Abstract
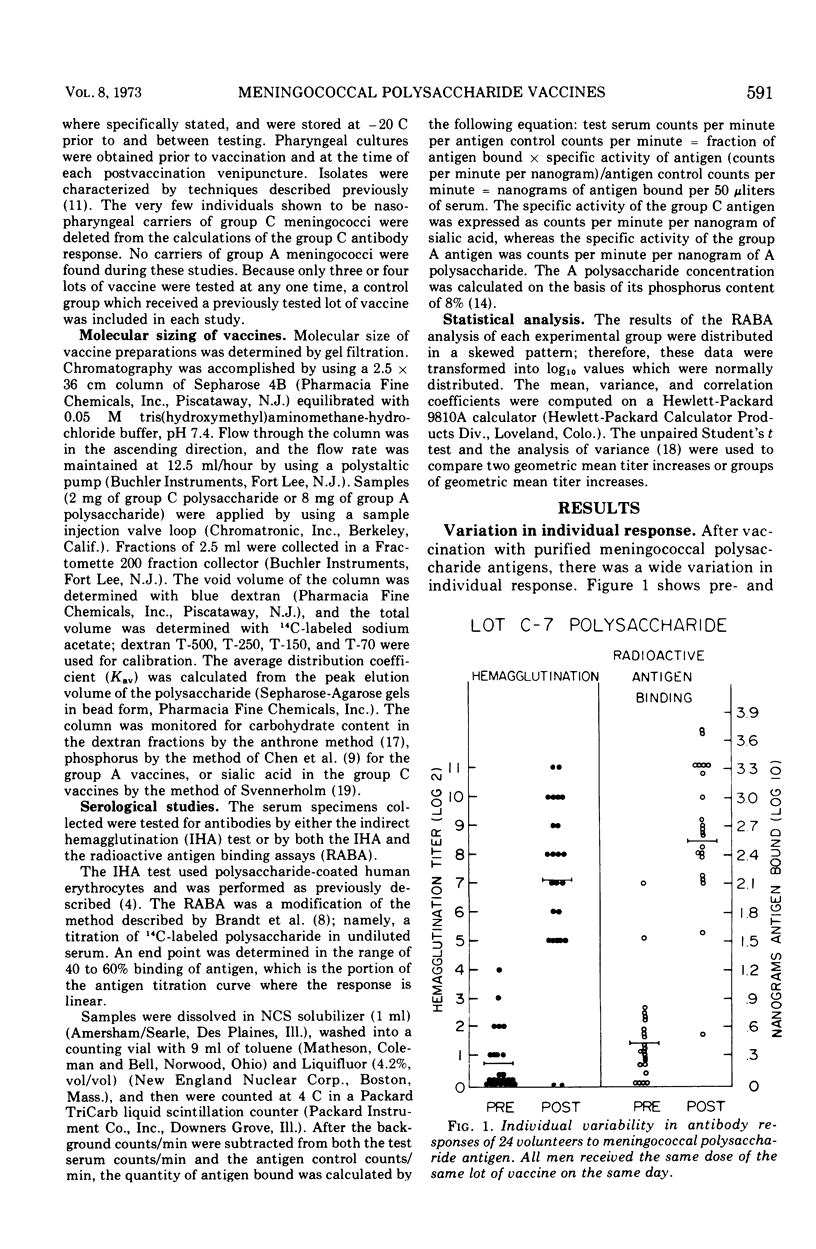
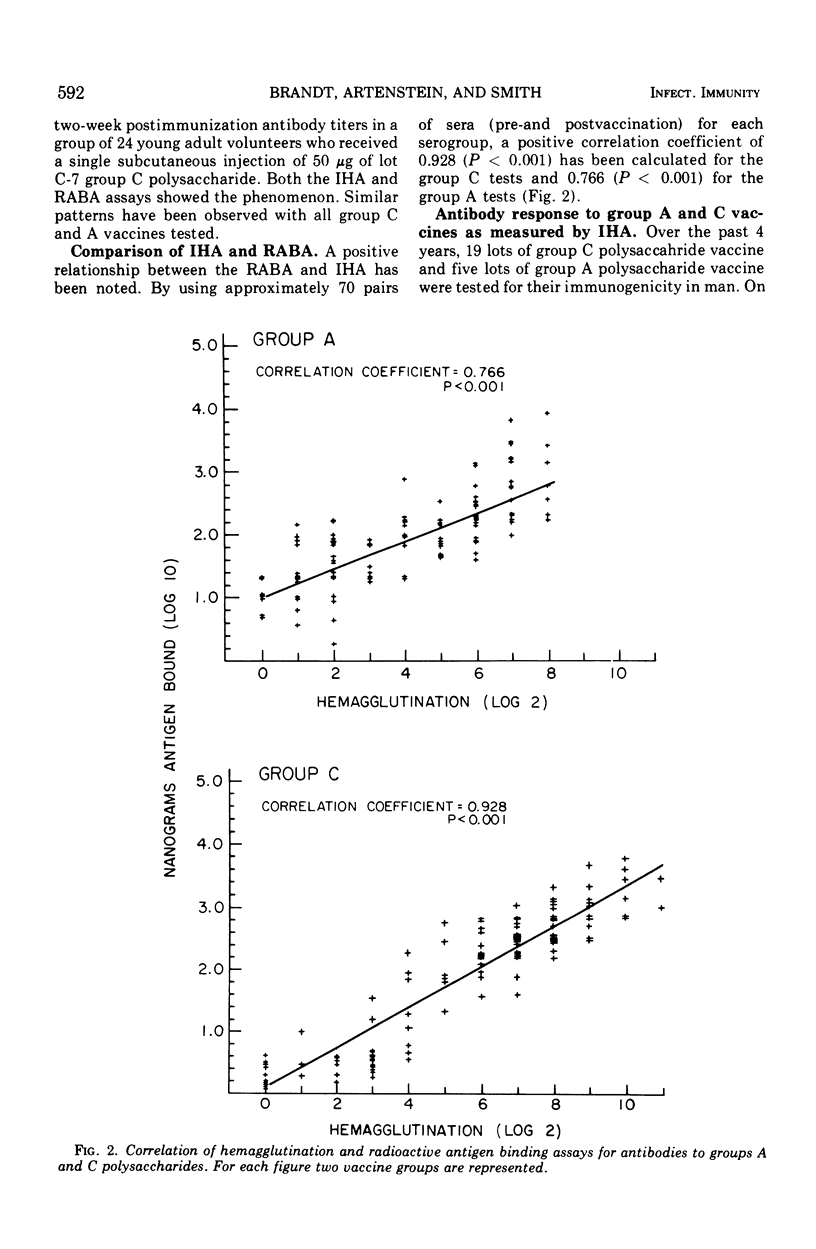
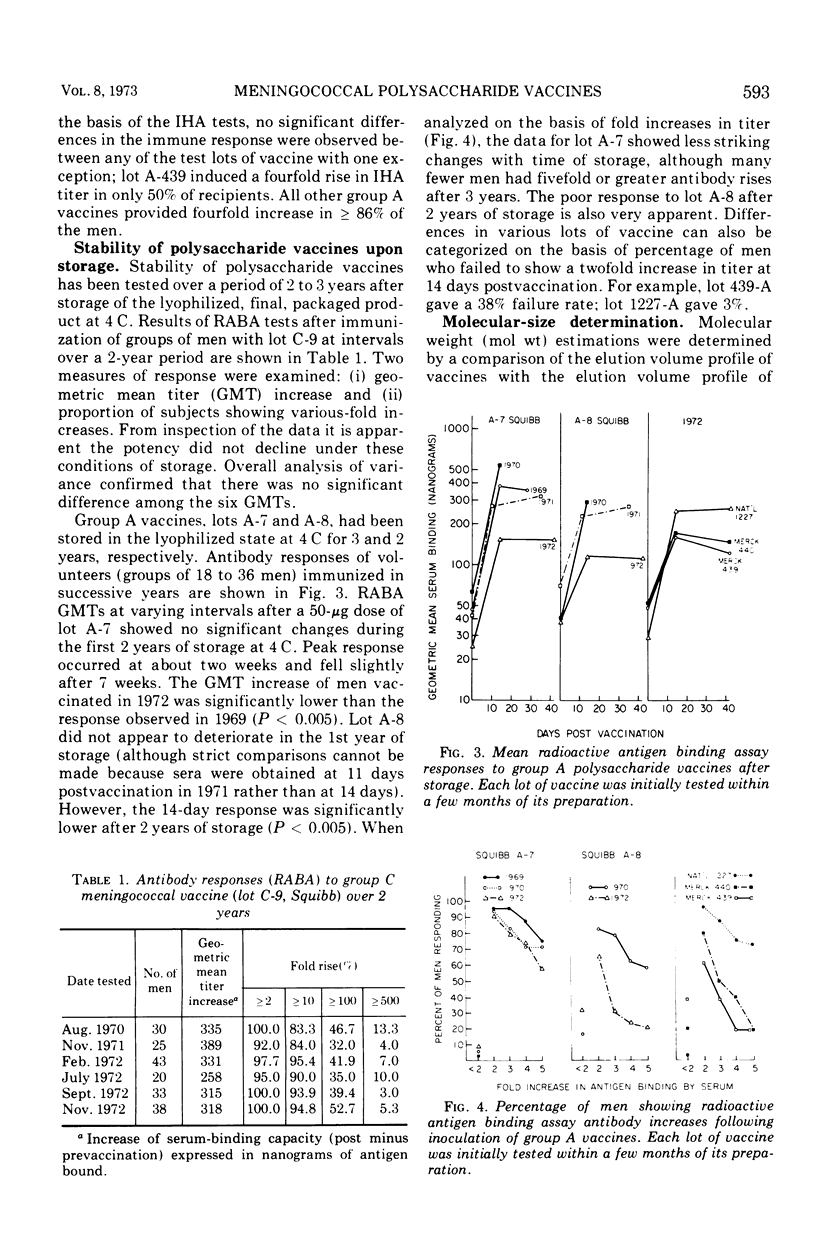
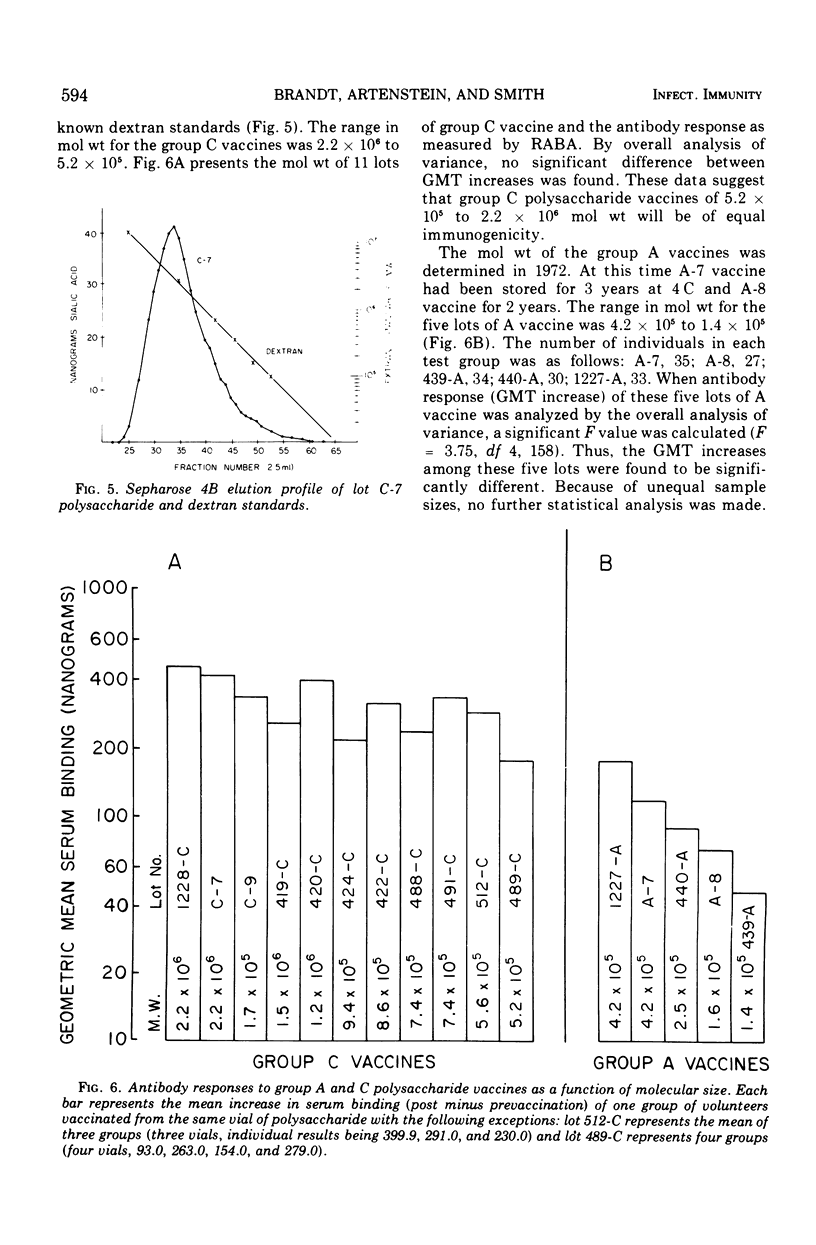
Over the past 4 years 19 lots of group C polysaccharide vaccine and five lots of group A polysaccharide vaccine have been tested for their immunogenicity in man. For each lot tested, groups of 18 to 50 men received 50 μg of vaccine subcutaneously. Sera were obtained prior to and 2 weeks after vaccination. The analytical and serological methods used in these studies were Sepharose 4B chromatography for the estimation of molecular size, the radioactive antigen binding assay, and the indirect hemagglutination (IHA) test for measuring the antibody response. Results have shown that the radioactive antigen binding assay is preferable to the IHA test as a measure of antibody response. Group C meningococcal vaccines have been highly stable when stored at 4 C in powdered form. All lots of group C vaccine tested to date have been of equal potency, with molecular weight varying from 520,000 to 2,000,000. Group A polysaccharides have been found to be unstable after 2 years of storage at 4 C. Optimal antibody response to the group A vaccines appears to be directly related to the molecular size of the preparation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artenstein M. S., Branche W. C., Jr, Zimmerly J. G., Cohen R. L., Tramont E. C., Kasper D. L., Harkins C. Meningococcal infections. 3. Studies of group A polysaccharide vaccines. Bull World Health Organ. 1971;45(3):283–286. [PMC free article] [PubMed] [Google Scholar]
- Artenstein M. S., Brandt B. L., Tramont E. C., Branche W. C., Jr, Fleet H. D., Cohen R. L. Serologic studies of meningococcal infection and polysaccharide vaccination. J Infect Dis. 1971 Sep;124(3):277–288. doi: 10.1093/infdis/124.3.277. [DOI] [PubMed] [Google Scholar]
- Artenstein M. S., Gold R., Zimmerly J. G., Wyle F. A., Branche W. C., Jr, Harkins C. Cutaneous reactions and antibody response to meningococcal group C polysaccharide vaccines in man. J Infect Dis. 1970 Apr;121(4):372–377. doi: 10.1093/infdis/121.4.372. [DOI] [PubMed] [Google Scholar]
- Artenstein M. S., Gold R., Zimmerly J. G., Wyle F. A., Schneider H., Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970 Feb 19;282(8):417–420. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- Artenstein M. S. Meningococcal infections. 4. Stability of group A and group C polysaccharide vaccines. Bull World Health Organ. 1971;45(3):287–290. [PMC free article] [PubMed] [Google Scholar]
- Artenstein M. S. Meningococcal infections. 5. Duration of polysaccharide-vaccine-induced antibody. Bull World Health Organ. 1971;45(3):291–293. [PMC free article] [PubMed] [Google Scholar]
- Berman S., Altieri P. L., Groffinger A., Lowenthal J. P. Pilot-scale production of group a and group C meningococcal polysaccharide immunogens. Infect Immun. 1970 Nov;2(5):640–643. doi: 10.1128/iai.2.5.640-643.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B. L., Wyle F. A., Artenstein M. S. A radioactive antigen-binding assay for Neisseria meningitidis polysaccharide antibody. J Immunol. 1972 Apr;108(4):913–920. [PubMed] [Google Scholar]
- Gold R., Artenstein M. S. Meningococcal infections. 2. Field trial of group C meningococcal polysaccharide vaccine in 1969-70. Bull World Health Organ. 1971;45(3):279–282. [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Lepow M. L., Gotschlich E. C. Immunogenicity of the group A and group C meningococcal polysaccharides in children. J Infect Dis. 1972 May;125(5):509–519. doi: 10.1093/infdis/125.5.509. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A. S., Brandt B. L., Artenstein M. S. Response of children to Neisseria meningitidis polysaccharide vaccines. J Infect Dis. 1973 Apr;127(4):394–400. doi: 10.1093/infdis/127.4.394. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]



