Abstract
Abscission is the last step of cytokinesis that leads to the physical separation of two daughter cells. An emerging picture is that abscission is a complex event that relies on changes in both lipid composition and cytoskeletal dynamics. These subcellular processes lead to the establishment of the abscission site and recruitment of the ESCRT-III protein complex to mediate the final separation event. It has become apparent that endocytic transport to the cleavage furrow during late cytokinesis mediates and coordinates lipid and cytoskeleton dynamics, thus playing a key role in abscission. Furthermore, new evidence suggests that endosomes may have additional roles in post-mitotic cellular events, such as midbody inheritance and degradation. Here, we highlight recent findings regarding the function of these endosomes in the regulation of cell division.
Keywords: endosome, phosphoinositide, Rho kinase, ESCRT-III complex
Abscission in animal cells
Cytoskeletal dynamics during abscission
Mitotic cell division ends in a very complex physical separation of daughter cells, known as cytokinesis. Cytokinesis starts during anaphase with the assembly and activation of the actomyosin contractile ring, leading to the formation and ingression of the cleavage furrow [1–3] (Figure 1A). The mechanisms regulating the actomyosin ring during early cytokinesis are well understood and rely on the centralspindlin-dependent activation of RhoA GTPase (Box 1) [4]. RhoA affects the actomyosin ring by regulating two distinct pathways (Figure 1B): i) RhoA stimulates the formation of unbranched actin filaments by activating Diaphanous-related formins [5, 6]; ii) RhoA activates ROCK kinase, which regulates the activation of myosin II, an event required for actomyosin ring contraction [3, 7]. RhoA is also known to bind anillin, a scaffolding protein that contributes to the attachment of the actomyosin network to the plasma membrane [8].
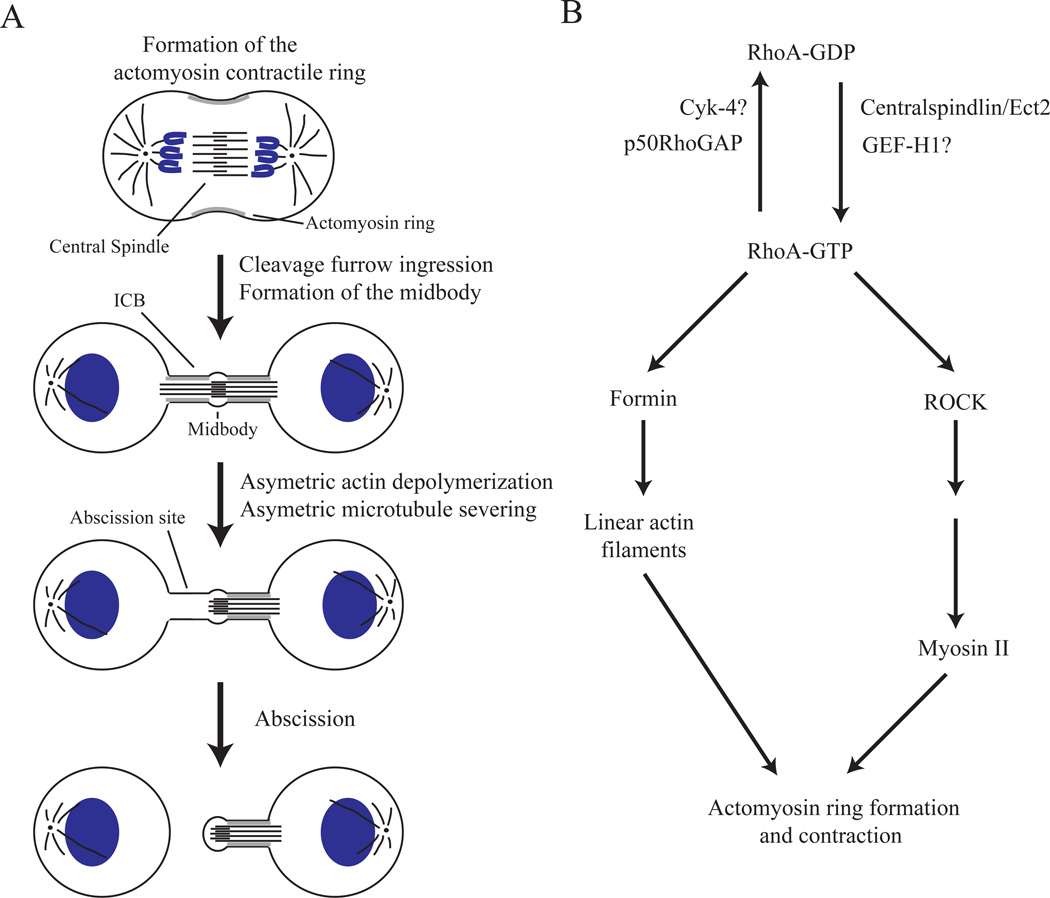
Figure 1. Cytoskeletal dynamics during cytokinesis.
(A) Schematic representation of actin and microtubule reorganization during cytokinesis. Cytokinesis is initiated by the formation of the cortical actomyosin network at the midzone of the dividing cell. Contraction of the actomyosin network leads to the cleavage furrow ingression and the formation of the intercellular bridge (ICB). ICB formation also leads to the compaction of the central spindle microtubules into a structure called the midbody. During late telophase, microtubules are severed and depolymerized in an asymmetric fashion, thus establishing the future abscission site. Microtubule severing is also accompanied by localized actin depolymerization at the abscission site. All of these localized changes in the cytoskeleton establish the abscission site and are required for the successful completion of cytokinesis.
(B) Schematic representation of RhoA-dependent regulation of the actomyosin contractile ring. The RhoA small GTPase exists in two states: GDP-bound (inactive) and GTP-bound (active). The transition of RhoA into its GTP-bound state is mediated by Ect2 and possibly by GEF-H1. Ect2 is recruited to the midzone of the dividing cell by binding to the Centralspindlin complex (consisting of Cyk-4 and MKLP1), which binds to the plus-ends of central spindle microtubules. Activated RhoA then induces the formation of non-branched actin filaments by activating formins. RhoA also activates myosin by inducing ROCK-dependent phosphorylation of the myosin light chain (MLC). At late telophase, RhoA is inactivated by p50RhoGAP and possibly Cyk-4, thus converting RhoA to its GDP-bound state.
Box 1. RhoA regulates the formation and activation of the actomyosin contractile ring.
RhoA GTPase is a well-established regulator of the formation and activation of the actomyosin contractile ring during cytokinesis. As do all small monomeric GTPases, RhoA cycles between GTP-bound (active) and GDP-bound (inactive) states. The localized activation of RhoA at the midzone of the dividing cells is mediated by the centralspindlin complex (reviewed in [4]). The centralspindlin complex consists of kinesin-like MKLP1 and Cyk-4 (also known as MgcRacGAP) proteins. The centralspindlin complex binds to the plus-ends of central spindle microtubules and functions by recruiting the Ect2 protein. Ect2 (also known as Pebble in fly) is a guanine-nucleotide exchange factor (GEF) for RhoA, resulting in the activation of RhoA at the midzone of the mitotic cell. Ect2 is also phosphorylated and inactivated by CDK1, thus temporally coordinating chromosomal segregation and the initiation of cytokinesis. In addition to Ect2, GEF-H1 was also shown to act as a RhoA GEF during cytokinesis, although the exact role of GEF-H1 during cytokinesis remains to be defined [60].
The inactivation of small monomeric GTPases is mediated by GTPase activating proteins (GAPs). The identity of the GAPs that regulate RhoA during cytokinesis remains controversial. Cyk-4 itself has a GAP domain and was suggested to also mediate RhoA inactivation [61, 62]. The functional significance of the presence of RhoA GAP (Cyk-4) and GEF (Ect2) within the same complex is unclear, but it was proposed that constant RhoA cycling between active and inactive states is required for the proper localization and function of RhoA [61]. Alternatively, Cyk-4 may be a GAP for Rac1. A recent study suggested that Cyk-4 acts by inactivating Rac1 and inhibiting Arp2/3-dependent branched actin filament formation, while allowing RhoA/formin-dependent linear actin filament assembly [63].
In addition to Cyk-4, p50RhoGAP was also shown to be required for RhoA inactivation and actin disassembly during cytokinesis [12]. Unlike Cyk-4, RhoA does not bind microtubules, but is activated by binding to FIP3/Rab11-containing endosomes and is delivered to the cleavage furrow during late telophase [12].
While formation of the actomyosin ring is crucial for successful furrow ingression, it is apparent that the actomyosin network is actually a very dynamic structure, constantly undergoing highly controlled cycles of polymerization and depolymerization. Contraction of the actin network is driven not only by myosin II motor activity, but also by the localized depolymerization of crosslinked actin filaments [9]. Furthermore, the actomyosin ring disassembles at a rate comparable to its contraction, because the concentration of actin and myosin II units remains constant throughout furrow ingression [10, 11]. This coordinated contraction and disassembly of actomyosin units during early cytokinesis is presumably required to allow a very rapid decrease in cleavage furrow width during division.
Once the ingression of the cleavage furrow is complete, daughter cells remain connected by a 1–3 µm thick intercellular bridge (ICB). Intriguingly, the actomyosin cortical network now needs to be disassembled to allow for the final abscission step to occur (Glossary, Figure 1A). Indeed, the inhibition of actin depolymerization within the ICB during late telophase inhibits the completion of cytokinesis [12, 13]. The machinery regulating this disassembly of cortical actin within the ICB remains poorly understood, but appears to involve the localized inactivation of RhoA [4] and the regulation of plasma membrane lipid composition of the ICB [12, 13].
In addition to actin, microtubules are intimately involved in cytokinesis. Both astral and central spindle microtubules are involved in the positioning and activation of the actomyosin ring (Box1). Furthermore, upon cleavage furrow ingression, central spindle microtubules become compacted and crosslinked to form a central spindle bundle that is located within the ICB (Figure 1A). The overlapping plus-ends of these microtubules, along with an amorphous electron-dense material, form a structure known as the midbody (MB). Currently it has been proposed that MBs can also serve as “recruiting platforms” that bind and/or activate many signaling proteins important in regulating cell division and post-mitotic cell fate [14, 15]. The composition, regulation and function of MBs have been the subject of recent review [16], hence, here we will only briefly describe the machinery regulating the function and inheritance of MBs during cell division.
Since abscission involves fusion of opposing plasma membranes, it became evident that microtubules within the ICB need to be disassembled on one side (or both sides in some cases) of the MB to allow for the completion of cytokinesis (Figure 1A). The regulation of this “nested” microtubule disassembly remains to be fully understood, but several studies have recently demonstrated that spastin appears to be required for microtubule severing during cytokinesis [17, 18]. In addition, microtubule bending and breaking also contributes to microtubule severing during abscission [19]. Interestingly, spastin was shown to preferentially act on bent microtubules, suggesting that both processes may act together to ensure efficient microtubule severing. Finally, at least in certain cell types, minus-end depolymerization also plays a role in abscission [19]. During interphase and early cytokinesis, microtubule minus-ends are anchored to the centrosomes, thus preventing their depolymerization. In contrast, during telophase central spindle microtubules are released from the centrosome, presumably by spastin/fidgetin-dependent severing [19, 20] (Figure 1A). How central spindle microtubule minus-end stability is regulated during late cytokinesis is not presently clear, but appears to involve kinesin 14 HSET and abnormal spindle (Asp) proteins [21, 22]. These proteins were shown to bind to γ-tubulin, are required for abscission and regulate the stability/bundling of the non-centrosomal minus-ends of central spindle microtubules [21, 22].
The ESCRT Complex mediates the abscission step of cytokinesis
Recently, the ESCRT complexes (Glossary) have emerged as key proteins required for abscission (Box 2). Originally, ESCRTs were implicated in regulating cytokinesis in light of the topological similarities between scission of the intraluminal vesicle necks during MVB maturation and fusion of the ICB plasma membrane. Consistent with their role during abscission, depletion of several ESCRT-III complex proteins results in a multinuclear phenotype, an indication of the failure of cytokinesis [23, 24]. The ESCRT-0, -I and -II complexes are largely dispensable during abscission, presumably because cytokinesis does not require the recruitment and accumulation of ubiquitinated cargo proteins. Instead, ESCRT-III is recruited directly to Cep55, which is localized at the midbody during cytokinesis [17, 24, 25]. ESCRT-III is perfectly suited to induce the scission of the ICB, since it can form oligomers that induce changes in liposome and cell membrane shape. Furthermore, ESCRT-III forms 17 nm filaments within the ICB during cytokinesis [26], and is recruited to the abscission site [17, 26]. Interestingly, Archaea cells possess only the ESCRT-III-like protein complex, which is also required for cell division [27]. This raises the possibility that the ESCRT complexes in eukaryotic cells originally evolved for cytokinesis and only later were adapted for the regulation of lysosomal targeting.
Box 2. ESCRTs and the formation of intraluminal vesicles.
Most membrane-embedded or luminal proteins are degraded via transport through the endocytic pathway to lysosomes. This targeting to the lysosomes is driven by protein ubiquitination and the formation of intraluminal vesicles (ILVs) within mid-stage endosomes, known as multivesicular bodies (MVBs) (reviewed in [64]). Among the proteins that mediate ILV formation, many were identified a couple of decades ago via genetic screens of vacuolar protein sorting in Saccharomyces cerevisiae. Most of the proteins identified in these screens were soluble proteins that regulated ILV formation by cycling on and off the limiting MVB membranes. Multiple studies from many laboratories have shown that these proteins form four complexes, known as endosomal sorting complexes required for transport (ESCRT). ESCRT complexes include ESCRT-0, -I, -II and –III, all of which work in a sequential manner to regulate the sorting of ubiquitinated proteins (ESCRT-0 and ESCRT-I) as well as the formation (ESCRT-I and ESCRT-II) and scission (ESCRT-III) of ILVs [64]. ESCRT-III is most enigmatic of these complexes and it remains unclear how ESCRT-III actually causes the scission of the ILV neck. The budding yeast ESCRT-III complex contains six proteins, with four of these proteins forming a core ESCRT-III complex (Vps2p, Vps20p, Vps24p and Snf7p). Snf7p is the functional unit of the complex, since it was shown to form long spiral filaments that are capable of deforming membranes in vitro and in vivo [30, 65]. Mammalian ESCRT-III consists of 11 proteins, which are often referred to as charged MVB proteins (CHMPs). In addition, there is some functional redundancy between different CHMPs. For example, CHMP4 (a Snf7p orthologue) comes in three different isoforms, CHMP4A, CHMP4B and CHMP4C. While all three isoforms clearly have some functional redundancy, recent studies also suggested that they may perform isoform-specific functions during ILV formation and cytokinesis [66]. It is clear that the assembly of ESCRT-III proteins in spiral, membrane-bound filaments is required for ILV formation and scission. However, what is missing from most of these models is an understanding of how the polymerization of CHMP4/Snf proteins actually drives membrane deformation and scission of the ILV necks.
While the role of the ESCRT-III complex as a scission factor is now firmly established, several questions remain about the machinery and regulation of ESCRT-III recruitment to the abscission site. One such question is in regards to the size of the ICB. During MVB maturation, ESCRT-III generates intraluminal vesicles that are ~30 nm in diameter as compared to 1–3 µm thick ICB [28]. Furthermore, ESCRT-III prefers to bind highly curved membranes [29]. Finally, CHMP4A/B-overexpression-induced assembly of circular arrays at the plasma membrane is inhibited by cortical actin network [30]. How can the ESCRT-III complex accommodate the drastic size difference between vesicle budding and scission of actin-supported ICB? It is also remains unclear how ESCRT-III moves from the midbody to the abscission site (Figure 2). One possibility is that the ESCRT-III complex forms spiral filaments that emanate from the midbody and cause the gradual constriction of the ICB, thus forming an abscission site [26]. However, the abscission site often forms a considerable distance away from the midbody [12]. Furthermore, it was shown that the thinning of the ICB at the abscission site, a process known as secondary ingression, precedes the recruitment of ESCRT-III [12]. An additional possibility is that ESCRT-III filaments are assembled de novo from an ESCRT-III cytosolic pool at the abscission site. Consistent with this model, the CHMP4B at the abscission site often forms a distinct pool, separated from the CHMP4B pool at the midbody [12, 17], although it remains to be determined whether the ESCRT-III “abscission pool” is assembled independently from the midbody ESCRT proteins. Another intriguing possibility is that ESCRT proteins may need to first be recruited to the MB to get activated before they can translocate to the abscission site. Many ESCRT-III complex proteins exist in the cytosol in a “closed” conformation [31, 32]. This closed conformation is dependent upon the highly charged C-terminal autoinhibitory domain present in proteins such as Vps24p and Snf7p [31]. The activation or “opening” of these proteins is needed before they can be recruited to the membranes and interact with other ESCRT-III components [33]. In addition, MBs are known to contain many activated mitotic kinases, such as Aurora B and Plk1, although it remains to be demonstrated whether any of these kinases directly phosphorylate and regulate ESCRT-III complex proteins.
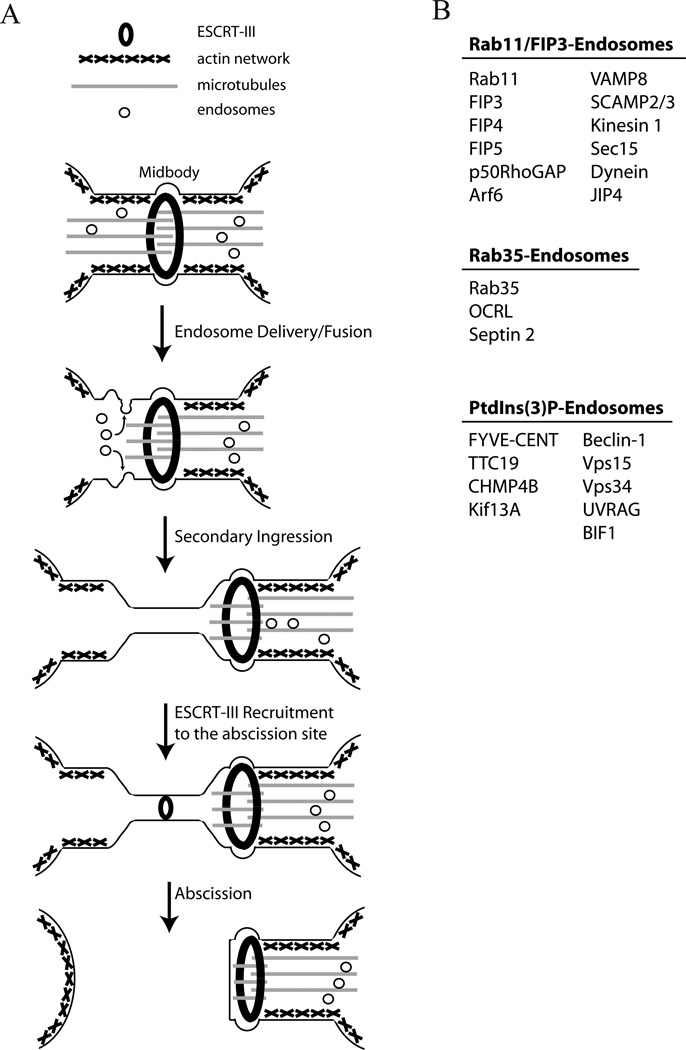
Figure 2. Endosomes and the ESCRT-III complex mediate abscission.
(A) Schematic representation of cell abscission. During telophase, endosomes are deliverer to the ICB by moving along central spindle microtubules. At the same time, ESCRT-III components accumulate at the midbody by binding Cep55. As the cell progresses through telophase, microtubules are depolymerized at the future abscission site. Localized microtubule depolymerization is mediated by several mechanisms, including spastin-dependent severing and bucking-induced breakage. Microtubule depolymerization is accompanied by endosome fusion with the ICB’s plasma membrane, leading to cortical actin disassembly and the formation of the secondary ingression. Finally, ESCRT-III is recruited from the midbody to the abscission site via mechanisms that remain to be determined. ESCRT-III recruitment to the abscission site then leads to the plasma membrane fusion and the separation of daughter cells.
(B) At least three types of endosomes are known to be transported to the ICB and be involved in regulating abscission. The cargo proteins that have been experimentally shown to reside and function within these organelles are listed.
Endocytic transport is required for the completion of cytokinesis
In addition to ESCRT proteins, endosomes have recently emerged as key regulators of late cytokinesis [1, 34]. The requirement for endosomes was first demonstrated in studies analyzing cellularization during the development of the fly embryo. It was demonstrated that Rab11 GTPase and its binding protein Nuf are both required for actin polymerization and furrow ingression during the process of cellularization process [35–37]. Soon after, it was demonstrated that recycling endosomes containing Rab11 GTPase are also required for cytokinesis in mammalian cells [38, 39]. However, in contrast to the flies, in mammalian cells Rab11 appears to be dispensable during furrow ingression, but instead is required for abscission [38–40].
All Rab GTPases function by activating and recruiting specific effector proteins to membranes. In a series of studies, it was discovered that Rab11-binding proteins FIP3 and FIP4 (Rab11 family interacting protein 3 and 4) localize to the ICB and are required for abscission [38, 39, 41]. Since FIP3 is a highly specific marker for recycling endosomes that accumulates within the ICB, this subset of endosomes is often referred to as FIP3-endosomes [40]. Spatiotemporal analysis of Rab11/FIP3-endosomes demonstrated that they are delivered via Kinesin-1-dependent transport along central spindle microtubules [42] and accumulate at the ICB by binding to the Exocyst and the Centralspindlin complexes [38, 40]. Furthermore, VAMP8-mediated fusion of FIP3-endosomes with the plasma membrane of the ICB was shown to be required for the formation of the secondary ingression and CHMP4B recruitment to the abscission site (Figure 2) [12, 19].
Recently it became apparent that Rab11/FIP3-organelles are not the only endosomes required for abscission. Rab35 has also emerged as a protein required for cytokinesis [43]. While the spatiotemporal dynamics of Rab35-endosomes during cytokinesis remains to be defined, it is now clear that Rab35-organelles are a distinct subtype of endosomes that carry cargo proteins, such as Septin 2 and oculocerebrorenal syndrome of Lowe (OCRL), which are not present in Rab11/FIP3-endosomes (Figure 2) [13]. Interestingly, while Rab35 is required for the abscission step of cytokinesis, some evidence suggests that Rab35 may additionally regulate the ingression and stability of the cleavage furrow [13].
PtdIns(3)P-rich endosomes also have emerged as important regulators of cytokinesis. The role of PtdIns(3)P was uncovered in a study using GFP-tagged 2xFYVE, a known sensor for PtdIns(3)P [44]. It was observed that GFP-2xFYVE-containing vesicles were present within the ICB during cytokinesis [44]. While the identity of these organelles is not clear, the evidence available so far suggests that they are organelles distinct from Rab35-endosomes (Figure 2). The relationship between FIP3-endosomes and PtdIns(3)P-rich endosomes still needs to be determined, especially since both were shown to contain the transferrin receptor [45]. Moreover, PtdIns(3)P-rich endosomes were shown to contain the protein known as FYVE-CENT (FYVE domain containing centrosomal protein) [46]. FYVE-CENT binds to tetratricopeptide repeat domain 19 protein (TTC19) and kinesin-like protein Kif13A, with all three of these proteins being required for cytokinesis [46]. The function of the FYVE-CENT/TTC19 complex remains unclear, but as it was shown that TTC19 binds to CHMP4B, it is possible that PtdIns(3)-Prich endosomes may be required for the assembly of ESCRT-III at the abscission site. What makes PtdIns(3)P-rich endosomes especially interesting is the finding that they contain a Vps15/Vps35/Beclin-1 protein complex, along with its interacting BIF-1 and UVRAG proteins [47]. All of these proteins together form the so-called Activating UVRAG complex, which is required for autophagosome maturation [48]. Since autophagosomes mediate the degradation of post-mitotic midbodies (see below), it is possible that PtdIns(3)P-rich endosomes may be involved in midbody inheritance.
Taken together, recent studies have led to the conclusion that the coordinated action of ESCRT-III along with several different types of endosomes is required for the successful completion of cytokinesis. The transport and fusion of endosomes with the ICB plasma membrane is likely required to induce the secondary ingression and “prepare” the abscission site for the eventual recruitment of the ESCRT-III for the final membrane scission event (Figure 2).
The role of endosomes in regulating lipids during cytokinesis
Endosomes and PtdIns(3)P during cytokinesis
Phoshoinositides are well known regulators of many cellular processes including signaling, cytoskeleton and membrane transport. One of them, PtdIns(3)P, was recently suggested to regulate cytokinesis, based on the observations that PtdIns(3)P-endosomes are present at the ICB, and that PI3K inhibitors block cytokinesis and lead to the accumulation of multinuclear cells (reviewed in [45]). The function of PtdIns(3)P during cytokinesis remains to be fully understood, but various ESCRT complex subunits bind PtdIns(3)P (reviewed in [49]). Thus, it is possible that the delivery of PtdIns(3)P to the abscission site may be required for the recruitment and assembly of the ESCRT-III filaments during abscission. PtdIns(3)P also recruits several effector proteins to endosomes, such as FYVE-CENT, thus ensuring their delivery to the ICB [46]. FYVE-CENT binds and recruits several other effector proteins, namely TTC19, Becklin1, Vps15, and UVRAG, which are known to regulate protein degradation and ESCRT-III recruitment [48]. Interestingly, FYVE-CENT also binds and recruits Vps34 (PI3KC3), which generates PI(3)P by phosphorylating PtdIns. Thus, the FYVE-CENT/Vps34 complex is likely involved in a positive-feedback loop that is needed to maintain high concentrations of PtdIns(3)P within the furrow.
Endosomes and PtdIns(4,5)P2 during cytokinesis
PtdIns(4,5)P2 also has emerged as a critical player in the regulation of cytokinesis. PtdIns(4,5)P2 is enriched at the cleavage furrow and was implicated in regulating inositol trisphosphate (IP3)-dependent calcium signaling during cytokinesis (reviewed in [50]). Importantly, the endocytic transport itself is dependent on PtdIns(4,5)P2. The Exocyst protein complex, known for its role in targeting endosomes to the ICB, can bind directly to PtdIns(4,5)P2, although the function of this binding during cytokinesis remains unclear (reviewed in [51]). The best-established role of PtdIns(4,5)P2 is its regulation of the actin cytoskeleton. It is clearly documented that the accumulation of PtdIns(4,5)P2 during early cytokinesis is required for the assembly and activation of the actomyosin ring (reviewed in [52]). Septins, filament-forming cytoskeletal proteins, were also shown to interact with PtdIns(4,5)P2 [8]. This interaction lowers the concentration of septin required for filament formation and mediates the cross-linking of the actomyosins and the septin cortical networks to the plasma membrane of the ICB. Interestingly, while high levels of PtdIns(4,5)P2 are required during early cytokinesis, the dramatic decrease in PtdIns(4,5)P2 concentration within the ICB is required for the cell to undergo abscission, presumably to allow for actin depolymerization in the furrow just before abscission [13]. Thus, PtdIns(4,5)P2 levels are dynamically regulated to ensure the fidelity of cytokinesis.
Recent studies have established that endosomes are directly involved in regulating PtdIns(4,5)P2 levels within the ICB. Arf6, a known cargo protein of Rab11-endosomes, binds and activates PIP5K, an enzyme that converts PtdIns(4)P to PtdIns(4,5)P2. Since Arf6 itself is activated by PtdIns(4,5)P2, it is possible that Arf6 provides a positivefeedback loop within the furrow to maintain high concentrations of PtdIns(4,5)P2. In contrast, Rab35-endosomes regulate the decrease in PtdIns(4,5)P2 levels during late cytokinesis. Rab35-endosomes deliver the PtdIns(4,5)P2 phosphatase OCRL to the ICB. OCRL then dephosphorylates PtdIns(4,5)P2, leading to a decrease in furrow PtdIns(4,5)P2 levels, the step that is a prerequisite for abscission [13, 53]. Interestingly, OCRL is mutated in patients with oculocerebrorenal syndrome of Lowe, and is required for cytokinesis.
The role of endosomes in regulating the actin cytoskeleton during cytokinesis
Recent work strongly implicates endosomes in regulating actin dynamics during furrow ingression and abscission. The first indications that Rab11-endosomes may play a role in regulating the actomyosin ring came from work in Drosophila embryos. It was observed that, during ingression of the cleavage furrow, membrane addition and simultaneous actin polymerization occurs at the leading edge of the ingressing cleavage furrow. The source of this membrane and these actin filaments has not been precisely defined, but evidence implicates the recycling endosome components, Rab11 and Nuclear-fallout (Nuf; FIP3 orthologue in mammals), in the delivery of membrane and the recruitment of actin to the cleavage furrow [36, 54, 55]. In addition, in Drosophila embryos, Nuf is required to recruit the membrane-associated discontinuous actin hexagon (DAH) protein to invaginating furrows during embryo cellularization [54]. When at the furrow, DAH interacts with the actin cytoskeleton, likely mediating the formation of the linkage between the actin cytoskeleton and the membrane of the cleavage furrow [56]. Interestingly, Nuf is not required for the recruitment of peanut (septin orthologue in fly) and anillin, and is likely delivering endosomal cargos to the cleavage furrow to regulate the actin cytoskeleton [57]. Consistent with this hypothesis, during live imaging of these Drosophila embryos, F-actin-associated endosomal vesicles were recruited to the cleavage furrow along central spindle microtubules [55], where they regulate the delivery of membranes and reorganization of the actin cytoskeleton at the furrow.
During late telophase in mammalian cells, constriction of the actomyosin contractile ring leads to the formation of the ICB, which is stabilized by a cortical actomyosin network at the plasma membrane. This actomyosin network is formed, in part, by the remnants of the actomyosin contractile ring and by the actin cytoskeleton regulator PtdIns(4,5)P2. While regulation of the actin cytoskeleton by PtdIns(4,5)P2 has been expertly covered elsewhere [50], it is important to note that high levels of PtdIns(4,5)P2 are associated with actin polymerization, while low levels of PtdIns(4,5)P2 are correlated with actin filament severing and disassembly.
At the end of cytokinesis, the stable ICB transitions into a more dynamic ICB, where plasma membrane waves are produced near the midbody, and the ICB undergoes further compaction and secondary ingression [12, 19, 58]. This transition is a prerequisite for the formation of the secondary ingression and abscission. To accomplish this transition, the cortical actomyosin network of the ICB is disassembled, priming the ICB for ESCRT-mediated abscission [12, 17]. When disassembly of F-actin is impaired in Drosophila twinstar mutants, abnormally large cleavage furrows develop and defects in cytokinesis occur. Reorganization of the actin network is accomplished through two different mechanisms, each performed by recycling endosomes. The first mechanism is mediated by Rab35-endosomes, which deliver OCRL to the ICB to dephosphorylate PtdIns(4,5)P2 [13]. Knock-down of either Rab35 or OCRL increases F-actin levels within the ICB and leads to abscission defects. The second mechanism is mediated by p50RhoGAP, which is delivered by FIP3-endosomes to the ICB during late cytokinesis [12]. The depletion of p50RhoGAP, a GAP for RhoA GTPase, also causes an increase in ICB cortical F-actin, as well as abscission delays. Together, Rab35 and Rab11/FIP3 endosomes likely cooperate to regulate the reorganization of the cortical actomyosin network inside the ICB, thus enabling the completion of cytokinesis.
Endosomes and midbody inheritance
The final abscission event of cytokinesis involves the severing of the ICB on one (asymmetric abscission) or on both sides of the MB (Figure 3). In the case of asymmetric abscission, one of the daughter cells retains the MB (midbody inheritance) (reviewed in [16]). This post-mitotic MB derivative (MBd) has been recently shown to be inherited by less differentiated, stem cell-like cell populations both in vitro and in vivo [14, 15, 59]. Similarly, MBd accumulation was shown in many cancer cell lines and was correlated with elevated aggressiveness and increased proliferation of cancer cells [14]. In addition, MBds are preferentially inherited by the daughter cell containing the older centrosome [14, 59]. Thus, it is clear that the establishment of the abscission site is not a stochastic event, but is tightly regulated by the dividing cell. The machinery mediating this asymmetric abscission and midbody inheritance remains to be understood. However, since Rab11/FIP3-endosomes were shown to asymmetrically fuse with the plasma membrane of the ICB [12, 19], it is tempting to speculate that FIP3-endosomes may play a role in determining the timing and location of abscission. During early cytokinesis Rab11/FIP3-endosomes associate with centrosomes and move to the ICB only during the late stages of division [19, 40]. Thus, Rab11/FIP3-endosomes may also govern the communication between centrosomes and the establishment of the abscission site.
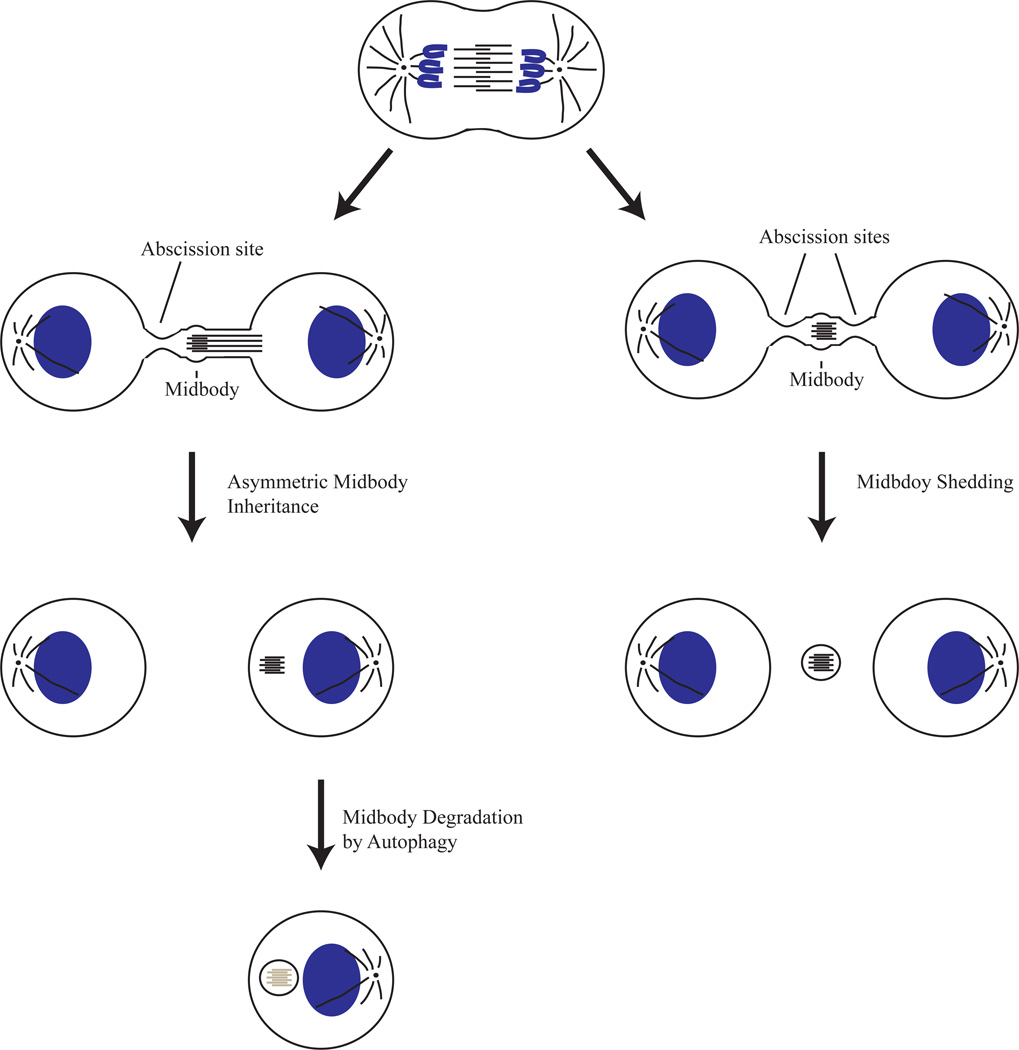
Figure 3. Mechanisms regulating midbody inheritance.
During late telophase cells can form an abscission site either on one side (asymmetric inheritance) or both sides (shedding) of the midbody. In the case of a single abscission site, only one of the cells inherits the midbody. Midbody retention within this cell is then regulated by autohphagy. In contrast, if the cell forms two abscission sites, the midbody is discarded.
Recent studies have shown that MBd retention is regulated by lysosomal degradation (Figure 3) [14, 59]. The MBd is degraded by Cep55-dependent scaffolding of NBR1 to activate the selective autophagy pathway and to encapsulate the midbody in the autophagic membrane. Rab11-positive and PtdIns(3)P-rich endosomes were both implicated in the delivery of membranes during autophagosome formation. In addition, PtdIns(3)P-rich endosomes contain the activating UVRAG protein complex (Beclin1/Vps34/Vps15/UVRAG/BIF1 proteins), which regulates autophagosome formation and maturation (reviewed in [48]). It is possible that these endosomes are not only involved in abscission, but also in the delivery of membrane and potentially cargo proteins during post-mitotic MBd degradation. Further experimentation will be needed to unravel the mechanisms behind the setting up and execution of MB degradation post-cell division.
Concluding remarks
Recent studies have begun to unveil the molecular composition, dynamics and regulation of the abscission process. We now understand the basic sequence of the events that lead to the severing of the ICB and separation of the daughter cells. It has also become apparent that abscission is an immensely complex process that relies on coordinated changes in the cytoskeleton, lipid composition and endocytic membrane transport. Thus, while our understanding of the molecular machinery regulating abscission is progressing rapidly, many open questions remain.
Two of the most important questions are: i) how the ESCRT-III complex relocates from the MB to the abscission site; and ii) what is the spatiotemporal regulation of ESCRT-III recruitment? Several theoretical models explaining ESCRT-III targeting have been proposed, however data unequivocally supporting either one of these models is still lacking. Additionally, we still do not understand what the molecular mechanism is that mediates ESCRT-based membrane scission. To a large extent the difficulties in understanding the timing and regulation of ESCRT-III recruitment is due to the resolution limits of light microscopy, which prevent image analysis of microtubule severing, endosome fusion and ESCRT-III recruitment during abscission. The emergence of super-resolution imaging techniques should allow the field to systematically start addressing these issues.
Another question is why are there so many different endocytic transport pathways required for abscission? In this review we focused only on the three best-characterized endosomes and their involvement in cytokinesis. The ever-expanding list of proteins regulating abscission includes several other Rab GTPases, namely Rab4, Rab10, Rab8 and Rab14, which may mark distinct endocytic transport pathways. One possibility is that some of these endosomes may only play a role in cytokinesis within specific tissues or cell types. Indeed, we already know that there are significant differences in the process of cytokinesis between polarized (e.g., MDCK) and non-polarized (e.g., HeLa) cells [17, 19]. Furthermore, it is likely that additional levels of regulation are needed in the case of asymmetric cell division. Experiments assessing the involvement of all these endosomes during different types of cell division will be needed.
Finally, recent studies demonstrating the role of MB inheritance on cell fate and proliferation clearly demonstrate that the establishment of the abscission site within the ICB is not a stochastic, but a highly regulated event. Yet, we know very little about what regulates asymmetric microtubule severing and asymmetric endosome transport and fusion. As the field progresses, it will be interesting to determine the machinery regulating the establishment of the abscission site and how this machinery is connected to cancer progression and cell differentiation. Finally, since most of the abscission studies have been done in vitro, it remains unclear whether similar machinery mediates abscission in vivo and how this machinery is regulated in different tissues. Some data already suggests that different machinery regulate cytokinesis in different cell types. Thus, further studies will be required to dissect the molecular mechanisms of abscission and midbody inheritance in vivo.
Acknowledgements
We are grateful to Dr. Gwyn Gould (University of Glasgow) for the critical reading of the manuscript. We apologize to all colleagues whose work could not be cited due to space limitations. We also acknowledge the financial support by NIH (to R.P.), Cancer League of Colorado (to R.P.) and Susan G. Komen for the Cure foundation (to R.P.).
Glossary
- Abscission
The final step of cytokinesis leading to the fusion of ICB plasma membrane and the separation of the daughter cells. Abscission is a very complex event that includes dramatic changes in ICB microtubules and the actin cytoskeleton. Abscission is also dependent on endosomal transport and ESCRT complex formation
- Autophagy
Autophagy or “self-eating” is used to describe several related pathways that degrade large cytosolic structures, such as mitochondria, midbodies or other membrane bound organelles. Autophagy relies on engulfing these cytosolic structures via the de novo formation of double-membrane organelles, known as autophagosomes. The formation of these autophagosomes depends on several protein complexes, namely autophagy-related genes (ATGs) and the Vps15/Vps34/Becklin 1 complex
- Centralspindlin complex
The centralspindlin complex is a heterodimer consisting of MKLP1 and Cyk4 (also known as MgcRacGAP) proteins. The centralspindlin complex is known to play a key role in the crosslinking of the microtubule cytoskeleton, as well as formation and activation of the actomyosin contractile ring. Centralspindlin regulates the actomyosin contractile ring by activating RhoA GTPase via recruiting Ect2, a known RhoA GEF. Centralspindlin can also bind to the FIP3/Rab11 complex, thus regulating FIP3-endosome accumulation at the ICB during late cytokinesis
- Endosomal sorting complex required for transport (ESCRT)
ESCRTs are protein complexes that were originally identified as regulators of protein targeting to lysosomes. ESCRTs work by mediating cargo recruitment and the formation of intraluminal vesicles during the maturation of MVBs. ESCRTs are actually four distinct complexes: ESCRT-0, -I, -II, -III. Due to topological similarities, ESCRTs were also suggested to mediate membrane scission during the budding of enveloped viruses and during abscission. Consistent with this, ESCRT-III was shown to be required for abscission
- Rab11 Family Interacting Proteins (FIPs)
FIPs are well-established effectors specific to Rab11 GTPases. The FIP family consists of five members, FIPs1-5. Based on sequence homology all FIPs can be divided into two sub-families. Class I FIPs (FIP1, FIP2 and FIP5) are known for their regulation of endocytic recycling during interphase, while Class II FIPs (FIP3 and FIP4) are required for endocytic transport during cytokinesis
- Intercellular bridge (ICB)
The thin cytoplasmic bridge connecting two daughter cells during late cytokinesis. The ICB is about 1–3 µm thick and contains remnants of the actomyosin contractile ring and central spindle microtubules. The overlapping plus-ends of these microtubules form a midbody at the middle of the ICB. The depolymerization of the actomyosin ring and localized cutting/depolymerization of central spindle microtubules are required for the abscission. The transport of various endosomes as well as the oligomerization of the ESCRT-III complex within the ICB are also required for abscission
- Midbody (MB)
The midbody (also known as the Flemming body) is a structure localized in the middle of the ICB. The midbody is formed from the overlapping plusends of central spindle microtubules, which recruit various cross-linking proteins to form a very dense structure during late cytokinesis. Abscission usually occurs asymmetrically on one side of the midbody, leading to the inheritance of the midbody only by one daughter cell
- Multivesicular Bodies (MVBs)
The endocytic organelle that mediates the lysosomal degradation of proteins. The key feature of MVBs is the formation of intraluminal vesicles that contain proteins destined for degradation. The formation and scission of these intraluminal vesicles are mediated by the ESCRT complexes
- Rab GTPases
Small monomeric GTPases belonging to a larger Ras GTPase super-family. All Rab GTPases are activated by binding GTP, and function by recruiting specific effector proteins which regulate distinct membrane transport steps
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131(5):847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol. 2010;22(1):50–56. doi: 10.1016/j.ceb.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fededa JP, Gerlich DW. Molecular control of animal cell cytokinesis. Nat Cell Biol. 2012;14(5):440–447. doi: 10.1038/ncb2482. [DOI] [PubMed] [Google Scholar]
- 4.Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15(12):651–658. doi: 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Severson AF, Baillie DL, Bowerman B. A Formin Homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr Biol. 2002;12(24):2066–2075. doi: 10.1016/s0960-9822(02)01355-6. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe S, et al. mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol Biol Cell. 2008;19(5):2328–2338. doi: 10.1091/mbc.E07-10-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15(7):371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Piekny AJ, Maddox AS. The myriad roles of Anillin during cytokinesis. Semin Cell Dev Biol. 2010;21(9):881–891. doi: 10.1016/j.semcdb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Kruse K, Julicher F. Self-organization and mechanical properties of active filament bundles. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;67(5 Pt 1):051913. doi: 10.1103/PhysRevE.67.051913. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho A, Desai A, Oegema K. Structural memory in the contractile ring makes the duration of cytokinesis independent of cell size. Cell. 2009;137(5):926–937. doi: 10.1016/j.cell.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Calvert ME, et al. Myosin concentration underlies cell size-dependent scalability of actomyosin ring constriction. J Cell Biol. 2011;195(5):799–813. doi: 10.1083/jcb.201101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiel JA, et al. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat Cell Biol. 2012;14(10):1068–1078. doi: 10.1038/ncb2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dambournet D, et al. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat Cell Biol. 2011;13(8):981–988. doi: 10.1038/ncb2279. [DOI] [PubMed] [Google Scholar]
- 14.Kuo TC, et al. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat Cell Biol. 2011;13(10):1214–1223. doi: 10.1038/ncb2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ettinger AW, et al. Proliferating versus differentiating stem and cancer cells exhibit distinct midbody-release behaviour. Nat Commun. 2011;2:503. doi: 10.1038/ncomms1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CT, et al. Resurrecting remnants: the lives of post-mitotic midbodies. Trends Cell Biol. 2012 doi: 10.1016/j.tcb.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elia N, et al. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci U S A. 2011;108(12):4846–4851. doi: 10.1073/pnas.1102714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connell JW, et al. Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic. 2009;10(1):42–56. doi: 10.1111/j.1600-0854.2008.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiel JA, et al. Endocytic membrane fusion and buckling-induced microtubule severing mediate cell abscission. J Cell Sci. 2011;124(Pt 9):1411–1424. doi: 10.1242/jcs.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang D, et al. Three microtubule severing enzymes contribute to the "Pacman-flux" machinery that moves chromosomes. J Cell Biol. 2007;177(2):231–242. doi: 10.1083/jcb.200612011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakefield JG, Bonaccorsi S, Gatti M. The drosophila protein asp is involved in microtubule organization during spindle formation and cytokinesis. J Cell Biol. 2001;153(4):637–648. doi: 10.1083/jcb.153.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai S, et al. Proper organization of microtubule minus ends is needed for midzone stability and cytokinesis. Curr Biol. 2010;20(9):880–885. doi: 10.1016/j.cub.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlton JG, Agromayor M, Martin-Serrano J. Differential requirements for Alix and ESCRT-III in cytokinesis and HIV-1 release. Proc Natl Acad Sci U S A. 2008;105(30):10541–10546. doi: 10.1073/pnas.0802008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316(5833):1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 25.Morita E, et al. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26(19):4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guizetti J, et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 2011;331(6024):1616–1620. doi: 10.1126/science.1201847. [DOI] [PubMed] [Google Scholar]
- 27.Samson RY, et al. A role for the ESCRT system in cell division in archaea. Science. 2008;322(5908):1710–1713. doi: 10.1126/science.1165322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wemmer M, et al. Bro1 binding to Snf7 regulates ESCRT-III membrane scission activity in yeast. J Cell Biol. 2011;192(2):295–306. doi: 10.1083/jcb.201007018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fyfe I, et al. Association of the endosomal sorting complex ESCRT-II with the Vps20 subunit of ESCRT-III generates a curvature-sensitive complex capable of nucleating ESCRT-III filaments. J Biol Chem. 2011;286(39):34262–34270. doi: 10.1074/jbc.M111.266411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson PI, et al. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J Cell Biol. 2008;180(2):389–402. doi: 10.1083/jcb.200707031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muziol T, et al. Structural basis for budding by the ESCRT-III factor CHMP3. Dev Cell. 2006;10(6):821–830. doi: 10.1016/j.devcel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Zamborlini A, et al. Release of autoinhibition converts ESCRT-III components into potent inhibitors of HIV-1 budding. Proc Natl Acad Sci U S A. 2006;103(50):19140–19145. doi: 10.1073/pnas.0603788103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shim S, Kimpler LA, Hanson PI. Structure/function analysis of four core ESCRT-III proteins reveals common regulatory role for extreme C-terminal domain. Traffic. 2007;8(8):1068–1079. doi: 10.1111/j.1600-0854.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 34.Schiel JA, Prekeris R. Membrane dynamics during cytokinesis. Curr Opin Cell Biol. 2012 doi: 10.1016/j.ceb.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riggs B, et al. The concentration of Nuf. a Rab11 effector. at the microtubuleorganizing center is cell cycle regulated. dynein-dependent. and coincides with furrow formation. Mol Biol Cell. 2007;18(9):3313–3322. doi: 10.1091/mbc.E07-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riggs B, et al. Actin cytoskeleton remodeling during early Drosophila furrow formation requires recycling endosomal components Nuclear-fallout and Rab11. J Cell Biol. 2003;163(1):143–154. doi: 10.1083/jcb.200305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelissier A, Chauvin JP, Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr Biol. 2003;13(21):1848–1857. doi: 10.1016/j.cub.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Fielding AB, et al. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. Embo J. 2005;24(19):3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson GM, et al. The FIP3-Rab11 Protein Complex Regulates Recycling Endosome Targeting to the Cleavage Furrow during Late Cytokinesis. Mol Biol Cell. 2004 doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon GC, et al. Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. Embo J. 2008 doi: 10.1038/emboj.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horgan CP, et al. Rab11-FIP3 localises to a Rab11-positive pericentrosomal compartment during interphase and to the cleavage furrow during cytokinesis. Biochem Biophys Res Commun. 2004;319(1):83–94. doi: 10.1016/j.bbrc.2004.04.157. [DOI] [PubMed] [Google Scholar]
- 42.Montagnac G, et al. ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr Biol. 2009;19(3):184–95. doi: 10.1016/j.cub.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 43.Kouranti I, et al. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16(17):1719–1725. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 44.Gillooly DJ, et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19(17):4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nezis IP, et al. Divide and ProsPer: the emerging role of PtdIns3P in cytokinesis. Trends Cell Biol. 2010;20(11):642–649. doi: 10.1016/j.tcb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Sagona AP, et al. PtdIns(3)P controls cytokinesis through KIF13A-mediated recruitment of FYVE-CENT to the midbody. Nat Cell Biol. 2010;12(4):362–371. doi: 10.1038/ncb2036. [DOI] [PubMed] [Google Scholar]
- 47.Thoresen SB, et al. A phosphatidylinositol 3-kinase class III sub-complex containing VPS15. VPS34. Beclin 1. UVRAG and BIF-1 regulates cytokinesis and degradative endocytic traffic. Exp Cell Res. 2010;316(20):3368–3378. doi: 10.1016/j.yexcr.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Abrahamsen H, Stenmark H, Platta HW. Ubiquitination and phosphorylation of Beclin 1 and its binding partners: Tuning class III phosphatidylinositol 3-kinase activity and tumor suppression. FEBS Lett. 2012;586(11):1584–1591. doi: 10.1016/j.febslet.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 49.Eathiraj S, Lambright DG. ESCRT complexes assembled and GLUEd. Structure. 2006;14(4):631–632. doi: 10.1016/j.str.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Brill JA, Wong R, Wilde A. Phosphoinositide function in cytokinesis. Curr Biol. 2011;21(22):R930–R934. doi: 10.1016/j.cub.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 51.He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21(4):537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–89. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 53.Ben El Kadhi K, et al. The inositol 5-phosphatase dOCRL controls PI(4,5)P2 homeostasis and is necessary for cytokinesis. Curr Biol. 2011;21(12):1074–1079. doi: 10.1016/j.cub.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 54.Rothwell WF, et al. The Drosophila centrosomal protein Nuf is required for recruiting Dah. a membrane associated protein. to furrows in the early embryo. J Cell Sci. 1999;112(Pt 17):2885–2893. doi: 10.1242/jcs.112.17.2885. [DOI] [PubMed] [Google Scholar]
- 55.Albertson R, et al. Vesicles and actin are targeted to the cleavage furrow via furrow microtubules and the central spindle. J Cell Biol. 2008;181(5):777–790. doi: 10.1083/jcb.200803096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang CX, et al. Discontinuous actin hexagon. a protein essential for cortical furrow formation in Drosophila. is membrane associated and hyperphosphorylated. Mol Biol Cell. 2000;11(3):1011–1022. doi: 10.1091/mbc.11.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rothwell WF, et al. Nuclear-fallout. a Drosophila protein that cycles from the cytoplasm to the centrosomes. regulates cortical microfilament organization. Development. 1998;125(7):1295–1303. doi: 10.1242/dev.125.7.1295. [DOI] [PubMed] [Google Scholar]
- 58.Byers TJ, Armstrong PB. Membrane protein redistribution during Xenopus first cleavage. J Cell Biol. 1986;102(6):2176–2184. doi: 10.1083/jcb.102.6.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pohl C, Jentsch S. Midbody ring disposal by autophagy is a post-abscission event of cytokinesis. Nat Cell Biol. 2009;11(1):65–70. doi: 10.1038/ncb1813. [DOI] [PubMed] [Google Scholar]
- 60.Birkenfeld J, et al. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell. 2007;12(5):699–712. doi: 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol. 2009;11(1):71–77. doi: 10.1038/ncb1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loria A, Longhini KM, Glotzer M. The RhoGAP domain of CYK-4 has an essential role in RhoA activation. Curr Biol. 2012;22(3):213–219. doi: 10.1016/j.cub.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canman JC, et al. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science. 2008;322(5907):1543–1546. doi: 10.1126/science.1163086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hurley JH. The ESCRT complexes. Crit Rev Biochem Mol Biol. 2010;45(6):463–87. doi: 10.3109/10409238.2010.502516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Babst M, et al. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell. 2002;3(2):271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- 66.Carlton JG, et al. ESCRT-III governs the Aurora B-mediated abscission checkpoint through CHMP4C. Science. 2012;336(6078):220–225. doi: 10.1126/science.1217180. [DOI] [PMC free article] [PubMed] [Google Scholar]