Abstract
Purpose
The association between physical activity and colorectal adenoma is equivocal. This study was designed to assess the relationship between physical activity and colorectal adenoma recurrence.
Methods
Pooled analyses from two randomized, controlled trials included 1,730 participants who completed the Arizona Activity Frequency Questionnaire at baseline, had a colorectal adenoma removed within 6 months of study registration, and had a follow-up colonoscopy during the trial. Logistic regression modeling was employed to estimate the effect of sedentary behavior, light-intensity physical activity, and moderate-vigorous physical activity on colorectal adenoma recurrence.
Results
No statistically significant trends were found for any activity type and odds of colorectal adenoma recurrence in the pooled population. However, males with the highest levels of sedentary time experienced 47% higher odds of adenoma recurrence. Compared to the lowest quartile of sedentary time, the ORs (95% CIs) for the second, third, and fourth quartiles among men were 1.23 (0.88, 1.74), 1.41 (0.99, 2.01), and 1.47 (1.03, 2.11) respectively (P trend=0.03). No similar association was observed for women.
Conclusions
This study suggests that sedentary behavior is associated with a higher risk of colorectal adenoma recurrence among men, providing evidence of detrimental effects of a sedentary lifestyle early in the carcinogenesis pathway.
Keywords: sedentary behavior, physical activity, colon cancer, colorectal adenoma
Introduction
The burden of colorectal cancer (CRC) in the United States includes more than 141,000 new cases of colorectal cancer each year and an estimated 50,000 deaths [1]. Sporadic cancers account for approximately 70 to 75% of the colorectal cancer disease burden [2] with an estimated 96% of sporadic colorectal cancers classified as carcinomas [1], arising from the adenomatous polyp [3]. Several modifiable lifestyle factors are associated with colorectal cancer, including physical activity [4]; yet physical activity levels are declining in the United States [5].
There is extensive evidence supporting an association between higher overall physical activity levels and reduced risk of colorectal cancer [4, 6, 7]. A recent meta-analysis investigated the association of sedentary behavior with colon and rectal cancer and found a statistically significant association between colon cancer and prolonged periods of sitting, but the same association was not found for rectal cancer [8]. Few studies have focused on the complex and emerging health consequences associated with sedentary behavior and its impact on early stages of the colorectal adenoma-to-carcinoma sequence. Common sedentary behaviors include watching television, computer and computer game use, workplace sitting, resting, reading, and lying down, all of which are activities that have increased over the past several decades [5, 9]. Recently, sedentary behavior was formally defined by the Sedentary Behavior Research Network as any waking behavior characterized by an energy expenditure ≤1.5 METs while in a sitting or reclining posture [10]. Sedentary behavior has been identified as a potential health risk in large part due to its association with central adiposity [9], weight gain, all-cause and cardiovascular mortality [5], as well as Type 2 diabetes and some cancers [8].
While non-exercisers have traditionally been characterized as sedentary, a distinct difference between physical inactivity and sedentary behavior is emerging [9, 11, 12]. Current recommendations for physical activity advocate moderate-intensity physical activity for 30 minutes per day on most days of the week [7, 13]. Even among those who fulfill these daily recommendations, lengthy periods of habitual sedentary behavior have been associated with early morbidity and mortality [14]. Referred to as the “active couch potato” paradigm [11], this model is currently under investigation in relation to several health risks associated with metabolic disturbances [11]. Studies have investigated the differences between sedentary and moderate-vigorous physical activity, indicating that sedentary behavior results in worse outcomes related to metabolic dysregulation including insulin resistance and unfavorable adipokines linked to obesity [11, 15]. Another study objectively measured light-intensity physical activity among older adults (who tend to spend the majority of their day engaged in light-intensity (e.g., easy walking activities) concluded that light-intensity activities are positively associated with physical health and overall well-being [16]. However, a range of activity levels from sedentary behavior to moderate-vigorous physical activity has not been explored among individuals at risk for colorectal adenoma recurrence. Two recent studies have focused on the impact of sedentary behavior on sub-site specific colorectal cancer risk [17, 18]. In one investigation, Howard et al. reported a 61% increase in colon cancer among men reporting higher daily sedentary behavior compared to those in the lowest category [18]. Results of another study reported that individuals who spent ≥10 years in an occupation that required sedentary behaviors had a two-fold risk of distal colon cancer compared to those participants who were employed in non-sedentary jobs [17]. While these studies support a role for sedentary behavior in the development of colorectal cancer, the degree to which sedentary behavior, light-intensity physical activity, and moderate-vigorous intensity physical activity may influence the risk of colorectal adenoma remains unknown. Therefore, the objective of this study was to investigate the association between sedentary behavior, light-intensity physical activity, and moderate-vigorous activity levels and colorectal adenoma recurrence in a pooled analysis of data from two randomized, controlled trials.
Materials and Methods
Study Participants
We conducted a pooled analysis among participants from the randomized, double blind, placebo-controlled, Wheat Bran Fiber (WBF) and Ursodeoxycholic Acid (UDCA) Phase III clinical trials conducted at the University of Arizona Cancer Center. Study participants were recruited between 1990 and 1999. A total of 2,502 participants completed the WBF or UDCA trials, including 1730 participants for whom data were available from the baseline Arizona Activity Frequency Questionnaire.
Data for both trials were collected in a network of gastroenterology practices in the Phoenix and Tucson metropolitan areas. Both trials have been described in detail previously [19, 20]; briefly, eligible participants were males and females ranging in age from 40 to 80 years who had one or more colorectal adenoma(s) removed (diameter ≥ 3 mm) during a colonoscopic evaluation within a six-month period prior to study registration. In the WBF trial, participants were randomized to a daily wheat-bran fiber supplement (13.5 g/d) or a low-fiber supplement (2.0 g/d). In the UDCA trial, participants were randomized to UDCA (administered at 8–10 mg/kg of body weight) or placebo. Both interventions were hypothesized to prevent colorectal adenoma recurrence, defined as the detection of one or more colorectal adenomas or adenocarcinomas at follow-up colonoscopy at least 6 months after randomization. The mean follow-up time from randomization to colonoscopy for the WBF and UDCA trials was 3.1 years and 3.2 years, respectively. The primary findings of these studies were that neither the WBF supplement nor the UDCA treatment prevented colorectal adenoma recurrence. However, UDCA was associated with a decreased odds of high-grade dysplasia [OR (95% CI), 0.61(0.39–0.96)] [19] and was later reported to decrease the odds of advanced lesions among men [OR (95% CI), 0.62 (0.43–0.89)] [21]. The University of Arizona Human Subjects Committee approved both studies and written informed consent was obtained from each participant prior to study enrollment.
Assessment of Physical Activity
At the baseline visit, participants were asked to complete the Arizona Activity Frequency Questionnaire (AAFQ). The AAFQ is a validated [22], self-administered, scannable activity questionnaire, including 59 items categorized by leisure, recreational, household, and “other” categories of activity. Questions included: “Did you do [this activity] in the last four weeks?” “How many times did you do [this activity] in the last four weeks?” and “How much time did you spend on this activity each time (on average) in the last four weeks?”
Three categories were created based on the self-reported data available from the AAFQ. Using the guidelines developed by the Sedentary Behavior Research Network, behaviors ≤1.5 METs were classified as “sedentary behavior,” physical activities between >1.5 METs and <3 METs were designated as “light-intensity physical activity,” and activities ≥ 3 METs were categorized as “moderate-vigorous physical activity”[10, 23]. Sedentary behavior included sitting activities such as eating, watching television, and reclining during waking hours. Light-intensity physical activity included grocery shopping, washing dishes, and doing the laundry; and moderate-vigorous physical activity included yard work, swimming, and jogging.
Each participant’s reported time spent in distinct activity types, not including sleep or work, was adjusted proportionally so that the duration of all reported activities plus time spent working and sleeping totaled 24 hours, in order to reduce measurement error [24].
Statistical Analysis
Baseline participant characteristics, including age, body mass index (BMI), waist circumference, race/ethnicity, family history of colorectal cancer in a first degree relative, smoking status, previous colorectal polyp(s), aspirin use, and nutrient intake, were described for the pooled sample. Means, medians, and standard deviations were calculated for continuous variables and frequencies and percentages were calculated for categorical variables. Participants were categorized according to sex-specific quartiles of sedentary behavior, light-intensity physical activity, and moderate-vigorous physical activity. The distribution of baseline characteristics was compared across the quartiles of sedentary time for the total sample. For sedentary behavior, light-intensity physical activity, and moderate-vigorous physical activity, logistic regression was used to compare the odds of adenoma recurrence for participants in second, third, and fourth quartiles to the odds of recurrence in the lowest sex-specific quartile of each category. P-for-trend was generated by including a continuous score based on the categorical quartile assignment in a logistic regression model.
All models were adjusted for trial arm. Confounding was assessed by considering whether adjusting for a potential confounder resulted in a greater than 10% change in the odds ratio [25]. Potential confounders included age, smoking, previous polyps, aspirin use, family history, dietary intake, various adenoma characteristics, sedentary behavior and physical activity. Only age was found to be a confounder. Waist circumference and BMI were correlated (r=0.80), and although neither materially altered the model, waist circumference was selected for inclusion in the final model because it captures visceral adiposity, an important risk factor for colorectal neoplasia [26].
To assess whether the association between each activity type and adenoma recurrence was modified by sex, sex-specific odds ratios were estimated. Tests for interaction between sex and quartile of behavior or activity were conducted by comparing a full model including interaction terms to a reduced model including only main effects, using the likelihood ratio test. Study heterogeneity was evaluated by generating trial arm-specific estimates and calculating the Q (chi squared) statistic and the I2 index [27]. All analyses were conducted using Stata, version 11.0, software (StataCorp LP, College Station, Texas).
Results
The majority of participants in the pooled population (n=1730) were male (68.0%), white (94.8%), and overweight (mean BMI; 28.0 ± 4.8 kg/m2). The mean age was 65.9 ± 8.6 years. Approximately 29.9% of the pooled population reported regular aspirin use, 12.7% were current smokers, 42.8% reported previous colorectal polyps, and 24.1% had a family history of CRC (Table 1).
Table 1.
Baseline participant characteristics for the pooled sample
| Baseline Characteristics1 | Pooled (n=1730) |
|---|---|
| Age (years) | 65.9 ± 8.6 |
| Male | 1176 (68.0) |
| White | 1624 (94.8) |
| Current Smoker | 219 (12.7) |
| Family History CRC2 | 403 (24.1) |
| Previous Polyps3 | 719 (42.8) |
| Aspirin use4 | 517 (29.9) |
| Waist circumference (in), males | 39.6 ± 4.3 |
| Waist circumference (in), females | 34.1 ± 5.3 |
| BMI (kg/m2) | 28.0 ± 4.8 |
| BMI categories | |
| Underweight or Normal <25 kg/m2 | 465 (27.1) |
| Overweight 25 – <30 kg/m2 | 760 (44.3) |
| Obese ≥30 kg/m2 | 490 (28.6) |
| Dietary Intake | |
| Energy (kcal/d) | 1963 ± 775.6 |
| Protein (g/d) | 73.2 ± 29.5 |
| Total fat (g/d) | 64.6 ± 31.0 |
| Saturated fat (g/d) | 21.1 ± 11.2 |
| Total fiber (g/d) | 22.3 ± 10.7 |
| Calcium (mg/d) | 959.7 ± 455.5 |
| Alcohol (g/d), median [25%ile, 75%ile] | 1.7 [0.0, 11.1] |
| Adenoma characteristics | |
| Multiple adenomas (3+) | 288 (16.7) |
| Large (≥1cm) | 702 (40.6) |
| Villous architecture | 362 (21.0) |
| Proximal location | 877 (52.0) |
Abbreviations: WBF, Wheat Bran Fiber; UDCA, Ursodeoxycholic Acid; CRC, Colorectal Cancer; BMI, Body Mass Index
Continuous variables are summarized with mean and standard deviation, unless otherwise noted. Categorical variables are summarized with counts and percent of non-missing total. Numbers may not add up to the total due to missing data. Data are missing for race/ethnicity (n=17), family history of colorectal cancer (n=56), previous polyps (n=51), large adenoma at baseline (n=1), villous architecture (n=2), proximal location (n=43), body mass index (n=15), and waist circumference (n=16).
Colorectal cancer in one or more first-degree relatives
History of colorectal polyps prior to qualifying colonoscopy
Regular use of aspirin four weeks prior to enrollment
Baseline participant characteristics were compared across the quartiles of sedentary behavior (Table 2). Mean age and percentage of participants reporting non-Hispanic white race/ethnicity, aspirin use, and previous colorectal polyps generally increased across the quartiles of sedentary behavior, as well as the percentage of participants who had a large or proximally located colorectal adenoma at baseline. Mean caloric intake and the percentage of participants reporting a family history of CRC generally decreased across the quartiles of sedentary behavior.
Table 2.
Baseline participant characteristics according to quartiles of baseline sedentary behavior
| Quartiles of sedentary time (hours per day) | ||||
|---|---|---|---|---|
| 1st (n=433) | 2nd (n=431) | 3rd (n=433) | 4th (n=431) | |
| 4.8 ± 1.3 | 7.6 ± 0.6 | 9.7 ± 0.7 | 12.6 ± 6.5 | |
| Baseline Characteristics1 | ||||
| Age (years) | 60.4 ± 9.4 | 65.4 ± 8.3 | 68.1 ± 7.4 | 69.4 ± 6.4 |
| Male | 294 (67.9) | 293 (68.0) | 294 (68.1) | 293 (68.0) |
| White | 393 (91.4) | 402 (94.4) | 418 (98.0) | 408 (95.6) |
| Current smoker | 59 (15.6) | 56 (13.0) | 47 (10.88) | 57 (13.2) |
| Family history CRC2 | 107 (25.2) | 100 (23.9) | 85 (20.6) | 110 (26.4) |
| Previous polyps3 | 135 (32.6) | 168 (39.9) | 198 (46.9) | 216 (51.6) |
| Aspirin use4 | 109 (25.2) | 109 (25.3) | 141 (32.6) | 155 (36.0) |
| Waist circumference (in), males | 39.6 ± 4.6 | 39.5 ± 4.3 | 39.6 ± 4.0 | 40.0 ± 4.4 |
| Waist circumference (in), females | 33.9 ± 5.7 | 33.7 ±5.5 | 34.2 ± 5.2 | 34.5 ± 4.9 |
| BMI (kg/m2) | 28.3 ± 5.1 | 27.9 ± 5.1 | 27.7 ± 4.2 | 28.1 ± 4.6 |
| BMI (kg/m2) categories | ||||
| Underweight or Normal <25 kg/m2 | 122 (28.5) | 112 (26.2) | 111 (25.8) | 108 (25.4) |
| Overweight 25 – <30 kg/m2 | 167 (39.0) | 199 (46.5) | 206 (47.8) | 186 (43.8) |
| Obese ≥30 kg/m2 | 136 (31.8) | 113 (26.4) | 112 (25.99) | 129 (30.4) |
| Dietary Intake | ||||
| Energy (kcal/d) | 2005.2 ±800 | 1974 ± 790 | 1938 ± 706 | 1939 ± 806 |
| Protein (g/d) | 76.2 ± 31.4 | 73.9 ± 30.5 | 71.6 ± 26.1 | 71.3 ± 29.6 |
| Total fat (g/d) | 66.9 ± 32.3 | 66.4 ± 31.6 | 63.7 ± 28.6 | 63.4 ± 31.2 |
| Saturated fat (g/d) | 21.8 ± 11.4 | 20.8 ± 11.3 | 20.8 ± 10.5 | 20.8 ± 11.5 |
| Total fiber (g/d) | 22.7 ± 10.8 | 22.9 ± 10.9 | 21.6 ± 9.9 | 21.9 ± 11.0 |
| Calcium (mg/d) | 958.92 ± 445.4 | 981.1 ± 483.71 | 949.7 ± 440.8 | 950.1 ±452.9 |
| Alcohol (g/d), median [25%ile, 75%ile] | 1.8 [0.0, 9.7] | 1.8 [0.0, 12.1] | 1.7 [0.0, 12.3] | 1.1 [0.0, 9.2] |
| Colorectal Adenoma characteristics | ||||
| Multiple adenomas (3+) | 67 (15.5) | 72 (16.7) | 69 (16.0) | 80 (18.6) |
| Large (≥1cm) | 162 (37.4) | 168 (39.0) | 193 (44.7) | 178 (41.4) |
| Villous architecture | 99 (22.9) | 88 (20.4) | 87 (20.1) | 87 (20.3) |
| Proximal location | 179 (42.1) | 218 (52.0) | 221 (52.3) | 258 (61.9) |
Abbreviations: CRC, Colorectal Cancer; BMI, Body Mass Index
Continuous variables are summarized with mean and standard deviation, unless otherwise noted. Categorical variables are summarized with counts and percent of non-missing total. Numbers may not add up to the total due to missing data. Data are missing for race/ethnicity (n=17), family history of colorectal cancer (n=56), previous polyps (n=51), large adenoma at baseline (n=1), villous architecture (n=2), proximal location (n=43), body mass index (n=15), and waist circumference (n=16).
Colorectal cancer in one or more first-degree relatives
History of colorectal polyps prior to qualifying colonoscopy
Regular use of aspirin four weeks prior to enrollment
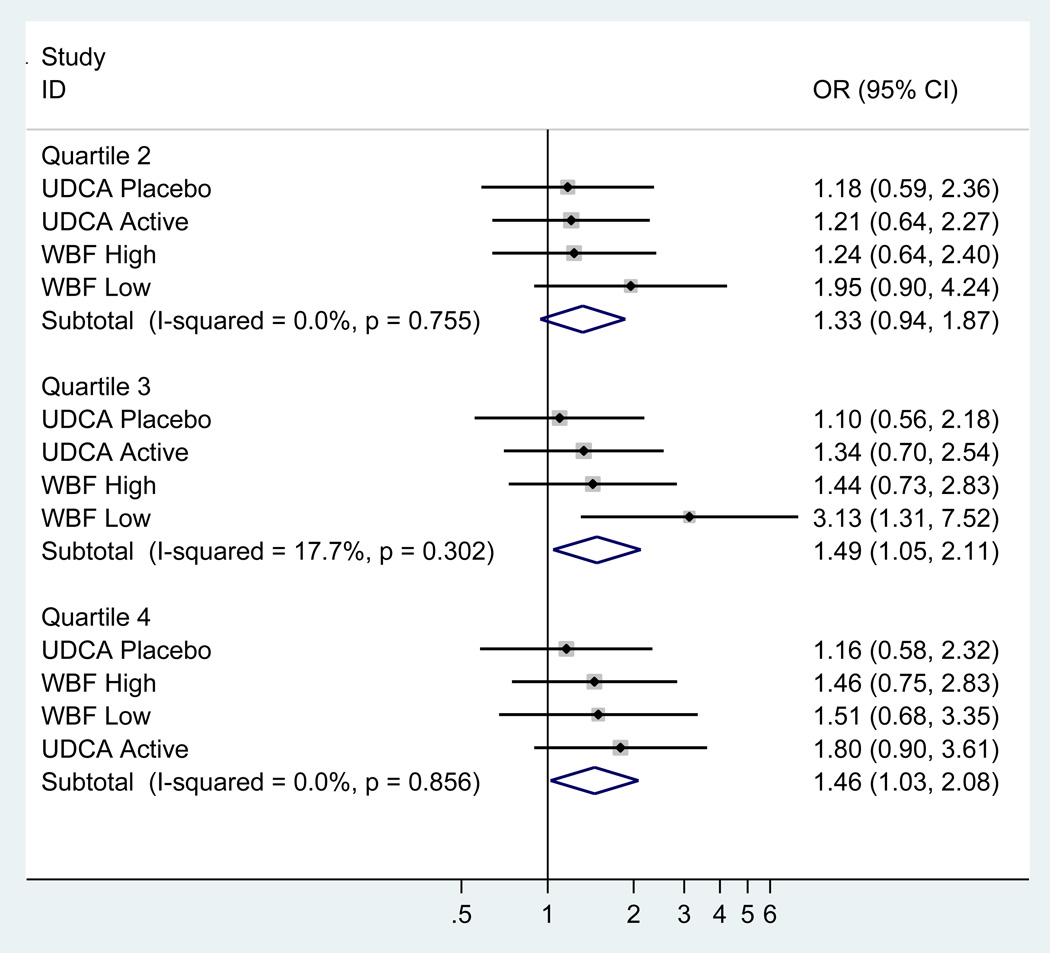
Adjusted odds ratios and 95% confidence intervals for adenoma recurrence for the sex-specific quartiles of sedentary behavior, light-intensity physical activity, and moderate-vigorous physical activity among all study participants are shown in Table 3. Participants in the second, third, and fourth quartiles of sedentary behavior had higher odds of colorectal adenoma recurrence compared to the first quartile; however, the differences were not statistically significant. No significant associations were found for light-intensity or moderate-vigorous physical activity. Sex-stratified analyses demonstrated statistically significant higher odds of adenoma recurrence among men in the fourth quartile of sedentary behavior, with an OR (95% CI) of 1.47 (1.03, 2.11) compared with the lowest quartile (P-trend=0.03) (Table 4). In contrast, no statistically significant association between sedentary behavior and adenoma recurrence was observed among women, though the p for interaction for sex was not statistically significant (P-interaction = 0.22). For light-intensity physical activity and moderate-vigorous physical activity, no notable associations were found for either sex. No evidence of heterogeneity by trial arm was observed for the association between sedentary behavior and adenoma recurrence among men, based on the I2 index or the Q statistic [22] (Figure 1).
Table 3.
Colorectal adenoma recurrence and sedentary behavior, light-intensity physical activity, and moderate-vigorous physical activity
| Colorectal Adenoma Recurrence | ||
|---|---|---|
| mean ± S.D. | n (%) | OR (95% CI)* |
| Sedentary behavior (hrs/day) | ||
| 4.80 ± 1.28 | 208 (48.0) | 1.00 (ref) |
| 7.57 ± 0.64 | 189 (43.9) | 1.04 (0.78, 1.38) |
| 9.71 ± 0.73 | 187 (43.3) | 1.19 (0.89, 1.59) |
| 12.63 ± 1.50 | 196 (45.5) | 1.28 (0.95, 1.72) |
| Ptrend =0.07 | ||
| Light-intensity physical activity (hrs/day) | ||
| 1.48 ± 0.95 | 209 (48.3) | 1.00 (ref) |
| 2.71 ± 1.14 | 188 (43.6) | 0.79 (0.61, 1.05) |
| 3.89 ± 1.29 | 195 (45.1) | 0.85 (0.65, 1.12) |
| 6.27 ± 2.02 | 188 (43.6) | 0.76 (0.58, 1.00) |
| Ptrend =0.09 | ||
| Moderate-vigorous physical activity (hrs/day) | ||
| 0.44 ± 0.32 | 208 (48.0) | 1.00 (ref) |
| 1.35 ± 0.48 | 189 (43.9) | 0.81 (0.61, 1.06) |
| 2.51 ± 0.73 | 187 (43.3) | 0.77 (0.58, 1.02) |
| 5.12 ± 1.97 | 196 (45.5) | 0.80 (0.60, 1.07) |
| Ptrend =0.12 | ||
Odds ratio and 95% confidence interval, adjusted for age, waist circumference, and trial arm
Table 4.
Sex-stratified analysis of sedentary behavior, light-intensity physical activity, and moderate-vigorous physical activity with colorectal adenoma recurrence
| Females (n=547) | Males (n=1167) | |||||
|---|---|---|---|---|---|---|
| Colorectal Adenoma Recurrence |
Colorectal Adenoma Recurrence |
|||||
| hours/day | count (%) |
OR (95% CI)* |
hours/day | count (%) |
OR (95% CI)* |
|
| Sedentary behavior | ||||||
| 0.00–6.21 | 54 (38.9) | 1.00 (ref) | 0.00–6.62 | 118 (40.1) | 1.00 (ref) | |
| 6.23–8.20 | 46 (33.3) | 0.71 (0.43, 1.12) | 6.64–8.80 | 139 (47.4) | 1.23 (0.88, 1.74) | |
| 8.21–10.28 | 54 (39.1) | 0.84 (0.50, 1.41) | 8.81–11.09 | 153 (52.0) | 1.41 (0.99, 2.01) | |
| 10.29–16.06 | 56 (40.6) | 0.96 (0.57, 1.63) | 11.10–19.37 | 160 (54.6) | 1.47 (1.03, 2.11) | |
| Ptrend=0.93 | Ptrend=0.03 | |||||
| Pinteraction†=0.22 | ||||||
| Light-intensity physical activity | ||||||
| 0.00–3.65 | 55 (39.6) | 1.00 (ref) | 0.00–1.53 | 154 (52.4) | 1.00 (ref) | |
| 3.66–5.00 | 47 (34.1) | 0.72 (0.44, 1.20) | 1.54–2.45 | 141 (48.1) | 0.82 (0.59, 1.15) | |
| 5.02–6.42 | 55 (39.9) | 0.92 (0.56, 1.52) | 2.46–3.76 | 140 (47.6) | 0.81 (0.58, 1.13) | |
| 6.43–16.44 | 53 (38.4) | 0.77 (0.46, 1.30) | 3.76–14.49 | 135 (46.1) | 0.75 (0.54, 1.04) | |
| Ptrend=0.53 | Ptrend=0.10 | |||||
| Pinteraction†=0.87 | ||||||
| Moderate-vigorous physical activity | ||||||
| 0.00–0.43 | 61 (43.9) | 1.00 (ref) | 0.00–1.10 | 147 (50.0) | 1.00 (ref) | |
| 0.44–1.07 | 46 (33.3) | 0.61 (0.37, 1.01) | 1.11–2.14 | 143 (48.8) | 0.89 (0.64, 1.25) | |
| 1.08–2.30 | 49 (35.5) | 0.67 (0.40, 1.09) | 2.15–3.86 | 138 (46.9) | 0.80 (0.57, 1.13) | |
| 2.31–9.49 | 54 (39.1) | 0.75 (0.46, 1.25) | 3.86–12.98 | 142 (48.5) | 0.79 (0.55, 1.12) | |
| Ptrend=0.33 | Ptrend=0.15 | |||||
| Pinteraction†=0.61 | ||||||
Odds ratio and 95% confidence interval, adjusted for age, waist circumference, and trial arm
Interaction between activity type and sex
Figure 1.
Sedentary behavior and colorectal adenoma recurrence among men, stratified by trial arm
Discussion
While our data do not support an association between light-intensity and moderate-vigorous physical activity and odds of colorectal adenoma recurrence in the pooled population, sex-stratified analyses revealed that increased sedentary behavior was significantly associated with odds of adenoma recurrence in men, but not women.
Of twelve published case-control studies, eight reported a significant inverse association [28–35], one reported a modest association [36], and three reported no association [37–39]. In addition, the duration and intensity of physical activity required to reduce risk of colorectal adenoma or colorectal cancer has not been well established [4, 40]; some studies indicate more vigorous-intensity activity is required [18, 41] while others indicate that moderate-intensity activity is sufficient [18, 42] to reduce risk. The findings of the present analysis are generally concordant with four studies that investigated the effects of sedentary behavior and colorectal cancer risk [17, 18, 43, 44]. In a study by Howard et al. [18], a higher risk of colorectal cancer in men who reported the highest levels of sedentary behavior was reported, but this elevation in risk was not statistically significant in women. In another study conducted among males only [44], a higher risk for colon [RR (95% CI), 2.22 (1.28, 3.85)] and rectal cancer [RR (95% CI), 2.0 (1.03, 3.85)] with greater sedentary behavior was observed.
The majority of studies investigating physical activity and risk of colorectal cancer and adenoma have compared individuals who are most active (engaged in activities that expend energy ≥3 METs) to least active (engaged in activities that expend energy <3 METs) [17, 45]. The least active group is often categorized as “sedentary” or “inactive” despite the fact that they can be participating in light-intensity activities (ranging from 1.9–2.9 METs); these individuals may otherwise not be classified as sedentary by other definitions [11, 17, 45, 46]. Sedentary behavior also may be defined simply as prolonged sitting time [45, 47] and is not necessarily analogous with an overall low physical activity level [17, 45, 47].
The physiologic effects of “sedentary physiology” versus “exercise physiology” have been elucidated in several studies with modulations in lipoprotein lipase activity cited as the central mechanism responsible for metabolic dysfunction [48]. Numerous metabolic events are dysregulated as a result of sedentary behavior [47], many of which may be related to increased adiposity and central adiposity specifically [47, 49]. The cascade of biologically plausible metabolic events that stem from adiposity to contribute to cancer risk include hyperglycemia [45, 50], hyperinsulinemia [47, 49], chronic inflammation [51], decreased bioavailability of vitamin D [52], and elevated endogenous sex hormones [47]. Each of these complex biological pathways contributes to carcinogenesis [47] and some mediate the steps associated with the adenoma-to-carcinoma sequence [53].
It remains unclear whether sedentary behavior and physical activity differentially impact the initiation and progression events along the pathway from adenoma to carcinoma. A large prospective cohort study (NIH-AARP Diet and Health Study) [18], which includes participants with similar demographics to those in the current study, found a statistically significant association between increased risk for colorectal cancer and sedentary behavior in men who spent ≥ 9 hours per day watching television compared to men who watched <3 hours per day [RR (95% CI), 1.61 (1.14–2.27); P for trend ≤0.001]. Congruent with evidence indicating a persistent deleterious effect of sedentary behavior independent of physical activity [11, 18], the study also concluded that participating in physical activity at any intensity reduced risk for colon [RR (95% CI), 0.79 (0.68–0.91); P for trend=0.001] and rectal cancer [RR (95% CI), 0.76 (0.7=0.61–0.94); P for trend=0.074], independent of the finding that sedentary behavior was associated with higher risk [18].
Reasons for a differential effect of sedentary behavior in men compared to women are unclear. While our interaction analysis does not confirm that sex is a modifier in the association between sedentary behavior and adenoma recurrence, our findings of a significant increased trend of higher colorectal adenoma recurrence rates among men are congruent with the literature [54]. The differential associations may reflect the potentially protective effects of estrogen, especially among overweight or obese females, which may play a role in reducing odds of colorectal cancer and adenoma [55]. In the present study, the average BMI of females was 27.5 ± 5.8 kg/m2, which is considered overweight, suggesting higher estrogen concentrations. However, given the mean age (65.6 ± 8.8 years), most women would be post-menopausal with lower circulating estrogen. Importantly, we did not have detailed data for hormone replacement therapy, which might also explain some of the variance shown between genders. The increase in inflammatory mediators [56] and insulin resistance [57] stemming from a high prevalence of obesity [58] and central obesity (visceral adiposity) may partly contribute to the increased odds found among males [59]; in addition, evidence suggests that adiposity may modify the association between sedentary behavior and cancer risk [47]. However, in the present study, inclusion of waist circumference or BMI in the models did not materially alter the association between sedentary behavior and adenoma recurrence, and obesity rates were similar for males and females. Nonetheless, we cannot exclude the possibility that the differential effect seen in men compared to women could be due to chance alone.
Existing evidence supports an association between higher overall physical activity levels and reduced risk of colorectal cancer and colorectal adenomas [4, 6, 7]. We did not find an association between light-intensity and moderate-vigorous physical activity and reduction in odds of colorectal adenoma recurrence. The difference in our results compared to prior studies is multifactorial. First, our outcome differs from prior studies; we measured colorectal adenoma recurrence and not risk of colorectal adenoma incidence or colorectal cancer. Our study was conducted over a relatively short period of time (3 years), which may not be sufficient time for physical activity to play a protective role in the adenoma to carcinoma sequence and thereby reducing risk for colorectal adenoma recurrence. Other studies that have investigated colorectal cancer and adenoma risk have taken into account a longer time period and also include large meta-analyses that provide a summary risk estimate over multiple studies.
Limitations of this study include self-reported activity data, and the potential for measurement error or misclassification bias. Although the three variables assessed in this paper (sedentary behavior, light-intensity activity, and moderate-vigorous activity) were categorized using METs and therefore provide a scientific basis for comparison. Because there is potential for measurement error and misclassification in studies of physical activity and sedentary behavior, exposure assessment and various levels of activity must be addressed before public health recommendations can be fully developed [43]; this is recognized as an area that warrants further investigation [47]. In order to accurately capture sedentary behavior, objective measures of physical activity (e.g., accelerometers) should be used to examine this relationship and self-reported questionnaires should include categories that describe and define sedentary time in accordance with the Sedentary Behavior Network definition should be incorporated into data collection [47].
In summary, the results of this study suggest that sedentary behavior increases risk of adenoma recurrence among men. While our findings support other studies that have reported a higher risk of colorectal cancer with increasing sedentary behavior, this appears to be the first published study to date to specifically investigate the association between sedentary behavior and recurrence of colorectal adenomas, the well-established precursor to colorectal cancer [3]. This study provides evidence of the detrimental effects of a sedentary lifestyle early in the carcinogenesis pathway, potentially mediated by the inflammatory response [11, 53].
Impact.
This work appears to be the first published study to date to specifically investigate the association between sedentary behavior and recurrence of colorectal adenomas, the well-established precursor to colorectal cancer. In addition, our findings support other studies that have reported a higher risk of colorectal cancer with increasing sedentary behavior.
Acknowledgments
Financial Support
NIH/NCI P01CA41108 Wheat Bran Fiber and Ursodeoxycholic Acid Phase III Clinical Trials conducted at the Arizona Cancer Center
NIH/NCI P30CA023074 Arizona Cancer Center Core grant
Footnotes
This study was completed at the University of Arizona Mel and Enid Zuckerman College of Public Health and the Arizona Cancer Center, in Tucson, Arizona.
The authors have no conflict of interest to report.
References
- 1.ACS. Colorectal Cancer Facts and Figures 2011–2013. 2011–2013 [Google Scholar]
- 2.Amersi F, Agustin M, Ko CY. Colorectal cancer: epidemiology, risk factors, and health services. Clinics in Colon and Rectal Surgery. 2005;18:133. doi: 10.1055/s-2005-916274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 4.Wolin K, Yan Y, Colditz G. Physical activity and risk of colon adenoma: a meta-analysis. British journal of cancer. 2011;104:882–885. doi: 10.1038/sj.bjc.6606045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthews CE, George SM, Moore SC, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. The American Journal of Clinical Nutrition. 2012;95:437–445. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WCRF/AICR. Food, Nutrition, and Physical Activity, and the Prevention of Cancer: A Global Perspective. 2007 [Google Scholar]
- 8.Cong Y, Gan Y, Sun H, et al. Association of sedentary behaviour with colon and rectal cancer: a meta-analysis of observational studies. British Journal of Cancer. 2013 doi: 10.1038/bjc.2013.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owen N, Sparling PB, Healy GvN, Dunstan DW, Matthews CE. Sedentary behavior: emerging evidence for a new health risk. Mayo Clinic Proceedings: Mayo Foundation. 2010:1138. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes J, Behrens TK, Benden ME, et al. Letter to the Editor: Standardized use of the terms "sedentary" and "sedentary behaviours". Applied Physiology Nutrition and Metabolism-Physiologie Appliquee Nutrition Et Metabolisme. 2012;37:540–542. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 11.Owen N, Healy GvN, Matthews CE, Dunstan DW. Too much sitting: the population-health science of sedentary behavior. Exercise and Sport Sciences Reviews. 2010;38:105. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle T. Physical Activity and Colon Cancer Timing, Intensity, and Sedentary Behavior. American Journal of Lifestyle Medicine. 2012;6:204–215. [Google Scholar]
- 13.Haskell WL, Lee I, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise. 2007;39:1423. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 14.Owen N, Bauman A, Brown W. Too much sitting: a novel and important predictor of chronic disease risk? British Journal of Sports Medicine. 2009;43:81–83. doi: 10.1136/bjsm.2008.055269. [DOI] [PubMed] [Google Scholar]
- 15.Yates T, Khunti K, Wilmot EG, et al. Self-reported sitting time and markers of inflammation, insulin resistance, and adiposity. American Journal of Preventive Medicine. 2012;42:1–7. doi: 10.1016/j.amepre.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Buman MP, Hekler EB, Haskell WL, et al. Objective light-intensity physical activity associations with rated health in older adults. American Journal of Epidemiology. 2010;172:1155–1165. doi: 10.1093/aje/kwq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle T, Fritschi L, Heyworth J, Bull F. Long-term sedentary work and the risk of subsite-specific colorectal cancer. Am J Epidemiol. 2011;173:1183–1191. doi: 10.1093/aje/kwq513. [DOI] [PubMed] [Google Scholar]
- 18.Howard RA, Freedman DM, Park Y, Hollenbeck A, Schatzkin A, Leitzmann MF. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2008;19:939–953. doi: 10.1007/s10552-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberts DS, Martinez ME, Hess LM, et al. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst. 2005;97:846–853. doi: 10.1093/jnci/dji144. [DOI] [PubMed] [Google Scholar]
- 20.Alberts DS, Martinez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians' Network. N Engl J Med. 2000;342:1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 21.Thompson PA, Wertheim BC, Roe DJ, et al. Gender modifies the effect of ursodeoxycholic acid in a randomized controlled trial in colorectal adenoma patients. Cancer Prev Res (Phila) 2009;2:1023–1030. doi: 10.1158/1940-6207.CAPR-09-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuhouser ML, Di C, Tinker LF, et al. Physical Activity Assessment: Biomarkers and Self-Report of Activity-Related Energy Expenditure in the WHI. American Journal of Epidemiology. 2013;177:576–585. doi: 10.1093/aje/kws269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and MET values. Medicine and science in sports and exercise. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 24.Wertheim BC, Martinez ME, Ashbeck EL, et al. Physical activity as a determinant of fecal bile acid levels. Cancer Epidemiology Biomarkers & Prevention. 2009;18:1591–1598. doi: 10.1158/1055-9965.EPI-08-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. American journal of epidemiology. 1989;129:125. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 26.Yamaji T, Iwasaki M, Sasazuki S, et al. Visceral fat volume and the prevalence of colorectal adenoma. American Journal of Epidemiology. 2009;170:1502–1511. doi: 10.1093/aje/kwp311. [DOI] [PubMed] [Google Scholar]
- 27.Green JPHaS. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. [Google Scholar]
- 28.Kato I, Tominaga S, Matsuura A, Yoshii Y, Shirai M, Kobayashi S. A comparative case-control study of colorectal cancer and adenoma. Jpn J Cancer Res. 1990;81:1101–1108. doi: 10.1111/j.1349-7006.1990.tb02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kono S, Shinchi K, Ikeda N, Yanai F, Imanishi K. Physical activity, dietary habits and adenomatous polyps of the sigmoid colon: a study of self-defense officials in Japan. J Clin Epidemiol. 1991;44:1255–1261. doi: 10.1016/0895-4356(91)90158-6. [DOI] [PubMed] [Google Scholar]
- 30.Little J, Logan R, Hawtin P, Hardcastle J, Turner I. Colorectal adenomas and energy intake, body size and physical activity: a case-control study of subjects participating in the Nottingham faecal occult blood screening programme. British Journal of Cancer. 1993;67:172. doi: 10.1038/bjc.1993.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benito E, Cabeza E, Moreno V, Obrador A, Bosch F. Diet and colorectal adenomas: A case-control study in majorca. International Journal of Cancer. 1993;55:213–219. doi: 10.1002/ijc.2910550208. [DOI] [PubMed] [Google Scholar]
- 32.Sandler RS, Pritchard ML, Bangdiwala SI. Physical activity and the risk of colorectal adenomas. Epidemiology. 1995;6:602–606. doi: 10.1097/00001648-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Neugut AI, Terry MB, Hocking G, et al. Leisure and occupational physical activity and risk of colorectal adenomatous polyps. Int J Cancer. 1996;68:744–748. doi: 10.1002/(SICI)1097-0215(19961211)68:6<744::AID-IJC9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Lubin F, Rozen P, Arieli B, et al. Nutritional and lifestyle habits and water-fiber interaction in colorectal adenoma etiology. Cancer Epidemiol Biomarkers Prev. 1997;6:79–85. [PubMed] [Google Scholar]
- 35.Boutron-Ruault M-C, Senesse P, Meance S, Belghiti C, Faivre J. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutrition and Cancer. 2001;39:50–57. doi: 10.1207/S15327914nc391_7. [DOI] [PubMed] [Google Scholar]
- 36.Terry MB, Neugut AI, Bostick RM, et al. Risk Factors for Advanced Colorectal Adenomas A Pooled Analysis. Cancer Epidemiology Biomarkers & Prevention. 2002;11:622–629. [PubMed] [Google Scholar]
- 37.Kono S, Handa K, Hayabuchi H, et al. Obesity, weight gain and risk of colon adenomas in Japanese men. Jpn J Cancer Res. 1999;90:805–811. doi: 10.1111/j.1349-7006.1999.tb00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauret KG, Bostick RM, Matthews CE, et al. Physical activity and reduced risk of incident sporadic colorectal adenomas: observational support for mechanisms involving energy balance and inflammation modulation. Am J Epidemiol. 2004;159:983–992. doi: 10.1093/aje/kwh130. [DOI] [PubMed] [Google Scholar]
- 39.Enger S, Longnecker M, Lee E, Frankl H, Haile R. Recent and past physical activity and prevalence of colorectal adenomas. British journal of cancer. 1997;75:740. doi: 10.1038/bjc.1997.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolin K, Yan Y, Colditz G, Lee I. Physical activity and colon cancer prevention: a meta-analysis. British journal of cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slattery ML. Physical activity and colorectal cancer. Sports Medicine. 2004;34:239–252. doi: 10.2165/00007256-200434040-00004. [DOI] [PubMed] [Google Scholar]
- 42.Mai PL, Sullivan-Halley J, Ursin G, et al. Physical activity and colon cancer risk among women in the California Teachers Study. Cancer Epidemiology Biomarkers & Prevention. 2007;16:517–525. doi: 10.1158/1055-9965.EPI-06-0747. [DOI] [PubMed] [Google Scholar]
- 43.Steindorf K, Jedrychowski W, Schmidt M, et al. Case-control study of lifetime occupational and recreational physical activity and risks of colon and rectal cancer. Eur J Cancer Prev. 2005;14:363–371. doi: 10.1097/00008469-200508000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Colbert LH, Hartman TJ, Malila N, et al. Physical activity in relation to cancer of the colon and rectum in a cohort of male smokers. Cancer Epidemiol Biomarkers Prev. 2001;10:265–268. [PubMed] [Google Scholar]
- 45.Pate RR, O'Neill JR, Lobelo F. The evolving definition of "sedentary". Exercise and Sport Sciences Reviews. 2008;36:173–178. doi: 10.1097/JES.0b013e3181877d1a. [DOI] [PubMed] [Google Scholar]
- 46.Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Applied Physiology, Nutrition, and Metabolism. 2010;35:725–740. doi: 10.1139/H10-079. [DOI] [PubMed] [Google Scholar]
- 47.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:2691–2709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 49.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA: the journal of the American Medical Association. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 50.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiology Biomarkers & Prevention. 2010;19:2691–2709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 51.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α, and IL-6. Diabetes research and clinical practice. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. The American Journal of Clinical Nutrition. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 53.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. e5. [DOI] [PubMed] [Google Scholar]
- 54.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 55.Jacobs ET, Martinez ME, Alberts DS, et al. Association between body size and colorectal adenoma recurrence. Clin Gastroenterol Hepatol. 2007;5:982–990. doi: 10.1016/j.cgh.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van den Borst B, Gosker HR, Koster A, et al. The influence of abdominal visceral fat on inflammatory pathways and mortality risk in obstructive lung disease. The American Journal of Clinical Nutrition. 2012;96:516–526. doi: 10.3945/ajcn.112.040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care. 2005;28:2322–2325. doi: 10.2337/diacare.28.9.2322. [DOI] [PubMed] [Google Scholar]
- 58.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA: the journal of the American Medical Association. 2010;303:235. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 59.Siddiqui A, Pena Sahdala HN, Nazario HE, et al. Obesity is associated with an increased prevalence of advanced adenomatous colon polyps in a male veteran population. Dig Dis Sci. 2009;54:1560–1564. doi: 10.1007/s10620-009-0811-7. [DOI] [PubMed] [Google Scholar]