Abstract
Vascular endothelial growth factor (VEGF) is essential for many angiogenic processes both in normal and pathological condition. However, the signaling pathways involved in VEGF-induced angiogenesis are incompletely understood. The protein kinase D1 (PKD1), a newly described the calcium/calmodulin-dependent serine/threonine kinase, has been implicated in cell migration, proliferation and membrane trafficking. Increasing evidence suggest critical roles for PKD1-mediated signaling pathways in the endothelial cells, particularly in the regulation of VEGF-induced angiogenesis. Recent studies show that class IIa histone deacetylases (HDACs) are PKD1 substrates and VEGF signal-responsive repressors of myocyte enhancer factor-2 (MEF2) transcriptional activation in endothelial cells. This review provides a guide on PKD1 signaling pathway and the direct downstream targets of PKD1 in VEGF signaling, and suggests important functions of PKD1 in angiogenesis.
Keywords: VEGF, PKD, PKC, CAMK, HDAC, MEF2, migration, angiogenesis, endothelial cells
INTRODUCTION
Protein kinase D1 (PKD1, also known as PKC-μ), a novel serine/threonine protein kinase, is composed of a zinc finger-like cysteine-rich motifs at its N-terminus that have high affinity for diacylglycerol, a pleckstrin homology domain, and a C-terminal catalytic domain (Rozengurt et al., 2005). Although PKD family kinases (PKD1, 2, 3) have a homologous catalytic domain, they vary in respect of their expression level, function, and subcellular localization (Auer et al., 2005; Rey et al., 2003; Rozengurt et al., 2005). PKD1 has been implicated in the regulation of a variety of cellular functions, including signal transduction, cell migration, and membrane trafficking (Avkiran et al., 2008; Johannes et al., 1994; Rozengurt et al., 2005; Valverde et al., 1994b). However, the direct downstream targets of PKD1 in VEGF signaling are not fully understood. Furthermore, a few PKD1 targeting genes have been identified so far.
Angiogenesis, the formation of new blood capillaries, is essential for embryonic vascular development, wound healing, and organ regeneration, as well as the pathological processes such as tumor growth, rheumatoid arthritis, and diabetic retinopathy, and atherosclerosis (Carmeliet, 2003; Ferrara et al., 2003; Folkman, 1995). Out of the many participants in the angiogenesis field, the vascular endothelial growth factors (VEGF) are by far the best characterized. VEGF (Folkman, 1995) is crucial for many angiogenic processes both in normal and pathological conditions (Carmeliet, 2003; Ferrara et al., 2003). VEGF receptors, VEGFR1 (Flt1) and VEGFR2 (mouse Flk1 or human KDR), are restricted in their tissue distribution primarily to endothelial cells (Yancopoulos et al., 2000). The binding of VEGF to its cognate receptors induces dimerization and subsequent intracellular signal cascades to mediate endothelial gene expression, cell migration, and angiogenesis (Claesson-Welsh, 2003; Sakurai et al., 2005; Takahashi et al., 2001; Zachary, 2003). In particular, VEGF receptor 2 (VEGFR2)-mediated phospholipase C γ(PLCγ)/protein kinase C (PKC) pathway regulates the activation of extracellular-regulated kinases and angiogenesis (Sakurai et al., 2005; Takahashi et al., 1999; Takahashi et al., 2001).
The importance of VEGF-induced signaling is demonstrated in that the genetic inactivation of either receptor leads to a complete lack of development of blood vessels in the embryo, and inactivation of VEGFR2 function dramatically impairs the growth of cancer cells in vivo (Carmeliet et al., 1996; Fong et al., 1995; Shalaby et al., 1995). However, the links from VEGF receptors intracellular signaling cascades to gene regulation remain largely elusive. This review will focus on VEGF-mediated PKD1 activation in endothelial cells and the potential role of PKD in angiogenesis.
VEGF signaling and Angiogenesis
The functions of VEGF (VEGFA) in angiogenesis have been studied for many years (Ferrara, 2002). Recent data indicate that angiogenic processes are highly complex and coordinated processes, requiring the subsequent activation of a chain of receptors by various ligands, but VEGF signaling shows a critical role in physiological angiogenesis (Carmeliet, 2000; Ferrara and Alitalo, 1999; Yancopoulos et al., 2000). VEGF also seems to be important in pathological angiogenesis, such as tumor growth (Ferrara and Davis-Smyth, 1997). VEGF stimulates proliferation and migration of endothelial cells (Ferrara et al., 2003). In addition, VEGF is a potent survival factor for endothelial cells during angiogenesis and it has been also shown to prevents apoptosis by inducing expression of the anti-apoptotic proteins such as Bcl-2 and A1 in the endothelial cells (Benjamin et al., 1999; Benjamin and Keshet, 1997; Gerber et al., 1998a; Gerber et al., 1998b; Yuan et al., 1996). VEGF (VEGF-A), a key regulator of blood vessel growth, is affiliated with a gene family that includes placental growth factor (PLGF), VEGF-B, VEGF-C and VEGF-D (Ferrara and Davis-Smyth, 1997; Neufeld et al., 1999). The human VEGF-A gene is composed of eight exons (Houck et al., 1991; Tischer et al., 1991). Four different isoforms of variable amino acid number are produced through alternative exon splicing (VEGF121, VEGF165, VEGF189, VEGF206) (Ferrara et al., 2003; Houck et al., 1991; Tischer et al., 1991). VEGF121, VEGF165 and VEGF189 are the major forms secreted by most cell types (Robinson and Stringer, 2001). The divergent functions of VEGF isoforms were studied by isoform specific VEGF knockout mice (Carmeliet et al., 1999; Stalmans et al., 2002). Mice expressing only VEGF165 are viable and healthy (Stalmans et al., 2002). These studies show the significance of VEGF165 as the principal effector of VEGF action. VEGF binds two related receptor tyrosine kinases (RTKs), VEGFR-1 and VEGFR-2 (Chen et al., 1997; Kolodkin et al., 1997). These VEGF receptors have seven immunoglobulin-like domains in the extracellular domain, a single trans-membrane region and a tyrosine kinase domain (Shibuya et al., 1990; Terman et al., 1991). VEGFR-1 (Flt-1) binds VEGF, VEGF-B and PLGF with high affinity (Shibuya et al., 1990), and VEGFR-2 (KDR or Flk-1) binds VEGF, VEGF-C and VEGF-D. However, recent studies show that VEGFR-2 is the major mediator of VEGF signals in endothelial cells (Gille et al., 2001; Meyer et al., 1999; Wise et al., 1999). VEGF induces the activation of various proteins such as PLC-γ, PI-3 kinase, and the Src family in endothelial cells through VEGFR-2 (Eliceiri et al., 1999; Guo et al., 1995).
The protein kinase (PKD) family
The protein kinase D (PKD) was made up of 3 isoforms (Fig. 1). Human and mouse PKD1, also known as protein kinase Cμ, was identified by two different group in 1994 (Johannes et al., 1994; Valverde et al., 1994a) and the more recently discovered isoforms PKD2 and PKD3 (PKCν) (Hayashi et al., 1999; Rey et al., 2003). PKD1 is a serine/threonine kinase with 918-amino acid that is composed of two cysteine-rich, zinc finger–like regions and a pleckstrin homology (PH) domain in N-terminal regulatory domain and a C-terminal catalytic domain (Johannes et al., 1994; Valverde et al., 1994a), as illustrated in Figure 1. Also, PKD2 and PKD3 have a similar structure and show a high homology to PKD1. The N-terminal regulatory regions of PKD inhibit the kinase activity of PKD and also control sublocalization of PKD (Rey and Rozengurt, 2001; Van Lint et al., 1995). The cysteine-rich domains plays a important role in mediating PKD translocation to the plasma membrane and nucleus in cells stimulated with various stimuli and also suppresses the kinase activity(Iglesias et al., 1998a). PKD also includes a PH domain, have been determined to play an autoregulatory role (Iglesias et al., 1998a; Iglesias and Rozengurt, 1998). Accordingly, PKD mutants with PH domain are constitutively active (Iglesias et al., 1998a; Waldron et al., 1999), showing that the PH domain, like the cysteine-rich domains, needs to maintain PKD in an inactive catalytic state.
Fig. 1. Diagram of functional domains and conserved phosphorylation sites in PKD families.
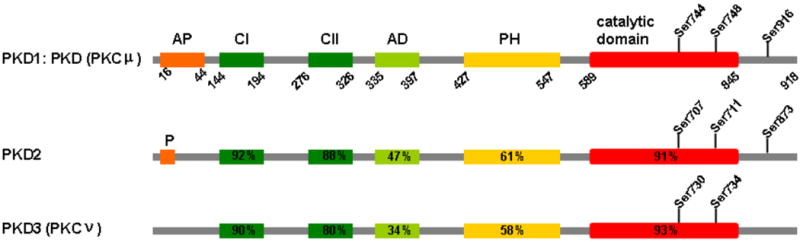
The percentages indicate amino acid sequence homology of PKD2 and PKD3 domains with corresponding mouse PKD1 domains. Abbreviations: AP, apolar region; C1 and C2, cysteine-rich and zinc finger–like domains (DAG-binding domain); AD, acidic domain; PH, pleckstrin homology domain; P, proline-rich region
The structural, enzymatic, and regulatory properties of PKD are different from PKC family (Rozengurt et al., 2005; Valverde et al., 1994a; Van Lint et al., 1995). PKD was initially divided to the AGC group (named for PKA, PKG, and PKC) (Mellor and Parker, 1998; Newton, 1997), but PKD are now classified as the calcium/calmodulin-dependent protein kinase (CAMK) group, separate from the AGC group (Hanks, 2003). This categorization is based on catalytic domain (Kunkel et al., 2007).
DAG triggers changes in localization, phosphorylation, and catalytic activation of PKD family. However, PKD family is not only a target of DAG (diacylglycerol) but also direct downstream of PKC family. Thus, as a second mechanism, PKCs activate PKD by phosphorylation of Ser-744 and Ser-748 within the activation loop of the PKD catalytic domain (Zugaza et al., 1996; Zugaza et al., 1997), and this PKC-mediated phosphorylation plays an important role in PKD activation by various stimuli in intact cell systems (Iglesias et al., 1998b; Waldron et al., 2001; Waldron and Rozengurt, 2003). The activated PKD also translocates from the plasma membrane into the cytoplasm and nucleus (Rey and Rozengurt, 2001), and undergoes autophosphorylation at Ser916 (Matthews et al., 1999). PKD can be activated by a variety of stimuli including biologically active phorbol esters, growth factors, bombesin, endothelin, vasopressin, and T- and B-cell receptor agonists via PKC-dependent pathways (Lint et al., 2002; Rozengurt et al., 2005; Zugaza et al., 1997). PKD has been implicated in the regulation of a variety of cellular responses, including signal transduction, membrane trafficking, protein transport, and cell survival, migration, differentiation, and proliferation (Baron and Malhotra, 2002; Hausser et al., 2002; Jamora et al., 1999; Liljedahl et al., 2001; Rozengurt et al., 2005; Storz and Toker, 2003; Yeaman et al., 2004). Furthermore, PKD activation has been studied in many normal cell types, including fibroblasts (Chiu and Rozengurt, 2001a; Matthews et al., 1997; Zhukova et al., 2001), intestinal and kidney epithelial cells (Chiu and Rozengurt, 2001b; Chiu et al., 2002; Rey et al., 2004; Rey et al., 2001), smooth muscle cells (Abedi et al., 1998), cardiomyocytes (Haworth et al., 2004; Haworth et al., 2000), and B and T lymphocytes (Matthews et al., 2000a; Matthews et al., 1999, 2000b; Sidorenko et al., 1996). However, the regulation of PKD activation and its function in endothelial cells are very poorly studied.
PKD1 functions in Angiogenesis
Recently, functional activities of PKD family in circulation system appear to mediate proliferation(Qin et al., 2006; Wong and Jin, 2005), and migration(Qin et al., 2006), in endothelial cells, hypertrophy in vascular smooth muscle cells(Xu et al., 2007), and activation in platelets(Stafford et al., 2003). In a recent report, PKD1 also mediates the VEGFR2-PLCγ-PKC pathway to ERK1/2 activation and EC proliferation (Wong and Jin, 2005). Furthermore, disturbing in PKD1 activity has been shown to block VEGF-stimulated angiogenesis in an in vivo model (Qin et al., 2006). Recent report also showed the physiological function of PKD2 in endothelial cells involved in angiogenesis (Hao et al., 2009). They found that PKD2 was a important PKD isoform mediating proliferation, migration, and in vitro angiogenesis in endothelial cells.
Although the cellular mechanism through which PKD1 mediates the pertinent process is unclear in many cases, several PKD1 downstream targets have been identified. Recent reports have revealed that class IIa histone deacetylase 5 (HDAC5) and 7 (HDAC7), an enzyme that induces chromatin modifications and act as signal responsive repressors for control of gene expression (Backs et al., 2006; Chang et al., 2006; McKinsey and Olson, 2005; Zhang et al., 2002), are direct downstream target of PKD1 in endothelial cells (Ha et al., 2008a; Ha et al., 2008b). VEGF stimulated PKD-dependent phosphorylation of two serine 259/498 residues in HDAC5 in endothelial cells. Furthermore, VEGF induced HDAC5 translocated from the nuclei to the cytoplasm after VEGF stimulation and that the PKD1-HDAC5 pathway is involved in VEGF-induced MEF2-dependent gene expression, NR4A1 expression, migration, and tube formation (Ha et al., 2008b). MEF2 family transcription factors have been implicated in blood vessel development and vascular integrity (Lin et al., 1998). Several MEF2-dependent genes, including NR4A1 and KLF2, have been identified (Chang et al., 2006; Youn and Liu, 2000).
In the recent study, it has been shown that VEGF stimulates PKD1-dependent HDAC7 phosphorylation at the sites of Ser178, Ser344, and Ser479 and cytoplasmic accumulation in endothelial cells, and that PKD1-HDAC7 pathway is involved in VEGF-induced angiogenic gene expression, including matrix metalloproteinases MT1-matrix metalloproteinase (MT1-MMP) and MMP10 expression, EC migration, tube formation, and microvessel sprouting (Ha et al., 2008a). It is increasingly apparent that PKD1 is a key player in the regulation of signaling transductions related to angiogenesis in endothelial cells.
Conclusion
Investigation of the regulation and functions of PKD1 in the endothelial cells is still in the beginning. Nevertheless, the recent advances already suggest that PKD is responsive to important stimuli such as VEGF and may control physiological and pathological angiogenesis by mediating class IIa HDACs such as HDAC5 and HDAC7. In conclusion, these discoveries may implicate PKD in mediating angiogenesis by VEGF and may suggest PKD as a potential therapeutic target for angiogenesis in various diseases.
Fig. 2. Schema for potential functions of PKD1 in VEGF-induced angiogenesis.
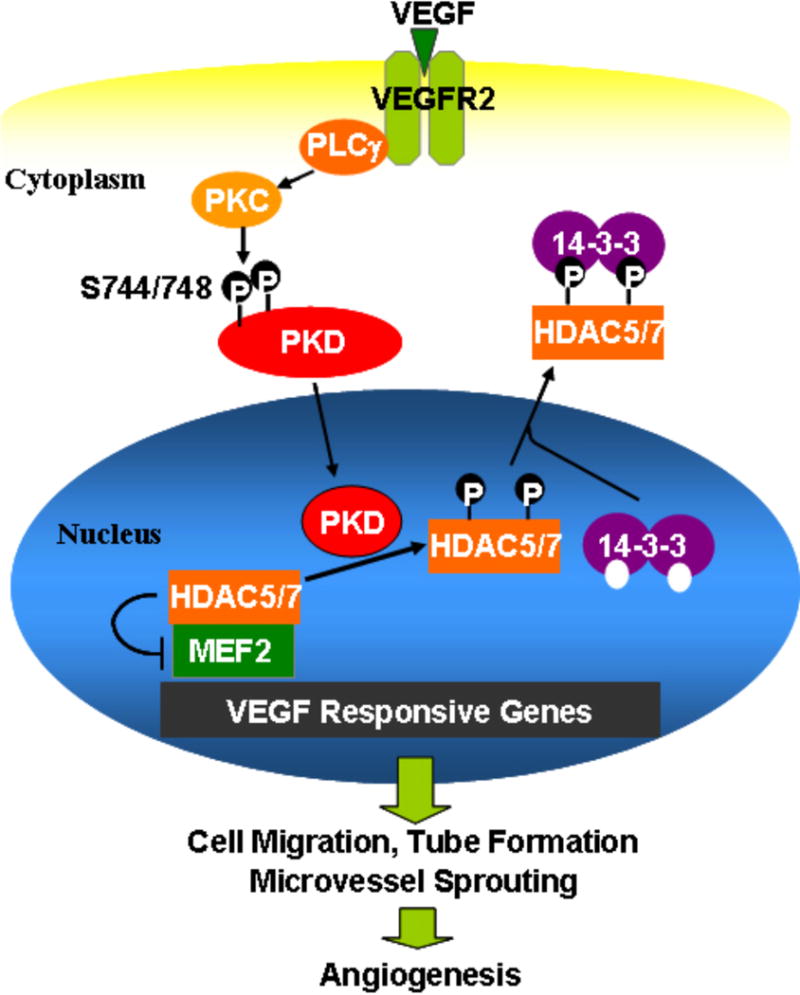
VEGF-activated PKD1 phosphorylates class IIa HDACs (HDAC 5 and 7) and induces those cytoplasmic accumulations in endothelial cells. Eventually, VEGF-induced PKD1 pathway is involved in VEGF-induced gene expressions, EC migration, tube formation and microvessel sprouting.
References
- Abedi H, Rozengurt E, Zachary I. Rapid activation of the novel serine/threonine protein kinase, protein kinase D by phorbol esters, angiotensin II and PDGF-BB in vascular smooth muscle cells. FEBS letters. 1998;427:209–212. doi: 10.1016/s0014-5793(98)00427-x. [DOI] [PubMed] [Google Scholar]
- Auer A, von Blume J, Sturany S, von Wichert G, Van Lint J, Vandenheede J, Adler G, Seufferlein T. Role of the regulatory domain of protein kinase D2 in phorbol ester binding, catalytic activity, and nucleocytoplasmic shuttling. Molecular biology of the cell. 2005;16:4375–4385. doi: 10.1091/mbc.E05-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avkiran M, Rowland AJ, Cuello F, Haworth RS. Protein kinase d in the cardiovascular system: emerging roles in health and disease. Circulation research. 2008;102:157–163. doi: 10.1161/CIRCRESAHA.107.168211. [DOI] [PubMed] [Google Scholar]
- Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. The Journal of clinical investigation. 2006;116:1853–1864. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science (New York, NY. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. The Journal of clinical investigation. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LE, Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8761–8766. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature medicine. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nature medicine. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell. 2006;126:321–334. doi: 10.1016/j.cell.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Chiu T, Rozengurt E. CCK2 (CCK(B)/gastrin) receptor mediates rapid protein kinase D (PKD) activation through a protein kinase C-dependent pathway. FEBS letters. 2001a;489:101–106. doi: 10.1016/s0014-5793(01)02076-2. [DOI] [PubMed] [Google Scholar]
- Chiu T, Rozengurt E. PKD in intestinal epithelial cells: rapid activation by phorbol esters, LPA, and angiotensin through PKC. American journal of physiology. 2001b;280:C929–942. doi: 10.1152/ajpcell.2001.280.4.C929. [DOI] [PubMed] [Google Scholar]
- Chiu T, Wu SS, Santiskulvong C, Tangkijvanich P, Yee HF, Jr, Rozengurt E. Vasopressin-mediated mitogenic signaling in intestinal epithelial cells. American journal of physiology. 2002;282:C434–450. doi: 10.1152/ajpcell.00240.2001. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem Soc Trans. 2003;31:20–24. doi: 10.1042/bst0310020. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nature reviews. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nature medicine. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocrine reviews. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature medicine. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. The Journal of biological chemistry. 1998a;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. The Journal of biological chemistry. 1998b;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, Pelletier N, Ferrara N. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. The Journal of biological chemistry. 2001;276:3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- Guo D, Jia Q, Song HY, Warren RS, Donner DB. Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. The Journal of biological chemistry. 1995;270:6729–6733. doi: 10.1074/jbc.270.12.6729. [DOI] [PubMed] [Google Scholar]
- Ha CH, Jhun BS, Kao HY, Jin ZG. VEGF stimulates HDAC7 phosphorylation and cytoplasmic accumulation modulating matrix metalloproteinase expression and angiogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2008a;28:1782–1788. doi: 10.1161/ATVBAHA.108.172528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, McKinsey TA, Olson EN, Jin ZG. Protein kinase D-dependent phosphorylation and nuclear export of histone deacetylase 5 mediates vascular endothelial growth factor-induced gene expression and angiogenesis. The Journal of biological chemistry. 2008b;283:14590–14599. doi: 10.1074/jbc.M800264200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK. Genomic analysis of the eukaryotic protein kinase superfamily: a perspective. Genome biology. 2003;4:111. doi: 10.1186/gb-2003-4-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q, Wang L, Zhao ZJ, Tang H. Identification of protein kinase D2 as a pivotal regulator of endothelial cell proliferation, migration, and angiogenesis. The Journal of biological chemistry. 2009;284:799–806. doi: 10.1074/jbc.M807546200. [DOI] [PubMed] [Google Scholar]
- Hausser A, Link G, Bamberg L, Burzlaff A, Lutz S, Pfizenmaier K, Johannes FJ. Structural requirements for localization and activation of protein kinase C mu (PKC mu) at the Golgi compartment. J Cell Biol. 2002;156:65–74. doi: 10.1083/jcb.200110047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth RS, Cuello F, Herron TJ, Franzen G, Kentish JC, Gautel M, Avkiran M. Protein kinase D is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circulation research. 2004;95:1091–1099. doi: 10.1161/01.RES.0000149299.34793.3c. [DOI] [PubMed] [Google Scholar]
- Haworth RS, Goss MW, Rozengurt E, Avkiran M. Expression and activity of protein kinase D/protein kinase C mu in myocardium: evidence for alpha1-adrenergic receptor-and protein kinase C-mediated regulation. Journal of molecular and cellular cardiology. 2000;32:1013–1023. doi: 10.1006/jmcc.2000.1143. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Seki N, Hattori A, Kozuma S, Saito T. PKCnu, a new member of the protein kinase C family, composes a fourth subfamily with PKCmu. Biochimica et biophysica acta. 1999;1450:99–106. doi: 10.1016/s0167-4889(99)00040-3. [DOI] [PubMed] [Google Scholar]
- Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Molecular endocrinology (Baltimore, Md. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Iglesias T, Matthews S, Rozengurt E. Dissimilar phorbol ester binding properties of the individual cysteine-rich motifs of protein kinase D. FEBS letters. 1998a;437:19–23. doi: 10.1016/s0014-5793(98)01189-2. [DOI] [PubMed] [Google Scholar]
- Iglesias T, Rozengurt E. Protein kinase D activation by mutations within its pleckstrin homology domain. The Journal of biological chemistry. 1998;273:410–416. doi: 10.1074/jbc.273.1.410. [DOI] [PubMed] [Google Scholar]
- Iglesias T, Waldron RT, Rozengurt E. Identification of in vivo phosphorylation sites required for protein kinase D activation. The Journal of biological chemistry. 1998b;273:27662–27667. doi: 10.1074/jbc.273.42.27662. [DOI] [PubMed] [Google Scholar]
- Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Johannes FJ, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. The Journal of biological chemistry. 1994;269:6140–6148. [PubMed] [Google Scholar]
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- Kunkel MT, Toker A, Tsien RY, Newton AC. Calcium-dependent regulation of protein kinase D revealed by a genetically encoded kinase activity reporter. The Journal of biological chemistry. 2007;282:6733–6742. doi: 10.1074/jbc.M608086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- Lin Q, Lu J, Yanagisawa H, Webb R, Lyons GE, Richardson JA, Olson EN. Requirement of the MADS-box transcription factor MEF2C for vascular development. Development. 1998;125:4565–4574. doi: 10.1242/dev.125.22.4565. [DOI] [PubMed] [Google Scholar]
- Lint JV, Rykx A, Vantus T, Vandenheede JR. Getting to know protein kinase D. The international journal of biochemistry & cell biology. 2002;34:577–581. doi: 10.1016/s1357-2725(01)00163-7. [DOI] [PubMed] [Google Scholar]
- Matthews SA, Iglesias T, Rozengurt E, Cantrell D. Spatial and temporal regulation of protein kinase D (PKD) The EMBO journal. 2000a;19:2935–2945. doi: 10.1093/emboj/19.12.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SA, Pettit GR, Rozengurt E. Bryostatin 1 induces biphasic activation of protein kinase D in intact cells. The Journal of biological chemistry. 1997;272:20245–20250. doi: 10.1074/jbc.272.32.20245. [DOI] [PubMed] [Google Scholar]
- Matthews SA, Rozengurt E, Cantrell D. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/Protein kinase Cmu. The Journal of biological chemistry. 1999;274:26543–26549. doi: 10.1074/jbc.274.37.26543. [DOI] [PubMed] [Google Scholar]
- Matthews SA, Rozengurt E, Cantrell D. Protein kinase D. A selective target for antigen receptors and a downstream target for protein kinase C in lymphocytes. J Exp Med. 2000b;191:2075–2082. doi: 10.1084/jem.191.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. The Journal of clinical investigation. 2005;115:538–546. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. The Biochemical journal. 1998;332(Pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Clauss M, Lepple-Wienhues A, Waltenberger J, Augustin HG, Ziche M, Lanz C, Buttner M, Rziha HJ, Dehio C. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. The EMBO journal. 1999;18:363–374. doi: 10.1093/emboj/18.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. Faseb J. 1999;13:9–22. [PubMed] [Google Scholar]
- Newton AC. Regulation of protein kinase C. Current opinion in cell biology. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- Qin L, Zeng H, Zhao D. Requirement of protein kinase D tyrosine phosphorylation for VEGF-A165-induced angiogenesis through its interaction and regulation of phospholipase Cgamma phosphorylation. The Journal of biological chemistry. 2006;281:32550–32558. doi: 10.1074/jbc.M604853200. [DOI] [PubMed] [Google Scholar]
- Rey O, Reeve JR, Jr, Zhukova E, Sinnett-Smith J, Rozengurt E. G proteincoupled receptor-mediated phosphorylation of the activation loop of protein kinase D: dependence on plasma membrane translocation and protein kinase Cepsilon. The Journal of biological chemistry. 2004;279:34361–34372. doi: 10.1074/jbc.M403265200. [DOI] [PubMed] [Google Scholar]
- Rey O, Rozengurt E. Protein kinase D interacts with Golgi via its cysteine-rich domain. Biochemical and biophysical research communications. 2001;287:21–26. doi: 10.1006/bbrc.2001.5530. [DOI] [PubMed] [Google Scholar]
- Rey O, Young SH, Cantrell D, Rozengurt E. Rapid protein kinase D translocation in response to G protein-coupled receptor activation. Dependence on protein kinase C. The Journal of biological chemistry. 2001;276:32616–32626. doi: 10.1074/jbc.M101649200. [DOI] [PubMed] [Google Scholar]
- Rey O, Yuan J, Young SH, Rozengurt E. Protein kinase C nu/protein kinase D3 nuclear localization, catalytic activation, and intracellular redistribution in response to G protein-coupled receptor agonists. The Journal of biological chemistry. 2003;278:23773–23785. doi: 10.1074/jbc.M300226200. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. Journal of cell science. 2001;114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. The Journal of biological chemistry. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- Sidorenko SP, Law CL, Klaus SJ, Chandran KA, Takata M, Kurosaki T, Clark EA. Protein kinase C mu (PKC mu) associates with the B cell antigen receptor complex and regulates lymphocyte signaling. Immunity. 1996;5:353–363. doi: 10.1016/s1074-7613(00)80261-7. [DOI] [PubMed] [Google Scholar]
- Stafford MJ, Watson SP, Pears CJ. PKD: a new protein kinase C-dependent pathway in platelets. Blood. 2003;101:1392–1399. doi: 10.1182/blood-2002-08-2384. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. The Journal of clinical investigation. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. The EMBO journal. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. The EMBO journal. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman BI, Carrion ME, Kovacs E, Rasmussen BA, Eddy RL, Shows TB. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene. 1991;6:1677–1683. [PubMed] [Google Scholar]
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. The Journal of biological chemistry. 1991;266:11947–11954. [PubMed] [Google Scholar]
- Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proceedings of the National Academy of Sciences of the United States of America. 1994a;91:8572–8576. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde AM, Sinnett SJ, Van LJ, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proceedings of the National Academy of Sciences of the United States of America. 1994b;91:8572–8576. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint JV, Sinnett-Smith J, Rozengurt E. Expression and characterization of PKD, a phorbol ester and diacylglycerol-stimulated serine protein kinase. The Journal of biological chemistry. 1995;270:1455–1461. doi: 10.1074/jbc.270.3.1455. [DOI] [PubMed] [Google Scholar]
- Waldron RT, Iglesias T, Rozengurt E. The pleckstrin homology domain of protein kinase D interacts preferentially with the eta isoform of protein kinase C. The Journal of biological chemistry. 1999;274:9224–9230. doi: 10.1074/jbc.274.14.9224. [DOI] [PubMed] [Google Scholar]
- Waldron RT, Rey O, Iglesias T, Tugal T, Cantrell D, Rozengurt E. Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. The Journal of biological chemistry. 2001;276:32606–32615. doi: 10.1074/jbc.M101648200. [DOI] [PubMed] [Google Scholar]
- Waldron RT, Rozengurt E. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. The Journal of biological chemistry. 2003;278:154–163. doi: 10.1074/jbc.M208075200. [DOI] [PubMed] [Google Scholar]
- Wise LM, Veikkola T, Mercer AA, Savory LJ, Fleming SB, Caesar C, Vitali A, Makinen T, Alitalo K, Stacker SA. Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3071–3076. doi: 10.1073/pnas.96.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Jin ZG. Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. The Journal of biological chemistry. 2005;280:33262–33269. doi: 10.1074/jbc.M503198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ha CH, Wong C, Wang W, Hausser A, Pfizenmaier K, Olson EN, McKinsey TA, Jin ZG. Angiotensin II stimulates protein kinase D-dependent histone deacetylase 5 phosphorylation and nuclear export leading to vascular smooth muscle cell hypertrophy. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2355–2362. doi: 10.1161/ATVBAHA.107.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Ayala MI, Wright JR, Bard F, Bossard C, Ang A, Maeda Y, Seufferlein T, Mellman I, Nelson WJ, et al. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nature cell biology. 2004;6:106–112. doi: 10.1038/ncb1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn HD, Liu JO. Cabin1 represses MEF2-dependent Nur77 expression and T cell apoptosis by controlling association of histone deacetylases and acetylases with MEF2. Immunity. 2000;13:85–94. doi: 10.1016/s1074-7613(00)00010-8. [DOI] [PubMed] [Google Scholar]
- Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukova E, Sinnett-Smith J, Rozengurt E. Protein kinase D potentiates DNA synthesis and cell proliferation induced by bombesin, vasopressin, or phorbol esters in Swiss 3T3 cells. The Journal of biological chemistry. 2001;276:40298–40305. doi: 10.1074/jbc.M106512200. [DOI] [PubMed] [Google Scholar]
- Zugaza JL, Sinnett-Smith J, Van Lint J, Rozengurt E. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. The EMBO journal. 1996;15:6220–6230. [PMC free article] [PubMed] [Google Scholar]
- Zugaza JL, Waldron RT, Sinnett-Smith J, Rozengurt E. Bombesin, vasopressin, endothelin, bradykinin, and platelet-derived growth factor rapidly activate protein kinase D through a protein kinase C-dependent signal transduction pathway. The Journal of biological chemistry. 1997;272:23952–23960. doi: 10.1074/jbc.272.38.23952. [DOI] [PubMed] [Google Scholar]


