft. In one small series, patients with a variety of wound types refractory to traditional therapies demonstrated improved healing following dHACM allografts treatment 5. Sheik et al. 6 also reported a case series demonstrating that refractory, non‐healing wounds treated with dHACM healed following dHACM therapy, and the healed wounds did not recur with long‐term follow‐up. Finally, a prospective randomised controlled trial for the treatment of diabetic foot ulcers published in this volume showed that 77% and 92% of the chronic wounds at 4 and 6 weeks, respectively, healed with a bi‐weekly treatment of dHACM. In contrast, standard of care resulted in healing in only 0% and 8% of patients 7. These clinical results clearly demonstrate that dHACM is efficacious for wound healing, while the results from this study help to explain the molecular basis and mode of action of dHACM in promoting healing in chronic wounds.
Nonetheless, given the complexity of growth factor composition and overlapping functions of the growth factors present, assignment of biological activity to specific molecules is not possible. The capacity of the material to influence distinct cell actions, that is, proliferation and migration in vitro and recruitment in vivo, suggests that more than one factor is active.
All of the growth factors presented in Table 1 are known to play critical roles in normal wound healing, including cell proliferation and chemotaxis, as well as promoting angiogenesis, deposition of ECM and regulating inflammation 22, 23. In chronic wounds, however, growth factor signalling in the wound is abnormal, likely contributing to an impaired physiological response required for healing. Growth factor levels in chronic wounds have been found to be significantly lower when compared with acute wounds 23, 24, and fluids from non‐healing wounds also have been shown to inhibit mitogenic activity of fibroblasts in vitro 25. As shown in this study, dHACM contains many of these relevant growth factors in varying amounts, including relatively high concentrations of PDGF‐AA, bFGF, TGF‐β1 and PLGF.
Growth factor treatments, including supplementation with PDGF, EGF and bFGF, have been used to promote healing of chronic wounds in a number of clinical trials; however, nearly all of these studies required large amounts of growth factor, generally on the order of 10–1000 µg, for clinical efficacy 26, 27, 28, 29. Unfortunately such large amounts of growth factor are generally highly expensive, and treatments are often ineffective 30. While the growth factor concentrations in dHACM are much lower than these clinically reported values, in the nanogram to picogram range, dHACM tissues also contain a vast range of cytokines, each with unique roles in the wound healing process, instead of a high dose of a single factor. Combinations of growth factors have also been shown to act synergistically to enhance the wound healing response 31, 32, 33, 34; so it is likely that a combination of multiple growth factors may be more effective in achieving the biological coordination required for accelerated healing. The unique combination of endogenous growth factors in dHACM tissues may act synchronously to regulate various cell responses in vivo in order to generate a healing response 34. As such, dHACM tissue grafts have been successfully used in treatment of diabetic foot ulcers, and other challenging chronic wounds, suggesting that the combination of growth factors present in dHACM are available in sufficient amounts to promote healing of chronic wounds 7.
Conclusion
PURION® processed dHACM retains biologically active growth factors and regulatory factors that are in part responsible for its clinical effectiveness in wound healing. dHACM is a multifaceted tissue graft that has the potential to positively affect at least four distinct physiological processes, including cell proliferation, inflammation, metalloproteinase activity and recruitment of stem cells, all of which are intimately involved in regenerative wound healing and soft tissue repair.
Acknowledgements
This study was sponsored and funded by MiMedx Group, Inc., Marietta, GA.
TJK, NZ, MM and JJL are employees of MiMedx. WWL is a consultant for MiMedx. RR, JST and GG report no conflict of interests.
Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, Li WW, Gurtner G. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing.
References
- 1. John T. Human amniotic membrane transplantation: past, present and future. Ophthalmol Clin North Am 2003;16:43–65. [DOI] [PubMed] [Google Scholar]
- 2. Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg 2009;26:507–23. [DOI] [PubMed] [Google Scholar]
- 3. Gruss JS, Jirsch DW. Human amniotic membrane: a versatile wound dressing. Can Med Assoc J 1978;118:1237–46. [PMC free article] [PubMed] [Google Scholar]
- 4. Sawhney CP. Amniotic membrane as a biological dressing in the management of burns. Burns 1989;15:339–42. [DOI] [PubMed] [Google Scholar]
- 5. Forbes J, Fetterolf D. Dehydrated amniotic membrane allografts for the treatment of chronic wounds: a case study. J Wound Care 2012;21:290–6. [DOI] [PubMed] [Google Scholar]
- 6. Sheikh ES, Sheikh ES, Fetterolf DE. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J 2013. DOI: 10.1111/iwj.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zelen C, Serena TE, Denoziere G, Fetterolf DE. A prospective randomized comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 2013. DOI: 10.1111/iwj.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serena T, Fetterolf D. Clinical Research: Dehydrated human amniotic membrane (dHAM) treatment of lower extremity venous ulceration (CR23). Poster presented at SAWC Annual Spring Meeting in Atlanta, GA. April 2012.
- 9.Ennis W, Sui A, Papineau E, Plummer M, Altman I, Meneses P. Clinical experience with a novel regenerative template for hard to heal wounds. SAWC Annual Spring Meeting in Atlanta, GA. April 2012.
- 10. Lopez‐Valladares MJ, Rodriguez‐Ares MT, Tourino R, Gude F, Teresa Silva M, Couceiro J. Donor age and gestational age influence on growth factor levels in human amniotic membrane. Acta Opththalmol 2010;88:e211–6. [DOI] [PubMed] [Google Scholar]
- 11. Russo A, Bonci P, Bonci P. The effects of different preservation processes on the total protein and growth factor content in a new biological product developed from human amniotic membrane. Cell Tissue Bank 2012;13:353–61. [DOI] [PubMed] [Google Scholar]
- 12.US Patent 8,357,403– Placenta Tissue Grafts.
- 13.US Patent 8,372,437‐Placenta Tissue Grafts.
- 14.US Patent 8,409,626‐Placenta Tissue Grafts.
- 15. Box GEP, Cox DR. An analysis of transformation. J R Stat Soc (B) 1964;26:211–52. [Google Scholar]
- 16. Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011;19:134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weber RL, Iacono VJ. The cytokines: a review of interleukins. Periodontal Clin Investig 1997;19:17–22. [PubMed] [Google Scholar]
- 18. Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 2010;316:2213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez‐Menocal L, Salgado M, Ford D, Van Badiavas E. Stimulation of skin and wound fibroblast migration by mesenchymal stem cells derived from normal donors and chronic wound patients. Stem Cells Transl Med 2012;1:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007;25:2648–59. [DOI] [PubMed] [Google Scholar]
- 21. Chen JS, Wong VW, Gurtner GC. Therapeutic potential of bone marrow‐derived mesenchymal; stem cells for cutaneous wound healing. Front Immunol 2012;3:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawrence WT, Diegelmann RF. Growth factors in wound healing. Clin Dermatol 1994;12:157–69. [DOI] [PubMed] [Google Scholar]
- 23. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601. [DOI] [PubMed] [Google Scholar]
- 24. Cooper DM, Yu EZ, Hennessey P, Ko F, Robson MC. Determination of endogenous cytokines in chronic wounds. Ann Surg 1994;219:688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schultz G, Rotatori DS, Clark W. EGF and TGF‐α in wound healing and repair. J Cell Biochem 1991;45:346–52. [DOI] [PubMed] [Google Scholar]
- 26. Pierce GF, Tarpley JE, Tseng J, Bready J, Chang D, Kenney WC, Rudolph R, Robson MC, Vande Berg J, Reid P. Detection of platelet‐derived growth factor (PDGF)‐AA in actively healing human wounds treated with recombinant PDGF‐BB and absence of PDGF in chronic nonhealing wounds. J Clin Invest 1995;96:1336–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robson MC, Phillips LG, Thomason A, Robson LE, Pierce GF. Platelet‐derived growth factor BB for the treatment of chronic pressure ulcers. Lancet 1992;339:23–5. [DOI] [PubMed] [Google Scholar]
- 28. Brown GL, Curtsinger L, Jurkiewicz MJ, Nahai F, Schultz G. Stimulation of healing of chronic wounds by epidermal growth factor. Plast Reconstr Surg 1991;88:189–94. [PubMed] [Google Scholar]
- 29. Robson MC, Phillips LG, Lawrence WT, Bishop JB, Youngerman JS, Hayward PG, Broemeling LD, Heggers JP. The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores. Ann Surg 1992;216:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steed DL. Clinical evaluation of recombinant human platelet—derived growth factor for the treatment of lower extremity diabetic ulcers. J Vasc Surg 1995;21:71–81. [DOI] [PubMed] [Google Scholar]
- 31. Brown RL, Breeden MP, Greenhalgh DG. PDGF and TGF‐α act synergistically to improve wound healing in the genetically diabetic mouse. J Surg Res 1994;56:562–70. [DOI] [PubMed] [Google Scholar]
- 32. Lynch SE, Colvin RB, Antoniades HN. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest 1989;84:640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hennessey PJ, Black CT, Andrassy RJ. Epidermal growth factor and insulin act synergistically during diabetic healing. Arch Surg 1990;125:926–9. [DOI] [PubMed] [Google Scholar]
- 34. Robson MC. The role of growth factors in the healing of chronic wounds. Wound Repair Regen 1997;5:12–7. [DOI] [PubMed] [Google Scholar]


