Abstract
Flavonoids, existing mainly as glycosides in nature, have multiple “claimed” beneficial effects in humans. Flavonoids are extensively metabolized in enterocytes and hepatocytes by phase II enzymes such as UGTs and SULTs to form glucuronides and sulfates, respectively. These glucuronides and sulfates are subsequently excreted via ABC transporters (e.g., MRP2 or BCRP). Therefore, it is the interplay between phase II enzymes and efflux transporters that affects the disposition of flavonoids and leads to the low bioavailability of flavonoid aglycones. Flavonoids can also serve as chemical regulators that affect the activity or expression levels of phase II enzymes including UGTs, SULTs and GSTs, and transporters including P-gp, MRP2, BCRP, OATP and OAT. In general, flavonoids may exert the inhibitory or inductive effects on the phase II enzymes and transporters via multiple mechanisms that may involve different nuclear receptors. Since flavonoids may affect the metabolic pathways shared by many important clinical drugs, drug-flavonoid interaction is becoming an increasingly important concern. This review article focused on the disposition of flavonoids and effects of flavonoids on relevant enzymes (e.g. UGTs and SULTs) and transporters (e.g. MRP2 and BCRP) involved in the interplay between phase II enzymes and efflux transporters. The effects of flavonoids on other metabolic enzymes (e.g. GSTs) or transporters (e.g. P-gp, OATP and OAT) are also addressed but that is not the emphasis of this review.
Keywords: flavonoids, ABC transporter, UGT, SULT, MRP2, BCRP, P-gp, OATP, OAT, disposition, glucuronidation, sulfonation, interplay
1. Introduction
Flavonoids are a class of phenolic and polyphenolic compounds produced as secondary plant metabolites. Flavonoids are widely distributed in nature. The typical sources of flavonoids include vegetables, fruits, beans, grains and herbs. Therefore, an average person could have a significant intake of flavonoids from daily diet. Published studies indicate that typical daily intake of flavonoids is in the range of 20–190 mg/day in typical Western populations1,2,3.
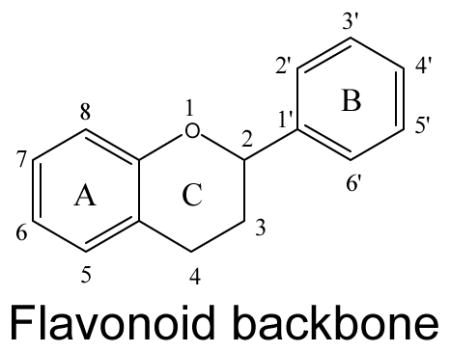
In nature, flavonoids are usually presented as glycosides and to a less extent as aglycones as well as their methylated derivatives. For aglycones, they share the backbone consisting of three carbon rings: a 1,4-benzopyran ring (A), in which the 2 position is linked to a phenyl ring (B) as a substituent4. For glycosides, the sugars are normally attached to the hydroxyl groups at position 3 or 7 of the A ring. The common sugars or carbohydrates linked to aglycones are D-glucose, glucorhamnose, galactose, L-rhamnose, or arabinose4. For methylated derivatives, the methylation usually occurs at position 5′ and 3′ of the B ring or at position 7 of the A ring5. According to their chemical structures, flavonoids (aglycones) are classified into several subfamilies: flavones, flavonols, flavanone, flavanonol, flavanol, isoflavones, anthocyanidin and chalcones6,7,8,9(Table 1).
Table 1.
Classification of flavonoids and typical compounds of each subfamily
| ||
|---|---|---|
| Subclass | Flavonoid | Substitution pattern |
Flavones
|
Chrysin | 5,7-OH |
| Apigein | 5,7,4′-OH | |
| Luteolin | 5,7,3′,4′-OH | |
| Eupatilin | 5,7-OH, 6,4′,5′-OMe | |
| Baicalein | 5,6,7-OH | |
| Nobiletin | 5,6,7,8,4′5′-OMe | |
| Tangeritin | 5,6,7,8,4′-OMe | |
| Tricin | 5,7,4′-OH,3′,5′-OMe | |
| Wogonin | 5,7-OH,8-OMe | |
|
| ||
Flavonol
|
Isorhamnetin | 3,5,7,4′-OH,3′-OMe |
| Kaempferol | 3,5,7,4′-OH | |
| Myricetin | 3,5,7,3′,4′,5′-OH | |
| Quercetin | 3,5,7,3′,4′-OH | |
| Morin | 3,5,7,2′,4′-OH | |
| Galangin | 3,5,7-OH | |
| Fisetin | 3,7,4′,5′-OH | |
|
| ||
Flavanone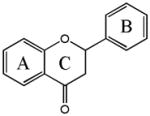
|
Naringenin | 5,7,4′-OH |
| Hesperetin | 5,7,3′-OH,4′-OMe | |
| Eriodictyol | 5,7,3′,4′-OMe | |
|
| ||
Flavanonol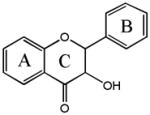
|
Silybin* | 3,5,7,-OH |
|
| ||
Flavanol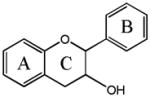
|
(−)-epicatechin (EC) | 3,5,7,3′,4′-OH (2-S, 3-R) |
| (+)-catechin | 3,5,7,3′,4′-OH (2-S, 3-S) | |
| Epigallocatechin gallate (EGCG) | 3,5,7,3′,4′,5′,-OH, 3-gallate | |
| Ellagic acid** | 2,3,7,8-OH | |
|
| ||
Isoflavones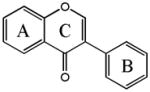
|
Daidzein | 7,4′-OH |
| Genistein | 5,7,4′-OH | |
| Glycitein | 6,4′-OH,7-OMe | |
| Formononetin | 7-OH,4′-OMe | |
| Biochanin A | 5,7-OH,4′-OMe | |
| Prunetin | 5,4′-OH,7-OMe | |
|
| ||
Anthocyanidin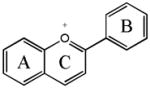
|
Cyanidin*** | 3,5,7,3′,4′-OH |
|
| ||
Chalcones
|
Phloretin**** | 2,4,6, 4′-OH |
Silybin: (2R,3R)-3,5,7-trihydroxy-2-[(2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydrobenzo[b][1,4]dioxin-6-yl]chroman-4-one
Ellagic acid: 2,3,7,8-tetrahydroxy-chromeno[5,4,3-cde]chromene-5,10-dione
Cyanidin: 2-(3,4-dihydroxyphenyl) chromenylium-3,5,7-triol
Phloretin: 3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one
There is growing scientific and public interests in flavonoids because of their potential uses for improving human health. Multiple investigations showed that higher consumption of flavonoids-rich dietary was associated with a lower incidence and mortality rates of various degenerative diseases such as cancer, cardiovascular disease and immune dysfunction1,10,11,12. Extensive research has uncovered that flavonoids has a myriad of biological activities (e.g. anti-cancer, anti-inflammation, antioxidant, anti- microbial, cardiotonic and lipid lowering activities), which could affect a plethora of enzyme systems and signaling cascades involved in the diseases4. Therefore, the beneficial impacts of flavonoids are likely to be derived from their pleiotropic biological activities.
To exert these biological activities, flavonoids must be absorbed and remain as aglycones in the body, since phase II metabolites of flavonoids are rarely reported as the active species13. It is believed that flavonoid aglycones are able to passively diffuse across the gut wall, though evidences suggest that the absorption of intact glycosides may also occur, albeit at a significantly slower rate14,15. For most flavonoid glycosides, they must be first hydrolyzed to aglycones either by microorganisms residing in intestine or by brush-border enzymes (e.g. lactase phloridzin hydrolase, LPH) before their absorption16,17,18, 19. Because the vast majority of flavonoids are present as glycosides in nature, absorption of flavonoids present in most fruits, vegetables, and herbs are slow and often incomplete. The exception to this rule is certain flavonoid mono-glucosides, which are rapidly hydrolyzed by intestinal LPH, releasing aglycones that are rapidly absorbed 20, 21, 22. On the other hand, for flavonoid aglycones, which are normally given at a dose that is intended to be pharmacologically active, the dose could be quite high (e.g., 1000 mg), which could result in higher concentration in the intestinal lumen. In general, the absorption of flavonoids (aglycones) is a rapid process. After entering the enterocytes, flavonoids are subjected to extensive metabolism (especially phase II) by intestine conjugating enzymes23. For flavonoids that have passed through the intestinal epithelium intact, they remain to be subjected to the equally rapid metabolism by the liver conjugating enzymes24.
After absorption, phase I metabolism (e.g. cytochrome P450) other than hydrolysis of glycosides makes only minor contribution to flavonoid clearance. In contrast, phase II metabolism plays a significant role in flavonoid clearance. Most of the flavonoids undergo glucuronidation by uridine-5′-diphosphate glucuronosyltransferases (UGTs) and/or sulfation (or sulfonation) by sulfotransferases (SULTs) in either enterocytes or hepatocytes, which convert them to glucuronides and sulfates, respectively25,26. These glucuronides and sulfates of flavonoids could be subsequently excreted into lumen or bile by efflux transporters such as multi-drug resistance protein 2 (MRP2) and breast cancer resistance protein (BCRP)26,27. Interestingly, the excreted glucuronides and sulfates in the intestine can be hydrolyzed by microflora β-glucuronidases and sulfatases back to aglycones, which can again undergo absorption and metabolism. Therefore, it is not surprising to speculate that the low bioavailability of flavonoids (usually, the oral bioavailability of flavonoid aglycones with a range of 2–20%) is due to the presence of a potent disposition network that is mainly consisted of phase II conjugating enzymes and efflux transporters of phase II enzyme conjugates28,29,30,31. In this paper, the bioavailability of flavonoids refers to intact aglycones in the systematic circulation, according to definition of the US Food and Drug Administration. Conjugates of flavonoids in the blood are not considered as bioavailable according to this definition.
Flavonoids are not only substrates of drug metabolizing enzymes (DMEs) and active efflux transporters; they are also regulators for some of those DMEs and transporters. Substantial amount of evidence demonstrated that flavonoids could inhibit the activities of phase II enzymes (e.g. UGTs, SULTs and glutathione-S-transferases (GSTs)) and active transporters such as P-gp (P-glycoprotein), BCRP, MRP2 or OATP (organic anion-transporting peptide) by a variety of mechanisms (e.g., competitive inhibition) 32,33,34,35. In addition, flavonoids can also modify the expression of phase II enzymes and transporters at both the mRNA and the protein levels. Thus, the enzymes and transporters involved in the flavonoid disposition could be affected by flavonoids. Due to the fact that many clinical drugs share the same metabolic pathways with flavonoids, the potential drug–flavonoids interactions are expected. Specifically, when large amounts of flavonoids, taken up from dietary or supplement sources, are administered with prescription or OTC drugs for a prolonged period of time, the pharmacokinetics of therapeutic drugs can be altered. As a result, it is meaningful to investigate the effects of flavonoids on functions and expression of the DMEs and transporters.
Based on the above considerations, we will present a current overview of flavonoid disposition and the effects of flavonoids on the phase II enzymes and transporters, most of which are involved in their disposition as well. The paper reviews the literature mainly from the period of 1990 to 2011. The effects of DMEs and transporters on flavonoid disposition will be addressed in details, the effects of flavonoids on the activity or expression of DMEs and transporters will be discussed, and lastly the potential impact of the interactions between flavonoids and DMEs as well as transporters on the approved drugs will be discussed. The focus of this review is on the in vitro or in vivo observations from human enzymes and transporters, though animal studies were also summarized.
Unless otherwise specified, flavonoids are referring to aglycones. Since phase I enzymes lead to minor metabolism of flavonoids and many other reviews have described their modulation by flavonoids on details32,35,36,37, the effects of flavonoids on phase I enzymes were not included in this review. Though phase II enzymes like GSTs and transporters like P-gp, OATP and OAT, are not mainly involved in flavonoid disposition on the basis of current knowledge, their modulation by flavonoids are still covered in this review, considering that many clinical drugs and endogenous compounds are their substrates. We believe that the information discussed in this review can be used as an effective and updated research references on the flavonoid study, especially for flavonoid disposition and drug-flavonoid interactions. Hopefully, the efforts made on flavonoid disposition will eventually lead to an effective way to improve flavonoid bioavailability, and allow flavonoids to exert more therapeutic or disease-prevention effects on humans.
2. Disposition of Flavonoids
2.1. Phase I metabolism of flavonoids
Flavonoids, like other drugs or xenobiotics, can be metabolized by cytochrome P450 (CYP450) enzymes. It was reported that CYP2C9 was the most efficient isoform for the oxidation of galangin followed by CYP1A3 and CYP1A138. For kaempferide, CYP1A2 was predominantly responsible for its oxidation followed by CYP2C9 and CYP1A139.
Compared with the phase II metabolic pathways, however, CYP450-mediated metabolism usually contributes little to overall metabolism of flavonoids. In addition, CYP-mediated metabolism of flavonoids has never been shown to be important in vivo or in intact cells. For example, apigenin was metabolized by rat liver microsomes to form three monohydroxylated derivatives, one of which was luteolin. Further investigation indicated the involvements of CYP1A1, CYP3B and CYP2E1 in apigenin hydroxylation in vitro24. However, when apigenin was perfused through an isolated rat liver, none of the phase I metabolites could be recovered in the effluent perfusate, suggesting that CYP-mediated metabolism has little impact on in vivo flavonoid clearance, when phase II metabolic pathway was functioning24. Therefore, metabolism via CYP is not a major clearance mechanism for flavonoids and not mainly responsible for their poor bioavailabilities in vivo.
2.2. Phase II metabolism of flavonoids
In contrast to minor contribution of phase I metabolism, phase II metabolism is the predominant pathway for the flavonoid disposition both in vitro and in vivo. There are two main types of phase II metabolites of flavonoids present in vivo: glucuronides and sulfates, which are produced by UGTs and SULTs, respectively. In a study of galangin metabolism, glucuronides are found to be the primary metabolites followed by sulfates, and then oxidative metabolites40. These results supported the hypothesis that the efficiency of glucuronidation of flavonoids was very high, followed by sulfation, with only a very minor contribution by CYP-mediated oxidation. This metabolism rank (glucuronidation>sulfation>oxidation) could be extended to most of flavonoids known in the literatures.
Both enterohepatic and enteric recyclings play a critical role in flavonoid disposition. Therefore, UGT and SULT isoforms expressed in the liver or intestine are functionally more important than other isoforms in flavonoid metabolism. It is expected that different UGT and SULT isoforms can convert the same flavonoid at different rates, and conjugations at different positions will produce regiospecific glucuronides and sulfates at different ratios41,42,43. On the other hand, flavonoids with different structural features are targets of different UGT or SULT isoforms41–44. Lastly, conditions of glucuronidation or sulfation reaction can also affect the structural preference of UGT or SULT isoforms.
2.2.1. UGT-mediated metabolism of flavonoids
Enzymes from UGT superfamily are located to the endoplasmic reticulum with molecular weight of 50–60 KD. These UGTs are able to transfer the glucuronyl group from uridine-5′-diphosphoglucuronic acid to the substrate molecules with a functional group of oxygen, nitrogen, sulphur or carbon45. As for flavonoids, which belong to polyphenolic compounds, glucuronidation occurs mostly at OH groups and rarely at the C position46.
Human UGT superfamily consists of UGT1, UGT2, UGT3 and UGT8 families. UGT1 family currently contains only UGT1A subfamily, while UGT2 family can be classified into UGT2A (including UGT2A1, 2A2 and 2A3) and UGT2B subfamilies. UGT3 family is currently composed of UGT3A subfamily (including UGT3A1 and 3A2). The only known member of the UGT8 family is UGT8A147.
Among all the UGT subfamilies, UGT1A and UGT2B subfamilies, which are classified according to their primary amino acid sequences, are predominantly responsible for metabolism of flavonoids and have more clinical relevance than other subfamilies with respect to drug metabolism. As the result of different exons’ splicing on the same gene, transcription of the UGT1A gene cluster can produce the following isoforms: UGT1A1, UGT1A3, UGT1A4, UGT1A5, UGT1A6, UGT1A7, UGT1A8, UGT1A9 and UGT1A1047. Unlike isoforms from UGT1A subfamily, each isoform (UGT2B4, UGT2B7, UGT2B10, UGT2B11, UGT2B15 and UGT2B17) from UGT2B subfamily is encoded by an individual gene47. UGTs are widely distributed in human body. Liver, intestines, kidney and stomach are enriched with various and sometime unique UGT isoforms48.
Flavonoids are extensively metabolized by varieties of UGT isoforms. It was reported that UGT1A1, UGT1A8, UGT1A9 and UGT1A10 mainly contributed to the glucuronidation of isoflavones including daidzein, genistein, glycitein, formononetin, biochanin A and prunetin in a concentration-dependent manner44. Under different concentrations, the glucuronidation pattern of each isoflavone by UGT isoforms was sometimes changed. For example, genistein was mostly metabolized by UGT1A9 at 2.5 μM, whereas it was catalyzed mainly by UGT1A8 at 10 and 35 μM44. In fact, isoflavones were the best substrates among all the 42 flavonoids tested on the basis of structure-function relationships of UGT1A10, suggesting that hydroxyl group at C6 or C7 other than C5 of the A-ring or on C4′ of the B ring was preferred49. UGT1A8, which had 94% similarity to primary amino acid sequence of UGT1A10, not only had maximally overlapping substrates with UGT1A10, but also displayed a higher glucuronidation rate toward flavones (7-hydroxyflavone, chrysin and apigenin), a flavanone (naringenin) and an isoflavone (genistein) than UGT1A1050. In another study, UGT1A9 and UGT1A3 were able to catalyze both flavonoid aglycones and glycoside, though their substrate preference was clearly aglycones51. The newly reported substrates of UGT1A3 included isorhamnetin, silybin, kaempferol, daidzein, morin, quercetin-3′, 4′-OCHO-, quercetin xylopyranoside, and avicularin, most of which were also the substrates of UGT1A9 except isorhamnetin, silybin and kaempferol51. With respect to shared substrates, UGT1A9 always had a higher catalyzing efficiency or intrinsic clearance (Vmax/Km) than UGT1A351. Other than the above UGT isoforms, UGT1A6 and UGT1A7 are also involved in glucuronidation of flavonoids. UGT1A6 was the predominant isoform responsible for glucuronidation of luteolin at 7 position52. It was also found that chrysin was metabolized by UGT1A6, an isoform highly expressed in Caco-2 cells53. In another study, UGT1A7 was found to be involved in glucuronidation of eupatilin, which was also the substrate of UGT1A1, UGT1A3, UGT1A8, UGT1A9 and UGT1A1054.
In addition to UGT1A isoforms, UGT2B isoforms can also metabolize flavonoids, though they may not be as efficient as UGT1A isoforms. Evidence showed that galangin, chrysin, 7-hydroxyflavone and naringin were substrates for UGT2B17, among which galangin had the highest conversion value, whereas apigenin, baicalein, fisetin, quercetin, genistein and biochanin A were not conjugated by UGT2B1755. Another example of flavonoids as UGT2B substrates was from the study of regioselectivity of phase II metabolism of luteolin and quercetin. It was demonstrated that luteoin and quercetin were substrates of UGT2B15 and UGT2B7 with glucuronidation primarily occurring at position 7 and 3′, respectively52. Between these two isoforms, UGT2B7 had a relatively higher conversion than UGT2B15. Generally, as substrates of UGT2B7, flavonol has higher glucuronidation activity than flavones and isoflavones56.
After glucuronidation mediated by various UGT isoforms, flavonoids are converted to corresponding glucuronides. These glucuronides are more hydrophilic and can be easily excreted via urine or feces. Usually, the biological activity of glucuronides is less than corresponding flavonoids with a few exceptions (e.g. quercetin-7-O-glucuronide)57.
2.2.2. SULT-mediated metabolism of flavonoids
SULTs, another important superfamily of phase II enzymes for flavonoid metabolism, can catalyze the formation of a flavonoid sulfate by transferring a sulfonate group from the coenzyme 3-phosphoadenosine-5′-phophosulfate (PAPS) to the substrate. SULTs expressed in the cytosol are responsible for the metabolism of flavonoids. In human, SULT superfamily can be divided into three subfamilies consisting of thirteen members: SULT1A family (SULT1A1, 1A2, 1A3, 1A4, 1B1, 1C2, 1C4 and 1E1), SULT 2B family (SULT2B1 v1 and v2), and SULT4A1 (SULT4A1 v1 and v2)58. Among all the SULTs, SULT1A1, SULT1A2, SULT1A3 and SULT1E1, which are distributed in liver and gastrointestinal tract, are well studied58,59,60,61.
Compared with the glucuronidation of flavonoids, research on sulfation of flavonoids, which is regarded as a metabolic pathway that can compete with glucuronidation, is relatively scant. Previously, sulfation primarily catalyzed by SULT1A1 and SULT1A3, was the major pathway in metabolism of (−)-epicatechin (EC) in human liver and intestine without detectable glucuronidation 62. This sulfation result was consistent with another report showing that SULT1A1 and SULT1A3 metabolized EC in addition to hesperetin, eriodictyol, (+)-catechin63. On the other hand, rapid sulfation and glucuronidation of EC in rats implied a large species difference in metabolism compared to humans 62. As mentioned previously, sulfation of galangin was one of metabolic pathways contributing to metabolism of galangin. Three SULT isoforms (SULT1A1, SULT1A3 and SULT1E1) were involved in sulfation of galangin, among which SULT1A1 displayed the most sulfation efficiency. On the contrary to above three SULT isoforms, SULT2A1 had no activity against galangin40. In Caco-2 cells, it is common to observe that flavonoids (e.g. genistein, apigenin and baicalein) are undergoing both glucuronidation and sulfation 23,64,65. And sulfation might be attributed to SULT isoforms expressing in Caco-2 cells such as SULT1A1, SULT1A2 and SULT1A366. Recently, Meng et al showed the SULT1A3 exclusively metabolize flavonoids at the 7-OH position41.
2.3. Efflux of flavonoids and their phase II metabolites
In addition to extensive metabolism of flavonoids, efflux of flavonoids and their metabolites is another factor that leads to low bioavailability of flavonoids. ATP-binding cassette (ABC) transporters are ubiquitous integral membrane proteins that outwardly transport various ligands actively across the membrane with hydrolysis of ATP67. Accumulating evidence showed that members of ABC such as P-gp, MRP2 and BCRP were involved in transportation of flavonoids or their phase II metabolites out of cells68,69,70,71. Experiments on Caco-2 cells demonstrated that both quercetin and naringenin were substrates of MRP2 while only naringenin was substrate of P-gp68. Moreover, glucuronides of quercetin (quercetin-7-O-β-D-glucuronide, -3-O-β-D-glucuronide, -3′-O-β-D-glucuronide and -4′-O-β-D-glucuronide) were also substrates of MRP269. Kaempferol, a major constituent of Ginkgo biloba extract, was found to be substrate of Bcrp by An and co-workers71. In fact, glucuronides and sulfates of flavonoids are more likely to be substrates of MRP2, MRP3 and BCRP26,27,69–70,72.
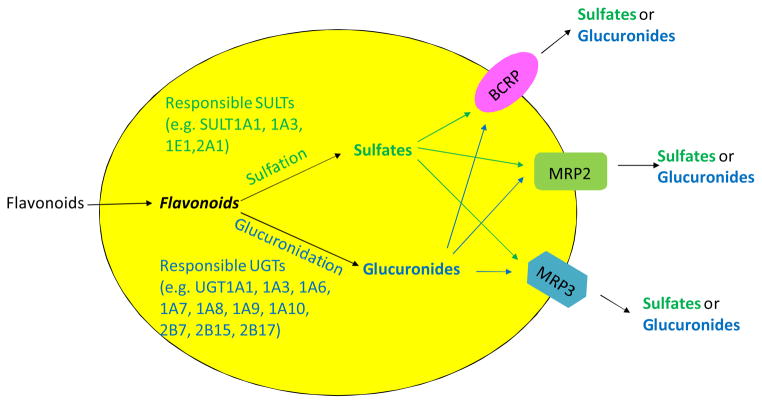
2.4. Interplay between phase II enzymes and efflux transporters in disposition of flavonoids
As an extension to the idea of interplay between CYP3A and P-gp, interplay between phase II enzymes and BCRP/MRP describes a sequential process in which substrates are metabolized by phase II enzymes (e.g., UGTs) and the produced hydrophilic phase II metabolites are transported out of cells by the efflux transporters (e.g., BCRP or MRP). This kind of interplay was identified commonly in the study of flavonoid disposition in both animal and cell models. This is because flavonoids as substrates can passively diffuse into cells and subsequently be metabolized to glucuronides and sulfates via glucuronidation and sulfation, respectively. The glucuronides and sulfates, which are too hydrophilic to diffuse across the cell membrane, can only exit cells via the action of the efflux transporters [Fig 1]. The engaged efflux transporters include BCRP, MRP2 and MRP326,27,69,70,72,. Previously, the efflux step of flavonoid conjugates (phase II metabolites) was regarded most likely as the rate-limiting step for the interplay due to the discrepancy of results between in vitro glucuronidation assay and Caco-2 cell model or rat perfusion model. For example, glucuronidation of biochanin A was found to be faster than formononetin in rat intestinal and liver microsomes; however, excretion of formononetin conjugates was more efficient than biochanin A in rat intestine perfusion model and Caco-2 cells model73. Recently, the idea that the rate-limiting step of efflux is challenged when it is applied to a simple model of one phase II enzyme (UGT1A9) and one dominant efflux transporter (BCRP). In this simple model, we showed clearly that the efflux transporter was simply a gate-keeper and kinetic interplay between UGT1A9 and BCRP ultimately determined how much metabolites were formed and then excreted74. Efforts are undergoing to understand more in details about the interplay between phase II enzymes and efflux transporters.
Fig. 1.
Cellular interplay between phase II enzymes and efflux transporters in flavonoid disposition.
3. Effects of flavonoids on phase II enzymes
Flavonoids are likely to be metabolized by phase II enzymes as described previously. Reciprocally, flavonoids can also exert some effects on phase II enzymes [Fig 2]. As an activator or inducer, a flavonoid may induce or activate the target enzymes via multiple mechanisms, which possibly require involvement of various nuclear receptors. For example, some flavonoids could induce the phase II enzymes by disrupting the interaction between Nrf2 and Keap1 and by making Nrf2 relocate to nucleus and bind to the antioxidant/electrophile response element (ARE) 35,75. In addition, flavonoids may interact with constitutive androstane receptor (CAR) and pregnane X receptor (PXR), which were reported to regulate phase II enzymes and transporters 76. On the other hand, flavonoids can act as inhibitors of phase II enzymes, more likely through simple mechanisms such as competitive inhibition. In the following parts, we will discuss the research developments on how flavonoids affect the phase II enzymes, including UGTs, SULTs and GSTs.
Fig. 2.
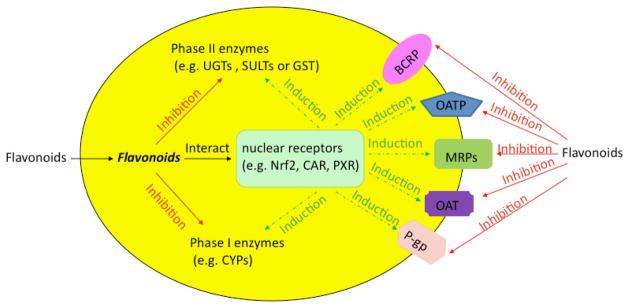
Effects of flavonoids on phase I and II enzymes and transporters in cells. The solid line represents the inhibition, whereas the dotted line represents the induction via various nuclear receptors.
3.1. Effects of flavonoids on UGTs
Early study on the prostate cancer cell line-LNCaP showed that certain glucuronidation activities (i.e., glucuronidation of testosterone via UGT2Bs) were increased after the cells were exposed to the flavonoids including biochanin A, daidzein, formononetin, genistein, prunetin, apigenin, galangin, kaempferol, naringenin and quercetin77. Among tested flavonoids, biochanin A was the most potent inducer of UGTs, increasing the glucuronidation activity by 10 folds77. At the same time, due to less availability of testosterone resulting from glucuronidation, the production and release of prostate specific antigen (PSA) as a prostate tumor marker were significantly decreased in the presence of biochanin A77. It suggested that flavonoids could play an important role in modulation of sex hormones in humans and provide us with a possible means to prevent and manage prostate cancers. In addition, chrysin was reported to be able to induce UGT1A1 specifically, both in Caco-2 and HepG2 cells78,79,80. However, UGT1A6, UGT1A9 and UGT2B7 were not affected by the chrysin treatment80. Further study from the same group showed that induction of UGT1A1 by chrysin was not dependent on aryl hydrocarbon receptor (AhR) since galangin and isorhamnetin, both of which are inducers of CYP1A1 via AhR, did not display any induction of UGT1A181. More recently, UGT1A1 was believed to be under the regulation of Nrf282. In addition to be an inducer of UGT1A1, chrysin was also an inhibitor for the glucuronidation of estradiol at substrate concentrations above 25 μM83. Similar inhibitory effects on glucuronidation of estradiol were also observed when nobiletin and silymarin were treated at substrate concentrations over 25 μM83. On the contrary, the same three flavonoids had little effect or possibly even slight stimulation effect on estradiol glucuronidation at the lower concentrations (5 and 10 μM). As for tangeritin and quercetin, both exerted the inhibitory effects on glucuronidation of estrodiol. Tangeritin had the most potent inhibitor, while quercetin was a relatively weaker inhibitor83. In addition, naringenin could inhibit glucuronidation of estradiol to a similar extent at all tested naringenin concentrations (5–100 μM), suggesting that it did not act as a competitive inhibitor83. Furthermore, the milk thistle extract silymarin and its major ingredient silybin could inhibit UGT1A6 and UGT1A9 in human hepatocytes at a concentration of 100 μM32,84. As for apigenin, it was found to be able to induce UGT1A1 transcription by 4 folds in undifferentiated Caco-2 cells85. The combination of apigenin and sulforaphane could further lead to a significant induction of UGT1A1 mRNA (up to 12 folds)85. Considering that SN50 (a NF-kB translocation inhibitor) could enhance the induction of UGT1A1 by apigenin, it was believed that UGT1A1 induction by apigenin might be associated with NF-kB translocation85. In addition to apigenin, quercetin was also shown to markedly increase the glucuronidation activity in Caco-2 cells80. The significant increase in glucuronidation activity might result from increasing expression levels of UGT1A6 (at 50 μM of quercetin) and UGT1A186,87.
Regulation studies done on the cell culture could be replicated in vivo, although the results were not always consistent. For example, feeding rats with diet containing 1% quercetin significantly increased the activity of UGTs in liver and to a lesser extent in intestine88. Similar results of increasing hepatic UGT activity were obtained after flavone, flavanone and tangeritin were fed to rats at a dietary concentration of 0.3% (w/w) or soy isoflavones (0.81 mg/kg diet)89,90. In contrast, hepatic UGT activity was inhibited after exposing mice with a liquid diet containing 5 mM baicalein or wogonin for 1 week91. In one study, where human jejunum was perfused with an onion and broccoli extract containing 60 μM quercetin for 2 hours, mRNA of UGT1A1 in shed enterocytes was increased87. The results from humans were consistent with the observation in Caco-2 cells treated with pure quercetin.
3.2. Effects of flavonoids on SULTs
It is not surprising to observe the inhibition of SULT activities at higher flavonoid concentrations (>10 μM). Indeed, auto-inhibition or substrate-inhibition is a very common phenomenon in SULT-mediated metabolism of flavonoids. For example, metabolism of genistein and daidzein by SULT1A1 followed the substrate-inhibition kinetic profile. When substrate concentrations were over 1 μM, the sulfation activities were substantially decreased 92. In addition to substrate-inhibition, a large body of studies showed that flavones (e.g. apigenin and chrysin), flavonols (e.g. quercetin, myricetin and kaempferol), isoflavones (e.g. genistein and daidzein), flavanone (e.g. hesperetin and eriodictyol) and flavanols (e.g. catechin and epicatechin) were potent inhibitors of SULT1A192,93,94,63.
In an investigation conducted by Harris and co-workers, 37 flavonoids were tested to determine their inhibition potency against SULT1A195. The results indicated that flavonoids could inhibit SULT1A1 significantly at submicromolar concentrations. Among all the flavonoids selected, 3, 4′-dihydroxyflavone was the most potent inhibitor with IC50 less than 1 nM, whereas luteolin was regarded as the most potent naturally occurring inhibitor with IC50 of 3 nM 95. In contrast to above flavones and flavonols, isoflavones such as genistein and daidzein had relatively high IC50, which was in the range of 500–600 nM except for 3′, 4′, 7-trihydroxyisoflavone, which had the IC50 of 20 nM 95. Therefore, 3′, 4′-dihydroxy motif might be a critical feature for flavonoids to have potent inhibitory effects of SULT1A1 based on the fact that flavonoids with above feature motif had an IC50 of 100 nM or less against SULT1A1-mediated sulfonation of the standard substrate 4-nitrophenol (3 μM). Though various flavonoids were shown to have potent inhibition of SULT1A1, few of them displayed the powerful inhibitory effects on SULT1A3. Baicalein was found to be the only one having substantial inhibition (IC50 500 nM) at less than 1 μM against 10 μM dopamine as the standard substrate 95,96. Recently, hesperetin and eriodictyol were also reported to be the potent inhibitors of SULT1A3. Hesperetin and eriodictyol had the IC50 values of 20 μM and 15 μM, respectively, against SULT1A3-mediated sulfonation of its typical substrate dopamine (50 μM)63. In case of SULT1E1, which was inhibited relatively easily by many simple phenols, tricin (a derivative of flavonoid, 3′, 5′-dimethoxy-4′, 5, 7-trihydroxyflavone) was found to be a most potent inhibitor97. It could competitively inhibit SULT1E1 with an inhibition constant of approximately 1 nM97. Another potent inhibitor of SULT1E1 was galangin with IC50 of < 1 μM against 10 nM estradiol as standard substrate95. Similarly, genistein and equol are as potent as galangin, they exhibited inhibitory constants at the active site of 400 and 500 nM and at an allosteric site of 2 and 5 μM, respectively (substrate 10 nM estradiol)95. On the other hand, daidzein was less potent than above flavonoids, and had IC50 of 5 μM against 10 nM estradiol even though its chemical structure was most close to equol and distantly related to genistein95. In the same system, formononetin was found to be the least potent inhibitor among all the tested compounds95. In another published study, quercetin had surprisingly 10 times more inhibitory potency for SULT activity in the intact cultured human mammary epithelial cells (HME) (an IC50 of about 0.1 μM) than in recombinant SULT isoform98. Last but not the least, SULT2A1, another SULT isoform expressed in intestine, was reported to be inhibited by apigenin with Ki value of around 2.4 μM. Similar to apigenin, myricetin, baicalein, galangin and 7-hydroxyflavone also had Ki values between 2–3 μM. Possibly, a 7-hydroxyflavone substituent seemed to be a requirement for some inhibition. On the contrary, fisetin, kaempferol, luteolin, hesperetin, morin, quecetin, rutin, catechin, daidzein did not show any inhibitory effect on SULT2A1 even at concentration of 25 μM96.
Flavonoids not only inhibit the SULT activity, but also induce the expressions of SULTs in various model systems. Recently in both HepG2 and Caco-2 cells, genistein was shown to induce SULT1A1 and SULT2A1 at both mRNA and protein levels in a dose- and time-dependent manner99. In Sprague-Dawley rats, biochanin A significantly induced expression of rat SULT1A1, SULT2A1 and SULT1E1 in liver and intestine after a 7-day treatment period (doses: 0, 2, 10, and 50 mg/kg/day)100.
3.3. Effects of flavonoids on GSTs
Glutathione-S-transferases (GSTs) are an another group of phase II enzymes. However, GSTs are not primarily responsible for flavonoid metabolism. They are able to catalyze the binding of a wide range of electrophiles to the sulfydryl group of glutathione in order to neutralize the reactive oxygen species. As a result, GSTs play an important role in detoxification of various chemical carcinogens. GSTs are widely distributed in many tissues and present in relatively large amounts in the epithelial tissues of the human gastrointestinal tract35,101. Volumes of evidence showed that considerable numbers of flavonoids contribute to the regulation of GSTs, either on gene expression or protein activities or both. An example from cell study in vitro indicated that effects of genistein on expression of GSTs through the ER/ARE signaling pathway were cell-type specific102. In COS1 I cells expressing ERα and ERβ, genistein (1 μM) repressed the GST Ya ARE-dependent gene expression; however, in C4-12-5 cells, which are ER-negative breast cancer cells derived from the MCF-7 cells, genistein (1 μM) had the modest induction of GST gene following transfection with ERα and ERβ102. In addition, evidence from an in vivo study showed that GSTs in stomach were significantly induced, when the mice were fed with citrus limonoids and citrus flavonoids (hesperidin, naringin and crude flavonoids mixture) for one week at dose of 20 mg103. Moreover, in another study, Swiss Webster mice were exposed to flavone, morin, naringenin, (+)-catechin and quercetin for 20 days at 2.5 g/kg diet104. It was revealed that only groups fed with a flavone showed the gender and isozyme-specific induction of GSTs. In female mice, GST activity was increased by 7 folds, while in male mice, GST activity was increased by 4 folds. With regard to enzyme specific assay, it was found that activities of GSTM and GSTP increased in a greater extent in females (8 folds and 4 folds respectively) than in males (6 folds and 2 folds)104. In humans, the modification of GSTs by flavonoids is more complicated than that in mice. In a study involving healthy volunteers, who drank juice containing several flavonoids (e.g., quercetin, quercetrin, myricetrin, kaempferol, hesperidin, eriodictyol and naringenin), GSTP1 protein in leucocytes was first suppressed at weeks 4 and 6. However, at week 8, expression of GSTP1, which was reversely linked with DNA damage, significantly increased105. The results were contrary to the in vitro experiments. In vitro, when leucocytes were treated with polyphenol mixtures containing flavonoids, no change of GST gene expression or activity was observed, though up-regulations of certain CYP450 and SULTs isoforms were observed105.
4. Effects of flavonoids on transporters
Currently, the interaction between flavonoids and ABC transporters is drawing more and more attention of the scientific researchers [Fig 2]. In addition to ABC transporters, another class of transporters called solute carrier family (SLC), is also involved in interaction with flavonoids [Fig 2]. In contrast to ABC transporters, which are mainly responsible for efflux, members of SLC primarily manage cellular uptake. Flavonoids and their metabolites might act as substrates or inhibitors of both ABC and SLC transporters. As an inducer of transporters either at the mRNA or at the protein level, long exposure to flavonoids is necessary. Various mechanisms underpinning the interaction between ABC transporters and flavonoids have been postulated. Some flavonoids can affect the ATPase activity by inhibition or stimulation; other flavonoids, which are substrates of transporters, can compete for the binding site of transporters and result in competitive inhibition; and still a more selected group of flavonoids can modulate the transporters through posttranslational modifications (e.g., phosphorylation).
4.1. Effects of flavonoids on P-gp
P-glycoprotein (P-gp), encoded by ABCB1 gene with a molecular weight of 170KD, is the first human ABC transporter that was cloned and characterized through its ability to confer a multi-drug resistant phenotype to cancer cells106. As an efflux transporter expressed in many tissues and apical membrane of epithelial cells, P-gp is mainly responsible for outward transportation (i.e., efflux) of hydrophobic, cationic or neutral compounds with a planar structure with some notable exception (e.g., methotrexate and phenytoin, both with negative charges)33,107. P-gp consists of two homologous segments, each of which contains six transmembrane domains (TMDs) and a nucleotide binding domain (NBDs) with ATPase activity108.
There were several controversial opinions with regard to effects of flavonoids on P-gp. First controversy is about the effects of green tea. One study supported the notion that P-gp activity was inhibited by green tea polyphenols109, whereas the other suggested that P-gp function was elevated by catechin in green tea110. Experiments from first group indicated that green tea polyphenols at concentration of 30 μg/ml could inhibit the photolabeling of P-gp by 75% and increase the accumulation of rhodamine-123 by 3 folds in the multidrug-resistant cell line CHRC5109. Moreover, EGCG as a representative of green tea polyphenols exhibited the inhibitory effect on P-gp activity not only in CHRC5 cells but also in Caco-2 cells109. In the second study, though some catechins like EGCG displayed inhibitory effects on P-gp, others like (−)-epicatechin were shown to facilitate the P-gp-mediated transport of the fluorescent markers LDS-751 via a heterotropic allosteric mechanism110. Currently, there is no explanation for these controversial findings.
Another controversy deals with the grapefruit juice effect111. Some studies show that there were some effects whereas other showed none. Earlier studies from Dr. Lown’s group indicated that twice daily consumption of grapefruit juice (8 oz) for 6 days resulted in 62% reduction of CYP3A4 levels without any change of P-gp level in 10 healthy volunteers112. This result was consistent with the conclusion that naringin and 6′ ′,7′-dihydroxybergamottin, both of which were components of grapefruit juice, improved saquinavir transport in Caco-2 cells by inhibition and down-regulation of the CYP3A4 rather than by modulation of P-gp113. On the contrary, some papers showed that grapefruit juice had either inhibitory or activating effect on P-gp. Dr. Benet’s group found that grapefruit juice stimulated the efflux of a couple of P-gp substrates across the MDCK-MDR1 cells114. In the study of vincristine transport across MBEC4 cells (cultured mouse brain capillary endothelial cells), quercetin and kaempherol were found to decrease the uptake of vincristine at low concentration (10 μM), but increased the uptake of vincristine at high concentration (50 μM)115. This biphasic in vitro results were further confirmed by the study on ddY mouse in vivo with co-administration of quercetin (0.1 mg/kg) and (1.0 mg/kg)115. The reasons were due to changes in P-gp functionality. At a low concentration, P-gp activity was stimulated as a result of enhancing its phosphorylation; while at a high concentration, P-gp function was hindered115. In addition to quercetin and kaempherol, other flavonoid components, including chrysin, flavone, hesperetin, naringenin, were found to increase the vincristine uptake in MBEC4 cells by inhibiting P-gp at the 10–50 μM,. However, their corresponding glycosides such as hesperidin, naringin and rutin did not affect P-gp115. Furthermore, P-gp was reported to be inhibited by kaempferol and naringenin in the human immortalized tubular cell line HK-2 cells by down-regulating the protein level and mRNA expressions of P-gp116.
Apart from above controversies, the majority of findings supported the inhibitory role of flavonoids against P-gp. It was reported that genistein (200 μM) could elicit an elevation in intracellular accumulation of rhodamine 123 and daunorubicin in P-gp-expressing cell lines. In addition, genistein could also decrease photoaffinity labeling of P-gp by [3H] azidopine117. Other related studies also pointed out that flavonoids had an inhibitory effect on P-gp in MDCKII-MDR1 cells, Caco-2 cells and K562 cells, based on their ability to the increase cellular uptake of various testing substrates118, 119, 120.
On the basis of accumulating interaction data, structure-function relationship between flavonoids and P-gp was established. More and more flavonoid derivatives were synthesized to modulate P-gp functions121. Recently, a 3D linear solvation energy model was developed to quantify the affinity of flavonoid derivatives toward P-gp. According to this 3D model, two major physicochemical parameters (shape parameters and hydrophobicity), were identified to be primarily responsible for the affinity of flavonoid derivatives towards P-gp. At the same time, hydrogen-bonding capacity was identified as a minor modulator122.
4.2. Effect of flavonoids on MRP2
MRP2 is an efflux transporter with molecular weight of 190KD, which belongs to ABCC (also called MRP) subfamily of ABC transporter. MRP2 has two nucleotide binding domains (NBDs) and seventeen transmembrane domains (TMDs)123. As a mechanism leading to the multidrug resistance, MRP2 is highly expressed in carcinoma cells or tumor tissues as well as normal tissues such as liver, kidney and intestine124. The main function of MRP2, which is located to apical membrane of polarized cells, is to actively transport endogenous (e.g. bilirubin and its polar conjugates) and xenobiotic (e.g. drugs) substances out of the cells124.
Early investigation showed that genistein inhibited the efflux of daunorubicin from small-cell lung cancer GL4/ADR cells, in which MRP was overexpressed, in a competitive manner 125. The same result of inhibition of daunorubicin by genistein competitively was also observed in plasma membrane vesicles from marine MRP-transfected NIH3T3 cells126. Follow-up study confirmed that genistein but not genistin, together with kaempferol and flavopiridol (synthetic flavonoid derivative) could affect MRP-mediated transport of anticancer drugs by a direct interaction with MRP127. Moreover, chrysin was reported to inhibit the accumulation of the MRP2 substrate-CMFDA in Caco-2 cells in a dose-dependent manner128. The maximal accumulation, which could also be achieved by specific MRP inhibitor-MK571, was observed in the presence of 250 μM chrysin. Interestingly, chrysin was also found to increase the expression of MRP2 by 5 folds in Caco-2 cells after a long-term treatment128. Over a time period of 48 hours, the inhibition of transporter function overtook the enhanced expression of MRP2 by chrysin, resulting in an increased accumulation of topotecan128. Therefore, chrysin seemed to play a dual role in regulating MRP2 in Caco-2 cells. In fact, the dual role of chrysin was also extended to other efflux transporters-P-gp and BCRP. Evidence from other investigators confirmed the inhibition od MRP2 by chrysin in Caco-2 cells129. It was found that chrysin, genistein, quercetin, biochanin A, catechin and EGCG (all at 50 μM) could increase transportation of ochratoxin A from apical side to basolateral side. The enhancement of ochratoxin A uptake by above flavonoids was also observed in Caco-2 cells. Moreover, quercetin displayed concentration-dependent inhibition on ochratoxin A absorption129. A later study further identified that major phase II metabolites of quercetin inhibited MRP2 functions with similar potency as quercetin itself130. The results by using Sf9 inside-out vesicles revealed that glucuronides, especially 7-O-glucuronosyl-quercetin significantly increased the potential of quercetin to inhibit MRP2-mediated calcein transport. On the contrary, methylation at 4′ position of quercetin resulted in the reduction of the potential to inhibit MRP2130.
Quantitative structure-function relationship has been built according to flavonoid-mediated inhibition of MRP2 by using MDCKII-MRP2 cells131. A total 29 flavonoids with various structures were used in the study. It was revealed that robinetin and myricetin, both of which had IC50 much lower than 50 μM (15 and 22 μM), respectively, were the two best inhibitors of MRP2 among all the tested flavonoids 131. Furthermore, the kinetic mechanism for robinetin to inhibit MRP2-mediated transportation of calcein was competitive. As a result, a flavonol B-ring pyrogallol group appeared to be an essential structure for inhibition of MRP2-mediated calcein efflux131.
4.3. Effect of flavonoids on BCRP
Breast cancer resistance protein (BCRP), which is encoded by ABCG2 gene, consists of 655 amino acids with molecular weight of 72KD132. Unlike many other members from ABC transporter family, BCRP is a “half ABC transporter” because it only contains one NBD and six TMDs133. To function properly, BCRP must form homodimers or homooligomers 134. In addition to its physiological function, BCRP has a role in limiting oral bioavailability of drugs and drug transport across the blood-brain barrier, blood–testis barrier and the maternal-fetal barrier of selected substrates135,136,137. Though BCRP was identified originally from cancer cells, it is broadly distributed in humans including kidney, liver and intestine138. The substrates of BCRP include various compounds from endogenous to exogenous, which are generally organic anion or neutral substances, often time similar to the substrates of MRP2.
Currently, the inhibition on BCRP by flavonoids was most extensively investigated among all the ABC transporters. Substantial amount of work has been done to study the interactions between flavonoids and BCRP. It was Zhang and coworker who firstly identified chrysin and biochanin A as the most potent inhibitors of BCRP among all the 20 naturally occurring flavonoids139. In fact, it was found that most tested flavonoids (e.g. apigenin, genistein, fisetin, kaempferol, hesteretin, naringenin and quercetin) could increase mitoxantrone accumulation in BCRP-overexpressing cells (MCF-7-MX100 and NCI-H460-MX20) at concentration of 50 μM, but none of flavonoid glycosides (e.g. naringin and phloridzin) was effective139. Specifically, the concentration-dependent enhancements of accumulations of mitoxantrone were observed for apigenin, biochanin A, chrysin, genistein and kaempferol 139. Furthermore, several combinations of multiple flavonoids were shown an additive (inhibitory) effect on BCRP 140. For example, apigenin (A), biochanin A (B) and chrysin (C) acted additively in inhibiting BCRP-mediated transport in combinations of AB, BC and ABC with equal molar concentration of each individual constituent 140. The combinations were not limited to 3 flavonoids. The additive inhibition of BCRP could be obtained when 5 or 8 flavonoids were combined together140. Recently, the pharmacokinetic and tissue distributions of mitoxantrone with or without co-administration of 5, 7-dimethoxyflavone (5,7-DMF) or multiple flavonoids with low EC50 (7, 8-benzoflavone, 5, 6, 7-trimethoxyflavone and 8-methylflavone) were studied in mice to evaluate the potentially additive or synergistic effect of multiple flavonoids on BCRP inhibition 141. It revealed that there was no significant change of pharmacokinetic parameters with or without flavonoids. However, significant changes in mitoxantrone distribution were observed in several tissues with co-administration of flavonoids. In the presence of 5, 7DMF, the AUC values of mitoxantrone were significantly increased in liver (94.5%), kidney (61.9%) and lung (18.4%); on the other hand, co-administration of multiple flavonoids resulted in increasing AUC values in heart (30.5%), liver (95.9%), kidney (63.3%), lung (30.7%) and muscle (33.8%) 141. Therefore, inhibition of BCRP by either individual flavonoid or multiple flavonoids can occur both in vitro and in vivo. However, conclusion from in vitro study was not always consistent with in vivo study. Discrepancy was observed when biochanin A was used as inhibitor to treat MDCKII-BCRP or MDCKII-Bcrp cells or given to mice 142. In vitro, accumulation of cellular mitoxantrone was enhanced with biochanin A at concentration of 2.5 or 25 μM in MDCK cells overexpressing human BCRP or murine Bcrp. In contrast to in vitro data, biochanin A administrated by i.v. at 10 mg/kg had no impact on the plasma concentration of mitoxantrone and AUC values in most tissues (brain, heart, liver and lung) 142. Thus, when extrapolating results of in vitro inhibition on BCRP by flavonoids to in vivo, we should pay particular attention to the fact the low bioavailability may severely limit their in vivo levels necessary to achieve observed inhibition effects (in vitro).
Unlike flavonoid aglycones, most flavonoid glycosides were reported to have little or no effects on BCRP-mediated drug resistance. Only a few flavonoid glycosides were shown to affect BCRP-mediated drug resistance. Naringenin-7-glucoside and luteolin-4-glucoside displayed moderate effects to reverse the resistance to SN-38 and mitoxantrone in BCRP-transduced K562 cells 143. However, the inhibition of BCRP by naringin might be controversial. Recently, experiments indicated that 6 isoflavonoid glucosides (daidzin, ononin, genistin, sissotrin, glycitin and coumestrin) had 10- to 100- fold lower inhibitory potency (against BCRP) than their corresponding aglycones144. Interestingly, it was also found that the inhibitory potency of daidzein-7-glucuronide was 100-fold lower than daidzein while daidzein-4-sulfate had the inhibitory potency comparable to daidzein144. It is very clear that there is a strong structure-inhibition relationship between BCRP and flavonoids. It appeared that flavonoids with following structural features might inhibit BCRP effectively: a hydroxyl group at position 5, double bond between position 2 and 3, and a methoxyl moiety at position 3 or 6145. With regard to the inhibition mechanisms, interference with substrate-binding sites of BCRP might account for flavonoid’s inhibition of BCRP since quercetin and daidzein were able to stimulate the vanadate-inhibitable ATPase activity in membranes prepared from bacteria expressing BCRP 146. In contrast to inhibition, expression of BCRP in Caco-2 cells can also be induced by flavonoids147. Evidence indicated that quercetin (25 μM) could strongly induce the protein expression of BCRP in Caco-2 cells. At mRNA level, quercetin, chrysin and flavone had a pronounced induction of BCRP, while genistein showed no effect. The induction of BCRP was also believed to be via regulation of AhR (aryl hydrocarbon receptor) 147.
4.4. Effects on OATP and OAT
Compared to abundant studies related to interactions of flavonoids with ABC transporters, little is known about interactions of flavonoids with transporters from solute carrier family (SLC). Organic anion-transporting peptide (OATP, also called SLC21A) and organic anion transporter (OAT, also called SLC22A) are two subfamilies, which are reported to have an interaction with flavonoids or flavonoid conjugates148, 149,150,151.
Members of OATP family mediate sodium-independent uptake of a broad spectrum of amphipathic organic anions, organic cations and conjugates. OATP2B1 and OATP1A2 are expressed in the intestine while OATP1B1 and OATP1B3 are expressed in liver 152, 153. According to the published papers, OATP1B1, OATP1B3 and OATP2B1 are localized on the basolateral membrane, while OATP1A2 is localized on the apical membrane 153. A large volume of evidence showed that various juices, which contained plenty of flavonoids, could inhibit the function of OATP. It was demonstrated that several citrus juice constituents (10 μM), including naringin, naringenin, quercetin, bergamottin and 6, 7-dihydroxybergamottin, tangeretin and nobiletin, significantly inhibited the OATP2B1-mediated uptake of estrone-3-sulfate by about 20–60% in OATP2B1-expressing HEK293 cells154. Another study also found that naringin (grapefruit) and hesperidin (orange) inhibit the OATP1A2-mediated transport of fexofenadine in vitro and in vivo 151. Further analysis of human study showed that consumption of grapefruit juice concomitantly or 2 hours before fexofenadine administration was linked with reduced oral fexofenadine plasma concentration without affecting protein expressions of OATP1A2 and P-gp in intestine152. In OATP1B1-expressing HeLa cells, 20 naturally occurring flavonoids were tested for their impacts on uptake of [3H]dehydroepiangrosterone sulfate (DHEAS)149. Most of flavonoids (e.g. biochanin A, genistein, and epigallocatechin-3-gallate) significantly inhibited DHEAS uptake in a concentration-dependent manner149. Not surprisingly, flavonoid glycosides showed comparable, opposite or no effect on uptake of DHEAS in contrast to their corresponding flavonoid aglycones. Genistin could not inhibit the uptake but genistein could; rutin, on the other hand, showed activation of uptake, whereas quercetin had no effect on uptake149. As the most potent inhibitor among 20 flavonoids, biochanin A was selected for in vitro kinetic study. The results suggested that biochanin A inhibited uptake of DHEAS in a noncompetitive way as it was not the substrate for OATP1B1149.
Members of OAT play an important role in the sodium-dependent renal uptake of organic anions. OAT1 and OAT3, which are highly expressed on the basolateral membrane of proximal tubules, are two relatively well-studied transporters within the OAT family155. Their substrates are highly variable from endogenous metabolites to drugs and toxicants. At present, only a few studies have focused on the interaction of OAT with flavonoids. In one study, ellagic acid was identified to be a substrate and potent inhibitor of OAT1156. In another, naringenin, morin, silybin and quercetin were reported to inhibit both OAT1 and OAT3157. Most recently, quercetin phase II conjugates (quercetin-3-O-glucuronide, -3′-O-glucuronide and -3′-O-sulfate) were found to potently inhibit OAT3-mediated transport of 5-carboxyfluorescein. In addition, quercetin-3′-O-sulfate could also strongly inhibited transport of p-aminohippuric acid, which is a substrate of OAT1148.
5. Clinical significance of flavonoid’s interaction with disposition machinery
Flavonoids can exert their various biological and pharmacological effects that benefit humans. This could be achieved since humans could easily ingest large amounts of flavonoids from daily dietary intake because of their ubiquitous distribution in vegetables, fruits, herbal supplements and beverages deriving from plants. A couple of clinical studies indicated that administration of flavonoids via i.v. or oral, either as individual components (e.g. quercetin alone) or mixtures, could inhibit lymphocyte tyrosine kinase activity (resulting in antitumor effects) or prevent bone loss in late postmenopausal women (without a detectable hyperplasia effect on the endometrium as the side effects as many drugs do)158,159. These and other clinical study results suggest that some of flavonoids can reach the effective concentrations in vivo at which their pharmacological activity was exhibited. Although there is extensive phase II metabolism mediated mainly by UGTs and SULTs, when multiple flavonoids are taken together, the bioavailability of one flavonoid might be improved. In an animal study, co-administration of quercetin and EGCG together with biochanin A significantly enhanced the bioavailability of biochanin A due to decrease of clearance by extending enterohepatic cycling160. Therefore, we should pay more attention to flavonoid-flavonoid interaction in pharmacokinetic and efficacy studies in vivo.
Drug – flavonoid interaction is another important issue, especially from the clinical point of view. In one instance, patients with long-term exposures to foods containing considerable amounts of flavonoids may experience changes in their physiological status including expression of drug metabolizing enzymes and transporters. On the other hand, the immediate inhibitory effects on drug metabolizing enzymes and transporters can also be observed when flavonoid – enriched edibles are consumed with medications. As a result, the pharmacokinetics of medications is changed. For example, an aqueous solution of naringin that contained the same amount of the flavanone as 300ml grapefruit juice decreased the bioavailability of co-administered fexofenadine 151. In another study, an intake of daidzein (200 mg b.i.d) for ten consecutive days increased bioavailability, maximal plasma concentration and elimination half–life of the bronchodilator theophylline in humans161. Since flavonoids can interact with multiple drug metabolizing enzymes and transporters, which are involved in absorption, distribution, metabolism and excretion of drugs, drug-flavonoid interaction has become and will remain an important area for clinical research and studies.
6. Conclusion
In conclusion, the mutual interactions between flavonoids and drug-metabolizing enzymes and transporters are very complex and multifaceted. Flavonoids can be substrates of and/or chemical modulators of certain enzymes and transporters that will impact the disposition processes of various compounds including flavonoids themselves. The extensive metabolism of flavonoids by phase II enzymes and subsequent excretion of conjugates by the associated efflux transporters, especially those from ABC transporter superfamily, explain why flavonoid aglycones are presents at very low level in the systemic circulation. Recent research further suggested that it was the interplay between phase II enzymes (e.g. UGTs and SULTs isoforms) and efflux transporters (e.g. MRP2 and BCRP) that drives the metabolic disposition and clearance processes.
On the other hand, flavonoids can also modify the expression or activity of those enzymes and transporters relevant for the interplay. For most flavonoids, these mean that they could inhibit the activity of phase II enzymes or transporters acutely; but induce the expression or activate the function of phase II enzymes or efflux transporters on a chronic basis. These interactions are often mediated via the action of various chemosensing mechanisms involving partners such as nucleic receptors and their co-regulators.
Therefore, the mutual interactions between flavonoids and enzymes as well as efflux transporters are critical factors that should be taken into considerations when flavonoids are co-administered with medications. This is because pharmacokinetics of medications can be altered. A better understanding of mutual interactions between disposition of flavonoids and elements responsible for their disposition will greatly help us to identify potential drug-flavonoid interactions and flavonoid-flavonoid interactions. In addition, the mutual interaction study can accelerate the process of building structure-metabolism or structure-activity relationships, allowing us to identify critical structural features that are not subjected to metabolism but may retain essential biological activities.
Table 2.
Interactions between UGTs and flavonoids
| Isoform | Expression*45,47,48 | Flavonoidsas substrates | Flavonoids as modulators | Ref. | |
|---|---|---|---|---|---|
| Inducer or activator | Inhibitor | ||||
| UGT1A1 | Liver Small intestine Colon Stomach |
Quercetin, fisetin, naringenin, luteolin, genistien, daidzein, eupatilin, glycitein, formononetin, biochanin A, prunetin | Chrysin Apigenin Quercetin |
Chrysin | 44, 54, 78–80, 85, 87 |
| UGT1A3 | Liver Small intestine Colon |
Isorhamnetin, sylibin, kaempferol, daidzein, morin, avicularin, eupatilin quercetin xylopyranoside quecetin-3′,4′-OCHO- | 51, 54 | ||
| UGT1A6 | Liver Small intestine Colon Stomach |
Luteolin and chrysin | Quercetin, | Silymarin | 52, 54, 32, 87 |
| UGT1A7 | Colon Stomach Esophagus |
Eupatilin | 54 | ||
| UGT1A8 | Small intestine Colon Esophagus |
Naringenin, genistein, daidzein, eupatilin, glycitein, formononetin, biochanin A, prunetin, apigenin, chrysin, 7-hydroxyflavone | 44, 50, 54 | ||
| UGT1A9 | Liver Colon Esophagus (Kidney) |
Luteolin, genistein, glycitein, formononetin, biochanin A, prunetin, daidzein, morin, avicularin, quercetin, eupatilin, quercetin xylopyranoside, quecetin-3′,4′-OCHO- | Silymarin | 44, 51, 54, 84 | |
| UGT1A10 | Small intestine Colon Stomach Esophagus |
Naringenin, genistein, apigenin, chrysin, 7-hydroxyflavone, eupatilin | 44, 49, 54, | ||
| UGT2B7 | Liver Small intestine Colon Esophagus |
Luteoin and quercetin | 52 | ||
| UGT2B15 | Liver Small intestine Colon Stomach Esophagus |
Luteoin and quercetin | 52 | ||
| UGT2B17 | Liver Small intestine Colon Stomach |
Galangin, chrysin, naringin, 7-hydroxyflavone | 55 | ||
| Unspecified Isoforms | - | Biochanin A Daidzein Formononetin Genistein Prunetin Apigenin Galangin Kaempferol Naringenin Quercetin Chrysin Nobiletin Silymarin Tangeritin |
Chrysin Nobiletin Silymarin Baicalein Wogonin |
77, 83, 80, 88, 89–91 | |
Expression was mostly referred to expression in those organs, which lead to metabolism and excretion.
Table 3.
Interactions between SULTs and flavonoids
| Isoform | Expression*58–61 | Flavonoidsas substrates | Flavonoids as modulators | Ref | |
|---|---|---|---|---|---|
| Inducer or activator | Inhibitor | ||||
| SULT1A1 | Liver Small intestine Colon Stomach |
EC, galangin, hesperetin, eriodictyol, (+)catechin | Genistein Biochanin A |
Apigenin, chrysin. quercetin, myricetin kaempferol, genistein daidzein, hesperetin eriodictyol, catechin EC, luteolin 3,4′-dihydroxyflavone 3′, 4′, 7-trihydroxyisoflavone | 62, 63, 40, 67, 92–96, 99, 100 |
| SULT1A3 | Liver Small intestine Colon |
Galangin, hesperetin, eriodictyol, (+)catechin, EC Similar to SULT1A1 | Baicalein, hesperetin, eriodictyol | 62, 63, 40, 66, 95, 97 | |
| SULT1E1 | Liver Small intestine Colon Stomach |
EC, galangin Similar to SULT1A1 | Biochanin A | Tricin, galangin, genistein, equol, daidzein, quercetin | 40, 95, 97, 98, 100 |
| Unspecified Isoform | Genistein, apigenin, baicalein | 23, 64, 65 | |||
| SULT2A1 | Liver Small intestine Colon |
Genistein Biochanin A |
Apigenin, myricetin, baicalein, galangin, 7-hydroxyflavone | 96, 99, 100 | |
Expression was mostly referred to expression in those organs, which lead to metabolism and excretion.
Table 4.
Interactions between transporters and flavonoids
| Transporters | Flavonoids as substrates | Flavonoids as modulators
|
|||
|---|---|---|---|---|---|
| Inducer or activator | Inhibitor | No effect | Ref. | ||
| P-gp | Flavonoid aglycone | Catechin, epicatechin, grapefruit juice, quercetin and kaempherol (low concentration) | EGCG, quercetin kaempherol (high concentraion), chrysin, flavones, hesperetin, naringenin, genistein | Grapefruit juice, narigin, hesperidin, rutin | 109–121 |
|
| |||||
| MRP2 | Flavonoid glucuronides and sulfates | Chrysin | Genistein, kaempferol, flavopiridol, chrysin, quercetin, biochanin A, catechin, EGCG, quercetin-7-o-glucuronide | Genistin | 125–131 |
|
| |||||
| BCRP | Flavonoid glucuronides and sulfates | Quercetin, chrysin and flavone | Chrysin, biochanin A, apigenin, genistein, fisetin, kaempferol, hesperetin, naringenin, quercetin, luteolin-4-glucoside, daidzein-7-glucuronide, daidzein-4-sulfate, daidzin, ononin, genistin, sissotrin, glycitin, coumestrin | Naringin and phloridzin | 139–147 |
|
| |||||
| OATP | Rutin | Naringin, naringenin, quercetin, hesperidin, biochanin A, genistein, EGCG | Genistin and quercetin | 149, 151, 153 | |
|
| |||||
| OAT | Ellagic acid quercetin-3-O-glucuronide, quercetin-3′-O-glucuronide, quercetin-3′-O-sulfate | Ellagic acid, naringenin, morin, silybin, quercetin, quercetin-3-o-glucuronide, quercetin-3′-O-glucuronide, quercetin-3′-O-sulfate | 156, 157, 148 | ||
Abbreviation lists
- ABC
ATP-binding cassette
- UGT
Uridine-5′-diphosphate glucuronosyltransferases
- SULT
Sulfotransferases
- CYP450
Cytochrome P450
- P-gp
P-glycoprotein
- MRP
Muti-drug resistance protein
- BCRP
Breast cancer resistance protein
- GST
Glutathione-S-transferases
- OATP
Organic anion-transporting peptide
- OAT
Organic anion transporter
- SLC
Solute carrier
- DME
Drug metabolizing enzymes
References
- 1.Chun OK, Chung SJ, Song WO. J Nutr. 2007;137:1244. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 2.de Vries JH, Janssen PL, Hollman PC, van Staveren WA, Katan MB. Cancer Lett. 1997;114:141. doi: 10.1016/s0304-3835(97)04645-4. [DOI] [PubMed] [Google Scholar]
- 3.Hertog MG, Hollman PC, Katan MB, Kromhout D. Nutr Cancer. 1993;20:21. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- 4.Narayana KR, Reddy MS, Chaluvadi MR, Krishna DR. Indian Journal of Pharmacology. 2001;33:2. [Google Scholar]
- 5.Schroder G, Wehinger E, Lukacin R, Wellmann F, Seefelder W, Schwab W, Schroder J. Phytochemistry. 2004;65:1085. doi: 10.1016/j.phytochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Wong YC, Zhang L, Lin G, Zuo Z. Expert Opin Drug Metab Toxicol. 2009;5:1399. doi: 10.1517/17425250903179300. [DOI] [PubMed] [Google Scholar]
- 7.Harborne JBaWCA. Nat Prod Rep. 1998;15:631. [Google Scholar]
- 8.Veitch NCaGRJ. Nat Prod Rep. 2011;28:1626. doi: 10.1039/c1np00044f. [DOI] [PubMed] [Google Scholar]
- 9.Harborne JB. The flavonoids: advances in research since 1986. Chapman&Hall/CRC; 1994. [Google Scholar]
- 10.Bosetti C, Spertini L, Parpinel M, Gnagnarella P, Lagiou P, Negri E, Franceschi S, Montella M, Peterson J, Dwyer J, Giacosa A, La Vecchia C. Cancer Epidemiol Biomarkers Prev. 2005;14:805. doi: 10.1158/1055-9965.EPI-04-0838. [DOI] [PubMed] [Google Scholar]
- 11.Grassi D, Desideri G, Croce G, Tiberti S, Aggio A, Ferri C. Curr Pharm Des. 2009;15:1072. doi: 10.2174/138161209787846982. [DOI] [PubMed] [Google Scholar]
- 12.Ames BN, Shigenaga MK, Hagen TM. Proc Natl Acad Sci U S A. 1993;90:7915. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koga T, Meydani M. Am J Clin Nutr. 2001;73:941. doi: 10.1093/ajcn/73.5.941. [DOI] [PubMed] [Google Scholar]
- 14.Walle T. Free Radic Biol Med. 2004;36:829. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Walgren RA, Lin JT, Kinne RK, Walle T. J Pharmacol Exp Ther. 2000;294:837. [PubMed] [Google Scholar]
- 16.Walle T, Otake Y, Walle UK, Wilson FA. J Nutr. 2000;130:2658. doi: 10.1093/jn/130.11.2658. [DOI] [PubMed] [Google Scholar]
- 17.Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJ, Morgan MR, Williamson G. FEBS Lett. 1998;436:71. doi: 10.1016/s0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- 18.Day AJ, Canada FJ, Diaz JC, Kroon PA, McLauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G. FEBS Lett. 2000;468:166. doi: 10.1016/s0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Liu Y, Dai Y, Xun L, Hu M. J Altern Complement Med. 2003;9:631. doi: 10.1089/107555303322524481. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Hu M. Drug Metab Dispos. 2002;30:370. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 21.Day AJ, Gee JM, DuPont MS, Johnson IT, Williamson G. Biochem Pharmacol. 2003;65:1199. doi: 10.1016/s0006-2952(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 22.Nemeth K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA. Eur J Nutr. 2003;42:29. doi: 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Lin H, Hu M. J Pharmacol Exp Ther. 2003;304:1228. doi: 10.1124/jpet.102.046409. [DOI] [PubMed] [Google Scholar]
- 24.Gradolatto A, Canivenc-Lavier MC, Basly JP, Siess MH, Teyssier C. Drug Metab Dispos. 2004;32:58. doi: 10.1124/dmd.32.1.58. [DOI] [PubMed] [Google Scholar]
- 25.Manach C, Donovan JL. Free Radic Res. 2004;38:771. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- 26.Zhu W, Xu H, Wang SW, Hu M. Aaps J. 2010;12:525. doi: 10.1208/s12248-010-9209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda SR, Lee JK, Brouwer KL, Wen Z, Smith PC, Hawke RL. Drug Metab Dispos. 2008;36:2219. doi: 10.1124/dmd.108.021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. J Nutr. 2001;131:1362S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 29.Crozier A, Del Rio D, Clifford MN. Mol Aspects Med. 2010;31:446. doi: 10.1016/j.mam.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Hu M. Mol Pharm. 2007;4:803. doi: 10.1021/mp7001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crozier A, Jaganath IB, Clifford MN. Nat Prod Rep. 2009;26:1001. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 32.Cermak R. Expert Opin Drug Metab Toxicol. 2008;4:17. doi: 10.1517/17425255.4.1.17. [DOI] [PubMed] [Google Scholar]
- 33.Morris ME, Zhang S. Life Sci. 2006;78:2116. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez AI, Real R, Perez M, Mendoza G, Prieto JG, Merino G. J Pharm Sci. 2010;99:598. doi: 10.1002/jps.21851. [DOI] [PubMed] [Google Scholar]
- 35.Moon YJ, Wang X, Morris ME. Toxicol In Vitro. 2006;20:187. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 36.Hodek P, Trefil P, Stiborova M. Chem Biol Interact. 2002;139:1. doi: 10.1016/s0009-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 37.Harris RZ, Jang GR, Tsunoda S. Clin Pharmacokinet. 2003;42:1071. doi: 10.2165/00003088-200342130-00001. [DOI] [PubMed] [Google Scholar]
- 38.Otake Y, Walle T. Drug Metab Dispos. 2002;30:103. doi: 10.1124/dmd.30.2.103. [DOI] [PubMed] [Google Scholar]
- 39.Walle UK, Walle T. Drug Metab Dispos. 2007;35:1985. doi: 10.1124/dmd.107.016782. [DOI] [PubMed] [Google Scholar]
- 40.Otake Y, Hsieh F, Walle T. Drug Metab Dispos. 2002;30:576. doi: 10.1124/dmd.30.5.576. [DOI] [PubMed] [Google Scholar]
- 41.Meng S, Wu B, Singh R, Yin T, Morrow JK, Zhang S, Hu M. Mol Pharm. 2012;9:862. doi: 10.1021/mp200400s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang L, Ye L, Singh R, Wu B, Lv C, Zhao J, Liu Z, Hu M. Mol Pharm. 2010;7:664. doi: 10.1021/mp900223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu B, Basu S, Meng S, Wang X, Hu M. Curr Drug Metab. 2011;12:900. doi: 10.2174/138920011797470100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang L, Singh R, Liu Z, Hu M. Mol Pharm. 2009;6:1466. doi: 10.1021/mp8002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tukey RH, Strassburg CP. Annu Rev Pharmacol Toxicol. 2000;40:581. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 46.Joseph TB, Wang SW, Liu X, Kulkarni KH, Wang J, Xu H, Hu M. Mol Pharm. 2007;4:883. doi: 10.1021/mp700135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. Pharmacogenet Genomics. 2005;15:677. doi: 10.1097/01.fpc.0000173483.13689.56. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura A, Nakajima M, Yamanaka H, Fujiwara R, Yokoi T. Drug Metab Dispos. 2008;36:1461. doi: 10.1124/dmd.108.021428. [DOI] [PubMed] [Google Scholar]
- 49.Lewinsky RH, Smith PA, Mackenzie PI. Xenobiotica. 2005;35:117. doi: 10.1080/00498250400028189. [DOI] [PubMed] [Google Scholar]
- 50.Cheng Z, Radominska-Pandya A, Tephly TR. Drug Metab Dispos. 1999;27:1165. [PubMed] [Google Scholar]
- 51.Chen Y, Xie S, Chen S, Zeng S. Biochem Pharmacol. 2008;76:416. doi: 10.1016/j.bcp.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Boersma MG, van der Woude H, Bogaards J, Boeren S, Vervoort J, Cnubben NH, van Iersel ML, van Bladeren PJ, Rietjens IM. Chem Res Toxicol. 2002;15:662. doi: 10.1021/tx0101705. [DOI] [PubMed] [Google Scholar]
- 53.Galijatovic A, Otake Y, Walle UK, Walle T. Xenobiotica. 1999;29:1241. doi: 10.1080/004982599237912. [DOI] [PubMed] [Google Scholar]
- 54.Lee HS, Ji HY, Park EJ, Kim SY. Xenobiotica. 2007;37:803. doi: 10.1080/00498250701534877. [DOI] [PubMed] [Google Scholar]
- 55.Turgeon D, Carrier JS, Chouinard S, Belanger A. Drug Metab Dispos. 2003;31:670. doi: 10.1124/dmd.31.5.670. [DOI] [PubMed] [Google Scholar]
- 56.Xie S, You L, Zeng S. Pharmazie. 2007;62:625. [PubMed] [Google Scholar]
- 57.Janisch KM, Williamson G, Needs P, Plumb GW. Free Radic Res. 2004;38:877. doi: 10.1080/10715760410001728415. [DOI] [PubMed] [Google Scholar]
- 58.Gamage N, Barnett A, Hempel N, Duggleby RG, Windmill KF, Martin JL, McManus ME. Toxicol Sci. 2006;90:5. doi: 10.1093/toxsci/kfj061. [DOI] [PubMed] [Google Scholar]
- 59.Nagata K, Yamazoe Y. Annu Rev Pharmacol Toxicol. 2000;40:159. doi: 10.1146/annurev.pharmtox.40.1.159. [DOI] [PubMed] [Google Scholar]
- 60.Ung D, Nagar S. Drug Metab Dispos. 2007;35:740. doi: 10.1124/dmd.106.013987. [DOI] [PubMed] [Google Scholar]
- 61.Nowell S, Falany CN. Oncogene. 2006;25:1673. doi: 10.1038/sj.onc.1209376. [DOI] [PubMed] [Google Scholar]
- 62.Vaidyanathan JB, Walle T. Drug Metab Dispos. 2002;30:897. doi: 10.1124/dmd.30.8.897. [DOI] [PubMed] [Google Scholar]
- 63.Huang C, Chen Y, Zhou T, Chen G. Xenobiotica. 2009;39:312. doi: 10.1080/00498250802714915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu M, Chen J, Lin H. J Pharmacol Exp Ther. 2003;307:314. doi: 10.1124/jpet.103.053496. [DOI] [PubMed] [Google Scholar]
- 65.Sun H, Zhang L, Chow EC, Lin G, Zuo Z, Pang KS. J Pharmacol Exp Ther. 2008;326:117. doi: 10.1124/jpet.108.137463. [DOI] [PubMed] [Google Scholar]
- 66.Meinl W, Ebert B, Glatt H, Lampen A. Drug Metab Dispos. 2008;36:276. doi: 10.1124/dmd.107.018036. [DOI] [PubMed] [Google Scholar]
- 67.Linton KJ. Physiology (Bethesda) 2007;22:122. doi: 10.1152/physiol.00046.2006. [DOI] [PubMed] [Google Scholar]
- 68.Nait Chabane M, Al Ahmad A, Peluso J, Muller CD, Ubeaud G. J Pharm Pharmacol. 2009;61:1473. doi: 10.1211/jpp/61.11.0006. [DOI] [PubMed] [Google Scholar]
- 69.Williamson G, Aeberli I, Miguet L, Zhang Z, Sanchez MB, Crespy V, Barron D, Needs P, Kroon PA, Glavinas H, Krajcsi P, Grigorov M. Drug Metab Dispos. 2007;35:1262. doi: 10.1124/dmd.106.014241. [DOI] [PubMed] [Google Scholar]
- 70.Brand W, van der Wel PA, Rein MJ, Barron D, Williamson G, van Bladeren PJ, Rietjens IM. Drug Metab Dispos. 2008;36:1794. doi: 10.1124/dmd.107.019943. [DOI] [PubMed] [Google Scholar]
- 71.An G, Gallegos J, Morris ME. Drug Metab Dispos. 2011;39:426. doi: 10.1124/dmd.110.035212. [DOI] [PubMed] [Google Scholar]
- 72.van de Wetering K, Feddema W, Helms JB, Brouwers JF, Borst P. Gastroenterology. 2009;137:1725. doi: 10.1053/j.gastro.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 73.Jia X, Chen J, Lin H, Hu M. J Pharmacol Exp Ther. 2004;310:1103. doi: 10.1124/jpet.104.068403. [DOI] [PubMed] [Google Scholar]
- 74.Jiang W, Xu B, Wu B, Yu R, Hu M. Drug Metab Dispos. 2011 doi: 10.1124/dmd.111.041467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Gordon GB. Mol Cancer Ther. 2004;3:885. [PubMed] [Google Scholar]
- 76.Urquhart BL, Tirona RG, Kim RB. J Clin Pharmacol. 2007;47:566. doi: 10.1177/0091270007299930. [DOI] [PubMed] [Google Scholar]
- 77.Sun XY, Plouzek CA, Henry JP, Wang TT, Phang JM. Cancer Res. 1998;58:2379. [PubMed] [Google Scholar]
- 78.Walle T, Otake Y, Galijatovic A, Ritter JK, Walle UK. Drug Metab Dispos. 2000;28:1077. [PubMed] [Google Scholar]
- 79.Galijatovic A, Walle UK, Walle T. Pharm Res. 2000;17:21. doi: 10.1023/a:1007506222436. [DOI] [PubMed] [Google Scholar]
- 80.Galijatovic A, Otake Y, Walle UK, Walle T. Pharm Res. 2001;18:374. doi: 10.1023/a:1011019417236. [DOI] [PubMed] [Google Scholar]
- 81.Walle UK, Walle T. Drug Metab Dispos. 2002;30:564. doi: 10.1124/dmd.30.5.564. [DOI] [PubMed] [Google Scholar]
- 82.Yueh MF, Tukey RH. J Biol Chem. 2007;282:8749. doi: 10.1074/jbc.M610790200. [DOI] [PubMed] [Google Scholar]
- 83.Williams JA, Ring BJ, Cantrell VE, Campanale K, Jones DR, Hall SD, Wrighton SA. Drug Metab Dispos. 2002;30:1266. doi: 10.1124/dmd.30.11.1266. [DOI] [PubMed] [Google Scholar]
- 84.Venkataramanan R, Ramachandran V, Komoroski BJ, Zhang S, Schiff PL, Strom SC. Drug Metab Dispos. 2000;28:1270. [PubMed] [Google Scholar]
- 85.Svehlikova V, Wang S, Jakubikova J, Williamson G, Mithen R, Bao Y. Carcinogenesis. 2004;25:1629. doi: 10.1093/carcin/bgh169. [DOI] [PubMed] [Google Scholar]
- 86.Bock KW, Eckle T, Ouzzine M, Fournel-Gigleux S. Biochem Pharmacol. 2000;59:467. doi: 10.1016/s0006-2952(99)00366-4. [DOI] [PubMed] [Google Scholar]
- 87.Petri N, Tannergren C, Holst B, Mellon FA, Bao Y, Plumb GW, Bacon J, O’Leary KA, Kroon PA, Knutson L, Forsell P, Eriksson T, Lennernas H, Williamson G. Drug Metab Dispos. 2003;31:805. doi: 10.1124/dmd.31.6.805. [DOI] [PubMed] [Google Scholar]
- 88.van der Logt EM, Roelofs HM, Nagengast FM, Peters WH. Carcinogenesis. 2003;24:1651. doi: 10.1093/carcin/bgg117. [DOI] [PubMed] [Google Scholar]
- 89.Canivenc-Lavier MC, Vernevaut MF, Totis M, Siess MH, Magdalou J, Suschetet M. Toxicology. 1996;114:19. doi: 10.1016/s0300-483x(96)03412-9. [DOI] [PubMed] [Google Scholar]
- 90.Appelt LC, Reicks MM. J Nutr. 1999;129:1820. doi: 10.1093/jn/129.10.1820. [DOI] [PubMed] [Google Scholar]
- 91.Ueng YF, Shyu CC, Lin YL, Park SS, Liao JF, Chen CF. Life Sci. 2000;67:2189. doi: 10.1016/s0024-3205(00)00809-2. [DOI] [PubMed] [Google Scholar]
- 92.Nakano H, Ogura K, Takahashi E, Harada T, Nishiyama T, Muro K, Hiratsuka A, Kadota S, Watabe T. Drug Metab Pharmacokinet. 2004;19:216. doi: 10.2133/dmpk.19.216. [DOI] [PubMed] [Google Scholar]
- 93.Eaton EA, Walle UK, Lewis AJ, Hudson T, Wilson AA, Walle T. Drug Metab Dispos. 1996;24:232. [PubMed] [Google Scholar]
- 94.Walle T, Eaton EA, Walle UK. Biochem Pharmacol. 1995;50:731. doi: 10.1016/0006-2952(95)00190-b. [DOI] [PubMed] [Google Scholar]
- 95.Harris RM, Wood DM, Bottomley L, Blagg S, Owen K, Hughes PJ, Waring RH, Kirk CJ. J Clin Endocrinol Metab. 2004;89:1779. doi: 10.1210/jc.2003-031631. [DOI] [PubMed] [Google Scholar]
- 96.Harris RM, Waring RH. Curr Drug Metab. 2008;9:269. doi: 10.2174/138920008784220637. [DOI] [PubMed] [Google Scholar]
- 97.Waring RH, Ayers S, Gescher AJ, Glatt HR, Meinl W, Jarratt P, Kirk CJ, Pettitt T, Rea D, Harris RM. J Steroid Biochem Mol Biol. 2008;108:213. doi: 10.1016/j.jsbmb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 98.Otake Y, Nolan AL, Walle UK, Walle T. J Steroid Biochem Mol Biol. 2000;73:265. doi: 10.1016/s0960-0760(00)00073-x. [DOI] [PubMed] [Google Scholar]
- 99.Chen Y, Huang C, Zhou T, Chen G. Basic Clin Pharmacol Toxicol. 2008;103:553. doi: 10.1111/j.1742-7843.2008.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Y, Huang C, Zhou T, Zhang S, Chen G. J Biochem Mol Toxicol. 2010;24:102. doi: 10.1002/jbt.20318. [DOI] [PubMed] [Google Scholar]
- 101.Peters WH, Kock L, Nagengast FM, Kremers PG. Gut. 1991;32:408. doi: 10.1136/gut.32.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ansell PJ, Espinosa-Nicholas C, Curran EM, Judy BM, Philips BJ, Hannink M, Lubahn DB. Endocrinology. 2004;145:311. doi: 10.1210/en.2003-0817. [DOI] [PubMed] [Google Scholar]
- 103.Patill BS, Brodbelt JS, Miller EG, Turner ND. In: Potential Health Benefits of Citrus. Patil BS, Turner ND, Miller EG, Brodbelt JS, editors. Vol. 936 2006. [Google Scholar]
- 104.Mitchell AE, Burns SA, Rudolf JL. Arch Toxicol. 2007;81:777. doi: 10.1007/s00204-007-0210-9. [DOI] [PubMed] [Google Scholar]
- 105.Hofmann T, Liegibel U, Winterhalter P, Bub A, Rechkemmer G, Pool-Zobel BL. Mol Nutr Food Res. 2006;50:1191. doi: 10.1002/mnfr.200600177. [DOI] [PubMed] [Google Scholar]
- 106.Dean M. The human ATP-binding cassette (ABC) transporter superfamily. National center for biotechnology information; 2002. [Google Scholar]
- 107.Gottesman MM, Pastan I. Annu Rev Biochem. 1993;62:385. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 108.Germann UA, Pastan I, Gottesman MM. Semin Cell Biol. 1993;4:63. doi: 10.1006/scel.1993.1008. [DOI] [PubMed] [Google Scholar]
- 109.Jodoin J, Demeule M, Beliveau R. Biochim Biophys Acta. 2002;1542:149. doi: 10.1016/s0167-4889(01)00175-6. [DOI] [PubMed] [Google Scholar]
- 110.Wang EJ, Barecki-Roach M, Johnson WW. Biochem Biophys Res Commun. 2002;297:412. doi: 10.1016/s0006-291x(02)02219-2. [DOI] [PubMed] [Google Scholar]
- 111.Zhou S, Lim LY, Chowbay B. Drug Metab Rev. 2004;36:57. doi: 10.1081/dmr-120028427. [DOI] [PubMed] [Google Scholar]
- 112.Lown KS, Bailey DG, Fontana RJ, Janardan SK, Adair CH, Fortlage LA, Brown MB, Guo W, Watkins PB. J Clin Invest. 1997;99:2545. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eagling VA, Profit L, Back DJ. Br J Clin Pharmacol. 1999;48:543. doi: 10.1046/j.1365-2125.1999.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soldner A, Christians U, Susanto M, Wacher VJ, Silverman JA, Benet LZ. Pharm Res. 1999;16:478. doi: 10.1023/a:1011902625609. [DOI] [PubMed] [Google Scholar]
- 115.Mitsunaga Y, Takanaga H, Matsuo H, Naito M, Tsuruo T, Ohtani H, Sawada Y. Eur J Pharmacol. 2000;395:193. doi: 10.1016/s0014-2999(00)00180-1. [DOI] [PubMed] [Google Scholar]
- 116.Romiti N, Tramonti G, Donati A, Chieli E. Life Sci. 2004;76:293. doi: 10.1016/j.lfs.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 117.Castro AF, Altenberg GA. Biochem Pharmacol. 1997;53:89. doi: 10.1016/s0006-2952(96)00657-0. [DOI] [PubMed] [Google Scholar]
- 118.Liu XL, Tee HW, Go ML. Bioorg Med Chem. 2008;16:171. doi: 10.1016/j.bmc.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 119.Hayeshi R, Masimirembwa C, Mukanganyama S, Ungell AL. Eur J Pharm Sci. 2006;29:70. doi: 10.1016/j.ejps.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 120.Ikegawa T, Ohtani H, Koyabu N, Juichi M, Iwase Y, Ito C, Furukawa H, Naito M, Tsuruo T, Sawada Y. Cancer Lett. 2002;177:89. doi: 10.1016/s0304-3835(01)00761-3. [DOI] [PubMed] [Google Scholar]
- 121.Hadjeri M, Barbier M, Ronot X, Mariotte AM, Boumendjel A, Boutonnat J. J Med Chem. 2003;46:2125. doi: 10.1021/jm021099i. [DOI] [PubMed] [Google Scholar]
- 122.Boccard J, Bajot F, Di Pietro A, Rudaz S, Boumendjel A, Nicolle E, Carrupt PA. Eur J Pharm Sci. 2009;36:254. doi: 10.1016/j.ejps.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 123.Leslie EM, Deeley RG, Cole SP. Toxicology. 2001;167:3. doi: 10.1016/s0300-483x(01)00454-1. [DOI] [PubMed] [Google Scholar]
- 124.Leslie EM, Deeley RG, Cole SP. Toxicol Appl Pharmacol. 2005;204:216. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 125.Versantvoort CH, Broxterman HJ, Lankelma J, Feller N, Pinedo HM. Biochem Pharmacol. 1994;48:1129. doi: 10.1016/0006-2952(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 126.Paul S, Belinsky MG, Shen H, Kruh GD. Biochemistry. 1996;35:13647. doi: 10.1021/bi9616615. [DOI] [PubMed] [Google Scholar]
- 127.Hooijberg JH, Broxterman HJ, Heijn M, Fles DL, Lankelma J, Pinedo HM. FEBS Lett. 1997;413:344. doi: 10.1016/s0014-5793(97)00940-x. [DOI] [PubMed] [Google Scholar]
- 128.Schumacher M, Hautzinger A, Rossmann A, Holzhauser S, Popovic D, Hertrampf A, Kuntz S, Boll M, Wenzel U. Biochem Pharmacol. 2010;80:471. doi: 10.1016/j.bcp.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 129.Sergent T, Garsou S, Schaut A, De Saeger S, Pussemier L, Van Peteghem C, Larondelle Y, Schneider YJ. Toxicol Lett. 2005;159:60. doi: 10.1016/j.toxlet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 130.van Zanden JJ, van der Woude H, Vaessen J, Usta M, Wortelboer HM, Cnubben NH, Rietjens IM. Biochem Pharmacol. 2007;74:345. doi: 10.1016/j.bcp.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 131.van Zanden JJ, Wortelboer HM, Bijlsma S, Punt A, Usta M, Bladeren PJ, Rietjens IM, Cnubben NH. Biochem Pharmacol. 2005;69:699. doi: 10.1016/j.bcp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 132.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. Proc Natl Acad Sci U S A. 1998;95:15665. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. Cancer Res. 1998;58:5337. [PubMed] [Google Scholar]
- 134.Ozvegy C, Litman T, Szakacs G, Nagy Z, Bates S, Varadi A, Sarkadi B. Biochem Biophys Res Commun. 2001;285:111. doi: 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- 135.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Nat Rev Drug Discov. 2010;9:215. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Herwaarden AE, Schinkel AH. Trends Pharmacol Sci. 2006;27:10. doi: 10.1016/j.tips.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 137.Vlaming ML, Lagas JS, Schinkel AH. Adv Drug Deliv Rev. 2009;61:14. doi: 10.1016/j.addr.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 138.Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. Adv Drug Deliv Rev. 2009;61:3. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang S, Yang X, Morris ME. Mol Pharmacol. 2004;65:1208. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- 140.Zhang S, Yang X, Morris ME. Pharm Res. 2004;21:1263. doi: 10.1023/b:pham.0000033015.84146.4c. [DOI] [PubMed] [Google Scholar]
- 141.An G, Wu F, Morris ME. Pharm Res. 2011;28:1090. doi: 10.1007/s11095-011-0368-y. [DOI] [PubMed] [Google Scholar]
- 142.An G, Morris ME. Biopharm Drug Dispos. 2010;31:340. doi: 10.1002/bdd.717. [DOI] [PubMed] [Google Scholar]
- 143.Imai Y, Tsukahara S, Asada S, Sugimoto Y. Cancer Res. 2004;64:4346. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- 144.Tamaki H, Satoh H, Hori S, Ohtani H, Sawada Y. Drug Metab Pharmacokinet. 2010;25:170. doi: 10.2133/dmpk.25.170. [DOI] [PubMed] [Google Scholar]
- 145.Pick A, Muller H, Mayer R, Haenisch B, Pajeva IK, Weigt M, Bonisch H, Muller CE, Wiese M. Bioorg Med Chem. 2011;19:2090. doi: 10.1016/j.bmc.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 146.Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA. Biochem Biophys Res Commun. 2004;317:269. doi: 10.1016/j.bbrc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 147.Ebert B, Seidel A, Lampen A. Toxicol Sci. 2007;96:227. doi: 10.1093/toxsci/kfl147. [DOI] [PubMed] [Google Scholar]
- 148.Wong CC, Botting NP, Orfila C, Al-Maharik N, Williamson G. Biochem Pharmacol. 2011;81:942. doi: 10.1016/j.bcp.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 149.Wang X, Wolkoff AW, Morris ME. Drug Metab Dispos. 2005;33:1666. doi: 10.1124/dmd.105.005926. [DOI] [PubMed] [Google Scholar]
- 150.Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Clin Pharmacol Ther. 2002;71:11. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 151.Bailey DG, Dresser GK, Leake BF, Kim RB. Clin Pharmacol Ther. 2007;81:495. doi: 10.1038/sj.clpt.6100104. [DOI] [PubMed] [Google Scholar]
- 152.Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, Kim RB. Clin Pharmacol Ther. 2007;81:362. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 153.Hagenbuch B, Meier PJ. Pflugers Arch. 2004;447:653. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 154.Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, Sawada Y. Drug Metab Dispos. 2005;33:518. doi: 10.1124/dmd.104.002337. [DOI] [PubMed] [Google Scholar]
- 155.Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, Endou H. J Am Soc Nephrol. 2002;13:848. doi: 10.1681/ASN.V134848. [DOI] [PubMed] [Google Scholar]
- 156.Whitley AC, Sweet DH, Walle T. Drug Metab Dispos. 2005;33:1097. doi: 10.1124/dmd.105.004275. [DOI] [PubMed] [Google Scholar]
- 157.Hong SS, Seo K, Lim SC, Han HK. Pharmacol Res. 2007;56:468. doi: 10.1016/j.phrs.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 158.Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, Baker J, Kerr DJ. Clin Cancer Res. 1996;2:659. [PubMed] [Google Scholar]
- 159.Zhang G, Qin L, Shi Y. J Bone Miner Res. 2007;22:1072. doi: 10.1359/jbmr.070405. [DOI] [PubMed] [Google Scholar]
- 160.Moon YJ, Morris ME. Mol Pharm. 2007;4:865. doi: 10.1021/mp7000928. [DOI] [PubMed] [Google Scholar]
- 161.Peng WX, Li HD, Zhou HH. Eur J Clin Pharmacol. 2003;59:237. doi: 10.1007/s00228-003-0596-0. [DOI] [PubMed] [Google Scholar]