Abstract
Background
The purpose of this study was to define the ultrasound imaging characteristics of adrenal tumors and to assess the performance of ultrasound in differentiating benign ‘leave-alone’ lesions from suspicious lesions.
Material/Methods
We enrolled 882 patients in this study. The nature of each lesion was determined by histopathology. Ultrasound finding of each lesion was compared with its corresponding histopathologic result. The final study group consisted of 911 adrenal masses in 882 patients. All images were reviewed by 2 experienced investigators in a double blind manner.
Results
There were 553 adenomas identified in the study, which constituted 60.70% of the lesions. There were 161 pheochromocytomas (17.67%), 49 myelolipomas (5.38%), 39 cysts (4.28%), 37 metastasis (4.06%), 35 ganglioneuromas (3.84%), 22 lymphomas (2.41%), and 15 cortical carcinomas (1.65%). The sensitivity, specificity, and accuracy of ultrasound-based diagnosis were 89%, 99%, and 93.9%, respectively. A positive predictive value of 90.9% and a negative predictive value of 94.2% were obtained in this study.
Conclusions
This large-sample study showed that ultrasound was a reliable method in differentiating benign ‘leave-alone’ lesions from suspicious lesions.
MeSH Keywords: Adrenal Medulla, Pharmacy, Ultrasonography
Background
Adrenal incidentaloma, characterized by hormonal activity and malignant histology, poses a great threat to the public health [1]. If hormone hypersecretion is ruled out in an adrenal tumor, a decision must be made between surgical excision and close observation [2]. The role of adrenal imaging is to differentiate benign ‘leave-alone’ lesions (e.g., adrenocortical adenoma, myelolipoma, and cyst) from suspicious lesions (e.g., pheochromocytoma, metastasis, adrenal cortical carcinoma, and adrenal lymphoma) that warrant further managements [2]. Simple and inexpensive, ultrasound has been widely used in the detection of adrenal masses [3–8]. However, most studies have relied on the follow-up (sometimes short-term follow-up) or results of CT/MRI imaging to confirm the nature of adrenal lesions. Histopathologic confirmation is available only in a small percentage (<20%) of adrenal masses [9]. Moreover, there is currently no consensus or guideline for adrenal tumor diagnosis based on sonographic imaging. Previous studies aimed primarily at differentiating benign adrenal masses from malignant adrenal masses, which did not consider suspicious lesions that warrant further management. Furthermore, some benign adrenal tumors, which were clinical presentations rather than pathologic entities, had been managed with surgical excision. Hence, establishing typical ultrasound imaging features is important for the therapeutic schedule. To the best of our knowledge, there are only a few datasets covering the ultrasound features of either adrenocortical adenomas or pheochromocytomas. Thus, we aimed to define reliable ultrasound imaging characterizations of adrenal tumors by analyzing a large number of cortical and non-cortical adrenal masses.
Material and Methods
The present study was a retrospective analysis of patients from a research protocol. The pathology database from January 2006 to March 2013 was searched after obtaining the approval of the institutional review board. Ultrasound records and hospital medical records of patients who underwent surgical resection or percutaneous ultrasound-guided adrenal biopsy were selected and reviewed retrospectively and 1823 patients (a total of 1905 adrenal masses) were considered for entry. We excluded 941 (51.6%) patients for the following reasons: (a) 829 (45.4%) patients had only 1 type of examination (CT or MRI only); (b) 95 (5.2%) patients had unreadable or lost ultrasound tapes; (c) 12 (0.6%) patients had masses less than 1 cm or had extra-adrenal lesions; (d) 5 (0.2%) patients had adrenal lesions that could not be characterized according to histologic features. After exclusions, we enrolled 882 (48.3%) patients who underwent ultrasound for the characterization of an adrenal mass: 853 (96.7%) patients had unilateral adrenal mass and 29 (3.3%) patients had lesions in both adrenal glands. There were a total of 429 males (48.64%) and 453 females (51.36%). The mean age of patients was 49.5±8.3 years (mean ±SD), ranging from 14 to 80 years.
Abdominal ultrasonography
All examinations were performed by physicians who were blind to the CT or MRI results. Ultrasonography (3.5–5 MHz convex probe, Phillips IU22, GE Logic 7, GE Logic 9, Simens Acuson 512) was used in all the cases. Patients underwent complete abdominal US examinations without any pre-procedure preparation.
For the adrenal gland examination, the patient was positioned in a slight left or right oblique position and lifted the opposite arm to widen the intercostal space. Using the liver as an acoustic window, the right adrenal gland was examined in an oblique position slightly to the left, with patient’s right arm lifted to widen the intercostal space. The transducer was placed on the 9th to 11th intercostal space between the middle and anterior axillary line, and the probe scanned along the intercostal space transversely and longitudinally. Using the margin of the liver as an anatomical landmark, the right adrenal gland was observed at the upper pole of the kidney, the inferior vena cava, and the right crus of the diaphragm. The left adrenal gland was examined in a decubitus and oblique position slightly to the right, with the patient’s left arm lifted to widen the intercostal space, using the spleen and left kidney as acoustic windows. Typically, the left adrenal gland was observed at the upper pole of the left kidney, the tail of the pancreas, the dorsal upper part of the spleen, and the left crus of the diaphragm. Conventional grey-scale US and color Doppler were both used to assess and record the location, size, shape, border, internal echogenicity of the lesion, and the intralesional blood supply.
Ultrasound imaging criteria
Adrenal adenoma is commonly diagnosed as a homogeneous and hypoechoic solid lesion with well-defined borders. Color Doppler examination reveals hypovascularization.
Myelolipoma has characteristic homogenous hyperechoic echogenicity and regular margins. Color Doppler examination reveals hypovascularization.
Adrenocortical carcinoma is generally a large heterogeneous and metastatic solid mass at the time of diagnosis. Color Doppler examination reveals hypervascularization or an afferent blood vessel.
Adrenal metastases vary considerably in diameter. They can be smaller than primary adrenal cancer lesions or larger than adrenal adenoma. Metastatic lesions usually are bilateral with irregular margins and inhomogeneous characters. Color Doppler examination reveals hypervascularization or an afferent blood vessel.
Pheochromocytomas are mostly heterogeneous in appearance, and have considerable variations in diameter. Pheochromocytoma is well encapsulated and color Doppler examination reveals hypervascularization.
Data analysis
All the ultrasound images were reviewed by 2 experienced investigators who did not know the results of CT/MRI imaging or the histopathologic examinations. Lesions without characteristic ultrasonographic features were assigned to the suspicious lesion group.
Standard of reference
Histopathologic examinations were performed after surgical resection (n=823) and percutaneous biopsy (n=59). Needle biopsy was performed under ultrasound guidance. Clinical and surgical operations were conducted according to the following rules. A functional lesion was operated on independent of its size. A non-functional lesion was operated on only if it was larger than 3 cm or complied with patient demand. For an invasive operation, informed consent should be obtained. Results of histopathologic examination were considered as the criterion standard.
Statistical analysis
Statistical analysis was performed using the SPSS 13.0 software package (Chicago, IL). Continuous data are expressed as mean ±SD. Sensitivity, specificity, positive and negative predictive values, and accuracy of the ultrasound examination were calculated using 2×2 contingency tables. Categorical variables such as sex were analyzed using frequency tables. P<0.05 was considered as significant in statistical difference.
Results
Size of masses
A total of 882 patients diagnosed with adrenal lesions were enrolled in this study. Characteristics of patients are shown in Table 1. The mean age was 49.5±8.3 (mean ±SD), ranging from 14 to 80 years. Nodule and sex laterality were distributed evenly. Mean adrenal nodule size was 3.43±2.16 cm (range, 1.0–21 cm). False-negative results of ultrasonography were encountered in 43 (4.8%) cases, which were caused by the small size of tumors (range, 1.0–2.8 cm).
Table 1.
Characteristics of adrenalectomy patients.
| Characteristics | Descriptions |
|---|---|
| Gender | 882 |
| Male | 429 |
| Female | 453 |
| Side of mass | 911 |
| Right | 558 |
| Left | 353 |
| Bilateral | 29 |
| Mean size of mass | |
| ≤4 cm | 609 |
| 4~6 cm | 137 |
| >6 cm | 165 |
| Indications for adrenal evaluation | |
| Incidental lesions for unrelated examinations | 732 |
| Symptoms of hormonal excess | 53 |
| Hypertension | 126 |
Characteristics of adrenal masses are listed in Table 2. The mean diameter of tumors with heterogeneous echostructure was 5.87±3.16 cm (range, 3.2–21 cm), and the mean diameter of tumors with relatively homogeneous echostructure was 2.95±1.91 cm (range, 1.0–6.3 cm). Adrenocortical carcinomas were significantly larger than adrenal adenomas (P<0.05), pheochromocytomas (P<0.05), and metastases (P<0.05), respectively. Only 3 of the 15 adrenocortical carcinomas (20%) were smaller than 6.0 cm in diameter. The mean diameter of pheochromocytomas was 5.70±3.38 cm, which was much greater than that of adrenal adenomas (2.76±1.69 cm) (P<0.01), but similar to that of metastases (6.88±2.88 cm). At a threshold diameter of 3 cm, the sensitivity and specificity for the diagnosis of adrenal adenomas were 83% (459 of 553 masses) and 86% (477 of 553 masses), respectively.
Table 2.
Differential diagnosis for adrenalomas.
| Histopathologic diagnosis | No. | Frequency (%) |
|---|---|---|
| Adenoma | 553 | 60.70 |
| Carcinoma | 15 | 1.65 |
| Pheochromocytoma | 161 | 17.67 |
| Myelolipoma | 49 | 5.38 |
| Metastasis | 37 | 4.06 |
| Cyst | 39 | 4.28 |
| Uncommon adrenal masses | 57 | 9.00 |
Features of masses
A total of 553 (60.70%) cases were diagnosed as adenomas, 161 (17.67%) cases were diagnosed as pheochromocytomas, 15 cases (1.65%) were diagnosed as carcinomas, 39 cases (4.28%) were diagnosed adrenal cysts, 49 cases (5.38%) were diagnosed as myelolipomas, 22 cases (2.41%) were diagnosed as lymphomas, and 37 cases (4.06%) were diagnosed as metastases. There were 43 tumors missed by US imaging-based diagnosis. Thirty (69.7%) of these 43 tumors were on the left adrenal and the other 13 (30.3%) were on the right adrenal. Forty-one (95.3%) of these 43 tumors were histologically adrenal adenomas, and the other 2 (4.7%) were a myelolipoma and a pheochromocytoma.
The adrenal metastases stemmed from 7 primary cancers. They were small-cell lung carcinomas in 6 patients, non-small-cell lung carcinomas in 12 patients, hepatocellular carcinomas in 13 patients, a breast carcinoma in 1 patient, renal cell carcinomas in 3 patients, esophagus carcinoma in 1 patient, and colorectal carcinoma in 1 patient. Demographic characteristics of patients are shown in Table 3.
Table 3.
Mean diameters of adrenal masses.
| Adrenal mass | No. | Diameter (cm) |
|---|---|---|
| Adenoma | 553 | 2.76±1.69 (1.0–17.2) |
| Pheochromocytoma | 161 | 5.70±3.38 (1.5–20) |
| Myelolipoma | 49 | 5.00±2.34 (1.5–9.3) |
| Carcinoma | 15 | 8.81±5.12 (3.4–21) |
| Metastasis | 37 | 6.88±2.88 (3.4–13.2) |
Diameters are given as means±standard deviations, with ranges in parentheses. The mean diameter of adrenocortical carcinomas was significantly larger than the mean diameters of adenomas, pheochromocytomas, and metastases (P<.001).
Tumors were classified as homogenous if they contained uniformly low levels of ultrasonic echoes; otherwise, they were classified as heterogenous. Although there were overlaps in the appearances of different tumors, several trends were observed. Pheochromocytomas were almost heterogeneous in appearance, whereas cortical adenomas were usually homogeneous.
Performance of the US detection
Using histology as the criterion standard, ultrasound imaging had 83 cases of (9.1% of 911 cases) misdiagnosis. The sensitivity of ultrasound imaging for the diagnoses of benign and suspicious adrenal lesions was 89.6%, and the specificity and accuracy were 94.9% and 91.0%, respectively. The positive predictive value (PPV) and negative predictive value (NPV) were calculated as 98.1% and 76.1%, respectively. The sensitivity, specificity, accuracy, PPV, and NPV for all entities are summarized in Table 4.
Table 4.
Accuracy of ultrasound imaging in characterization of adrenal masses.
| Finding | Number | Sensitivity | Specificity | Accuracy | Predictive value | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Adenoma | 553 | 89.8% | 96.1% | 92.3% | 97.3% | 83.9% |
| Pheochromocytoma | 161 | 91.3% | 97.1% | 96.0% | 87.0% | 98.1% |
| Myelolipoma | 49 | 91.8% | 100.0% | 99.6% | 97.9% | 99.7% |
| Carcinoma | 15 | 80.0% | 98.4% | 98.7% | 46.2% | 99.7% |
| Metastasis | 37 | 83.8% | 99.9% | 99.2% | 96.9% | 99.3% |
Differential diagnosis and ultrasound features
Adrenal adenoma
Adrenal adenoma is the most common type of adrenal mass, which represents up to 80% of all adrenal masses [10]. Adrenal adenomas usually are small (<3 cm) and have hypoechoic patterns on sonography, with smooth, oval, well-defined margins. Their homogeneous cellular structures allow specific diagnosis, but imaging still needs to be performed to confirm the diagnosis. Calcifications, necrosis, and hemorrhage are atypical features of adrenal adenoma, but they can occur, particularly in larger lesions [11]. In our study, 476 (86.07%) out of 553 adrenal adenomas appeared as small, well-defined, sharp, marginal, and hypoechoic solid nodules. However, a small number of adrenal adenomas were heterogeneous due to cystic degeneration or hemorrhage, and were difficult to diagnose. These lesions varied in size, but most of them were large (>5 cm) and had necrotic areas. Thirty-three (6.5%) out of 553 adrenal adenomas were larger than 5 cm, but lacked typical ultrasound features, resulting in incorrect ultrasound diagnoses. Most of these lesions were interpreted as pheochromocytoma or malignant tumors based on ultrasound imaging. There were 41 (7.4%) false-negative cases. Thirteen of these cases occurred in the early series due to lack of experience, and 28 false-negative cases occurred due to excessive fat in the adrenal region in obese patients.
Adrenocortical carcinoma
Primary adrenocortical carcinoma is a rare type of lesion. Adrenal cortical cancer can be diagnosed incidentally by ultrasound imaging (Figure 1). The prognosis is generally very poor. The risk increases with greater lesion size. Therefore, the lesion size is an important parameter of malignancy in the differential diagnosis. The US appearance varies depending on the lesion size. Most adrenocortical carcinomas are larger than 6 cm when first detected clinically. In the vast majority of cases, the first imaging presents a large, inhomogeneous mass, with necrosis, hemorrhages, and calcifications [12]. In the present study, the mean diameter was 8.8 cm (range, 3.4–21 cm), and only 3 of 15 adrenocortical carcinomas were smaller than 6 cm. A large right adrenal cortical carcinoma can be misdiagnosed as an exophytic hepatocellular carcinoma on CT and MRI. US can precisely indicate the right adrenal origin of the mass by showing the anterior displacement of the retroperitoneal fat reflection. Therefore, US can sometimes be more informative than CT or MRI.
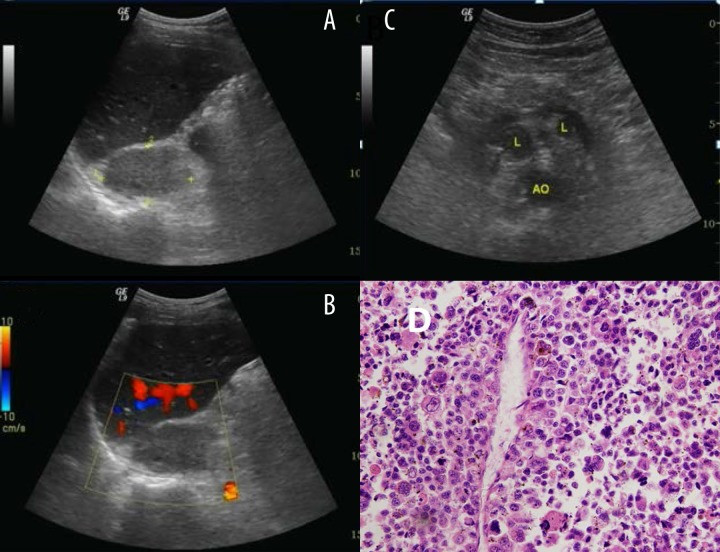
Figure 1.
Typical ultrasound imaging of adrenal carcinoma. (A) Right adrenal mass with 6.1×3.6 cm size, middle uniform echo, and unclear boundary. (B) No notable vascularization is shown within the mass on color Doppler sonography. (C) Multiple enlarged lymph nodes were found retroperitoneally (D) Histopathology result showed adrenal adenomas.
Although only a small fraction of adrenocortical carcinomas are smaller than 6 cm, smaller lesions are commonly homogeneous and can be difficult to differentiate from adenomas. In our study, 6 patients with adrenal cortical carcinomas were detected with a low level of steroids (cortisol and androgens) production. Five cases of distant metastases were found at the time of diagnoses. This indicates that large adrenal carcinomas tended to invade the adrenal vein and inferior vena cava. The results of our study were consistent with the previous studies showing that on average, 55% of adrenal-cortical carcinomas were functional [13]. Therefore, we highly recommend that a primary adrenocortical carcinoma should be suspected when multiple adrenal hormones are elevated. Calcification was observed in 2 cases in the present study.
Pheochromocytoma
Pheochromocytoma is usually a benign tumor of the adrenal gland, but 9% of the tumors can be malignant [14,15]. It has been reported to be asymptomatic in 10–17% of patients in previous clinical studies [16] and in 76% of an autopsy series [17]. Pheochromocytomas exhibit variability in size, which ranges from 1.2 cm to 15 cm with a mean size of 5.5 cm [11]. To evaluate for recurrence and/or malignancy, comprehensive clinical, biochemical, and imaging investigations should be conducted [16,17]. In our study, 161 (17.67%) pheochromocytomas had a mean size of 5.70±3.38 cm, ranging from 1.5 cm to 20.0 cm. We found that adrenal pheochromocytomas were large, highly vascularized, and well-capsulated tumors. They can be heterogeneous or homogeneous solid masses, depending on the degree of internal hemorrhage or necrosis (Figure 2). Unlike most of the adrenal adenomas, pheochromocytomas commonly have regions of necrosis (Figure 3). Most of these lesions have intratumoral cystic regions under gross or microscopic pathologic examination. In our study, 149 of the 161 adrenal pheochromocytomas appeared to have mild to severe heterogeneity. Larger tumors tended to be more heterogeneous, which had a high rate of hemorrhage or pathological necrosis. Furthermore, they had predominant cystic lesions, filling with old blood and necrotic debris. Small pheochromocytomas were usually larger than 3 cm with homogeneous appearances, and had necrosis or hemorrhage in the internal areas (Figure 4).
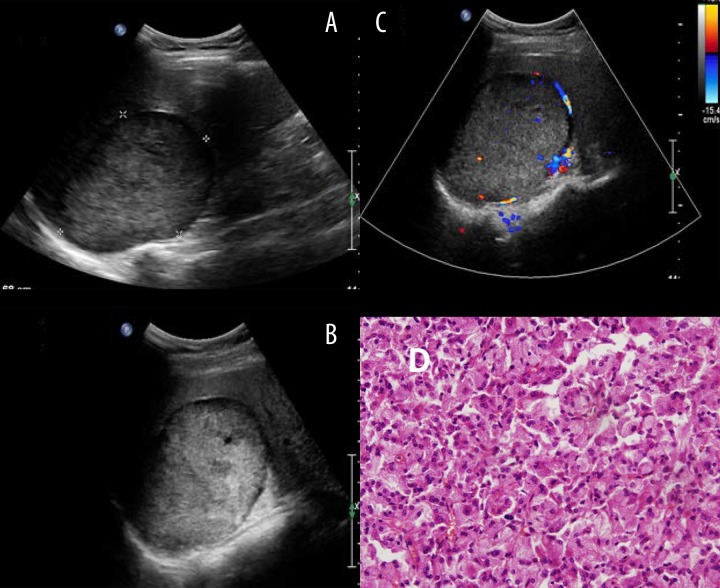
Figure 2.
Typical ultrasound imaging of no necrosis pheochromocytomas. (A) Right adrenal mass with 9.5×5.4 cm size, middle echo, and complete capsule. (B) Visible internal sonolucent area in multiple sections. (C) Blood flow signals were found both inside and around the lesion by color Doppler examination. (D) Histopathology result showed pheochromocytomas.
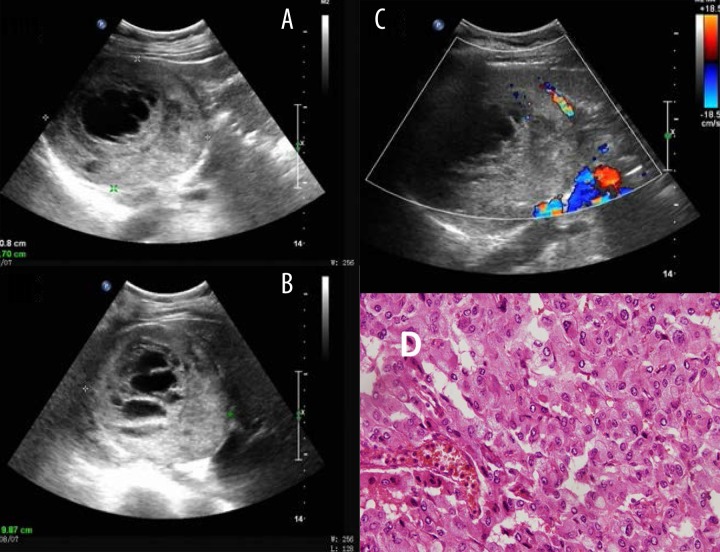
Figure 3.
_Typical ultrasound imaging of pheochromocytomas with necrosis in a 59-year-old female with no history of hypertension. (A) Right adrenal mass with 10.8×8.7 cm size, middle and uneven echo, and visible liquefied area. (B) 1/3 above the tumor volume was a necrotic area in different scanning section. (C) Blood flow signals were found mainly around the lesion but also in the lesion by color Doppler examination. (D) Histopathology result showed pheochromocytomas.
Figure 4.
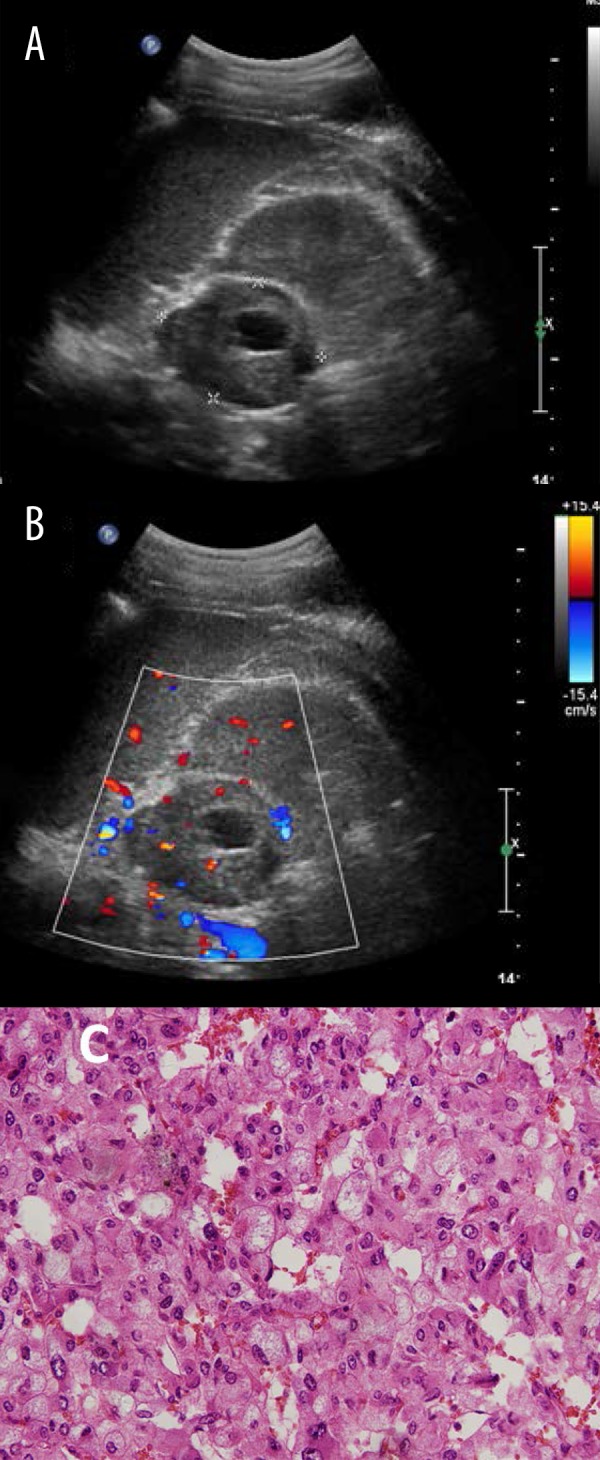
Typical ultrasound imaging of pheochromocytomas with necrosis in a 31-year-old man with a 5-year history of hypertension. (A) Left adrenal mass with 5.5×4.1 cm size, middle and uneven echo, and visible liquefied area. (B) Blood flow signals were found mainly in the lesion but also around the lesion by color Doppler examination. (C) Histopathology result showed pheochromocytomas.
Gray-scale ultrasound is helpful in confirming cystic-necrotic changes within pheochromocytomas. The cyst fluid may be anechoic or contain echogenic debris, and posterior acoustic enhancement may be an accompanying feature [18]. In our study, atypical sonographic images were found in 13 cases, which were histologically proven to be misdiagnosed as adrenal adenomas (8 cases), adrenal carcinomas (2 cases), and ganglioneuromas (3 cases). The misdiagnosis by ultrasound imaging could have been avoided if our study protocol had included knowledge of clinical history, as well as blood and urine analysis.
Ganglioneuroma
Ganglioneuromas are benign and well-differentiated tumors in the sympathetic nervous system. Ganglioneuromas in the adrenal gland are extremely rare. These tumors preferentially affect young people before the age of 20. Their most relevant clinical characteristics are that they are hormonal inactive, slow growing, and incidentally discovered neoplasms, which is consistent with our results. Ganglioneuromas commonly contain calcifications, necrosis, or hemorrhage. Adrenal calcification can be seen on plain abdominal radiographs in patients with carcinoma or ganglioneuromas, but is unusual in pheochromocytoma and rare in adrenal adenoma [19]. In our series, calcifications appeared in 21/35 (60%) of ganglioneuromas.
Myelolipoma
Myelolipoma is an uncommon and benign tumor composed of matured adipose tissue and hematopoietic tissue. Most of these cases are discovered incidentally. Currently, sonography enables efficient diagnosis of these tumors preoperatively. A fat-filled mass with high echogenicity is the key marker for both identification and preoperative diagnosis (Figure 5). However, different proportions of fat and myeloid tissue may lead to difficult diagnosis. In this study, sonography detected 48 adrenal masses in 49 cases. These masses can be categorized into 2 groups (the homogeneous group and the heterogeneous group) based on their ultrasound features. Homogeneous and hyperechoic tumors were seen in 37 cases and heterogeneous (mostly hyperechoic) tumors were seen in 11 cases. One tumor with diameter of 1.5 cm on the left side was missed due to the masking of small hyperechoic lesions by the echogenicity of adjacent retroperitoneal fat.
Figure 5.
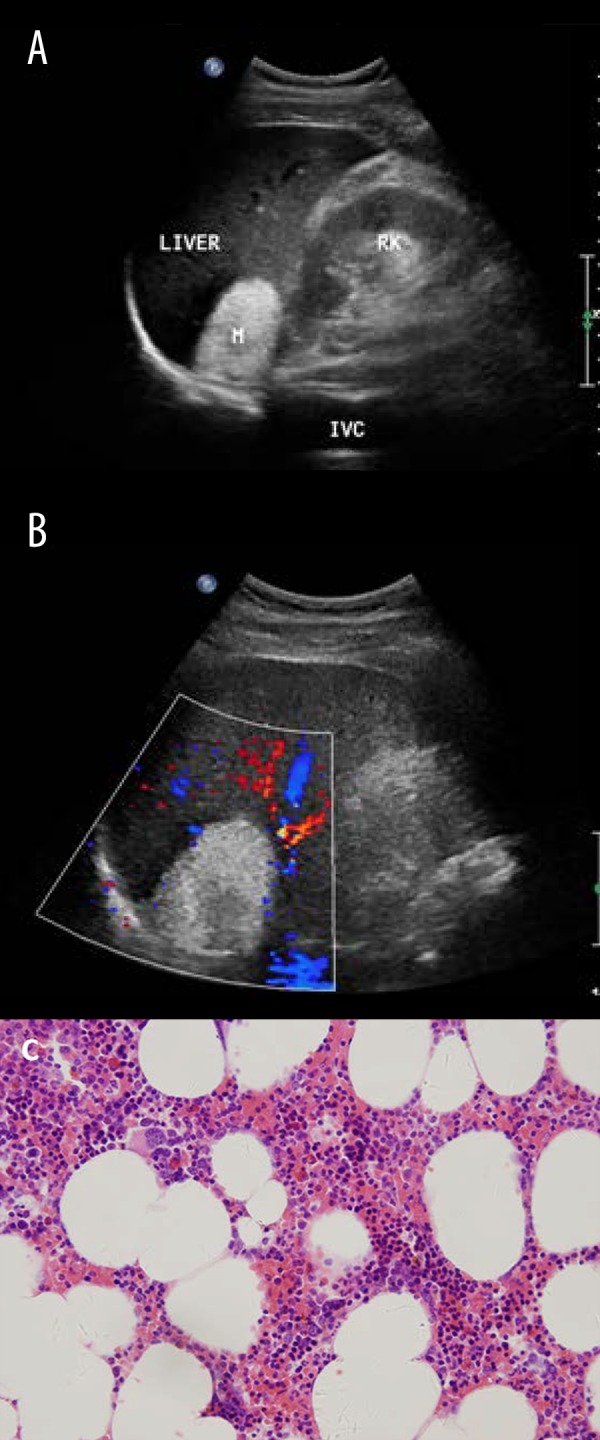
Typical ultrasound imaging of myelolipoma in a 59-year-old man. (A) Right adrenal mass 52×39 mm in size and homogenous hyperechoic. (B) No notable vascularization is shown within the mass (arrows) on color Doppler sonography. (C) Histopathology result showed myelolipoma.
Metastases to the adrenal
Metastases are the most common malignant lesions involving the adrenal gland. Adrenal metastases are found in up to 27% of patients with malignant epithelial tumors at autopsy [20]. Common tumors, including carcinomas of the breast, lung, kidney, colon, esophagus, pancreas, liver, and stomach, as well as melanoma, can metastasize to the adrenal gland [21,22]. Metastases are usually bilateral but can also be unilateral. In patients with known primary malignancy, metastatic disease should first be excluded since distant metastatic disease can deteriorate patient health and has a profound effect on management decisions. In our study, adrenal metastases varied considerably in diameter. They could be smaller than primary adrenal cancer lesions or larger than adrenal adenoma and pheochromocytomas. Metastases were commonly bilateral, large, heterogeneous, and poorly defined masses on sonography. However, our study found that small metastatic lesions could be homogeneous and well-defined, and were indistinguishable from the primary adrenal neoplasm. Thus, the diagnosis was difficult in these cases. Therefore, ultrasound was unable to distinguish unilateral small adrenal masses, and other examinations were required to differentiate adrenal adenomas from metastases.
Adrenal lymphoma
Lymphoma occasionally involves the adrenal glands, and non-Hodgkin lymphomas are more common than Hodgkin disease at this site. Bilateral involvement is seen in 50% of patients [23], and bilateral involvement of the adrenal gland is considered as a single-site extranodal involvement [24]. In the present study, bilateral lesions were seen in 6 patients. According to previous reports, the most common presenting symptoms are abdominal or back pain, fever of unknown origin, anorexia, weight loss, and signs of adrenal insufficiency such as hypoglycemia, hyponatremia, and addisonian crisis [25,26]. In our series of 16 patients, the most common symptoms were pain, fever, or both. Lymphoma usually presents as a homogeneous, markedly hypoechoic mass on sonography. This is because lymphoma is a highly homogeneous tumor and generates few internal reflections [27]. However, there have been a few reports of lymphomas with unusual sonographic features such as lesions of mixed echogenicity, target lesions, and heterogeneous lesions [28]. In adrenal lymphoma diagnosis, one should pay attention to the differential diagnosis of patients presenting with unilateral or bilateral huge adrenal malignant masses.
Discussion
Because more cases of adrenal lesions are being detected incidentally [1], it is important for the radiologists to be aware of the appearances of adrenal masses and know how to perform proper diagnosis. If adrenal lesions are observed incidentally, the differential diagnosis based on ultrasound appearances should consider malignant adrenal lesions, particularly adrenal cortical carcinomas and pheochromocytomas [3,5–8]. The present study showed the reliability of ultrasound imaging in the characterization of adrenal masses. It is a simple and effective way to assess the origin of adrenal masses, which has important implications in guiding treatment.
However, the present study has several limitations. First, it is unknown whether ultrasound is able to identify adrenal adenoma smaller than 1 cm, due to the lack of such tumors in our series. Second, the unavailability of data from other imaging methods, such as CT or MRI, prevents systematically comparing the performance of ultrasound with other methods.
Conclusions
Our study demonstrates the important role of ultrasound in the evaluation of adrenal diseases. Certain imaging features were established for the diagnoses of adrenal tumors. In future studies, a combination of ultrasound and contrast-enhanced ultrasound examination can be used to improve the performance of diagnosis.
Footnotes
Source of support: Departmental sources
References
- 1.Young WF., Jr Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356(6):601–10. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 2.Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentaloma”): NIH conference. Ann Intern Med. 2003;138(5):424–29. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki Y, Sasagawa, Suzuki H, et al. The role of ultrasonography in the detection of adrenal masses: comparison with computed tomography and magnetic resonance imaging. Int Urol Nephrol. 2001;32:303–6. doi: 10.1023/a:1017583211460. [DOI] [PubMed] [Google Scholar]
- 4.Lumachi F, Borsato S, Brandes AA, et al. Fine needle aspiration cytology of adrenal masses in noncancer patients: clinicoradiologic and histologic correlations in functioning and nonfunctioning tumors. Cancer. 2001;93:323–29. doi: 10.1002/cncr.9047. [DOI] [PubMed] [Google Scholar]
- 5.Trojan J, Schwarz W, Sarrazin C, et al. Role of ultrasonography in the detection of small adrenal masses. Ultraschall Med. 2002;23:96–100. doi: 10.1055/s-2002-25190. [DOI] [PubMed] [Google Scholar]
- 6.Yip L, Tublin ME, Falcone JA, et al. The Adrenal Mass: Correlation of Histopathology with Imaging. Ann Surg Oncol. 2010;17:846–52. doi: 10.1245/s10434-009-0829-2. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich-Rust M, Glasemann T, Polta A, et al. Differentiation between benign and malignant adrenal mass using contrast-enhanced ultrasound. Ultraschall Med. 2011;32(5):460–71. doi: 10.1055/s-0031-1273408. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich CF, Ignee A, Barreiros AP, et al. Contrast-enhanced ultrasound for imaging of adrenal masses. Ultraschall Med. 2010;31(2):163–68. doi: 10.1055/s-0028-1109357. [DOI] [PubMed] [Google Scholar]
- 9.Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85:637–44. doi: 10.1210/jcem.85.2.6372. [DOI] [PubMed] [Google Scholar]
- 10.Jacques AE, Sahdev A, Sandrasagara M, et al. Adrenal phaeochromocytoma: correlation of MRI appearances with histology and function. Eur Radiol. 2008;18:2885–92. doi: 10.1007/s00330-008-1073-z. [DOI] [PubMed] [Google Scholar]
- 11.Lyon SM, Lee MJ. Imaging the non-hyperfunctioning adrenal mass. Imaging. 2002;14:137–46. [Google Scholar]
- 12.Wajchenberg BL, Albergaria Pereira MA, Medonca BB, et al. Adrenocortical carcinoma: clinical and laboratory observations. Cancer. 2000;88(4):711–36. [PubMed] [Google Scholar]
- 13.Lucon AM, Pereira MA, Mendonca BB, et al. Pheochromocytoma: study of 50 cases. J Urol. 1997;157:1208–12. doi: 10.1016/s0022-5347(01)64925-5. [DOI] [PubMed] [Google Scholar]
- 14.Koch CA, Pacak K, Chrousos GP. The molecular pathogenesis of hereditary and sporadic adrenocortical and adrenomedullary tumors. J Clin Endocrinol Metab. 2002;87(12):5367–84. doi: 10.1210/jc.2002-021069. [DOI] [PubMed] [Google Scholar]
- 15.Miehle K, Kratzsch J, Lenders JW, et al. Adrenal incidentaloma diagnosed as pheochromocytoma by plasma chromogranin A and plasma metanephrines. J Endocrinol Invest. 2005;28(11):1040–42. doi: 10.1007/BF03345347. [DOI] [PubMed] [Google Scholar]
- 16.Singer J, Koch CA, Kassahun W, et al. A patient with a large recurrent pheochromocytoma demonstrating the pitfalls of diagnosis. Nat Rev Endocrinol. 2011;7(12):749–55. doi: 10.1038/nrendo.2011.132. [DOI] [PubMed] [Google Scholar]
- 17.Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma: review of a 50-year autopsy series. Mayo Clin Proc. 1981;56:354–60. [PubMed] [Google Scholar]
- 18.Bowerman RA, Silver TM, Jaffe MH, et al. Sonography of adrenal pheochromocytomas. Am J Roentgenol. 1981;137(6):1227–31. doi: 10.2214/ajr.137.6.1227. [DOI] [PubMed] [Google Scholar]
- 19.Kahn PC. The radiologic identification of functioning adrenal tumors. Radiol Clin North Am. 1967;5:221–30. [PubMed] [Google Scholar]
- 20.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:601–10. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 22.Terzolo M, Bovio S, Pia A, et al. Management of adrenal incidentaloma. Best Pract Res Clin Endocrinol Metab. 2009;23:233–43. doi: 10.1016/j.beem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Tomoyose T, Nagasaki A, Uchihara JN, et al. Primary adrenal adult T-cell leukemia/lymphoma: a case report and review of the literature. Am J Hematol. 2007;82(8):748–52. doi: 10.1002/ajh.20856. [DOI] [PubMed] [Google Scholar]
- 24.Kim YR, Kim JS, Min YH, et al. Prognostic factors in primary diffuse large B-cell lymphoma of adrenal gland treated with rituximab-CHOP chemotherapy from the Consortium for Improving Survival of Lymphoma (CISL) J Hematol Oncol. 2012;13:49. doi: 10.1186/1756-8722-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh D, Kumar L, Sharma A, et al. Adrenal involvement in non-Hodgkin’s lymphoma: four cases and review of literature. Leuk Lymphoma. 2004;45:789–94. doi: 10.1080/10428190310001615756. [DOI] [PubMed] [Google Scholar]
- 26.Mantzios G, Tsirigotis P, Veliou F, et al. Primary adrenal lymphoma presenting as Addison’s disease: case report and review of the literature. Ann Hematol. 2004;83:460–63. doi: 10.1007/s00277-003-0838-3. [DOI] [PubMed] [Google Scholar]
- 27.Kim H, Kim KW, Park MS, Kim H. Lymphoma presenting as an echogenic periportal mass: sonographic findings. J Clin Ultrasound. 2008;36(7):437–39. doi: 10.1002/jcu.20453. [DOI] [PubMed] [Google Scholar]
- 28.Castroagudin JF, Molina E, Abdulkader I, et al. Sonographic features of liver involvement by lymphoma. J Ultrasound Med. 2007;26:791–96. doi: 10.7863/jum.2007.26.6.791. [DOI] [PubMed] [Google Scholar]