Abstract
Being overweight or obese, as measured with body mass index (BMI) or central adiposity (waist circumference), and evolving trajectory of BMI over the life course, have been associated with brain atrophy, white matter changes, blood brain barrier integrity and risk of all-cause late-onset dementia and Alzheimer’s Disease (AD). This observation leads us to question what it is about BMI that is associated with health of the brain and dementia risk. If high BMI and central adiposity represent an increase in adipose tissue, then the endocrine aspect of adipose tissue, mediated by adipose tissue hormones and adipokines, may be a clue to understanding the association with dementia and AD. Hundreds of adipokines have been identified, creating a complexity that is challenging to simplify. Nonetheless, adipokines are being investigated in association with clinical dementia outcomes, as well as imaging-based measures of brain volume, structure and function in preclinical and human models of clinical dementia.
Introduction
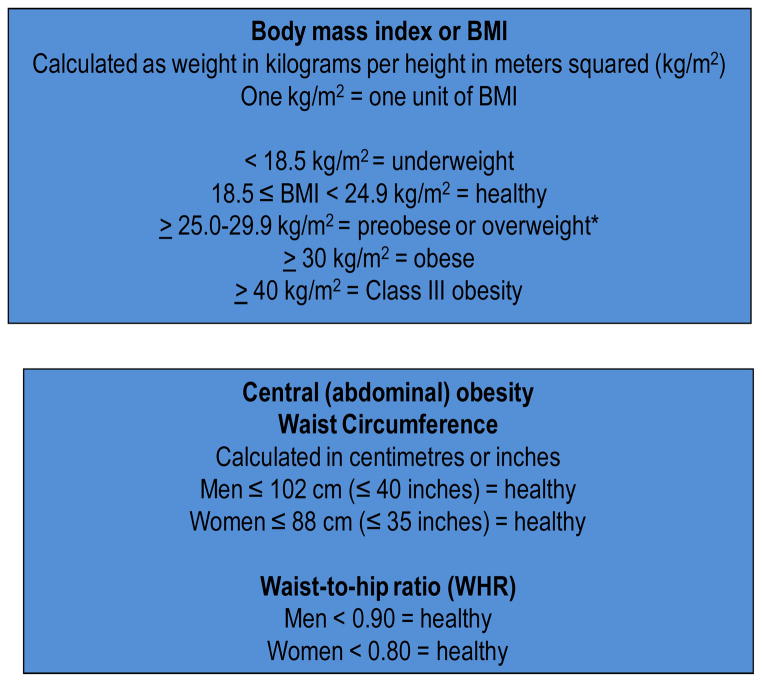
Since 2003, when the first report was published of a risk association in women between AD and higher body mass index (BMI, kg/m2), a common and simple measure of overweight and obesity,1 there have been many epidemiologic reports relating higher mid-life and late-life BMI to dementia1–8 There is an approximate 2-fold higher risk associated with mid-life BMI or central adiposity measures.3, 4, 6, 7 The levels of mid-life adiposity that are associated with dementia or AD are in overweight and obese ranges based on traditional anthropometric cutpoints for BMI, waist circumference and waist-to-hip ratio (WHR) and used to denote risk for cardiovascular disease and overall mortality. (See Figure 1). Following mid-life, a decrease in BMI tends to occur, such that those who have clinical dementia have lower BMI or body weight compared to those who do not.9, 10 This reverse epidemiology phenomenon has been a topic of debate. 11 It has been suggested that the higher mid-life adiposity that is associated with higher dementia risk may be due to vascular mechanisms, whereas the decline in BMI and body weight is reflective of neurodegeneration and interruption of homeostatic feedback mechanisms in later life.11 An overarching challenge in linking anthropometric measures with dementia has been to answer the question, ‘What is BMI, waist circumference or WHR measuring that translates to differences in dementia risk?’ One answer to this is the quantity and secretory capacity of peripheral white adipose tissue (WAT).
Figure 1.
Anthropometric measures and corresponding cutpoints of overweight and obesity in adults.
WAT is an endocrine tissue that secretes hundreds of compounds, which are called adipokines when WAT is thought to be the major source. The endocrine function of adipose tissue may provide insight as to underlying mechanisms linking adipose tissue to the major neurodegenerative and vascular disease of aging - cognitive impairments and dementia. There are other potential factors associated with risk for dementia and adipose tissue, including physical activity, dietary constituents (nutrients and non-nutrients), dietary patterns, health and disease status, as well as genetic background, but discussion of these is beyond the scope of this review.12
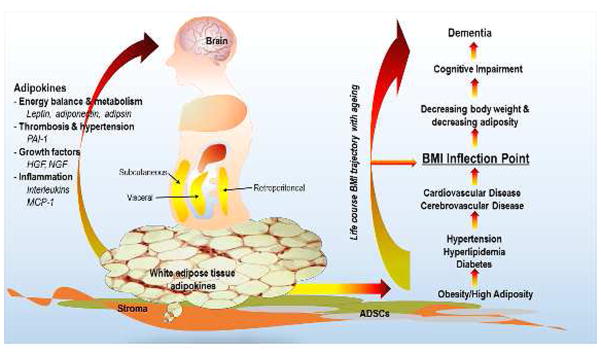
In this Review we will define and discuss the role of the adipokines that have shown associations with dementia from human observational and clinical studies. We will also discuss examples of adipokines that may be important for the brain and dementia that have been identified from experimental models and characteristic of the obese condition. (See Figure 2) The hypotheses described are related to selected adipokines, and their potential association with vascular events and neurodegeneration. This review is not comprehensive, but provides an overview for how certain adipokines could be associated with late-onset dementia risk. In light of this evolving literature and the paucity of data on associations between adipokines and dementia and dementia-related brain outcomes, we will also discuss the limitations of the data. Search Strategy and selection criteria
Figure 2.
WAT is an endocrine organ that secretes hundred of adipokines that influence multiple physiological processes to maintain homeostasis and respond to injury. WAT exists as subcutaneous, visceral and retroperitoneal depots, and has a stromal layer containing ADSCs. Amount of WAT is measured crudely in humans as BMI. BMI, on average, exhibits a dynamic trajectory with ageing. This trajectory is characterized by an adult life increase, followed by later life decrease. Generally, higher BMI or BMI increase is associated with the vascular risk that is common among overweight and obese adults. BMI decrease is associated with ageing and neurodegeneration. This reverse association is reflected in changing risk associations commonly observed between BMI and dementia/AD over the life course.
We searched PubMed using the following terms: dementia, Alzheimer, adiposity, body mass index (BMI), and the following adipokines: leptin, adiponectin, plasminogen activator inhibitor-1 (PAI-1), hepatocyte growth factor (HGF), nerve growth factor (NGF), adipsin (Complement Factor D), monocyte chemotactic protein (MCP-1), and interleukins. A comprehensive literature search was performed for studies reporting on associations between adipokines and dementia with no date restrictions. Studies of biological mechanism focused on 2010–2013.
ADIPOKINES AND ADIPOSE TISSUE
WAT is a complex tissue consisting of multiple cell types with multiple cellular phenotypes depending on cell composition and location.13 WAT consists of a stromal layer and a mature adipocyte layer. The stromal layer consists of Adipose Derived Stem Cells (ADSCs) or pre-adipocytes, fibroblasts, blood vessels, and nerve cells. ADSCs are self-renewing and can differentiate along several mesenchymal lineages into adipocytes, osteoblasts, myocytes, chondrocytes, endothelial cells, cardiomyocytes, and even neuronal-like cells.14 The mature adipocyte layer consists of fully differentiated adipocytes.15
Adipokines include hundreds of polypeptides secreted by the different cells of WAT, sometimes referred to as the adipose secretome or adipokinome,16 In the periphery, adipokine release is fat depot-specific, which is coherent with differences in adipocyte morphology and the local milieu. 14, 17, 18 More adipokines are released from visceral versus subcutaneous WAT. In addition, brown, epicardial and pancreatic adipose tissue appear to have unique adipokine profiles.19–21 Since the amount of visceral adipose is associated with higher waist circumference or WHR,22–24 this has been one rationale for using relatively unsophisticated methods of determining body adiposity, such anthropometric measures, to pinpoint mechanisms of action. Similarly BMI grossly reflects total adipose tissue during adulthood, and is a relatively good correlate, regardless of changing body composition with aging 25
The word adipokine or adipocytokine means ‘adipo-, adipose; -cyto-, cell; -kinos, movement. The term, ‘adipokine’ was derived to refer to immunomodulatory cytokines secreted by adipose tissue. However, the popular use of this term has expanded beyond this to include compounds which are technically not cytokines, adipokine instead refers to16 cytokines, acute phase reactants, growth factors and other inflammatory mediators, adipose tissue hormones such as leptin, and other chemical messengers.11, 26, 27 Adipokines act in autocrine, paracrine, or endocrine ways,28 and many affect processes in the periphery and the central nervous system (CNS).
Leptin, adiponectin, and interleukin-6 (IL-6), are the best known examples of adipokines, broadly defined. In 2011, experiments with isolated human adipocytes, serum, and adipose tissue biopsies from lean and obese individuals, identified 347 protein components (including 44 never before reported) of the adipokinome.16 However, over 700 adipose tissue-derived proteins have been reported in response to specific chronic or acute stimuli or at rest.16 One may also surmise that fat in the CNS plays a role, since the CNS has the highest lipid content in the human body after adipose tissue. However, CNS lipid does not exist as adipose tissue, but as layers of fatty acid-containing myelin sheaths that surround the axons of the brain and spinal cord.29 CNS-derived adipokines are instead, produced in various brain regions, by a variety of cell types30 and nuclei, such as the arcuate nucleus in the hypothalamus, for specific purposes such as regulating feeding behavior.
Since neurodegenerative and vascular processes observed in dementia affect several brain regions and nuclei,31 while not proven, the action of adipokines could be altered during neurodegenerative and vascular events and may even feedback to contribute to neurodegeneration. However, much remains to be discovered as to the relative source, brain versus periphery, and mechanistic actions of these adipokines in each compartment.
Adipokine release may be dysregulated in obesity and in aging, possibly because the adipose tissue may be ‘diseased’. The terms inflammaging32 and adiposopathy have been used to describe unhealthy adipose tissue. Adiposopathy describes the excessive hypertrophy of adipocytes33, 34 that leads to the dysregulated paracrine and endocrine adipose tissue activity that is associated with cardiovascular disease. Alterations in adipose tissue also contribute to the frailty syndromes observed in aging, to encompass physical (e.g., weight loss, sarcopenia) and functional (e.g., diminishing Activities of Daily Living) frailty in the periphery, as well as cognitive frailty in the brain.35 As such, while not proven, there is potentially a role for dysregulated adipose tissue in the development of dementia, brain pathology and/or clinical symptoms of dementia. While there is no known pharmacologic intervention targeting the link between adipose tissue and dementia, the link is indirectly suggested via the evidence presented in this review.
The adipokines most studied in humans are listed in Table 1. These molecules may be grouped according to primary function as shown; however, each may possess more than one function, and these functions do overlap. For example, leptin, adiponectin, resistin, plasminogen activator inhibitor-1 (PAI-1), hepatocyte growth factor (HGF), and nerve growth factor (NGF) are involved in dysregulation of nutrient utilization as well as inflammation, endothelial dysfunction, hypertension and atherogenesis.36 In addition, adipose and non-adipose hormones, for example, leptin and insulin, respectively, interact to augment each other.37 Insulin interacts directly with hypothalamic nuclei, and it appears that both leptin and insulin are mediators in insulin resistance, as illustrated by observations that pro-opiomelanocortin (POMC) neurons in the hypothalamus express both leptin and insulin receptors and regulate energy balance and glucose homeostasis. Experimental mouse models lacking both leptin and insulin receptors in POMC neurons display systemic insulin resistance. Thus, direct action of both insulin and leptin on POMC neurons appears required to maintain normal glucose homeostasis.38
Table 1.
A sampling of adipokines involved in energy balance and metabolism, inflammation, and thrombosis and hypertension and that may have relevance for AD119
| Energy balance and metabolism |
| Adiponectin |
| Adipsin (complement Factor D) |
| Apelin |
| Chemerin |
| Dipeptidyl peptidase-4 (DPP-4) adenosine deaminase complexing protein 2 or CD26 |
| Leptin |
| Lipocalin |
| Omentin |
| Resistin |
| Retinol binding protein-4 |
| Vaspin |
| Visfatin, also pre-B cell enhancing factor (PBEF) |
| Inflammation |
| IL-6 |
| IL-1 |
| IL-10 |
| IL-8 |
| monocyte chemotactic protein-1 (MCP-1) |
| TNF-alpha |
| Thrombosis & hypertension |
| Serum amyloid A (SAA) |
| CRP |
| PAI-1, total, active |
| Proteins of the Renin Angiotensin System |
| Growth factors |
| NGF |
| HGF |
| Brown fat |
| fibroblast growth factor-21 |
| interleukin-6 |
| insulin-like growth factor-1 |
We now review eight adipokines or classes of adipokines, grouped according to a primary function: energy balance and metabolism (leptin, adiponectin, and adipsin [Complement Factor D]); thrombosis and hypertension (plasminogen activator inhibitor-1 [PAI-1]); growth factors (HGF1, NGF1); and inflammation (interleukins and monocyte chemotactic protein [MCP-1]). First are the adipokines that have been reported in epidemiologic studies in association with dementia (Table 2); second we present a selection of adipokines that may be associated with dementia due to their biological roles and/or associations with brain processes.
Table 2.
A selection of epidemiologic studies associating blood adipokine levels with clinical dementia, AD or cognitive impairment.
| Study Name | N | Avg age (y) | Obs time | Study Type | Results |
|---|---|---|---|---|---|
| Leptin | |||||
| Prospective Population Study in Gothenburg, Sweden 32 | N=1462 | 38–60 | 24y | Long | No association of mid-life leptin with late-onset dementia in women (multivariate adj HR 1.01 95% CI 0.98–1.03, p=0.620) |
| Framingham Heart Study, USA120 | N=785, no dementia | 79 +/−5 | 8.3y | Long | Higher leptin levels associated with lower risk of incident dementia and AD in multivariable models (HR per 1SD log leptin, 0.68, 95% CI, 0.54–0.87, for all- cause dementia; HR per 1SD log leptin, 0.60, 95% CI, 0.46–0.79, for AD). A 1SD elevation in plasma leptin level associated with higher total cerebral brain volume (p=0.005) |
| Study of Osteoporotic Fractures121 | N=579 no dementia | 82.6 | 5y | long | Among women with BMI <25 kg/m2 a 1SD difference in log leptin (0.91 ng/mL) was associated with 32% lower odds of dementia/MCI (OR =0.68; 95% CI = .46,0.99) compared to those with BMI ≥ 25 kg/m2 |
| Health ABC Study, USA122 | N=2871 | 73.7 | 4y | Long | Compared to those in the lower leptin groups, elders in the high leptin group had less likelihood of cognitive decline, OR= 0.66 (95% CI 0.480.91). The lowest leptin group: mean leptin 2.3 ng/ml (SD 1.0 ng/ml), range 03.7 ng/ml; the middle leptin group: mean leptin 10.9 ng/ml (SD 5.2 ng/ml), range 3.722.8 ng/ml; the highest leptin group: mean leptin 32.3 ng/ml (SD 8.0 ng/ml), range 22.84.7 ng/ml. |
| Case-control study, in Japan123 | N=60, 20 Vascular Dementia, 40 age- matched controls | 79 | 0 | XS | Average leptin levels not different between VaD and controls (5.2 ± 0.9 vs 4.5 ± 0.6 ng/ml, p=0.548) |
| Adiponectin | |||||
| Framingham Heart Study124 | N=826 | Median 76 | 13y | Total adiponectin levels associated with increased all- cause dementia risk (HR 1.29; 95% CI, 1.00–1.66; P=0.054) and AD (HR 1.33; 95% CI, 1.00–1.76; P=.050) in women; baseline adiponectin > median at higher risk for all cause dementia (HR 1.63; 95% CI, 1.03–2.56; P=.04) and AD (HR 1.87; 95% CI, 1.13–3.10; =.01) | |
| Rochester Epidemiology Project, USA125 | N=890 no dementia | Median 80 | 0 | XS | Total adiponectin not related to MCI in a case-control study; average level MCI 14.4 (9.8, 19.6) mg/L vs no MCI: 14.5 (9.8, 19.6) p=0.97 |
| Case-control study, in Japan, Vascular dementia vs age-matched controls123 | N=60, 20VaD, 40 controls | 79 | 0 | XS | Average total adiponectin levels not different between VaD and controls (14 ±2 vs 12 ± 1(mcg/ml), p=0.387) |
| Clinical case series in Japan126 | N=28 controls, 18 MCI, 27 AD | 74.7 | 0 | XS | Higher plasma adiponectin in MCI and AD compared to controls (p<0.05), and higher CSF adiponectin in MCI compared to controls (p<0.05) |
| Clinical case series of MCI and AD, Brazil127 | N=157; 41 AD, 65 MCI, 51 controls | 71 | 0 | XS | Lower total adiponectin levels among those with MCI and AD (F = 18.2, df = 2, p=0.001); adiponectin did not predict progression of MCI to AD |
| Interleukins | |||||
| Framingham Heart Study128 | N=691 | 79 | 7y | long | Compared to the lowest tertile, individuals in the top two tertiles of PBMC production of IL-1 at higher risk for AD: for tertile 2, HR 2.84, 95% CI 1.09, 7.43, p=0.03, for tertile 3, HR 2.61, 95% CI 0.96, 7.07, p=0.06. |
| Health ABC Study109 | N=665 high, N=1967 low inflammation | 73.5 | 4y | long | Among those with metabolic syndrome, “high inflammation” defined as higher than the median for both CRP (≥2.0 mg/L) and IL-6 (≥2.0 pg/mL) associated with risk of developing cognitive impairment (RR = 1.66, 95% CI 1.19–2.32). |
| Rochester Epidemiology Project, USA125 | N=890 no dementia | Median 80 | 0 | XS | Total IL-6 not related to MCI in a case-control study; average level MCI 4.8 (2.5, 14.0) pg/ml vs 4.0 (2.4, 11.0), p=0 .35 |
| Dutch family study129 | N=206 children of AD, N=200 children of no AD | 50.3 | 0 | XS | In middle-aged children of late-onset AD cases, higher mean production capacity of IL-1beta (13 091 (380) vs 10 548 (580) pg/ml P < .001), IL-1beta to IL-1ra ratio (1.38 (0.06) vs 1.10 (0.05) P < .001), IL-6 (96 031 (2809) vs 88 226 (2827), P = .04); production capacity after stimulation with 10-ng/mL lipopolysaccharide. |
| Case-control study, in Japan, Vascular dementia vs age-matched controls123 | N=60, 20VaD, 40 controls | 79 | 0 | XS | Average IL-6 levels suggested higher among VAD vs controls (7.5 ± 1.7 vs 4.6 ± 0.7 pg/ml, p= 0.078) |
| PAI-1 | |||||
| Case-control study, in Japan, Vascular dementia vs age-matched controls123 | N=60, 20VaD, 40 controls | 79 | 0 | XS | Average PAI-1 levels suggested higher among VaD vs controls 26 ±5 vs 18 ±2 ng/ml, p=0.064 |
Abbreviations: Obs, observation; avg, average; y, years; RR, relative risk; HR, hazards ratio; OR, odds ratio; VaD, Vascular dementia; vs, versus; Long, longitudinal; XS, cross-sectional;
Energy Balance and Metabolism
Leptin;
Leptin is a 16 kDa protein hormone39–41 that is primarily secreted by adipose tissue and positively correlated with BMI.42, 43 Correlations of approximately r=0.7 between BMI and blood leptin levels are observed in adults, even among those with obesity syndromes.2, 44
Peripheral leptin enters the CNS and CSF and interacts with specific areas of the brain such as the hypothalamus and hippocampus.45, 46 However, beside leptin transport into the CSF and other brain regions, several studies indicate that leptin is also produced in human and rodent brains, for example in the hypothalamus, cortex and cerebellum.47–51 Leptin transport across the BBB occurs via a mechanism involving leptin receptor a (LepRa), and a second, not yet characterized, transport mechanism.52
Leptin regulates food intake and energy expenditure, improves insulin sensitivity, facilitates lipolysis, and inhibits lipogenesis.53, 54 In addition, leptin plays a permissive role in neuroendocrine immune function.55 Leptin is the most studied adipokine in association with brain structure and function, and has numerous effects on brain development56 and potentially on brain health in cognition and aging. Leptin affects hypothalamic function, and learning and memory processes controlled by the hippocampus.56–59 Experimental data show that leptin, and other adipokines, interact directly with hypothalamic nuclei, such as the arcuate nucleus, and regulate energy expenditure and food intake through production of orexigenic (NPY, agrp) and anorectic (aMSH) peptides.60, 61 In addition, leptin appears to facilitate pre- and postsynaptic transmitter release and sensitivity, respectively, in hippocampal CA1 neurons.58 This translates to improved performance related to spatial learning and memory function. Leptin may both shape the hypothalamus in the earliest stages of development and enhance cognition.56 Leptin reduces beta-secretase activity in vitro, increases APOE-dependent Abeta uptake, and via lipolytic mechanisms, affects Abeta turnover in experimental models.59
Observations from human population studies in late life suggest that high leptin levels and high BMI are associated with lower dementia risk when measured within 10 years of clinical dementia diagnosis (Table 2).56, 62 Longer term follow-up and cross-sectional studies show no association between leptin and dementia. Since leptin levels are higher among adults who do not develop dementia during the approximate 10 year prodromal phase, it has been suggested that leptin may be a cognitive enhancer when given at the time of cognitive impairment or dementia.63 While leptin levels may trend with BMI in relation to dementia, given the ctrong correlation between them,, leptin may also have an independent role in health of the ageing brain.
Adiponectin;
Adiponectin (ACRP30) exists as complex multimeric isoforms comprised of High Molecular Weight (HMW) hexamers and trimers.64 Adiponectin modulates inflammatory responses, energy expenditure in the CNS and periphery, central food intake, and a number of metabolic processes, including glucose regulation and fatty acid catabolism in the periphery. It is an effective insulin sensitizer, and circulating levels are inversely correlated with insulin resistance, metabolic syndrome, obesity, type 2 diabetes, and cardiovascular diseases.65 HMW adiponectin or the ratio of HMW adiponectin to total adiponectin may be better indicators of insulin sensitivity than total adiponectin in obesity, diabetes, and cardiovascular disease.64 While adiponectin is produced solely by adipose tissue, its receptors are not.66 The peripheral effects of adiponectin are mediated mainly via 2 receptors, AdipoR1 and AdipoR2. Expression of these receptors is reported in adipose, brain, ovaries, endometrium, and placenta.67 Both AdipoR1 and AdipoR2 are widely found throughout the CNS in brain microvessels, hippocampus, hypothalamus and brainstem in humans and rodent models.51, 68–71 However, in humans, a 1000-fold lower cerebrospinal fluid (CSF)/serum adiponectin ratio is observed.71 Thus, the origin of brain adiponectin is debated. Trimeric and low molecular weight adiponectins are detectable in the CSF of humans and rodents.61, 69, 72 In combination with a lack of HMW adiponectin observed in CSF, this may imply that only smaller forms of adiponectin cross the blood brain barrier (BBB).52, 69, 72
The Prospective Study of Women in Gothenburg, Sweden show late-life (age 70 years and older) correlations of r= −0.29, between BMI and blood HMW adiponectin levels.2 Similar correlations are observed in mid-life for women with or at risk for HIV infection.2 Given the inverse association of adiponectin with BMI in adults, one may expect higher adiponectin levels to be associated with prevalent dementia and AD, since individuals with dementia tend to have lost weight prior to a clinical diagnosis, and subsequently weigh less than those without dementia.9, 73 Of the studies summarized in Table 2, conclusions are mixed. Adiponectin is suggested to be a visceral adiposity marker 74, 75 and only moderately correlated with BMI (compared to leptin for example), and, as aforementioned, BBB transport mechanisms are unclear. Thus blood levels may not provide adequate indication of potential interaction between adiponectin and the brain.76 Studies evaluating adiponectin in association with dementia, have reported on total adiponectin levels. Isolating HMW adiponectin and the smaller adiponectin fragments can present problems in the laboratory and inter- and intra-assay variability can be high,64 making laboratory assay optimization difficult.
Adipsin (Complement Factor D)
Plasma adipsin is inversely correlated with age and positively associated with BMI, WHR, and overweight and obesity.77 There are no observational human studies showing an association between adipsin and dementia. However, it is interesting to note that adipsin is elevated in animal models that have been treated with functional gamma secretase inhibitors.78 However, gamma secretase inhibition elevates adipsin secretion from ileum crypt cells only (measured via higher levels of fecal adipsin), not from adipose tissue.78
Thrombosis and Hypertension
PAI-1
In humans, plasminogen activator inhibitor-1 (PAI-1) is elevated in plasma of obese children, adolescents and adults,79–82 primarily due to adipocyte secretion. PAI-1 influences vascular health by inhibiting fibrinolysis83 via inhibition of tissue type plasminogen activator (tPA) and urokinase plasminogen activator (uPA).83, 84 Furthermore, excess adipose tissue, especially central obesity in adults is associated with decreased fibrinolysis, possibly due to elevated PAI- 1.85 Despite the association of peripheral PAI-1 with obesity, peripheral PAI-1 may not be capable of affecting brain processes, since no transport mechanism for PAI-1 across the BBB has been discovered. PAI-1 produced within the brain by microglia and astrocytes may regulate apoptosis, survival of neurites and migration of microglia.86–89 Moreover, in vitro studies show that PAI-1 contributes to the survival of neurites, axons and/or dendrites.89
There are several investigations of the interactions between PAI-1 and t-PA in the brain; however, the data are not clear. t-PA is produced by endothelial cells, mediates fibrinolysis, and crosses intact BBB.11 As aforementioned, PAI-1 inhibits t-PA. Therefore, related to aging and dementia processes, the role of endogenous t-PA in the brain may be protective and includes clot dissolution or amyloid degradation.90–92 It has also been shown that brain amyloid induces t-PA formation, thus increasing plasmin levels, which may lead to Abeta-42 degradation.93 However, it has also been suggested that in the brain, t-PA may be neurotoxic, lead to tau hyperphosphorylation, destabilize microtubules, mediate amyloid toxicity, and shift apoptosis in a stressed brain, such as observed in strokes without clot formation.93 Only one study was identified on PAI-1 and vascular dementia (Table 2); and a study of brain tissue from AD and control brains, revealed higher tPA mRNA expression in AD brains.94
Inflammation
A variety of adipokines, particularly interleukins, are associated with inflammatory processes and implicated in dementia. Obesity is characterized by a chronic low inflammation state partly mediated via production of pro-inflammatory adipokines such as IL-1 and IL-6.95 This has been reviewed extensively.30 For example, IL-6 is an immunoregulatory cytokine that activates a cell surface signaling assembly composed of IL-6, IL-6RA, and the shared signaling receptor gp130, a common mechanism in inflammation.96 IL-6 crosses the BBB by a saturable transport mechanism, entering both CSF and brain parenchyma. Approximately 50% of IL-6 in the CSF and 16% in brain parenchyma represent intact peripheral cytokine in male mice.97 Since there is excessive degradation of IL-6 in the brain, the influence of peripheral IL-6 in the CNS is unclear; small amounts of intact IL-6 may be effective.97 Rodent studies show that IL-6 is produced in the brain by glial cells, astrocytes and endothelial cells of the brain’s microvessels.98–100 In addition, amyloid deposition, and other neuropathological events in dementia are associated with local inflammatory events in the brain characterized by interleukin release, as well as release of TNF-alpha and other inflammatory agents.30
The hippocampus is particularly vulnerable to adverse effects of IL-6, affecting brain functions like synaptic plasticity and neurogenesis.101–104 In the hypothalamus, IL-6 modifies leptin signaling and other anorexic signals.105 An early onset of IL-6 elevation due to childhood and adolescent obesity, and its persistence in aging obese adults, has been proposed to negatively affect brain functioning by inhibiting neurogenesis, decreasing synaptic plasticity and subsequently disrupting learning and memory processes, particularly in the hippocampus, which increases the risk of cognitive deficits in obese individuals.106 In middle-aged adults, higher plasma IL-6 levels are associated with lower hippocampal grey matter volume.107 Studies of interleukins in association with dementia and mild cognitive impairment (MCI) are summarized in Table 2. While different interleukins are measured across studies, in general, they are suggested to increase with MCI and dementia.
MCP-1 in the blood is another marker of systemic inflammation. Insulin induces substantial expression and secretion of MCP-1 in vitro in insulin-resistant adipocytes, and in vivo in insulin-resistant obese mice (ob/ob).108 The MCP-1 gene functionally resembles other genes that are sensitive to insulin in insulin-resistant states, such as PAI-1. MCP-1 is overexpressed in obesity and included in the family of genes like PAI-1 and SREBP-1c that continue to respond to exogenous insulin in insulin resistant mice and adipocytes. Mice remain sensitive to insulin in terms of PAI-1 gene expression, possibly because glucose homeostasis and PAI-1 gene expression are regulated by different insulin signaling pathways.109 Consistent with this hypothesis, it has been demonstrated that in leptin-deficient states, insulin signaling in murine liver also diverges along two pathways; and the transcription factor SREBP-1c is another gene that remains sensitive to insulin in these IR mice.108, 110 Similar selective insulin resistance also has been described in human and rodent muscle.111, 112
Collectively, these observations raise the possibility that in metabolic insulin resistance accompanied by hyperinsulinemia and obesity, the expression of certain insulin-responsive genes may dramatically increase in insulin target tissues. Higher levels of MCP-1 protein may induce adipocyte dedifferentiation and contribute to pathologic states associated with hyperinsulinemia and obesity, including type 2 diabetes. Increased MCP-1 mRNA in adipose tissue and MCP-1 protein in plasma are observed in genetically obese diabetic (db/db) mice and in wild-type mice with obesity induced by a high-fat diet.113 Interestingly, plasma MCP-1 is correlated with severity of Traumatic Brain Injury (TBI) and an index of compromised axonal fiber integrity in the frontal cortex. MCP-1 is suggested as a marker of AD risk in TBI.114
Growth Factors
Hepatocyte Growth Factor (HGF)
HGF, also known as scatter factor and hematopoietin A, is elevated in obese adults and adolescents.115 In vitro, HGF secretion from adipocytes of obese versus lean individuals is greater.116 HGF is a multifunctional trophic factor that binds to its receptor, MET, and activates a tyrosine kinase signaling cascade. While HGF is produced by neurons and nonneuronal cells, MET is highly expressed in neurons. During embryogenesis, HGF acts as a neural inducer, an interneuron motogen, axonal chemoattractant, angiogenic factor and as neuroprotective survival factor.117, 118 In adults, HGF production is induced by ischemic injury119 and AD.120
HGF enhances long-term potentiation121 and improves memory deficits due to ischemia.122 HGF mRNA is found in the brain; HGF-like immunoreactivity is observed in both the cerebral cortex and white matter; and confocal microscopy confirms that HGF is present in GFAP positive astrocytes and LN3 positive microglia cells, as well as rare scattered cortical neurons.120 These studies also indicate that HGF is increased within senile plaques as a function of gliosis and microglial proliferation that occurs in association with AD.120 HGF and other growth factors are also shown to accelerate neuroprotection, angiogenesis, and regeneration in the brain.123 However, it is unclear the role of central versus peripheral HGF.
Nerve Growth Factor (NGF)
NGF is a neurotrophin secreted by adipose tissue, and associated with neuronal survival, differentiation of target neurons, and growth of nerve fibers and their guidance (tropism) toward the source of production.124 With application for the brain and important for AD, is that NGF inhibits the amyloidogenic processing of amyloid precursor protein (APP) in vitro.125, 126 The source of this NGF may be central or peripheral production, since NGF has been shown to cross the BBB. There are few studies evaluating circulating NGF with cognitive outcomes, although it is hypothesized that NGF repletion may be a treatment for AD via protection of the cholinergic system.127 Serum NGF has been suggested to be lower among those with AD.128 In contrast, CSF levels of NGF were higher in AD patients versus healthy controls.129 NGF has also been proposed as a therapy for traumatic brain injury (TBI),130 a risk factor for dementia.
In relationship to adiposity, a study in China assessing the correlations between anthropometric indices and adipokines, showed that WHR was associated with NGF (r = 0.48), and leptin (r = 0.53) levels. In this study, BMI and WHR were also correlated with mean HGF levels (r = 0.34 and 0.51, respectively) and PAI-1 levels (r = 0.42 and 0.56, respectively).131
CONCLUSION
The association between adipokines and clinical dementia or cognitive impairment is largely unexplored, despite published epidemiologic data supporting associations between adiposity, measured via anthropometry, and dementia and AD.
Considerations and Limitations
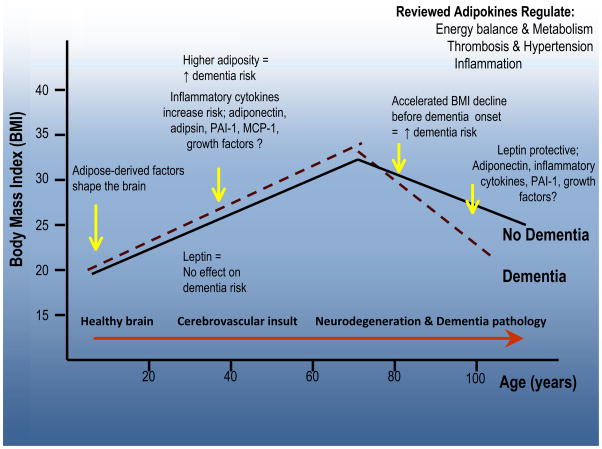
There are several considerations and/or limitations when evaluating the adipokine-dementia literature. 1) Adipokines are not secreted from adipose tissue only. While hundreds of adipokines potentially reflect the adipose tissue exposure and comprise the adipokinome, depending on the mechanisms of action of interest and tissues involved, different adipokines may play a role. In this case, adipokines may function as biomarkers for systems biology approaches or be good statistical markers of risk, but poor indicators of neurodegenerative or vascular mechanisms that are coupled to adipose tissue. Definitive dementia risk estimates remain to be elucidated for any adipokine. 2) Many adipokines are not associated with anthropometric measures of overweight and obesity. Reasons for this may be that anthropometric measures are not be good estimates of amount of adipose tissue during adulthood; adipose tissue is not a primary source of these particular adipokines; or adipokine release is not associated with quantity of adipose tissue. 3) Adipokine functions in the periphery are not necessarily similar to those in the brain, e.g., PAI-1. This has challenged understanding of adipokine actions in each compartment, as well as requiring more research on their movement across the BBB and interactions with other adipokines. 4) There are sex and race/ethnic differences in adult body composition and adipokine levels, and these differences do not correspond to differences in dementia occurrence.132, 133 5) Potential influencers of blood adipokine levels, such as medications, type of metabolic syndrome (e.g., in type 2 diabetics, adults with HIV infection), degree of overweight and obesity, and other factors are not well understood. 6) The trajectory of BMI over the life course suggested in Figure 3,10 and variations in the relationship of BMI to general aging, dementia, and mortality,134 emphasize the importance of age of BMI measurements and proximity to occurrence of clinical dementia onset, for example, mid- versus late-life. While higher levels of adult BMI may increase risk for chronic neurodegenerative and vascular diseases of aging, some studies show that the direction of the BMI-dementia relationship changes direction, and declines, later in life.2, 7, 10 Perhaps higher BMI, and more central adiposity, are mid-life markers of vascular risk dominating the dementia risk equation, while declining, and lower levels of BMI, denote predominant neurodegenerative events in latest life. This latter point is illustrated by data from the National Alzheimer Coordinating Center (NACC) in U.S., showing that among those with MCI, a higher baseline level of BMI is associated with a worse clinical dementia rating, but greater subsequent body weight decline is associated with faster clinical progression.9 Not all observations, however, evidence a similar trajectory. The Gothenburg Birth Cohort Studies showed that there is lesser BMI increase prior to the inflection point indicated in Figure 3, followed by a similar rate of BMI decline among those with and without dementia.10 This suggests different biological mechanisms, perhaps mediated by adipokines, underlying the evolution of aging and dementia, as well as heterogeneity in the dementia outcome reflecting both vascular and neurodegenerative processes in the brain. 7) Survival bias may be influencing observed mid-life, late-life obesity-dementia associations, since competing risk analyses tend to show that those who are overweight or obese die before the age at which they are at risk for dementia.5, 135 However, this may change as survivorship with multiple co-morbidities, many associated with overweight and obesity, increases. These considerations can be best addressed, and limitations overcome, by additional research.
Figure 3.
The trajectory of BMI over the life course by chronological age and potential roles of adipokines. Observational data suggest that higher levels of adult BMI may increase risk for late-onset dementia. Mid-life inflammatory cytokine levels measured in blood are higher in relationship to dementia. A null association with blood leptin levels has been reported. The BMI-dementia association changes direction later in life. BMI declines such that lower levels of BMI are observed among those with dementia compared to those without. During this period, lower blood leptin levels have been reported and reports on other adipokines are mixed. Other BMI trajectories have been observed, suggesting perhaps different adipokine-related mechanisms underlying the evolution of aging and dementia, as well as heterogeneity in the dementia outcome.
In summary
The focus of this review has been on adipokines associated with excess adiposity, a risk factor for late-onset dementia. We are unaware of published data on changes in adipokine levels over the life course. As additional chapters on the epidemiology and biological mechanisms linking adipokines and dementia are written, there will be greater understanding of inter- and intra-individual differences in adipokine metabolism; dysregulation of metabolism that occurs with aging, neurodegeneration and/or dementia; and differences in metabolism given differences in diet, physical activity, race/ethnicity and genetics. For example, whether changes in body composition with physical activity interventions among elderly ‘improve’ the adipokine profile for the aging brain, is unknown. Given the vast number of adipokines, one approach for exploration could be directed toward their cumulative role as classes of adipokines versus single adipokines. In addition, therapeutic strategies related to the use of single or combined adipokines may be an avenue for exploration in prevention of cognitive impairments and dementia. This has been suggested for leptin.63
Given the immense secretory capacity of adipose tissue, the often acute nature of the adipose secretome in response to various stimuli, and changing body composition with aging, it remains a challenge to unravel the influence of this organ over the life course. From bench to bedside, adipokines as biomarkers may enhance understanding of late-onset dementia risk over the life course, as well as the clinical progression of prodromal and manifest dementias. This will allow for identification of populations at risk, and for the design of better clinical trials to target vascular and metabolic risk associated with adipose tissue both centrally and peripherally.
Acknowledgments
Role of funding source
Dr. Gustafson is supported by the EU 7th framework LipiDiDiet project (FP7/2007–2015) under grant agreement no211696; NIH/NIAID U01 318345; Swedish Research Council Diarienummer: 523-2005-8460; and the State University of New York Research Foundation.
Dr. Kiliaan is supported by the EU 7th framework LipiDiDiet project (FP7/2007-2015) under grant agreement no211696
The funding sources had no role in the content of this review.
Footnotes
Authors' contributions Dr. Gustafson received the invitation to write the review as a follow-up to her 2006 Lancet Neurology review on BMI and dementia. For this review, she conducted literature searches, helped to design the figure and tables, and drafted the text.
Ms. Arnoldussen conducted literature searches, helped to design the figure, and drafted parts of the text.
Dr. Kiliaan conducted literature searches, helped to design the figure, and drafted parts of the text.
Conflict of interest statements
The authors have no conflicts of interest to declare.
Ethics committee approval
NA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amanda J Kiliaan, Email: A.Kiliaan@anat.umcn.nl.
Ilse AC Arnoldussen, Email: I.Arnoldussen@anat.umcn.nl.
Deborah R Gustafson, Email: deborah.gustafson@downstate.edu, deborah.gustafson@neuro.gu.se.
References
- 1.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Archives of internal medicine. 2003;163(13):1524–8. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 2.Gustafson DR. personal communication. 2013.
- 3.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4(2):103–9. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 4.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71 (14):1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 7.Gustafson DR, Backman K, Waern M, et al. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73(19):1559–66. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20 (2):93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 9.Besser LM, Gill DP, Monsell SE, et al. Body Mass Index, weight change, and clinical progression in Mild Cognitive Impairment and Alzheimer’s Disease. Alz Dis Assoc Dis. doi: 10.1097/WAD.0000000000000005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafson DR. Adiposity and cognitive decline: underlying mechanisms. Journal of Alzheimer's disease : JAD. 2012;30 (Suppl 2):S97–112. doi: 10.3233/JAD-2012-120487. [DOI] [PubMed] [Google Scholar]
- 11.Arnoldussen IAC, Kiliaan AJ, Gustafson DR. Obesity and dementia: Adipokines interact with the brain. Eur J Neuropsychopharmacology. doi: 10.1016/j.euroneuro.2014.03.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JH, Lin KP, Chen YC. Risk factors for dementia. Journal of the Formosan Medical Association = Taiwan yi zhi. 2009;108(10):754–64. doi: 10.1016/S0929-6646(09)60402-2. [DOI] [PubMed] [Google Scholar]
- 13.Prunet-Marcassus B, Cousin B, Caton D, Andre M, Penicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Experimental cell research. 2006;312(6):727–36. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Vindigni V, Tonello C, Lancerotto L, et al. Preliminary report of in vitro reconstruction of a vascularized tendonlike structure: a novel application for adipose-derived stem cells. Annals of plastic surgery. 2013;71(6):664–70. doi: 10.1097/SAP.0b013e3182583e99. [DOI] [PubMed] [Google Scholar]
- 15.Adipose tissue protocols. New York, NY: Humana Press; 2010. [Google Scholar]
- 16.Lehr SHS, Lamers D, et al. Mol Cell Proteomics. 2012;11(1):M111 010504. doi: 10.1074/mcp.M111.010504. Identification and validation of novel adipokines released from primary human adipocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avram AS, Avram MM, James WD. Subcutaneous fat in normal and diseased states: 2. Anatomy and physiology of white and brown adipose tissue. J Amer Acad Dermatology. 2005;53 (4):671–83. doi: 10.1016/j.jaad.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Sbarbati A, Accorsi D, Benati D, et al. Subcutaneous adipose tissue classification. European journal of histochemistry : EJH. 2010;54(4):e48. doi: 10.4081/ejh.2010.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villarroya J, Cereijo R, Villarroya F. An endocrine role for brown adipose tissue? American journal of physiology Endocrinology and metabolism. 2013;305(5):E567–72. doi: 10.1152/ajpendo.00250.2013. [DOI] [PubMed] [Google Scholar]
- 20.Esteve Rafols M. Adipose tissue: Cell heterogeneity and functional diversity. Endocrinologia y nutricion : organo de la Sociedad Espanola de Endocrinologia y Nutricion. 2013 doi: 10.1016/j.endonu.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Feng B, Zhang T, Xu H. Human adipose dynamics and metabolic health. Annals of the New York Academy of Sciences. 2013;1281:160–77. doi: 10.1111/nyas.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferland M, Despres JP, Tremblay A, et al. Assessment of adipose tissue distribution by computed axial tomography in obese women: association with body density and anthropometric measurements. The British journal of nutrition. 1989;61(2):139–48. doi: 10.1079/bjn19890104. [DOI] [PubMed] [Google Scholar]
- 23.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. The American journal of cardiology. 1994;73 (7):460–8. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 24.Seidell JC, Oosterlee A, Deurenberg P, Hautvast JG, Ruijs JH. Abdominal fat depots measured with computed tomography: effects of degree of obesity, sex, and age. European journal of clinical nutrition. 1988;42(9):805–15. [PubMed] [Google Scholar]
- 25.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? American journal of epidemiology. 1996;143(3):228–39. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 26.Campbell DR, Kurzer MS. Flavonoid inhibition of aromatase enzyme activity in human preadipocytes. The Journal of steroid biochemistry and molecular biology. 1993;46(3):381–8. doi: 10.1016/0960-0760(93)90228-o. [DOI] [PubMed] [Google Scholar]
- 27.Gustafson DR. Adiposity hormones and dementia. Journal of the neurological sciences. 2010;299(1–2):30–4. doi: 10.1016/j.jns.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 28.Stedman's Medical Dictionary. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 29.Morell PaQRH. Myelin formation, structure, and biochemistry Basic neurochemistry: molecular, cellular, and medical aspects. 5. Philadelphia: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 30.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. International journal of biological sciences. 2012;8(9):1254–66. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenk GL. Neuropathologic changes in Alzheimer's disease. The Journal of clinical psychiatry. 2003;64 (Suppl 9):7–10. [PubMed] [Google Scholar]
- 32.Michaud M, Balardy L, Moulis G, et al. Proinflammatory Cytokines, Aging, and Age-Related Diseases. Journal of the American Medical Directors Association. 2013 doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Van de Voorde J, Pauwels B, Boydens C, Decaluwe K. Adipocytokines in relation to cardiovascular disease. Metabolism: clinical and experimental. 2013 doi: 10.1016/j.metabol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Bays HE. Adiposopathy is "sick fat" a cardiovascular disease? Journal of the American College of Cardiology. 2011;57(25):2461–73. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 35.Catindig JA, Venketasubramanian N, Ikram MK, Chen C. Epidemiology of dementia in Asia: insights on prevalence, trends and novel risk factors. Journal of the neurological sciences. 2012;321(1–2):11–6. doi: 10.1016/j.jns.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Duvnjak L, Duvnjak M. The metabolic syndrome - an ongoing story. J Physiol Pharmacol. 2009;60 (Suppl 7):19–24. [PubMed] [Google Scholar]
- 37.Figlewicz DP. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2003;284(4):R882–92. doi: 10.1152/ajpregu.00602.2002. [DOI] [PubMed] [Google Scholar]
- 38.Hill JW, Elias CF, Fukuda M, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell metabolism. 2010;11(4):286–97. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Proenca R, Maffel M, Barone M, Leopold L, Friedman J. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 40.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 41.Banks W. Is obesity a disease of the blood-brain barrier? Physiological, pathological, and evolutionary considerations. Curr Pharm Des. 2003;9:801–9. doi: 10.2174/1381612033455350. [DOI] [PubMed] [Google Scholar]
- 42.Friedman J, Halaas L. Leptin and the regulation of body weight in mammals. Nature. 1998;22:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 43.Lissner L, Karlsson C, Lindroos AK, et al. Birth weight, adulthood BMI, and subsequent weight gain in relation to leptin levels in Swedish women. Obes Res. 1999;7(2):150–4. doi: 10.1002/j.1550-8528.1999.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 44.Gustafson DR, Backman K, Joas E, et al. 37 years of body mass index and dementia: observations from the prospective population study of women in Gothenburg, Sweden. Journal of Alzheimer's disease : JAD. 2012;28(1):163–71. doi: 10.3233/JAD-2011-110917. [DOI] [PubMed] [Google Scholar]
- 45.Peiser C, McGregor GP, Lang RE. Binding and internalization of leptin by porcine choroid plexus cells in culture. Neuroscience letters. 2000;283(3):209–12. doi: 10.1016/s0304-3940(00)00942-3. [DOI] [PubMed] [Google Scholar]
- 46.Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology. 2000;141(4):1434–41. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]
- 47.Wiesner G, Vaz M, Collier G, et al. Leptin is released from the human brain: influence of adiposity and gender. The Journal of clinical endocrinology and metabolism. 1999;84(7):2270–4. doi: 10.1210/jcem.84.7.5854. [DOI] [PubMed] [Google Scholar]
- 48.Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140(12):5995–8. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- 49.Brown R, Imran SA, Belsham DD, Ur E, Wilkinson M. Adipokine gene expression in a novel hypothalamic neuronal cell line: resistin-dependent regulation of fasting-induced adipose factor and SOCS-3. Neuroendocrinology. 2007;85(4):232–41. doi: 10.1159/000104248. [DOI] [PubMed] [Google Scholar]
- 50.Brown R, Thompson HJ, Imran SA, Ur E, Wilkinson M. Traumatic brain injury induces adipokine gene expression in rat brain. Neuroscience letters. 2008;432(1):73–8. doi: 10.1016/j.neulet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkinson M, Brown R, Imran SA, Ur E. Adipokine gene expression in brain and pituitary gland. Neuroendocrinology. 2007;86(3):191–209. doi: 10.1159/000108635. [DOI] [PubMed] [Google Scholar]
- 52.Schulz C, Paulus K, Lehnert H. Adipocyte-brain: crosstalk. Results and problems in cell differentiation. 2010;52:189–201. doi: 10.1007/978-3-642-14426-4_16. [DOI] [PubMed] [Google Scholar]
- 53.Bluher M. Adipose tissue dysfunction in obesity. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2009;117(6):241–50. doi: 10.1055/s-0029-1192044. [DOI] [PubMed] [Google Scholar]
- 54.Abizaid A, Horvath TL. Brain circuits regulating energy homeostasis. Regulatory peptides. 2008;149(1–3):3–10. doi: 10.1016/j.regpep.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bluher S, Mantzoros CS. Leptin in humans: lessons from translational research. The American journal of clinical nutrition. 2009;89(3):991S–7S. doi: 10.3945/ajcn.2008.26788E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harvey J, Shanley LJ, O'Malley D, Irving AJ. Leptin: a potential cognitive enhancer? Biochem Soc Trans. 2005;33(Pt 5):1029–32. doi: 10.1042/BST20051029. [DOI] [PubMed] [Google Scholar]
- 57.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiology & behavior. 2005;86(5):731–46. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Oomura Y, Aou S, Fukunaga K. Prandial increase of leptin in the brain activates spatial learning and memory. Pathophysiology : the official journal of the International Society for Pathophysiology / ISP. 2010;17(2):119–27. doi: 10.1016/j.pathophys.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer's Abeta. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(15):1870–8. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- 60.Kishi T, Elmquist JK. Body weight is regulated by the brain: a link between feeding and emotion. Mol Psychiatry. 2005;10(2):132–46. doi: 10.1038/sj.mp.4001638. [DOI] [PubMed] [Google Scholar]
- 61.Qi Y, Takahashi N, Hileman SM, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10(5):524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 62.Lieb W, Beiser AS, Vasan RS, et al. Association of plasma leptin levels with incident Alzheimer's disease and MRI measures of brain aging. JAMA. 2009;302(23):2565–72. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carro EM. Therapeutic approaches of leptin in Alzheimer's disease. Recent Pat CNS Drug Discov. 2009;4(3):200–8. doi: 10.2174/157488909789104848. [DOI] [PubMed] [Google Scholar]
- 64.Sinha MK, Songer T, Xiao Q, et al. Analytical validation and biological evaluation of a high molecular-weight adiponectin ELISA. Clinical chemistry. 2007;53(12):2144–51. doi: 10.1373/clinchem.2007.090670. [DOI] [PubMed] [Google Scholar]
- 65.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes care. 2003;26(8):2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 66.Kang KH, Higashino A, Kim HS, Lee YT, Kageyama T. Molecular cloning, gene expression, and tissue distribution of adiponectin and its receptors in the Japanese monkey, Macaca fuscata. Journal of medical primatology. 2009;38(2):77–85. doi: 10.1111/j.1600-0684.2008.00298.x. [DOI] [PubMed] [Google Scholar]
- 67.Angelidis G, Dafopoulos K, Messini CI, et al. The emerging roles of adiponectin in female reproductive system-associated disorders and pregnancy. Reprod Sci. 2012 doi: 10.1177/1933719112468954. [DOI] [PubMed] [Google Scholar]
- 68.Qiu G, Wan R, Hu J, et al. Adiponectin protects rat hippocampal neurons against excitotoxicity. Age (Dordr) 2011;33(2):155–65. doi: 10.1007/s11357-010-9173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell metabolism. 2007;6(1):55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Fry M, Smith PM, Hoyda TD, et al. Area postrema neurons are modulated by the adipocyte hormone adiponectin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(38):9695–702. doi: 10.1523/JNEUROSCI.2014-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kos K, Harte AL, da Silva NF, et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. The Journal of clinical endocrinology and metabolism. 2007;92(3):1129–36. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 72.Kusminski CM, McTernan PG, Schraw T, et al. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50(3):634–42. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- 73.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65(6):892–7. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 74.Lenchik L, Register TC, Hsu FC, et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33(4):646–51. doi: 10.1016/s8756-3282(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 75.Ryan AS, Berman DM, Nicklas BJ, et al. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes care. 2003;26(8):2383–8. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 76.Drolet R, Belanger C, Fortier M, et al. Fat depot-specific impact of visceral obesity on adipocyte adiponectin release in women. Obesity. 2009;17(3):424–30. doi: 10.1038/oby.2008.555. [DOI] [PubMed] [Google Scholar]
- 77.Blogowski W, Budkowska M, Salata D, et al. Clinical analysis of selected complement-derived molecules in human adipose tissue. Journal of translational medicine. 2013;11:11. doi: 10.1186/1479-5876-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Searfoss GH, Jordan WH, Calligaro DO, et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional gamma-secretase inhibitor. The Journal of biological chemistry. 2003;278(46):46107–16. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- 79.Giordano P, Del Vecchio GC, Cecinati V, et al. Metabolic, inflammatory, endothelial and haemostatic markers in a group of Italian obese children and adolescents. European journal of pediatrics. 2011;170(7):845–50. doi: 10.1007/s00431-010-1356-7. [DOI] [PubMed] [Google Scholar]
- 80.Mantovani RM, Rios DR, Moura LC, et al. Childhood obesity: evidence of an association between plasminogen activator inhibitor-1 levels and visceral adiposity. Journal of pediatric endocrinology & metabolism : JPEM. 2011;24(5–6):361–7. doi: 10.1515/jpem.2011.015. [DOI] [PubMed] [Google Scholar]
- 81.Singh A, Foster GD, Gunawardana J, et al. Elevated circulating tissue factor procoagulant activity, factor VII, and plasminogen activator inhibitor-1 in childhood obesity: evidence of a procoagulant state. British journal of haematology. 2012;158(4):523–7. doi: 10.1111/j.1365-2141.2012.09160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. The American journal of clinical nutrition. 2006;83(2):461S–5S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 83.Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes & metabolism. 2004;30(1):13–9. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- 84.Loskutoff DJ, Sawdey M, Mimuro J. Type 1 plasminogen activator inhibitor. Progress in hemostasis and thrombosis. 1989;9:87–115. [PubMed] [Google Scholar]
- 85.Skurk T, Hauner H. Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2004;28(11):1357–64. doi: 10.1038/sj.ijo.0802778. [DOI] [PubMed] [Google Scholar]
- 86.Ahn MY, Zhang ZG, Tsang W, Chopp M. Endogenous plasminogen activator expression after embolic focal cerebral ischemia in mice. Brain research. 1999;837(1–2):169–76. doi: 10.1016/s0006-8993(99)01645-5. [DOI] [PubMed] [Google Scholar]
- 87.Jeon H, Kim JH, Kim JH, Lee WH, Lee MS, Suk K. Plasminogen activator inhibitor type 1 regulates microglial motility and phagocytic activity. Journal of neuroinflammation. 2012;9:149. doi: 10.1186/1742-2094-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soeda S, Koyanagi S, Kuramoto Y, et al. Anti-apoptotic roles of plasminogen activator inhibitor-1 as a neurotrophic factor in the central nervous system. Thrombosis and haemostasis. 2008;100(6):1014–20. doi: 10.1160/th08-04-0259. [DOI] [PubMed] [Google Scholar]
- 89.Soeda S, Oda M, Ochiai T, Shimeno H. Deficient release of plasminogen activator inhibitor-1 from astrocytes triggers apoptosis in neuronal cells. Brain research Molecular brain research. 2001;91(1–2):96–103. doi: 10.1016/s0169-328x(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 90.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. The New England journal of medicine. 2013;368(10):904–13. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kasza A, Kowanetz M, Poslednik K, Witek B, Kordula T, Koj A. Epidermal growth factor and pro-inflammatory cytokines regulate the expression of components of plasminogen activation system in U373-MG astrocytoma cells. Cytokine. 2001;16(5):187–90. doi: 10.1006/cyto.2001.0957. [DOI] [PubMed] [Google Scholar]
- 92.Murakami K, Suzuki M, Suzuki N, Hamajo K, Tsukamoto T, Shimojo M. Cerebroprotective effects of TAK-937, a novel cannabinoid receptor agonist, in permanent and thrombotic focal cerebral ischemia in rats: Therapeutic time window, combination with t-PA and efficacy in aged rats. Brain research. 2013;1526:84–93. doi: 10.1016/j.brainres.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 93.Medina MG, Ledesma MD, Dominguez JE, et al. Tissue plasminogen activator mediates amyloid-induced neurotoxicity via Erk1/2 activation. The EMBO journal. 2005;24(9):1706–16. doi: 10.1038/sj.emboj.7600650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barker R, Kehoe PG, Love S. Activators and inhibitors of the plasminogen system in Alzheimer's disease. Journal of cellular and molecular medicine. 2012;16(4):865–76. doi: 10.1111/j.1582-4934.2011.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17(11–12):953–66. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 96.Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300(5628):2101–4. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 97.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neuroscience letters. 1994;179(1–2):53–6. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 98.Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. European journal of immunology. 1989;19(4):689–94. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- 99.Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(16):6348–52. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. Journal of neuroimmunology. 1993;47(1):23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- 101.Balschun D, Wetzel W, Del Rey A, et al. Interleukin-6: a cytokine to forget. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(14):1788–90. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- 102.Nelson TE, Olde Engberink A, Hernandez R, et al. Altered synaptic transmission in the hippocampus of transgenic mice with enhanced central nervous systems expression of interleukin-6. Brain, behavior, and immunity. 2012;26(6):959–71. doi: 10.1016/j.bbi.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Richwine AF, Parkin AO, Buchanan JB, et al. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology. 2008;33(10):1369–77. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 104.Tancredi V, D'Antuono M, Cafe C, et al. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. Journal of neurochemistry. 2000;75(2):634–43. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- 105.Ropelle ER, Flores MB, Cintra DE, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS biology. 2010;8(8) doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292(18):2237–42. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 107.Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biological psychiatry. 2008;64(6):484–90. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(12):7265–70. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Samad F, Pandey M, Bell PA, Loskutoff DJ. Insulin continues to induce plasminogen activator inhibitor 1 gene expression in insulin-resistant mice and adipocytes. Molecular medicine. 2000;6(8):680–92. [PMC free article] [PubMed] [Google Scholar]
- 110.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Molecular cell. 2000;6(1):77–86. [PubMed] [Google Scholar]
- 111.Cusi K, Maezono K, Osman A, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. The Journal of clinical investigation. 2000;105(3):311–20. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang ZY, Lin YW, Clemont A, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. The Journal of clinical investigation. 1999;104(4):447–57. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation. 2006;116(6):1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ho L, Zhao W, Dams-O'Connor K, et al. Elevated plasma MCP-1 concentration following traumatic brain injury as a potential "predisposition" factor associated with an increased risk for subsequent development of Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2012;31(2):301–13. doi: 10.3233/JAD-2012-120598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jung C, Fritzenwanger M, Fischer N, Figulla HR. Hepatocyte growth factor is elevated in obese adolescents. Journal of pediatric endocrinology & metabolism : JPEM. 2009;22(7):645–51. doi: 10.1515/jpem.2009.22.7.645. [DOI] [PubMed] [Google Scholar]
- 116.Bell LN, Ward JL, Degawa-Yamauchi M, et al. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity. American journal of physiology Endocrinology and metabolism. 2006;291(4):E843–8. doi: 10.1152/ajpendo.00174.2006. [DOI] [PubMed] [Google Scholar]
- 117.Jung W, Castren E, Odenthal M, et al. Expression and functional interaction of hepatocyte growth factor-scatter factor and its receptor c-met in mammalian brain. The Journal of cell biology. 1994;126(2):485–94. doi: 10.1083/jcb.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(2):622–31. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nagayama T, Nagayama M, Kohara S, et al. Post-ischemic delayed expression of hepatocyte growth factor and c-Met in mouse brain following focal cerebral ischemia. Brain research. 2004;999(2):155–66. doi: 10.1016/j.brainres.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 120.Fenton H, Finch PW, Rubin JS, et al. Hepatocyte growth factor (HGF/SF) in Alzheimer's disease. Brain research. 1998;779(1–2):262–70. doi: 10.1016/s0006-8993(97)00958-x. [DOI] [PubMed] [Google Scholar]
- 121.Akimoto M, Baba A, Ikeda-Matsuo Y, et al. Hepatocyte growth factor as an enhancer of nmda currents and synaptic plasticity in the hippocampus. Neuroscience. 2004;128(1):155–62. doi: 10.1016/j.neuroscience.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 122.Date I, Takagi N, Takagi K, et al. Hepatocyte growth factor improved learning and memory dysfunction of microsphere-embolized rats. Journal of neuroscience research. 2004;78 (3):442–53. doi: 10.1002/jnr.20263. [DOI] [PubMed] [Google Scholar]
- 123.Shimamura M, Sato N, Nakagami H, Taniyama Y, Morishita R. Development of nucleic acid drugs for neurological disorders. Current topics in medicinal chemistry. 2012;12(15):1621–9. doi: 10.2174/156802612803531405. [DOI] [PubMed] [Google Scholar]
- 124.Manni L, Rocco ML, Bianchi P, et al. Nerve growth factor: basic studies and possible therapeutic applications. Growth factors. 2013;31(4):115–22. doi: 10.3109/08977194.2013.804073. [DOI] [PubMed] [Google Scholar]
- 125.Cattaneo A, Calissano P. Nerve growth factor and Alzheimer's disease: new facts for an old hypothesis. Molecular neurobiology. 2012;46(3):588–604. doi: 10.1007/s12035-012-8310-9. [DOI] [PubMed] [Google Scholar]
- 126.Fragkouli A, Tzinia AK, Charalampopoulos I, Gravanis A, Tsilibary EC. Matrix metalloproteinase-9 participates in NGF-induced alpha-secretase cleavage of amyloid-beta protein precursor in PC12 cells. Journal of Alzheimer's disease : JAD. 2011;24(4):705–19. doi: 10.3233/JAD-2011-101893. [DOI] [PubMed] [Google Scholar]
- 127.Giacobini E, Becker RE. One hundred years after the discovery of Alzheimer's disease. A turning point for therapy? Journal of Alzheimer's disease : JAD. 2007;12(1):37–52. doi: 10.3233/jad-2007-12105. [DOI] [PubMed] [Google Scholar]
- 128.Konukoglu D, Andican G, Firtina S, Erkol G, Kurt A. Serum brain-derived neurotrophic factor, nerve growth factor and neurotrophin-3 levels in dementia. Acta neurologica Belgica. 2012;112(3):255–60. doi: 10.1007/s13760-012-0101-6. [DOI] [PubMed] [Google Scholar]
- 129.Blasko I, Lederer W, Oberbauer H, et al. Measurement of thirteen biological markers in CSF of patients with Alzheimer's disease and other dementias. Dementia and geriatric cognitive disorders. 2006;21(1):9–15. doi: 10.1159/000089137. [DOI] [PubMed] [Google Scholar]
- 130.Tian L, Guo R, Yue X, et al. Intranasal administration of nerve growth factor ameliorate beta-amyloid deposition after traumatic brain injury in rats. Brain research. 2012;1440:47–55. doi: 10.1016/j.brainres.2011.12.059. [DOI] [PubMed] [Google Scholar]
- 131.Lee SA, Kallianpur A, Xiang YB, et al. Intra-individual variation of plasma adipokine levels and utility of single measurement of these biomarkers in population-based studies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16(11):2464–70. doi: 10.1158/1055-9965.EPI-07-0374. [DOI] [PubMed] [Google Scholar]
- 132.Azrad M, Gower BA, Hunter GR, Nagy TR. Racial differences in adiponectin and leptin in healthy premenopausal women. Endocrine. 2013;43(3):586–92. doi: 10.1007/s12020-012-9797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khan UI, Wang D, Sowers MR, et al. Race-ethnic differences in adipokine levels: the Study of Women's Health Across the Nation (SWAN) Metabolism: clinical and experimental. 2012;61(9):1261–9. doi: 10.1016/j.metabol.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. The New England journal of medicine. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]