Abstract
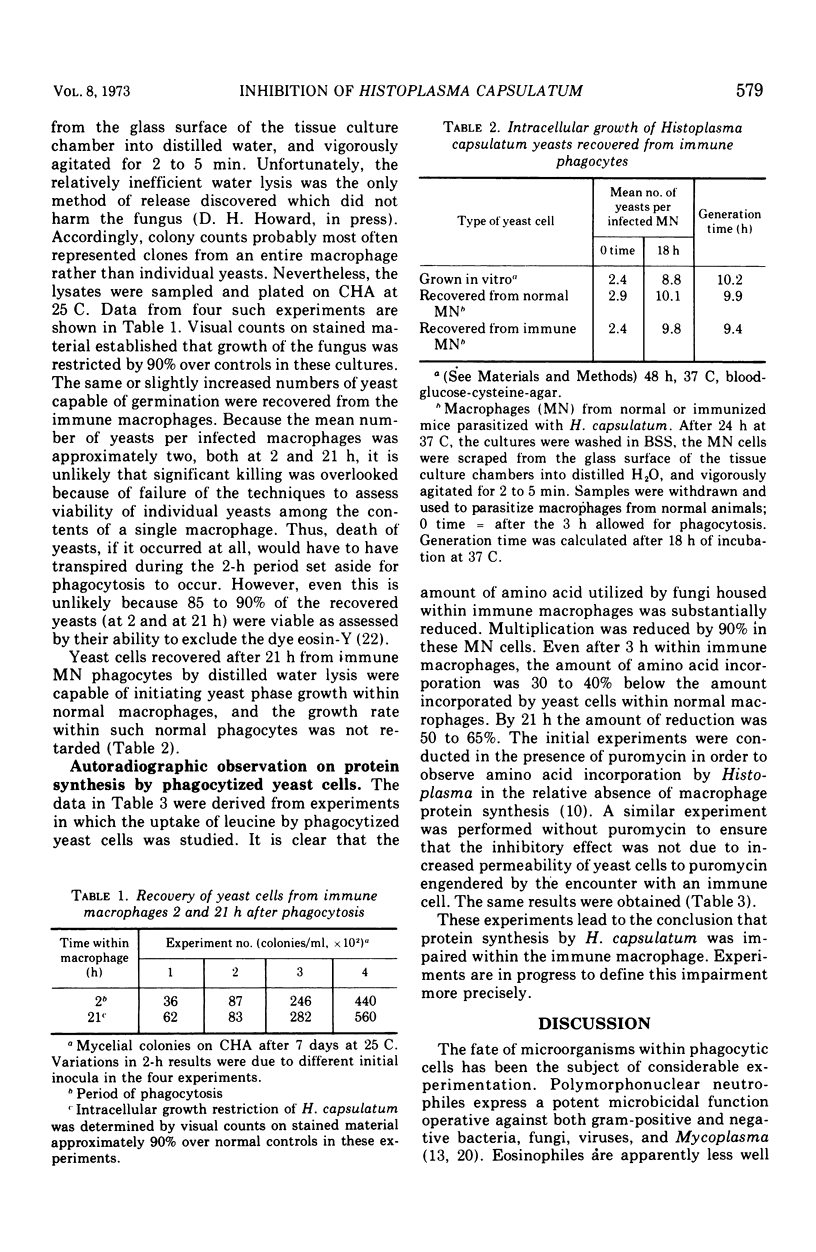
Mononuclear phagocytes freshly harvested from immunized animals restrict the intracellular growth of Histoplasma capsulatum. The fate of the inhibited fungus is the subject of this report. Macrophages harvested from mice immunized by sublethal infection were parasitized in vitro with yeast cells of H. capsulatum. The subsequent fate of the fungus was assessed by staining characteristics, viability on subculture, and radioisotopic techniques. No tinctorial distinction could be made between yeasts within normal or immune phagocytes stained by the May Greenwald-Giemsa or by the Gridley techniques. Yeast cells recovered after 24 h of residence within the cytoplasm of immune monocytes excluded the dye eosin-Y, germinated on Casamino Acid agar incubated at 30 C, and initiated growth at a normal rate within phagocytes from normal animals. Thus even though the growth of H. capsulatum was inhibited within macrophages from immunized animals, the fungus was not killed by the encounter. However, autoradiographic studies established that protein synthesis by the fungus in situ was impaired.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cline M. J. Microbicidal activity of human eosinophils. J Reticuloendothel Soc. 1972 Sep;12(3):332–339. [PubMed] [Google Scholar]
- Dabrowa N., Howard D. H., Landau J. W., Shechter Y. Synthesis of nueic acids and proteins in the dimorphic forms of Candida albicans. Sabouraudia. 1970 Nov;8(3):163–169. doi: 10.1080/00362177085190831. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973 Feb;7(2):231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry L. O., Remington J. S. Resistance against Cryptococcus conferred by intracellular bacteria and protozoa. J Infect Dis. 1971 Jan;123(1):22–31. doi: 10.1093/infdis/123.1.22. [DOI] [PubMed] [Google Scholar]
- HOWARD D. H. INTRACELLULAR BEHAVIOR OF HISTOPLASMA CAPSULATUM. J Bacteriol. 1964 Jan;87:33–38. doi: 10.1128/jb.87.1.33-38.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD D. H. Some factors which affect the initiation of growth of Cryptococcus neoformans. J Bacteriol. 1961 Sep;82:430–435. doi: 10.1128/jb.82.3.430-435.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. H. Effect of temperature on the intracellular growth of Histoplasma capsulatum. J Bacteriol. 1967 Jan;93(1):438–444. doi: 10.1128/jb.93.1.438-444.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. H., Otto V., Gupta R. K. Lymphocyte-mediated cellular immunity in histoplasmosis. Infect Immun. 1971 Nov;4(5):605–610. doi: 10.1128/iai.4.5.605-610.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. H., Otto V. Protein synthesis by phagocytized yeast cells of Histoplasma capsulatum. Sabouraudia. 1969 Oct;7(3):186–194. [PubMed] [Google Scholar]
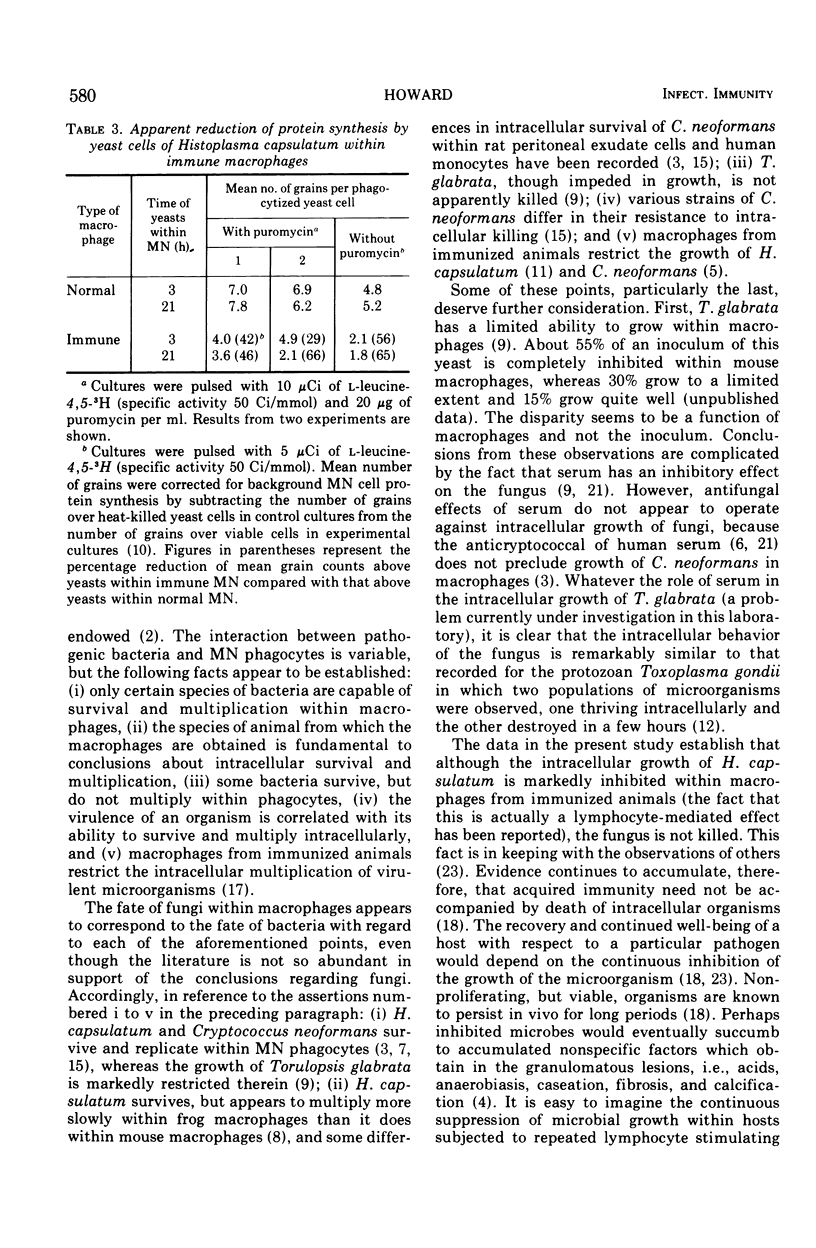
- Howard D. H., Otto V. The intracellular behavior of Torulopsis glabrata. Sabouraudia. 1967 Feb;5(3):235–239. [PubMed] [Google Scholar]
- In vitro methods in cell-mediated immunity: a progress report. Cell Immunol. 1973 Mar;6(3):331–347. doi: 10.1016/0008-8749(73)90034-8. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Yeh S., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. I. Mechanism of entry and intracellular fate of the parasite. J Exp Med. 1972 Nov 1;136(5):1157–1172. doi: 10.1084/jem.136.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- LUND E., LYCKE E., SOURANDER P. A cinematographic study of Toxoplasma gondii in cell cultures. Br J Exp Pathol. 1961 Aug;42:357–362. [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. G., Friedman L. In vitro phagocytosis and intracellular fate of variously encapsulated strains of Cryptococcus neoformans. Infect Immun. 1972 Apr;5(4):491–498. doi: 10.1128/iai.5.4.491-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMMERS D. F., HASENCLEVER H. F. IN VITRO INHIBITION OF YEAST GROWTH BY MOUSE ASCITES FLUID AND SERUM. J Bacteriol. 1964 Jan;87:1–7. doi: 10.1128/jb.87.1.1-7.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarra A. J., Paul B. B., Jacobs A. A., Strauss R. R., Mitchell G. W., Jr Role of the phagocyte in host-parasite interactions. 38. Metabolic activities of the phagocyte as related to antimicrobial action. J Reticuloendothel Soc. 1972 Aug;12(2):109–126. [PubMed] [Google Scholar]
- Youmans G. P. The role of lymphocytes and other factors in antimicrobial cellular immunity. J Reticuloendothel Soc. 1971 Jul;10(1):100–119. [PubMed] [Google Scholar]



