Abstract
Background
Low serum magnesium (Mg) has been associated with an increased risk of cardiovascular disease (CVD), including ventricular arrhythmias. However, the association between serum or dietary Mg and AF has not been investigated.
Methods and Results
We studied 14,290 men and women (75% white, 53% women, mean age 54) free of AF at baseline participating in the Atherosclerosis Risk in Communities study in the United States. Incident AF cases through 2009 were ascertained from electrocardiograms, hospital discharge codes, and death certificates. Multivariable Cox proportional hazards regression was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for AF associated with serum and dietary Mg quintiles. Over a median follow-up time of 20.6 years, 1,755 incident AF cases were identified. In multivariable models, lower serum Mg was associated with higher AF risk: compared to individuals in the middle quintile (≥1.60–1.65 mEq/L), the HR (95% CI) of AF in quintiles 1, 2, 4, and 5 were 1.34 (1.16–1.54), 0.99 (0.85–1.16), 1.04 (0.90–1.22), and 1.06 (0.91–1.23), respectively. There was no evidence of significant interactions between serum Mg and sex or race. No association between dietary Mg and AF risk was observed.
Conclusions
Lower serum Mg was associated with a higher AF risk, and this association was not different between whites and African Americans. Dietary Mg was not associated with AF risk.
Keywords: atrial fibrillation, serum magnesium, dietary magnesium
Introduction
Atrial fibrillation (AF), a common cardiac arrhythmia, affects over two million individuals in the United States.1 AF is also associated with an increased risk of cardiovascular disease (CVD), heart failure (HF), stroke, and overall mortality.2 Numerous studies have identified risk factors for AF, including older age, male sex, white race, cigarette smoking, hypertension, obesity, diabetes, HF, coronary heart disease (CHD), left ventricular hypertrophy, metabolic syndrome, and inflammation;2–7 however, a large proportion of the attributable risk of AF (44%) remains unexplained after accounting for one or more borderline or elevated risk factors.8
Magnesium (Mg) is an abundant cation in the body that plays a crucial role as a modulator of vasomotor tone, blood pressure, and peripheral blood flow. Consequently, Mg deficiency can affect the cardiovascular system. Previous epidemiologic studies have found that higher levels of serum Mg were associated with a lower risk of hypertension,9 CHD,10,11 diabetes,12 sudden cardiac death,13 and ischemic stroke.14 Results from studies examining dietary Mg intake with cardiometabolic outcomes, though, have been conflicting with some identifying an inverse association and others finding no association.9–17 In addition, there is some evidence that serum Mg deficiency complicates cardiac surgery and is associated with increased risk of postoperative AF.18 As a result, Mg has been suggested as a possible prophylactic treatment to prevent postoperative AF events, but evidence of the effectiveness of this intervention is mixed.19–23 The association between serum or dietary Mg and incident AF in the general population, however, has not been investigated.
Using data from the Atherosclerosis Risk in Communities (ARIC) study, a community-based cohort including middle-aged whites and African Americans, we explored whether serum and dietary Mg were associated with the risk of incident AF. We hypothesized that individuals with lower serum Mg levels or dietary intake would have a higher risk for AF independent of other cardiovascular risk factors and that the association of Mg with AF risk would be of a similar magnitude in both white and African-American individuals.
Methods
Study design and subjects
The ARIC study is a prospective cohort study aimed to study risk factors of atherosclerosis and CVD.24 In 1987–89, an initial baseline examination including a home interview and clinical visit was conducted with 45- to 64-year-old participants in four United States communities: Forsyth County, NC; Jackson, MS (African Americans only); the northwest suburbs of Minneapolis, MN; and Washington County, MD. At that time, 15,792 participants were enrolled in the study, and three further examinations occurred over an interval of approximately three years between examinations through 1998. Response rates for the follow-up triennial exams were 93%, 86%, and 80%, respectively. Participants are contacted yearly by telephone interviews to obtain follow-up information related to hospital admissions and to determine vital status. Telephone interviews after exam 4 have seen an average response rate of over 90%. Surveillance of death certificates and hospital discharges within pre-specified catchment areas is also continuous. The ARIC Study protocol was approved by the institutional review board of each participating university, and written informed consent was gathered from all enrolled participants.
Assessment of Mg and Covariates
Most risk factors for this analysis were ascertained at the baseline examination. Before the clinical examination, participants were asked to fast for 12 hours, and blood was drawn from an antecubital vein of seated participants into vacuum tubes containing thylenediaminetetraacetic acid (for measurement of lipids) or a serum separator gel (Mg, potassium, creatinine, and glucose). Aliquots were stored at −70°C and were shipped to central laboratories for analyses. Serum Mg was measured at visits 1 and 2 and was based on the Gindler and Heth’s procedure of using the metallochromic dye calmagite (1-[1-hydroxy-4-methyl-2-phenylazo]-2-napthol-4sulfonic acid). Using split samples sent one week apart, laboratories were blindly tested for the laboratory coefficient of variation for Mg, and the result was 3%.24 The correlation coefficient of serum Mg measured at visit 1 versus visit 2 was 0.46.
Serum potassium (K) was assessed with a Coulter DACOS analyzer (Coulter Instruments, Hialeah, FL) using a direct ion-selective electrode. Serum creatinine was measured using a modified kinetic Jaffe method described previously.24,27 Over several weeks, 40 participants were repeatedly tested yielding a reliability coefficient of 0.69 for Mg, 0.66 for K, and 0.68 for creatinine.28 Serum glucose was assayed by a hexokinase/glucose-6-phosphate dehydrogenase method. Prevalent diabetes mellitus was characterized by a fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, a self-reported physician diagnosis, or a current treatment for diabetes. High-density lipoprotein cholesterol (HDL-c) was determined using enzymatic methods while low-density lipoprotein cholesterol (LDL-c) was calculated using the Friedewald equation.29
Blood pressure was measured with a random-zero sphygmomanometer after 5 minutes of rest in the sitting position and was defined as the average of the last 2 of 3 measurements. Questionnaires were used to assess education, smoking status, drinking status, and dietary information. Using an adapted version of the Willett 61-item food frequency questionnaire, usual dietary intake from the previous year was collected at baseline and at visit 3.30 Dietary Mg intake was computed by multiplying the Mg content of each food item by the frequency of its daily consumption and summing over all items. In addition, all medications used in the preceding 2 weeks before each clinic visit were recorded from prescription bottles brought by the participant. At baseline, prevalent myocardial infarction (MI) was characterized by a previously reported MI event or from electrocardiograms (ECG) while prevalent HF was defined by Gothenburg criteria 3 or the intake of HF medications. Prevalent stoke was designated by a self-reported history of physician-diagnosed stroke. Incident cases of HF, MI, or stroke were identified through annual telephone interviews, triennial field center examinations, and the ongoing surveillance of ARIC community hospitals for any hospitalizations or deaths of any cohort participants and were then validated.
Incident AF events
Incident AF cases were ascertained from three sources: ECGs completed during the study exams, hospital discharges codes, and death certificates. ECGs during study visits were performed with MAC PC Personal Cardiographs (Marquette Electronics Inc, Milwaukee, WI). A standard supine 12-lead resting ECG was transmitted to the ARIC ECG Reading Center for coding, interpretation, and storage. A trained cardiologist visually rechecked the ECGs automatically coded as AF to confirm the diagnosis.25 Those identified with prevalent AF at visit 1 by ECG were excluded from further analysis.
Annual follow-up phone calls to the study participants were used to identify hospitalizations and deaths through the end of 2009,26 and discharge lists from local hospitals were reviewed for any cardiovascular events. Using International Classification of Diseases, ninth revision, clinical modification (ICD-9-CM) codes, AF was identified when 427.31 or 427.32 was listed as a discharge diagnostic code. Any AF event identified during a hospitalization for cardiac surgery was excluded. In a physician review of discharge summaries from 125 possible AF cases, approximately 90% of the cases were confirmed.3 Lastly, AF cases were identified as a cause of death if ICD-9 code 427.3 or ICD-10 code I48 was listed on the death certificates. Most incident AF cases (>98%) in this analysis were identified from hospital discharge codes while less than 1% of AF cases were identified from death certificates.
AF incidence date was defined as the date of the first ECG showing AF, the first hospital discharge date for an AF or atrial flutter diagnosis, or date when death occurred due to AF, whichever occurred first.
Statistical analysis
From the initial 15,792 ARIC participants, the following were excluded: those who were races other than white or African-American (n=48); African Americans located in Minneapolis and Washington County (n=55); those with prevalent AF or atrial flutter identified by the baseline ECG (n=37); those with missing or unreadable ECG at baseline (n=309); those with missing baseline serum Mg data measurements (n=124); those not fasting at least eight hours for the baseline examination (n=536); and those missing any information on covariates (n=393).
Initially, we explored the shape of the association between Mg and AF risk using restricted cubic splines. Next, both serum and dietary Mg were divided into ranked quintiles. For serum Mg, ranked quintiles were generated based on the mean two visit measurements (visit 1 and 2). For individuals who did not attend visit 2 (or were censored before the visit), their visit 1 serum Mg measurement was used for the purposes of ranking. For dietary Mg, visit 1 and visit 3 measurements were used to generate the ranked quintiles.
Adjusted hazard ratios (HRs) and their 95% confidence interval (CI) for the association of serum Mg with incident AF were calculated using Cox proportional hazards regression. Follow-up time was defined as the time between the baseline visit and the date of AF incidence, death, loss to follow-up, or December 31, 2009, whichever came first. The initial model was adjusted for age, sex, race, and ARIC field center site at baseline. The second model was additionally adjusted for other baseline variables including smoking status (current, former, never), drinking status (current, former, never), education level (less than high school, high school graduate, at least some college), diabetes (yes, no), use of antihypertensive medications (angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, other medications, none), and the following continuous variables: body mass index, HDL-c, LDL-c, serum K, serum creatinine, and systolic blood pressure. The third model further adjusted for prevalent MI, HF, or stroke at baseline. Finally, the fourth model was adjusted for incident MI, HF, and stroke as time-dependent covariates and potential mediators of the association between Mg and AF risk. Test for linear trend using the serum quintile number as an ordinal, continuous variable was examined for each model. Effect modification was evaluated by age, sex, and race conducting stratified analysis and including multiplicative terms between the effect modifier and Mg measures in the models. All potential effect modifiers were found to be non-significant to a p-value cutoff of 0.05 although a modest interaction was identified between serum Mg and race (p=0.04). The proportional hazards assumption of the Cox models was tested with the inspection of log-negative log survival curves and was found not to be violated.
Dietary Mg and its association with incident AF was evaluated using the same Cox proportional hazard models. All four models were additionally adjusted for total energy intake (kcal), and models 2 through 4 included adjustment for other dietary covariates that are correlated with Mg intake: K, calcium, dietary fiber, protein, caffeine, and polyunsaturated to saturated fat ratio. All statistical analyses were performed using SAS v 9.2 (SAS Inc, Cary, NC).
Results
At baseline, 7,911 women and 6,379 men aged 45–64 years free of AF met the inclusion criteria. The range for serum Mg levels from both visit 1 and 2 was 0.6 to 3.1 mEq/L and followed a normal distribution with 98% of individuals having serum Mg between 1.2 to 2.0 mEq/L. Dietary Mg intake from both visit 1 and 3 also had a fairly normal distribution that ranged from 41.2 to 807 mg/day and included 98% of individuals being between 101 and 505 mg/day. There was no linear association between serum and dietary Mg levels (Pearson correlation coefficient = 0.04).
Table 1 shows the characteristics of the ARIC participants at baseline by serum Mg quintile. LDL-c, serum K, current drinker, high school degree, and intake of K, Ca, and total energy were all positively associated with serum Mg levels. Many of the risk factors for CVD such as African-American race, diabetes mellitus, systolic blood pressure, use of antihypertensive medications, and a history of CVD were inversely associated with serum Mg levels.
Table 1.
Baseline Characteristics by Serum Magnesium Quintile, Atherosclerosis Risk in Communities, 1987 to 1989
| Serum Magnesium (mEq/L)
|
|||||
|---|---|---|---|---|---|
| <1.55 (n=3118) | 1.55–1.60 (n=2620) | ≥1.60–1.65 (n=3100) | ≥1.65–1.75 (n=2739) | ≥1.75 (n=2713) | |
| Magnesium median, mEq/L | 1.45 | 1.55 | 1.65 | 1.70 | 1.80 |
| Age, y | 54.2 (5.9) | 53.9 (5.8) | 54.1 (5.7) | 54.2 (5.7) | 54.5 (5.7) |
| Females, % | 59.6 | 57.8 | 54.0 | 52.5 | 52.6 |
| African Americans, % | 41.0 | 23.3 | 22.5 | 18.3 | 16.0 |
| Body mass index, kg/m2 | 28.9 (6.2) | 27.5 (5.2) | 27.4 (5.1) | 27.2 (5.0) | 26.8 (4.7) |
| Low density lipoprotein cholesterol, mg/dL | 133.5 (39.7) | 136.6 (38.8) | 139.0 (39.5) | 139.1 (37.9) | 140.9 (39.2) |
| High density lipoprotein cholesterol, mg/dL | 52.1 (18.0) | 52.3 (17.1) | 51.5 (16.4) | 51.9 (16.7) | 52.0 (16.6) |
| Serum potassium, mmol/L | 4.2 (0.4) | 4.3 (0.3) | 4.3 (0.4) | 4.4 (0.4) | 4.4 (0.4) |
| Serum creatinine, mg/dL | 1.1 (0.3) | 1.1 (0.2) | 1.1 (0.2) | 1.1 (0.3) | 1.2 (0.7) |
| Current smoker, % | 27.8 | 24.5 | 26.3 | 24.3 | 25.0 |
| Current drinker, % | 49.2 | 57.0 | 57.2 | 61.4 | 60.5 |
| High school degree, % | 38.6 | 43.2 | 43.2 | 42.5 | 40.0 |
| Diabetes, % | 20.3 | 9.4 | 8.6 | 6.2 | 4.8 |
| Systolic BP*, mmHg | 125.2 (20.3) | 120.2 (17.6) | 120.3 (18.5) | 119.2 (18.2) | 119.0 (17.6) |
| Use of antihypertensive medications, % | 43.0 | 28.0 | 27.4 | 24.3 | 30.0 |
| Previous myocardial infarction, % | 4.9 | 3.4 | 3.5 | 3.8 | 3.6 |
| Previous heart failure, % | 6.6 | 4.1 | 3.9 | 3.2 | 3.2 |
| Previous stroke, % | 2.0 | 1.7 | 1.7 | 2.1 | 1.2 |
| Dietary magnesium, mg/day | 247.1 (87.2) | 252.9 (87.1) | 253.4 (85.7) | 255.9 (88.3) | 257.6 (87.3) |
| Dietary potassium, mg/day | 2596.7 (929.8) | 2646.7 (885.0) | 2641.0 (879.9) | 2665.9 (896.7) | 2687.8 (892.5) |
| Dietary calcium, mg/day | 641.3 (343.8) | 660.9 (342.0) | 657.3 (332.0) | 669.2 (340.4) | 663.7 (340.2) |
| Total energy intake, kcal/day | 1614.1 (573.7) | 1615.4 (550.4) | 1619.6 (547.2) | 1616.5 (557.8) | 1626.7 (553.4) |
BP indicates blood pressure. Values are mean (SD) when appropriate.
Serum Mg and AF risk
Over a median follow-up time of 20.6 years, 1,755 incident AF cases were identified through December 31, 2009. The association of serum Mg with incident AF approximately followed an L-shaped curve, with the highest AF risk among individuals with low serum Mg levels and a lower risk among individuals with average or higher Mg levels. After adjustment for age, race, sex, and field center, individuals in the lowest quintile (<1.55 mEq/L) had approximately a 60% higher risk of AF compared to those in the middle quintile of serum Mg (≥1.60–1.65 mEq/L) (HR, 1.60 [95% CI, 1.39 to 1.84]; table 2, model 1). Additional adjustment for multiple confounders, including a history of CVD (table 2, models 2 and 3) partially attenuated the association (HR of AF comparing the lowest and middle quintile: 1.34 [95% CI, 1.16 to 1.54]). Additional adjustment for incident CVD as a potential mediator further attenuated the association (HR of AF the lowest and middle quintile: 1.24 [95% CI, 1.08 to 1.43]; table 2, model 4). Further adjustment for PR interval provided similar results (data not shown). Results were similar in men and women (figure 1), whites and African Americans (figure 2), and younger and older individuals (figure 3).
Table 2.
Hazard Ratio (HR) and 95% Confidence Interval (CI) of Atrial Fibrillation by Serum Magnesium Quintile, Atherosclerosis Risk in Communities, 1987 to 2009
| Serum Magnesium (mEq/L)
|
P for Trend* | |||||
|---|---|---|---|---|---|---|
| <1.55 (n=3118) | 1.55–1.60 (n=2620) | ≥1.60–1.65 (n=3100) | ≥1.65–1.75 (n=2739) | ≥1.75 (n=2713) | ||
| Cases | 466 | 297 | 351 | 313 | 328 | |
| Person-years | 53,896 | 49,347 | 57,131 | 50,218 | 50,427 | |
| Crude Incidence Rate (per 1000 person-years) | 8.65 | 6.02 | 6.14 | 6.23 | 6.50 | |
| Model 1 HR (95% CI) | 1.60 (1.39–1.84) | 1.02 (0.87–1.19) | 1.00 (Reference) | 0.99 (0.85–1.15) | 1.00 (0.86–1.17) | <0.0001 |
| Model 2 HR (95% CI) | 1.34 (1.16–1.54) | 1.00 (0.85–1.16) | 1.00 (Reference) | 1.04 (0.89–1.21) | 1.05 (0.90–1.22) | 0.005 |
| Model 3 HR (95% CI) | 1.34 (1.16–1.54) | 0.99 (0.85–1.16) | 1.00 (Reference) | 1.04 (0.90–1.22) | 1.06 (0.91–1.23) | 0.008 |
| Model 4 HR (95% CI) | 1.24 (1.08–1.43) | 1.00 (0.85–1.16) | 1.00 (Reference) | 1.10 (0.94–1.28) | 1.10 (0.94–1.28) | 0.26 |
Model 1: Cox proportional hazards model adjusted for age, sex, race, and study site.
Model 2: Model 1 with additional adjustment for body mass index, high density lipoprotein cholesterol, low density lipoprotein cholesterol, serum potassium, serum creatinine, smoking status, drinking status, educational level, systolic blood pressure, diabetes, and antihypertensive medication.
Model 3: Model 2 with additional adjustment for prevalent myocardial infarction, heart failure, or stroke at baseline.
Model 4: Model 3 with additional adjustment for incident myocardial infarction, heart failure, or stroke as time-varying covariates.
Linear trend in quintile number.
Figure 1.
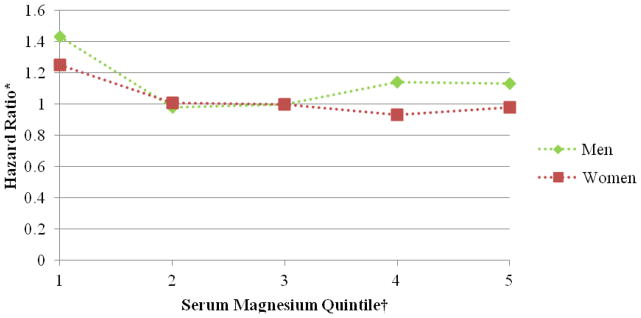
Association between serum magnesium quintile and atrial fibrillation stratified by sex, Atherosclerosis Risk in Communities, 1987 to 2009. *Cox proportional hazards model adjusted for age, sex, race, study site, body mass index, high density lipoprotein cholesterol, low density lipoprotein cholesterol, serum potassium, serum creatinine, smoking status, drinking status, educational level, systolic blood pressure, diabetes, antihypertensive medication, and prevalent myocardial infarction, heart failure, or stroke. †The reference group is the third serum magnesium quintile.
Figure 2.
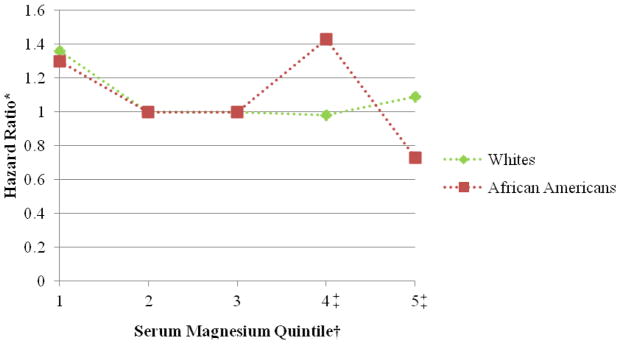
Association between serum magnesium quintile and atrial fibrillation stratified by race, Atherosclerosis Risk in Communities, 1987 to 2009. *Cox proportional hazards model adjusted for age, sex, race, study site, body mass index, high density lipoprotein cholesterol, low density lipoprotein cholesterol, serum potassium, serum creatinine, smoking status, drinking status, educational level, systolic blood pressure, diabetes, antihypertensive medication, and prevalent myocardial infarction, heart failure, or stroke. †The reference group is the third serum magnesium quintile. ‡Please note that there was a reduced total of atrial fibrillation events in the fourth and fifth serum magnesium quintiles among African Americans (52 and 26, respectively), and a modest interaction between serum Mg and race was identified (p=0.04).
Figure 3.
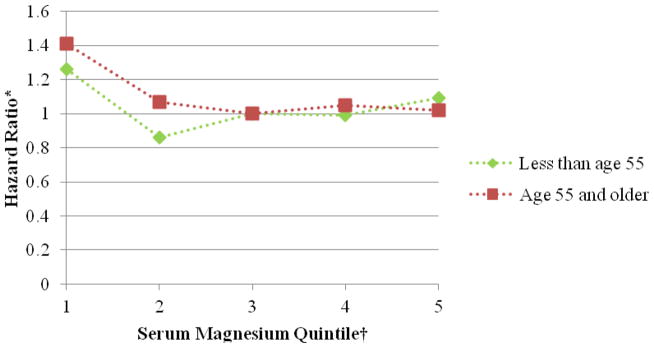
Association between serum magnesium quintile and atrial fibrillation stratified by age, Atherosclerosis Risk in Communities, 1987 to 2009. *Cox proportional hazards model adjusted for age, sex, race, study site, body mass index, high density lipoprotein cholesterol, low density lipoprotein cholesterol, serum potassium, serum creatinine, smoking status, drinking status, educational level, systolic blood pressure, diabetes, antihypertensive medication, and prevalent myocardial infarction, heart failure, or stroke. †The reference group is the third serum magnesium quintile.
Several sensitivity analyses were conducted to examine the association between serum Mg and AF in more detail. First, since most cases were identified through hospitalizations, we repeated the analysis including only the 117 incident AF cases identified by ECGs performed in the 3 ARIC follow-up visits. The adjusted HRs of ECG-defined AF were 1.60 (95% CI, 0.88 to 2.91) and 1.47 (95% CI, 0.81 to 2.68) in those in the first quintile and fifth quintile, respectively, compared to those in the middle quintile (online table A1). Second, because diuretics have been shown to decrease serum Mg concentration, the association between serum Mg and incident AF was examined excluding the 2426 individuals who were using diuretics. These results were similar to the results of the full cohort (online table A2). In the fully-adjusted models, the risk of AF was 27% higher in those in the lowest quintile of serum Mg (HR, 1.27; 95% CI, 1.07 to 1.50) and 11% higher in those in the fifth quintile (HR, 1.17; 95% CI, 0.93 to 1.32) compared to those in the middle quintile. Third, due to an increase in measurement error the further away a Mg measurement is from the AF event, an analysis was performed limited to the first ten years of follow-up. With the occurrence of 526 incident AF cases, the resulting HRs were of similar magnitude to the full follow-up time analysis (results not shown). Fourth, we conducted an additional analysis excluding individuals with hypertension, diabetes, or a history of heart failure or myocardial infarction at baseline to reduce potential residual confounding, and the results remained unchanged (data not shown). In a final analysis to examine the risk of AF among those with the lowest Mg levels in more detail, the lowest serum Mg quintile was divided into two categories (≤1.45 and 1.46–1.54 mEq/L) (online table A3). This analysis resulted in the same association between serum Mg and AF risk, with a higher AF risk among individuals with lower serum Mg.
Dietary Mg and AF risk
Table 3 shows the HRs of AF by quintile of dietary Mg. Overall, there was no association between dietary Mg and incident AF. When compared to those with a dietary Mg intake of 223.2–264.8 mg/day (middle quintile), those with a Mg intake of 320.1 mg/day or more (highest quintile) had a HR of 1.09 (95% CI, 0.86 to 1.36) while those with a Mg intake of less than 180.9 mg/day (lowest quintile) had a HR of 1.02 (95% CI, 0.83 to 1.24) in the multivariable models. No significant interaction was identified between dietary Mg and sex or race.
Table 3.
Hazard Ratio (HR) and 95% Confidence Intervals (CI) of Atrial Fibrillation by Dietary Magnesium Quintile, Atherosclerosis Risk in Communities, 1987 to 2009
| Dietary Magnesium (mg/day)
|
P for Trend* | |||||
|---|---|---|---|---|---|---|
| <180.90 (n=2711) | 180.90–223.16 (n=2711) | 223.17–264.76 (n=2712) | 264.77–320.08 (n=2711) | ≥320.08 (n=2711) | ||
| Cases | 295 | 335 | 315 | 343 | 359 | |
| Person-years | 49,579 | 49,852 | 50,303 | 50,474 | 48,473 | |
| Crude Incidence Rate (per 1000 person-years) | 5.95 | 6.72 | 6.26 | 6.80 | 7.41 | |
| Model 1 HR (95% CI) | 1.09 (0.92–1.30) | 1.10 (0.94–1.28) | 1.00 (Reference) | 1.03 (0.88–1.20) | 1.05 (0.88–1.26) | 0.51 |
| Model 2 HR (95% CI) | 1.07 (0.88–1.31) | 1.10 (0.93–1.29) | 1.00 (Reference) | 1.05 (0.89–1.24) | 1.09 (0.87–1.37) | 0.80 |
| Model 3 HR (95% CI) | 1.10 (0.91–1.34) | 1.12 (0.95–1.32) | 1.00 (Reference) | 1.04 (0.88–1.23) | 1.07 (0.86–1.35) | 0.55 |
| Model 4 HR (95% CI) | 1.02 (0.83–1.24) | 1.04 (0.89–1.23) | 1.00 (Reference) | 1.05 (0.89–1.24) | 1.09 (0.86–1.36) | 0.82 |
Model 1: Cox proportional hazards model adjusted for age, sex, race, study site, and total energy intake.
Model 2: Model 1 with additional adjustment for body mass index, high density lipoprotein cholesterol, low density lipoprotein cholesterol, smoking status, drinking status, educational level, systolic blood pressure, diabetes, antihypertensive medication, and intake of potassium, calcium, dietary fiber, protein, caffeine, and polyunsaturated to saturated fat ratio.
Model 3: Model 2 with additional adjustment for prevalent myocardial infarction, heart failure, or stroke at baseline.
Model 4: Model 3 with additional adjustment for incident myocardial infarction, heart failure, or stroke as time-varying covariates.
Linear trend in quintile number.
Discussion
In this prospective analysis of the ARIC cohort, an L-shaped association between serum Mg and incident AF was identified, with the highest risk of AF in individuals at the low end of the serum Mg distribution range and a lower risk at more normal and elevated levels. This association was evident even after adjustment for the most important risk factors of AF and possible mediators of the serum Mg-AF relationship. The results did not differ significantly by race, sex, or age. Despite the association between serum Mg and AF, dietary Mg was not associated with the risk of AF, which may be due to limitations associated with the food frequency questionnaire being a less accurate measurement yielding a higher degree of exposure misclassification. Serum Mg levels also reflect intracellular levels and renal handing of whole-body Mg, and these factors may have a stronger effect on serum Mg than dietary Mg intake, especially since only some dietary Mg is absorbed during digestion. Overall, our results are consistent with previous analyses conducted on the ARIC cohort that have found an association of different cardiometabolic outcomes with serum Mg but not with dietary Mg. 9,10,12–14
Although this is the first study to report an association between serum Mg and AF in the general population to our knowledge, numerous studies, including randomized trials, have assessed the effect of intravenous administration of Mg on prevention of AF in a post-operative setting. However, the findings are inconclusive,19,31 and the potential effect mechanism is still not entirely understood.19–23 Intracellular Mg is frequently depleted after surgery,32,33 and this reduction in Mg has been linked to an increase in supraventricular and ventricular arrhythmias, including AF.34 Kaplan et al. have suggested that the decrease in Mg may be linked to an increase in the sensitivity of the atrial myocardium and the increased possibility of arrhythmias.23 Indeed, intravenous administration of Mg prolongs sinoatrial node conduction time, atrioventricular conduction and atrial refractoriness,35,36 which potentially lowers the risk of AF. Mg also plays a role in cell membrane stabilization, modulation of calcium and potassium channels, and acts as a cofactor in numerous enzymatic reactions.37
Some potential limitations related to the exposure in this study should be noted. First, serum Mg only accounts for less than 1% of all Mg in the body38 and the correlation coefficient of 0.46 between visit 1 and visit 2 serum Mg suggests that there may be a large, intraindividual variation of serum Mg concentration. However, there is moderate correlation (r=0.54) between serum Mg level and the intracellular free Mg level.39 Both circumstances likely lead to non-differential misclassification of serum Mg exposure. We expect this misclassification to bias the results toward the null and possibly underestimate the true association. Second, because the use of diuretics has been shown to reduce not only blood pressure but also serum Mg concentration,37,40 hypertension may be a potential confounder between serum Mg and AF incidence. However, in a sub-analysis excluding individuals on diuretics, the association between serum Mg and incident AF risk remained unchanged. Third, serum Mg was only measured during early follow-up; therefore, this may not be reflective of usual serum Mg or of the level immediately preceding an AF event, which may be more relevant as a predictor of AF. In an analysis restricted to the first 10 years of follow-up, though, results were similar to those for the entire period.
In addition, the ascertainment of AF in the ARIC cohort was not without limitations. The majority of AF cases were identified through hospitalization discharges, which does not account for asymptomatic AF or AF cases managed in outpatient settings. As a result, there may be an underascertainment of incident AF cases. To examine the differential ascertainment of AF, a sensitivity analysis including AF cases identified from study ECGs of all study participants was performed and showed an association between serum Mg and AF of a similar magnitude. Also, the ascertainment of AF cases through hospitalizations in ARIC and other cohorts has shown acceptable validity. 3,41 Finally, AF incidence rates in the ARIC cohort are fairly consistent with other cohort studies, further supporting the case ascertainment validity.3
Unmeasured or residual confounding could partly explain the observed association. Individuals with low levels of serum Mg were more likely to be hypertensive, diabetic or present a previous history of cardiovascular disease, all established risk factors for AF. However, we adjusted for these covariates and others in the multivariable analysis, making confounding a less likely explanation for our results. Also, in a sensitivity analysis, the results remained unchanged even after excluding individuals with hypertension, diabetes, or a history of heart failure or myocardial infarction at baseline.
Despite these limitations, some important strengths related to our analysis should be highlighted: the large, biracial sample with a long follow-up time, a large number of AF events, and the detailed measurement of key covariates in this cohort.
Conclusion
Overall, this study has identified an association between low serum Mg levels and higher AF risk but no association between dietary Mg and incidence of AF. Future studies are needed to examine the specific mechanisms underlying this association and to explore the effect of changes in serum Mg levels over time on AF risk.
Supplementary Material
Acknowledgments
Sources of funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C from the NHLBI, Bethesda, Maryland. This study was additionally funded by grants 09SDG2280087 from the American Heart Association (AHA) in Dallas, Texas, and RC1-HL099452 and R01-HL103706S1 from the National Institutes of Health. This research was conducted while Dr. Chen was a recipient of a Scientist Development Grant (10SDG3420031) from the AHA.
We thank the staff and participants of the ARIC study for their important contributions.
Footnotes
The authors have reported that they have no relationships to disclose.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort, The Framingham Heart Study. JAMA. 1994;271(11):840–844. [PubMed] [Google Scholar]
- 3.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2009;158(1):111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang TJ, Parise H, Levy D, D’Agostino RB, Sr, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Chen P-S, Bild DE, Mascette AM, Albert CM, Alonso A, et al. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2009;119(4):606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;159(5):850–856. doi: 10.1016/j.ahj.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108(24):3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 8.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2011;123(14):1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peacock JM, Folsom AR, Arnett DK, Eckfeldt JH, Szklo M. Relationship of serum and dietary magnesium to incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 1999;9(3):159–165. doi: 10.1016/s1047-2797(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 10.Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 1998;136(3):480–490. doi: 10.1016/s0002-8703(98)70224-8. [DOI] [PubMed] [Google Scholar]
- 11.Ma J, Folsom AR, Melnick SL, Eckfeldt JH, Sharrett AR, Nabulsi AA, et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol. 1995;48(7):927–940. doi: 10.1016/0895-4356(94)00200-a. [DOI] [PubMed] [Google Scholar]
- 12.Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Arch Intern Med. 1999;159(18):2151–2159. doi: 10.1001/archinte.159.18.2151. [DOI] [PubMed] [Google Scholar]
- 13.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk of Communities (ARIC) Study. Am Heart J. 2010;160(3):464–470. doi: 10.1016/j.ahj.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohira T, Peacock JM, Iso H, Chambless LE, Rosamond WD, Folsom AR. Serum and dietary magnesium and risk of ischemic stroke: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2009;169(12):1437–1444. doi: 10.1093/aje/kwp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y, Manson JE, Buring JE, Liu S. Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care. 2004;27(1):59–65. doi: 10.2337/diacare.27.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Song Y, Sesso HD, Manson JE, Cook NR, Buring JE, Liu S. Dietary magnesium intake and risk of incident hypertension among middle-aged and older US women in a 10-year follow-up study. Am J Cardiol. 2006;98(12):1616–1621. doi: 10.1016/j.amjcard.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 17.Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr. 2012;95(2):362–366. doi: 10.3945/ajcn.111.022376. [DOI] [PubMed] [Google Scholar]
- 18.Kiziltepe U, Eyileten ZB, Sirlak M, Tasoz R, Aral A, Eren NT, et al. Antiarrhythmic effect of magnesium sulfate after open heart surgery: effect on blood levels. Int J Cardiol. 2003;89:153–158. doi: 10.1016/s0167-5273(02)00449-7. [DOI] [PubMed] [Google Scholar]
- 19.Miller S, Crystal E, Garfinkle M, Lau C, Lashevsky I, Connolly SJ. Effects of magnesium on atrial fibrillation after cardiac surgery: a meta analysis. Heart. 2005;91(5):618–623. doi: 10.1136/hrt.2004.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhart K, Baker WL, Siv ML. Review: Beyond the Guidelines: New and novel agents for the prevention of atrial fibrillation after cardiothoracic surgery. J Cardiovasc Pharmacol Ther. 2011;16(1):5–13. doi: 10.1177/1074248410378120. [DOI] [PubMed] [Google Scholar]
- 21.Koniari I, Apostolakis E, Rogkakou C, Baikoussis NG, Dougenis D. Pharmacologic prophylaxis for atrial fibrillation following cardiac surgery: a systematic review. J Cardiothorac Surg. 2010;5:121. doi: 10.1186/1749-8090-5-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho KM, Lewis JP. Prevention of atrial fibrillation in cardiac surgery: time to consider a multimodality pharmacological approach. Cardiovasc Ther. 2010;28(1):59–65. doi: 10.1111/j.1755-5922.2009.00117.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan M, Kut MS, Icer UA, Demirtas MM. Intravenous magnesium sulfate prophylaxis for atrial fibrillation after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2003;125(2):344–352. doi: 10.1067/mtc.2003.108. [DOI] [PubMed] [Google Scholar]
- 24.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 25.Soliman EZ, Prineas RJ, Case D, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2009;40(4):1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 27.National Heart, Lung, and Blood Institute. Operations Manual No 10. Bethesda: National Heart, Lung, and Blood Institute; 1987. Atherosclerosis Risk in Communities (ARIC) Study: Clinical chemistry determinations; pp. 25–28. [Google Scholar]
- 28.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118(5):496–500. [PubMed] [Google Scholar]
- 29.National Heart, Lung, and Blood Institute. Operations Manual No 8. Bethesda: National Heart, Lung, and Blood Institute; 1987. Atherosclerosis Risk in Communities (ARIC) Study: Lipid and lipoprotein determination. [Google Scholar]
- 30.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 31.Cook RC, Humphries KH, Gin K, Janusz MT, Slavik RS, Bernstein V, et al. Prophylactic intravenous magnesium sulphate in addition to oral β-blockade does not prevent atrial arrhythmias after coronary artery or valvular heart surgery: a randomized, controlled trial. Circulation. 2009;120:S163–169. doi: 10.1161/CIRCULATIONAHA.108.841221. [DOI] [PubMed] [Google Scholar]
- 32.Aglio LS, Stanford GG, Maddi R, Boyd JL, 3rd, Nussbaum S, Chernow B. Hypomagnesaemia is common following cardiac surgery. J Cardiothrorac Vasc Anesth. 1991;5(3):201–208. doi: 10.1016/1053-0770(91)90274-w. [DOI] [PubMed] [Google Scholar]
- 33.Booth JV, Phillips-Bute B, McCants CB, Podgoreanu MV, Smith PK, Mathew JP, et al. Low serum magnesium level predicts major adverse cardiac events after coronary artery bypass great surgery. Am Heart J. 2003;145(6):1108–1113. doi: 10.1016/S0002-8703(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 34.Zaman AG, Alamgir F, Richens T, Williams R, Rothman MT, Mills PG. The role of signal averaged P wave duration and serum magnesium as a combined predictor of atrial fibrillation after elective coronary artery bypass surgery. Heart. 1997;77(6):527–531. doi: 10.1136/hrt.77.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulick DL, Hong R, Ryzen E, Rude RK, Rubin JN, Elkayam U, et al. Electrophysiologic effects of intravenous magnesium in patients with normal conduction systems and no clinical evidence of significant cardiac disease. Am Heart J. 1988;115(2):367–373. doi: 10.1016/0002-8703(88)90483-8. [DOI] [PubMed] [Google Scholar]
- 36.Christiansen EH, Frost L, Andreasen F, Mortensen P, Thomsen PE, Pedersen AK. Dose-related cardiac electrophysiological effects of intravenous magnesium. A double-blind placebo-controlled dose-response study in patients with paroxysmal supraventricular tachycardia. Europace. 2000;2(4):320–326. doi: 10.1053/eupc.2000.0123. [DOI] [PubMed] [Google Scholar]
- 37.Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S. Protective role of magnesium in cardiovascular diseases: a review. Mol Cell Biochem. 2002;238:163–179. doi: 10.1023/a:1019998702946. [DOI] [PubMed] [Google Scholar]
- 38.Elin RJ. Assessment of magnesium status. Clin Chem. 1987;33(11):1965–1970. [PubMed] [Google Scholar]
- 39.Ryzen E, Servis KL, DeRusso P, Kershaw A, Stephen T, Rude RK. Determination of intracellular free magnesium by nuclear magnetic resonance in human magnesium deficiency. J Am Coll Nutr. 1989;8(6):580–587. doi: 10.1080/07315724.1989.10720330. [DOI] [PubMed] [Google Scholar]
- 40.Altura BM, Altura BT. Role of magnesium in the pathogenesis of hypertension: relationship to its actions on cardiac and vascular smooth muscle. New York, NY: Raven Press; 1990. [Google Scholar]
- 41.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


