Abstract
The steady increase in the incidence and mortality of hepatocellular carcinoma (HCC) signifies a crucial need to understand better its pathogenesis to improve clinical management and prevention of the disease. The aim of this study was to investigate molecular mechanisms for the chemopreventive effects of folic acid and tributyrin alone or in combination on rat hepatocarcinogenesis. Male Wistar rats were subjected to a classic “resistant hepatocyte” model of liver carcinogenesis and treated with folic acid and tributyrin alone or in combination for 5 weeks during promotion stage. Treatment with folic acid and tributyrin alone or in combination strongly inhibited the development of glutathione-S-transferase placental form (GSTP)-positive foci. Microarray analysis showed significant changes in gene expression. A total of 501, 655, and 940 of differentially expressed genes, involved in cell cycle, p53-signaling, angiogenesis, and Wnt pathways, was identified in the livers of rats treated with folic acid, tributyrin or folic acid and tributyrin. A detailed analysis of these differentially expressed genes revealed that treatments inhibited angiogenesis in the preneoplastic livers. This was evidenced by the fact that 30 out of 77 differentially expressed genes common to all three treatments are involved in the regulation of the angiogenesis pathway. The inhibition of angiogenesis was confirmed by reduced levels of CD34 protein. In conclusion, the tumor-suppressing activity of folic acid and tributyrin is associated with inhibition of angiogenesis at early stages of rat liver carcinogenesis. Importantly, the combination of folic acid and tributyrin has stronger chemopreventive effect than each of the compounds alone.
Keywords: Hepatocarcinogenesis, chemoprevention, angiogenesis, folic acid, tributyrin
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent life-threatening human cancers and the incidence is rapidly increasing globally.1–5 Despite significant progress in clinical management of HCC, it remains the third greatest cause of cancer-related deaths worldwide.4,6 It is widely-believed that prevention of cancer is the most promising strategy for reducing both cancer incidence and cancer-related mortality.7 Currently, the prevention of HCC focuses on eliminating of exposure to liver carcinogens, e.g., aflatoxin B1, and vaccinating against hepatitis B virus infection. This approach has substantially reduced the incidence of HCC associated with these risk factors; however, there is no effective strategy to inhibit hepatocarcinogenesis once preneoplastic lesions are established.6
One promising approach to cancer prevention is an active intervention with agents that are expected to suppress or attenuate the initial phases of carcinogenesis and/or the progression of premalignant lesions to full-fledged tumors.7,8 In previous studies, using a classic “resistant hepatocyte” model of liver carcinogenesis in rats, we demonstrated a potent chemopreventive effect of tributyrin, a butyric acid prodrug, and folic acid on hepatocarcinogenesis.9,10 The cancer-suppressing effect of tributyrin and folic acid has been linked to the ability of these agents to induce apoptotic cell death and impede cell proliferation, especially in preneoplastic GSTP-positive hepatic foci, resulting in inhibition of their clonal expansion.9–12
The efficiency of cancer prevention strategies relies on a better understanding of molecular and cellular biology of carcinogenesis, and the identification of molecular targets to inhibit the carcinogenic process.7,8 In this regard, the mechanisms of the anticancer activities of folic acid and tributyrin are only partially understood. Furthermore, in the field of cancer chemoprevention research, there is a great need to investigate combination strategies in addition to single agent approaches.8 In view of this, the goals of the present study were (a) to investigate the underlying molecular mechanisms associated with the chemopreventive activity of folic acid and tributyrin, or their combination on rat liver carcinogenesis when administered during the promotion stage of the hepatocarcinogenic process; and (b) determine, whether or not this chemopreventive effect can be achieved at the lower doses of folic acid and tributyrin.
Material and Methods
Animals and experimental design
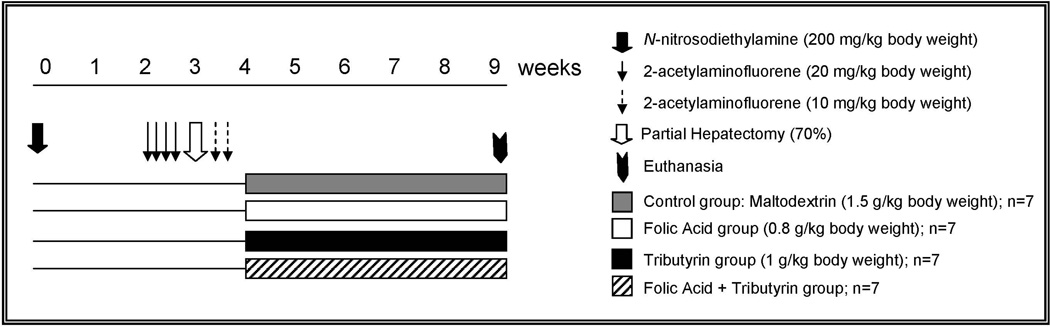
Male Wistar rats (50 g) were obtained from the Faculty of Pharmaceutical Sciences of the University of São Paulo (São Paulo, Brazil) breeding facility, housed in a temperature-controlled (24°C) room, with a 12-h light-dark cycle, and given ad libitum access to water and commercially prepared diet (Purina Nutrimentos Ltda, Paulinia, Brazil). Twenty eight rats were subjected to a “resistant hepatocyte” model of hepatocarcinogenesis.13 Briefly, the rats received a single intraperitoneal injection of N-nitrosodiethylamine (DEN; Sigma-Aldrich, St Louis, MO; 200 mg/kg body weight; dissolved in 0.9% of NaCl) to initiate hepatocarcinogenesis. After a recovery period of 2 weeks, the rats were gavaged with 2-acetylaminofluorene (2-AAF; Sigma-Aldrich; 20 mg/kg body weight; dissolved in corn oil) for four consecutive days, and then subjected to a partial hepatectomy. Two and four days after the partial hepatectomy, the rats were gavaged with 2-AAF (10 mg/kg body weight). One week after the partial hepatectomy, the rats were allocated randomly to control and three experimental groups. Rats (n = 7) in the experimental groups were treated by gavage with folic acid (0.8 g/kg body weight), tributyrin (1 g/kg body weight), or combination of folic acid and tributyrin (same folic acid and tributyrin doses) daily for 5 weeks. These doses of folic acid and tributyrin were two times lower from those used in previous studies.9–12 Rats (n = 7) in control group were subjected to the “resistant hepatocyte” model of carcinogenesis only (Figure 1). Rats in control and folic acid groups received maltodextrin (Nestlé, São Paulo, Brazil; 1.5 g/kg body weight) at the isocaloric dose to tributyrin group. All experimental procedures were performed in accordance with an animal study protocol approved by the Faculty of Pharmaceutical Sciences of the University of São Paulo Ethics Committee for Animal Research (Protocol number 320).
Figure 1. Experimental design.
Sample collection and biochemical measurements
Rats from control and experimental groups were euthanized by exsanguination under light ether anesthesia 9 weeks after DEN initiation. The livers were excised and a slice of each lobe was fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin, sectioned at 5 μm, and mounted on a glass slide for histopathological and immunohistochemical evaluations. The remaining liver was frozen immediately in liquid nitrogen and stored at −80°C for subsequent analyses.
Folic acid concentrations in serum were determined by a standard microbiological microtiter assay.14 The content of butyric acid, a tributyrin metabolite, in liver tissue extracts was determined using gas chromatography/mass spectrometry as described by Su et al.15
Immunohistochemistry and image analysis
The status of GSTP expression and the extent of cell proliferation in the livers of control and experimental rats were assessed by a double immunostaining technique. Briefly, formalin-fixed paraffin-embedded liver sections were deparaffinized, rehydrated, and immunostained with anti-proliferating cell nuclear antigen (PCNA) antibody (1:200; Dako, Glostrup, Denmark) followed by immunostaining for anti-GSTP antibody (1:500, Medical and Biological Laboratories, Tokyo, Japan), as previously described.9
Immature vessels and/or vessels undergoing angiogenesis were detected by CD34 staining.16 Liver sections were deparaffinized, rehydrated, and immunostained with anti-CD34 antibody (1:100; R&D Systems, Minneapolis, USA). All CD34 positive vessels in each liver section were counted. All sections were examined by light microscopy (Axio Imager 2, Carl Zeiss, Jena, Germany); AxioVision LE 4.8.2.0 digital image processing software (Carl Zeiss) was used for quantification. The image analysis was conducted by pathologists blinded to the treatments. All immunohistochemical analyses were conducted in duplicate and the experiments were repeated twice.
Apoptosis evaluation
Liver sections stained with hematoxylin and eosin were analyzed for the presence of apoptotic bodies as described previously,17 based on the method of Stinchcombe et al.18
RNA extraction and gene expression analysis using microarray technology
Total RNA was extracted from liver tissue using RNeasy Mini kits (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The gene expression profiles in the livers of control male Wistar rats and those treated with folic acid, tributyrin, or the combination of folic acid and tributyrin groups were determined utilizing Agilent whole genome 4×44K rat microarrays (Agilent Technologies, Santa Clara, CA). Sample labeling and microarray processing were performed as detailed in the "One-Color Microarray-Based Gene Expression Analysis" Version 5.5 (Agilent Technologies) protocol. The hybridized slides were scanned with an Agilent DNA Microarray scanner (Agilent Technologies) at 5 µm resolution. The resulting images were analyzed by determining the Cy3 fluorescence intensity of all gene spots (features) on each array using the Agilent Feature Extraction Software (Version 10.7). The raw data were then uploaded into the ArrayTrack database.19 The median fluorescence intensity of all the pixels within one feature was taken as the intensity value for that feature. The raw intensity values were then normalized using 75 percentile channel scaling normalization within ArrayTrack. A list of differentially expressed genes was generated using a Student’s t-test at P-value < 0.05 and a fold change > 1.5.
Functional analysis of significant genes
The Kyoto Encyclopedia of Genes and Genomes (KEGG) database20 and Ingenuity Pathway Analysis software (IPA, IPA version 9.0; Ingenuity Systems, Redwood City, CA) were used to determine pathways that were enriched for the significant mRNA transcripts identified from the t-test analysis using ArrayTrack. Significance values were calculated based on a right-tailed Fisher’s exact test that determined whether a pathway was overrepresented by calculating whether or not the genes in a given pathway were enriched within the dataset compared to all genes on the array in the same pathway; P < 0.05 was selected as the cutoff for significance based on KEGG and IPA threshold recommendations. Only those pathways with a P-value above the threshold and having more than three representative genes in the dataset were considered to be significant.
Western blot
Total liver tissue lysates were prepared by homogenization of 30 mg of tissue in 500 μl of lysis buffer (50 mM Tris-HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM PMSF; 1 μg per ml each of aprotinin, leupeptin, and pepstatin; 1 mM Na3VO4; and 1 mM NaF) and incubation at 4°C for 30 min, followed by centrifugation at 10,000 × g at 4°C for 20 min. The protein level was measured using the Bio-Rad Protein Assay kits (Bio-Rad Laboratories, Hercules, CA). Extracts containing equal quantities of proteins were separated by SDS-PAGE on 7% polyacrylamide gels and transferred to PVDF membranes. Membranes were probed with primary antibody against CD34 (1:1000; R&D Systems). Alkaline phosphatase-coupled donkey anti-goat antibodies (Santa Cruz Biotechnology) were used for visualization. Images are representative of three independent immunoblots. Signals were quantified using ImageQuant 5.1 Software (Molecular Dynamics, Sunnyvale, CA). The western blot experiments were repeated twice.
Statistical analyses
Results are presented as mean ± S.D. Data were analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s test for pair-wise comparisons. When appropriate, Student’s t-test or Fisher’s exact test was used. P-values < 0.05 were considered significant.
Results
Concentrations of folic acid and butyric acid
Treatment of rats with folic acid alone or in combination with tributyrin during the promotion stage of hepatocarcinogenesis resulted in a significant increase, 2.9- and 2.5-fold, respectively, in the levels of folic acid in serum as compared to control rats (Supplementary Figure 1A). Similarly, the hepatic content of butyric acid in rats treated with tributyrin alone or in combination with folic acid was 5.1- and 3.2-times greater than in the control rats (Supplementary Figure 1B).
Histopathological analysis of preneoplastic livers
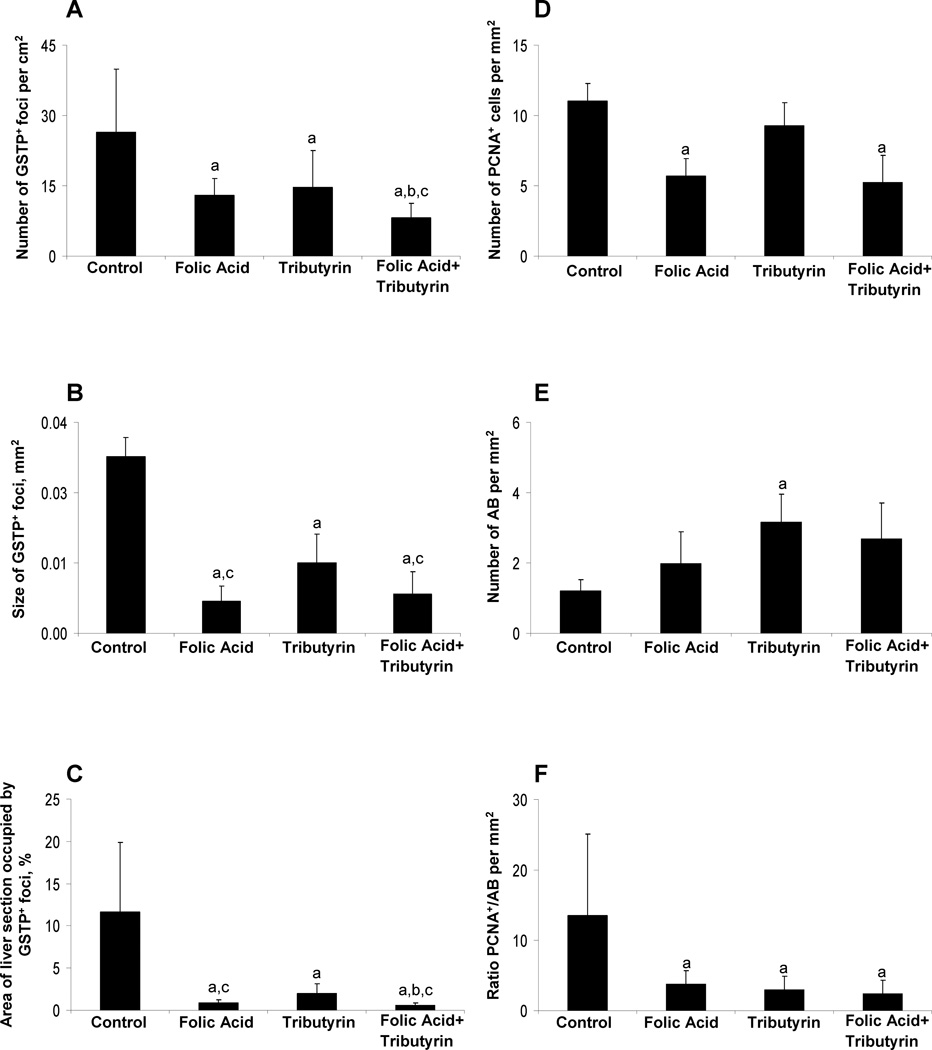
The formation of altered GSTP-positive hepatic foci is a well-accepted end-point indicator of rat liver carcinogenesis.21,22 Immunohistochemical staining of liver sections of control rats revealed the presence of large uniformly stained GSTP-positive foci that were evenly distributed throughout the entire section of the liver (Supplementary Figure 2). In the livers of rats treated with folic acid, tributyrin, or a combination of folic acid and tributyrin during the promotion stage of liver carcinogenesis, the number (Figure 2A) and size of GSTP-positive foci (Figure 2B and Supplementary Figure 2) and the area of liver section occupied by them (Figure 2C and Supplementary Figure 2) were significantly smaller than in control rats. In animals treated with folic acid alone, the size and area of liver section occupied by GSTP-positive foci were smaller as compared to animals treated with tributyrin. Importantly, the combined treatment with folic acid and tributyrin reduced the number (Figure 2A) and the area (Figure 2C) of the liver sections occupied by GSTP-positive foci, as compared to rats treated with folic acid or tributyrin only, and the size (Figure 2B) as compared to rats treated with tributyrin only.
Figure 2. Histopathological changes in the in the livers of control rats and rats treated with folic acid, tributyrin, or the combination of folic acid and tributyrin.
(A) Morphometric analysis of GSTP-positive foci in the livers. The numbers of GSTP-positive foci were evaluated in liver sections using a two-dimensional approach. The data are presented as an average number of foci per cm2 of the liver. (B) Cell proliferation and apoptotic cell death in the livers. PCNA-positive cells and apoptotic bodies (AB) were counted as described in “Material and methods” and labeling indices were expressed per mm2 of the liver. a - Significantly different from control rats; b –significantly different from rats treated with folic acid only; c – significantly different from rats treated with tributyrin only; n = 7, means ± S.D.
A double-labeling immunohistochemical staining approach was used to examine the extent of cell proliferation in the persistent GSTP-positive foci in the livers of control and experimental rats. Treatment of rats with folic acid alone or in combination with tributyrin significantly reduced the magnitude of cell proliferation as compared to that in the livers of control rats. This was evidenced by an approximately 50% decrease in the number of PCNA-stained hepatocyte nuclei in S-phase in the GSTP-positive foci in the livers of rats treated with folic acid alone or in combination with tributyrin (Figure 2D). Interestingly, treatment of rats undergoing hepatocarcinogenesis with tributyrin alone did not affect the extent of cell proliferation. In contrast, tributyrin significantly induced apoptosis in the GSTP-positive foci, while there was no difference in apoptotic cell death in the livers of rats treated with folic acid alone or in combination of tributyrin (Figure 2E). Nevertheless, treatment of rats with folic acid, tributyrin, or a combination of folic acid and tributyrin resulted in a marked 80% reduction of a ratio between cell proliferation and apoptosis, as compared to control rats (Figure 2F).
Microarray gene expression analysis
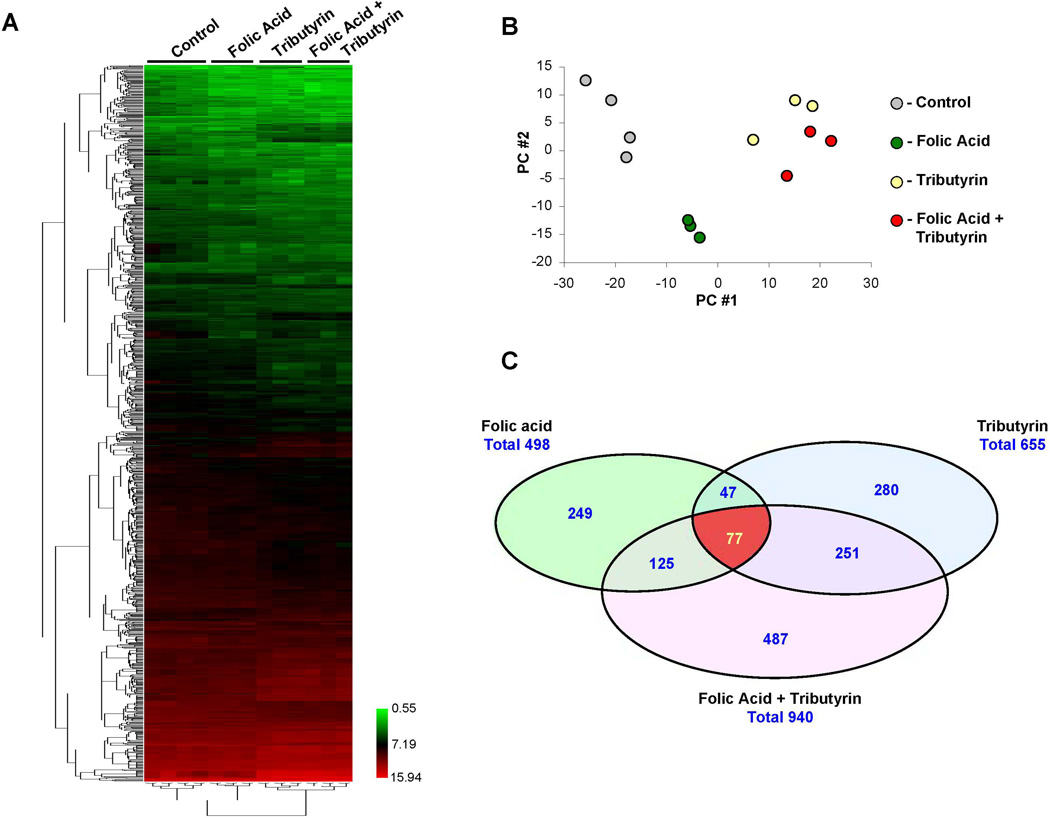
To determine the mechanistic basis of the chemopreventive effects of folic acid, tributyrin, or the combination of folic acid and tributyrin on liver carcinogenesis, hepatic gene expression profiles were examined using high-throughput Agilent whole genome 4×44K rat microarrays. An unsupervised hierarchical clustering of the gene expression data showed that each treatment could be distinguished by its hepatic gene expression profile (Figure 3A). The tight clustering of samples within each group (Figure 3B) indicated high quality data that would allow subtle differences in gene expression to be identified.
Figure 3. Whole genome microarray analysis of gene expression in the livers of control rats and rats treated with folic acid, tributyrin, or the combination of folic acid and tributyrin.
(A) Heat map illustrating differences in global gene expression among control rats and rats treated with folic acid, tributyrin, or combination of folic acid and tributyrin. The color bar identifies high-expressed (red) and low-expressed (green) genes. (B) Principal component analysis illustrating similarities within groups, as evidenced by tight clustering of samples within groups, and differences among control rats (n =4) and rats treated with folic acid, tributyrin, or the combination of folic acid and tributyrin (n =3). (C) Venn diagram showing significant differences among control rats and rats treated with folic acid, tributyrin, or the combination of folic acid and tributyrin.
Principal component (PC) analysis utilizing the entire gene expression dataset showed the relatively tight clustering of each treatment group and the clear separation of control rats from rats in experimental groups (Figure 3B). Interestingly, the profiles of rats treated with tributyrin alone or in combination with folic acid clustered differently from rats treated with folic acid only.
To identify genes that were differentially expressed between the control and the each of the experimental groups, a t-test P < 0.05 coupled with a fold-change cut-off > 1.5 was applied. A total of 498, 655, and 940 genes was found to be differentially expressed in the livers of rats treated with folic acid, tributyrin, or the combination of folic acid and tributyrin, respectively (Figure 3C). Similarly to the PC analysis, gene expression results (Figure 3C) and the pathway enrichment analysis of differentially expressed genes (Figure 4) clearly indicate that folic acid and tributyrin exert their chemopreventive activity on rat hepatocarcinogenesis via different mechanisms. This was evidenced by the fact that among 498 and 655 differentially expressed individual genes affected by either folic acid or tributyrin treatments, respectively, only 124 genes were common for both agents (Figure 3C). A combined treatment of rats undergoing liver carcinogenesis with folic acid and tributyrin substantially increased the number of differentially expressed genes in the livers. Specifically, the number of differentially expressed genes in this experimental group was 1.89 and 1.44 times greater, respectively, than in rats treated with folic acid or tributyrin alone (Figure 3C).
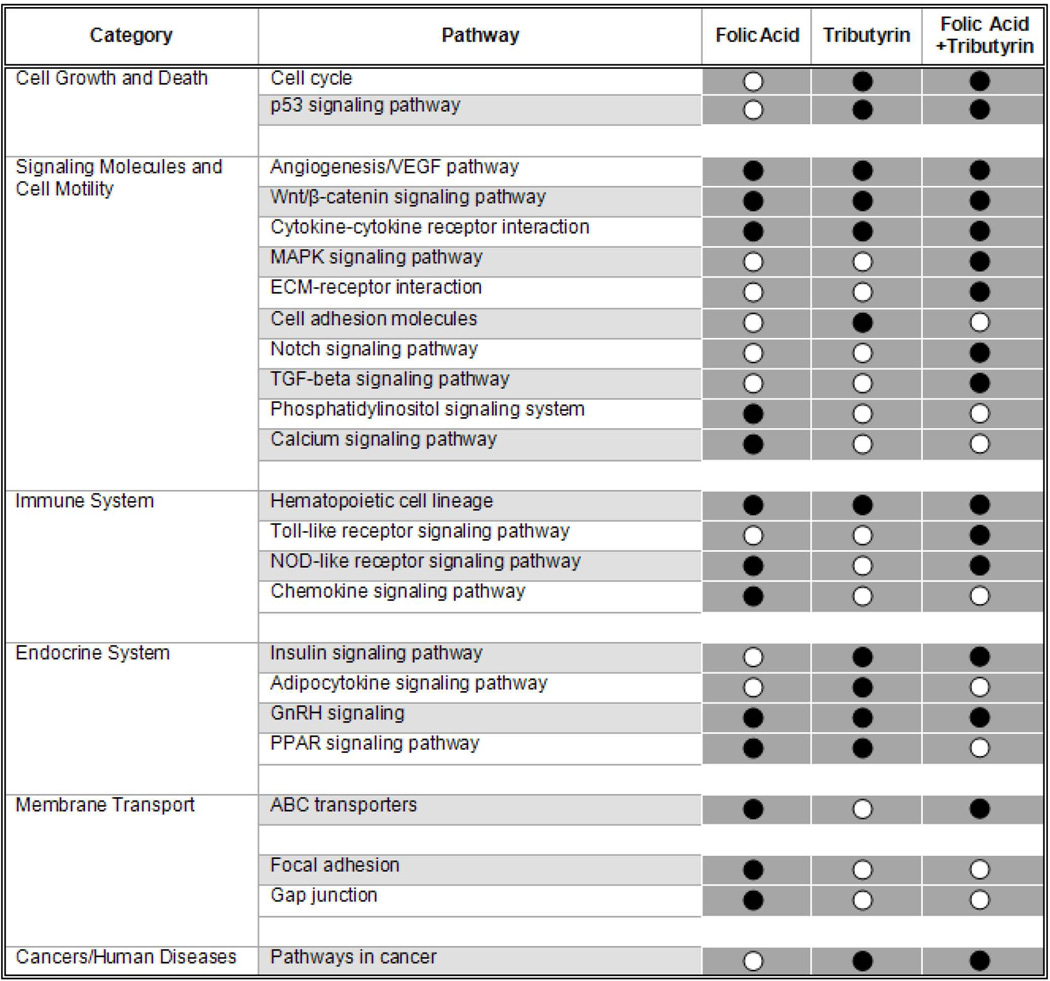
Figure 4. Summary of molecular pathways affected by folic acid, tributyrin or combined folic acid and tributyrin treatments.
Whether the percentage of genes with an altered expression in a certain pathway differed from the percentage of altered genes not represented in the pathway was tested by Fisher’s exact test. KEGG pathways with at least three differentially regulated genes and a P-value of <0.05 were considered “enriched”. Each grey square represent a significant enrichment (black circuits) or under-representation (white circuits) of differentially regulated genes of the particular pathway in the analyzed data set.
Effect of tributyrin and folic acid treatment on angiogenesis
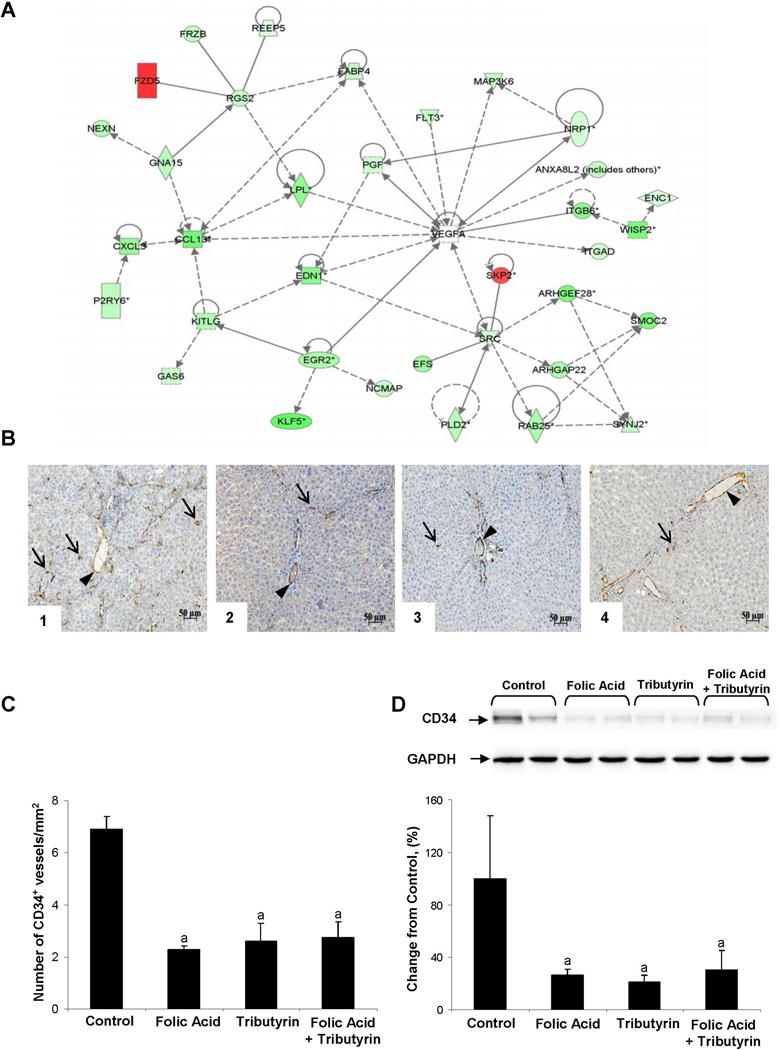
Despite the noticeable difference in the number of differentially expressed genes in experimental groups, there were 77 genes common to all three treatment groups (Figure 3C). The pattern of common changes in gene expression shows a prominent treatment-related down-regulation of gene expression. Pathway enrichment analysis of common genes demonstrated a strong enrichment in genes involved in Wnt/β-catenin and angiogenesis pathways (Table 1). Interestingly, the greatest effect of folic acid, tributyrin, or the combined folic acid and tributyrin treatment was on the angiogenesis pathway. This was evidenced by the fact that 30 out of 77 (39%) common differentially expressed genes are involved in the regulation of the angiogenesis signaling network (Table 1, Figure 5A).
Table 1.
Expression of common genes in the livers of rats treated with folic acid, tributyrin, or a combination of folic acid and tributyrin (p<0.05).
| # | Gene name | Gene bank ACC# | Description | Fold change | ||
|---|---|---|---|---|---|---|
| Folic acid | Tributyrin | Folic acid +tributyrin |
||||
| 1 | Sctra,b | NM_031115 | Secretin receptor | −5.65 | −3.55 | −4.00 |
| 2 | Wisp2b | NM_031590 | WNT1 inducible signaling pathway protein 2 | −4.67 | −3.91 | −4.40 |
| 3 | Lpl | NM_012598 | Lipoprotein lipase | −4.27 | −2.62 | −3.96 |
| 4 | Rgnefa | NM_001108542 | Rho-guanine nucleotide exchange factor | −4.06 | −2.70 | −4.05 |
| 5 | Ly49i6 | NM_001009718 | Ly49 inhibitory receptor 6 | −3.72 | −3.27 | −2.65 |
| 6 | Fabp4b | NM_053365 | Fatty acid binding protein 4, adipocyte | −3.69 | −3.06 | −2.27 |
| 7 | Ampd3 | NM_031544 | Adenosine monophosphate deaminase 3 | −3.68 | −3.44 | −3.47 |
| 8 | Ccl2a | NM_031530 | Chemokine (C-C motif) ligand 2 | −3.53 | −4.01 | −4.59 |
| 9 | Lrrcc1 | NM_001100645 | Leucine rich repeat and coiled-coil domain containing 1 | −3.51 | −2.98 | −4.41 |
| 10 | Mgpa | NM_012862 | Matrix Gla protein | −3.50 | −2.45 | −3.33 |
| 11 | Klf5b | NM_053394 | Kruppel-like factor 5 | −3.45 | −2.77 | −4.30 |
| 12 | Efs | NM_001106033 | Embryonal Fyn-associated substrate | −3.32 | −2.74 | −3.61 |
| 13 | Itgb6a | NM_001004263 | Integrin, beta 6 | −3.26 | −3.12 | −4.02 |
| 14 | Spdef | NM_001109530 | SAM pointed domain containing ets transcription factor | −3.24 | −3.14 | −3.52 |
| 15 | Rab25a | NM_001107687 | RAB25, member RAS oncogene family | −3.07 | −2.77 | −3.14 |
| 16 | Tmeff2 | NM_001108795 | Transmembrane protein with EGF-like and two follistatin-like domains 2 | −3.03 | −3.01 | −4.15 |
| 17 | Smoc2a | NM_001106215 | SPARC related modular calcium binding 2 | −3.02 | −3.65 | −4.72 |
| 18 | Gcnt3 | NM_173312 | Glucosaminyl (N-acetyl) transferase 3, mucin type | −2.91 | −5.35 | −8.47 |
| 19 | Tlr11 | NM_001144779 | Toll-like receptor 11 | −2.87 | −3.82 | −4.81 |
| 20 | Anxa8a | NM_001031654 | Annexin A8 | −2.87 | −2.15 | −2.39 |
| 21 | Ccdc8 | NM_001009533 | Coiled-coil domain containing 8 | −2.87 | −2.77 | −3.81 |
| 22 | Slpi | NM_053372 | Secretory leukocyte peptidase inhibitor | −2.79 | −2.02 | −2.30 |
| 23 | Clec9a | NM_001109354 | C-type lectin domain family 9, member A | −2.78 | −2.17 | −2.63 |
| 24 | Tns4a | NM_001024881 | Tensin 4 | −2.75 | −2.46 | −4.31 |
| 25 | Edn1a,b | NM_012548 | Endothelin 1 | −2.73 | −3.20 | −3.75 |
| 26 | Stk39 | NM_019362 | Serine threonine kinase 39 | −2.65 | −2.34 | −1.69 |
| 27 | Slco3a1 | NM_177481 | Solute carrier organic anion transporter family, member 3a1 | −2.64 | −1.84 | −2.39 |
| 28 | Flt3a | NM_001100822 | Fms-related tyrosine kinase 3 | −2.57 | −2.01 | −2.35 |
| 29 | Map3k6a,b | NM_001107909 | Mitogen-activated protein kinase kinase kinase 6 | −2.57 | −2.11 | −2.25 |
| 30 | Pacrg | NM_001077677 | Park2 co-regulated | −2.55 | −2.03 | −2.75 |
| 31 | Arhgap22a | NM_001107297 | Rho gtpase activating protein 22 | −2.51 | −2.75 | −3.18 |
| 32 | Kitlga,b | NM_021843 | KIT ligand (Kitlg), transcript variant 1 | −2.49 | −2.34 | −3.00 |
| 33 | Plxdc2 | NM_001108422 | Plexin domain containing 2 | −2.48 | −2.40 | −2.34 |
| 34 | Asb2 | NM_001011984 | Ankyrin repeat and SOCS box-containing 2 | −2.41 | −1.87 | −2.11 |
| 35 | Egr2b | NM_053633 | Early growth response 2 | −2.35 | −2.40 | −2.76 |
| 36 | Mmp24a,b | NM_031757 | Matrix metallopeptidase 24 | −2.26 | −1.82 | −2.75 |
| 37 | Bcl2l14 | NM_001024338 | Bcl2-like 14 (apoptosis facilitator) | −2.21 | −2.60 | −2.32 |
| 38 | Cxcl2a | NM_053647 | Chemokine (C-X-C motif) ligand 2 | −2.18 | −2.39 | −4.02 |
| 39 | St14 | NM_053635 | Suppression of tumorigenicity 14 (colon carcinoma) | −2.13 | −1.98 | −2.16 |
| 40 | P2ry6 | NM_057124 | Pyrimidinergic receptor P2Y, G-protein coupled, 6 | −2.12 | −1.64 | −2.42 |
| 41 | Dctd | NM_001013882 | Dcmp deaminase (Dctd), transcript variant 1 | −2.12 | −2.33 | −2.50 |
| 42 | Lrrn4cl | NM_001109579 | LRRN4 C-terminal like | −2.12 | −2.20 | −2.53 |
| 43 | Frzba,b | NM_001100527 | Frizzled-related protein | −2.08 | −1.92 | −2.27 |
| 44 | Gas6a | NM_057100 | Growth arrest specific 6 | −2.08 | −1.72 | −2.07 |
| 45 | Pgfa | NM_053595 | Placental growth factor | −2.01 | −1.56 | −1.94 |
| 46 | Itgada | NM_031691 | Integrin, alpha D | −1.98 | −1.86 | −1.78 |
| 47 | Nexn | NM_139230 | Nexilin (F actin binding protein) | −1.96 | −1.92 | −2.90 |
| 48 | Synj2a | NM_032071 | Synaptojanin 2 | −1.92 | −1.53 | −2.13 |
| 49 | Rgs10 | NM_019337 | Regulator of G-protein signaling 10 | −1.90 | −1.62 | −1.81 |
| 50 | Gorab | NM_001100563 | Golgin, RAB6-interacting | −1.89 | −2.97 | −2.85 |
| 51 | Lsp1 | NM_001025420 | Lymphocyte-specific protein 1 | −1.87 | −1.61 | −1.80 |
| 52 | Atp10a | NM_001141935 | Atpase, class V, type 10A | −1.87 | −1.97 | −1.92 |
| 53 | St6galnac2 | NM_001031652 | ST6 (alpha-N-acetyl-neuraminyl-2,3-beta-galactosyl-1,3)-N-acetylgalactosaminide alpha-2,6-sialyltransferase 2 | −1.85 | −1.98 | −2.53 |
| 54 | Mrvi1 | NM_001105210 | Murine retrovirus integration site 1 homolog | −1.83 | −1.61 | −2.00 |
| 55 | Pld2a,b | NM_033299 | Phospholipase D2 | −1.83 | −1.94 | −2.03 |
| 56 | Fbxw17 | NM_001082409 | F-box and WD-40 domain protein 17 | −1.80 | −1.97 | −2.33 |
| 57 | Pfkp | NM_206847 | Phosphofructokinase, platelet | −1.78 | −1.52 | −1.59 |
| 58 | Gna15 | NM_053542 | Guanine nucleotide binding protein, alpha 15 | −1.78 | −2.54 | −2.68 |
| 59 | Col4a4 | NM_001008332 | Collagen, type IV, alpha 4 | −1.75 | −2.38 | −1.58 |
| 60 | Nap1l3 | NM_133402 | Nucleosome assembly protein 1-like 3 | −1.74 | −2.18 | −2.49 |
| 61 | Prkar2ba | NM_001030020 | Protein kinase, camp dependent regulatory, type II beta | −1.73 | −1.64 | −2.00 |
| 62 | Fktn | NM_001108667 | Fukutin | −1.71 | −2.06 | −1.77 |
| 63 | Enc1a,b | NM_001003401 | Ectodermal-neural cortex 1 | −1.68 | −1.58 | −1.55 |
| 64 | Srca,b | NM_031977 | V-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog | −1.66 | −1.75 | −1.72 |
| 65 | Glsa | NM_012569 | Glutaminase | −1.66 | −1.55 | −1.52 |
| 66 | Cd200a | NM_031518 | Cd200 molecule | −1.65 | −2.00 | −2.35 |
| 67 | Spats2l | NM_001014102 | Spermatogenesis associated, serine-rich 2-like | −1.62 | −1.70 | −2.06 |
| 68 | Fut4a | NM_022219 | Fucosyltransferase 4 (alpha (1,3) fucosyltransferase, myeloid-specific) | −1.61 | −1.82 | −2.20 |
| 69 | Spock2 | NM_001108533 | Sparc/osteonectin, cwcv and kazal-like domains proteoglycan 2 | −1.59 | −1.76 | −2.01 |
| 70 | Nrp1a,b | NM_145098 | Neuropilin 1 | −1.59 | −1.53 | −1.64 |
| 71 | Reep5 | NM_001108888 | Receptor accessory protein 5 | −1.58 | −1.68 | −1.62 |
| 72 | Ap1s2 | NM_001127531 | Adaptor-related protein complex 1, sigma 2 subunit | −1.51 | −1.52 | −1.68 |
| 73 | Il4ra | NM_133380 | Interleukin 4 receptor, alpha | 1.51 | 1.91 | 1.90 |
| 74 | Rtn4rl1 | NM_181377 | Reticulon 4 receptor-like 1 | 1.62 | 1.99 | 2.41 |
| 75 | Skp2b | NM_001106416 | S-phase kinase-associated protein 2 (p45) | 1.73 | 1.83 | 2.66 |
| 76 | Fzd5a,b | NM_173838 | Frizzled family receptor 5 | 1.90 | 2.75 | 3.16 |
| 77 | Per2 | NM_031678 | Period homolog 2 | 2.25 | 2.46 | 2.73 |
- genes related to cell proliferation and angiogenesis.
- genes related to WNT pathway.
Figure 5. Effect of folic acid, tributyrin, or combined folic acid and tributyrin treatments on angiogenesis.
(A) Molecular network interactions between the common differentially expressed genes significantly different between control rats and rats treated with folic acid, tributyrin, or the combination of folic acid and tributyrin were visualized using the Ingenuity Pathway Analysis database (version 9.0). Up-regulated genes are identified in red, down-regulated in green. (B) Representative CD34 immunohistochemical staining of liver tissues from control rats (1) and rats treated with folic acid (2), tributyrin (3), or the combination of folic acid and tributyrin (4). Arrows point to some of the representative CD34-positive microvessel staining; arrowhead points to non-specific staining that was excluded from the analysis. (C) Quantitative analysis of CD34 immunohistochemical staining. a - Significantly different from control rats (mean ± SD, n = 5). (D) Western blot analysis of CD34 protein. The results are presented as an average percent change in the level of CD34 protein in the livers of rats treated with treated with folic acid, tributyrin, or the combination of folic acid and tributyrin relative to that in control rats, which was assigned a value 100%. a - Significantly different from control rats (mean ± SD, n = 5). Representative western blot images are shown.
To confirm further the inhibitory effect of folic acid, tributyrin, or combined folic acid and tributyrin treatment on angiogenesis, the level of CD34 protein in the livers was examined by immunohistochemical and western blot analyses. CD34 is an endothelial cell marker that stains immature vessels undergoing neovascularization but not mature blood vessels.16 Treatment of rats undergoing hepatocarcinogenesis with folic acid, tributyrin, or the combination of folic acid and tributyrin resulted in a significant reduction of angiogenesis, especially neovascularization. This was evidenced by a significantly lower CD34-positive staining (Figure 5B and 5C) and a diminished level of CD34 protein (Figure 5D) in the livers of rats from the experimental groups.
Discussion
Recent studies have established that treatment of rats undergoing liver carcinogenesis with folic acid or the butyric acid prodrug tributyrin results in a strong inhibition of the carcinogenic process;9,10 however, this effect was achieved by an administration of relatively high doses of both compounds. The results of this study demonstrated that chemopreventive effect of folic acid and tributyrin can be reached at lower doses administered alone or in combination. Additionally, we demonstrated that the combined folic acid and tributyrin treatment of rats undergoing liver carcinogenesis had a more pronounced chemopreventive effect than treatment with either agent alone.
Previously we reported that the cancer-suppressing activity of tributyrin and folic acid is associated with enhancing of apoptosis and suppressing sustained cell proliferation;9,10 however, there is a lack of consensus on the impact of these compounds on other hallmarks of cancer. In the present study we demonstrate that, in addition to those previously demonstrated effects, chemopreventive activity of folic acid and tributyrin is associated with their ability to affect other critical cancer-related molecular pathways (Figure 4). Interestingly, there is a substantial degree of difference in pathways affected by folic acid and tributyrin when these agents were administered separately (Figure 4). One of the common pathways inhibited by folic acid and tributyrin treatment administered alone or in combination during promotion stage of rat hepatocarcinogenesis is angiogenesis. Angiogenesis is one of the fundamental hallmarks in tumor biology.23 It is well-established that HCC is one of the most vascularized solid human tumors24 and the extent of angiogenesis in HCC correlates tightly with the progression of the disease.25,26 More importantly, it has been demonstrated that angiogenesis is critical not only in the progression of full-fledged tumors, but also in the preneoplastic conditions. Specifically, Folkman et al.27 have reported the induction of the angiogenic activity at early preneoplastic stage of mouse multistage carcinogenesis during transition from hyperplasia to neoplasia. Likewise, Wang et al.28 demonstrated that an elevated level of VEGF occurred at early stages of rat chemical liver carcinogenesis and increased progressively with the advancement of the carcinogenic process. Furthermore, Park et al.29 and Nascimento et al.30 have reported an aberrant gradual stage-dependent increase in the degree of vascular endothelial growth factor A (VEGFA) and CD34 expression during hepatocarcinogenesis in humans.
The results of our study demonstrating that treatment of rats undergoing hepatocarcinogenesis with folic acid and tributyrin alone or in combination caused a marked inhibition of de novo angiogenesis in the preneoplastic livers correspond to report by Bergers et al.31 that has shown a strong tumor-suppressing effect of inhibitors of angiogenesis at early stages of carcinogenesis. Several mechanisms may contribute to the observed suppression of angiogenesis in the livers of rats treated with folic acid and tributyrin. First, treatment of rats with folic acid and tributyrin alone or in combination caused down-regulation of several key genes encoding activators of the angiogenesis signaling pathway, including Itgb6, Itgad, Ftl3, Map3k6, Mgp, and Src.32–37 It has been demonstrated that these proteins stimulate a VEGFA secretion through activation of the MAPK pathway (integrins, FLT3, MAP3K6, and SRC) or induction of the TGF-β1 pathway (MGP). Second, treatment of rats with folic acid and tributyrin alone, or a combination of folic acid and tributyrin resulted in marked down-regulation of the Edn1 gene. EDN1 modulates different stages of neovascularization by acting directly on endothelial cells, or indirectly through the induction of VEGFA pathway.38,39 Importantly, there is overwhelming evidence showing that targeted inhibition of any of these genes results in attenuation of VEGF expression, endothelial proliferation, and capillary network formation.32,34–38 Additionally, a number of Rho GTPases genes, including Rgnef, Arhgap22, and Rab25, that play an important role in angiogenesis and vascular physiology,40 were down-regulated by folic acid and/or tributyrin chemopreventive treatment.
Currently, tumor anti-angiogenic therapy is considered as a promising and high-priority approach in cancer treatment, including HCC.41,42 Specifically, sorafenib, the first chemotherapeutic agent to demonstrate a significant effect on survival in patients with advanced HCC and currently the standard treatment of HCC,42–44 prevents tumor-associated angiogenesis by inhibiting the VEGF-signaling pathway.42,44 A critical role of neovasculizarion in early preneoplastic liver lesions, suggests that blocking angiogenesis during carcinogenesis may be a promising and unique opportunity to prevent or attenuate cancer development.24,31,45 The results of our study, which show that the chemopreventive activity of folic acid and tributyrin on rat hepatocarcinogenesis is associated with a strong anti-angiogenic effect in preneoplastic livers, provide experimental support for this suggestion.
The Wnt signaling cascade, a fundamental developmental pathway, was another critical cellular pathway affected by the folic acid and tributyrin treatment. The Wnt pathway controls tissue development in embryos and tissue maintenance in adult organisms; however, aberrant activation of the Wnt pathway plays a crucial role in the pathogenesis of multiple types of cancer, including the development of HCC. Accumulating evidence has clearly established that abnormal activation of the Wnt pathway is an early event in hepatocarcinogenesis and linked to formation of an aggressive HCC phenotype.46 This has led to a suggestion that targeting of the Wnt signaling cascade may be an important therapeutic strategy for HCC treatment.46 The observed marked down-regulation of members of the Wnt pathway by folic acid and tributyrin (Table 1) provides further evidence for the importance of this pathway in prevention of hepatocarcinogenesis.
In conclusion, the results of our study demonstrate that the tumor-suppressing activity of tributyrin and folic acid on rat hepatocarcinogenesis is associated with the ability of these agents to inhibit angiogenesis at early stages of rat liver carcinogenesis. Importantly, a combined treatment with folic acid and tributyrin exhibited a stronger chemopreventive effect as compared to either folic acid or tributyrin treatment. This may be linked to the ability of these agents to complement each other and affect a greater number of independent cancer-linked pathways than each of the agents by themselves (Figure 4). In the present study we observed a substantial chemopreventive effect of folic acid and tributyrin at significantly lower doses, as compared to those used in previous studies (9.10,12). Despite the fact that doses of folic acid and tributyrin used in this study did not cause adverse effect and were well tolerated, it is important to investigate further chemopreventive effect of both of these agents at doses relevant to a human population.
Supplementary Material
Description.
This study reinforces the chemopreventive effect of folic acid and tributyrin on hepatocarcinogenesis. The results demonstrate that tumor-suppressing activity of folic acid and tributyrin, in addition to well-established inhibition of cell proliferation and activation of apoptosis, is associated with an inhibition of angiogenesis at early stages of rat liver carcinogenesis. Importantly, the study emphasizes a key role of angiogenesis at early stages of hepatocarcinogenesis and identifies angiogenesis as an imperative target for chemoprevention.
Abbreviations
- 2-AAF
2-acetylaminofluorene
- GSTP
glutathione-S-transferase placental form
- HCC
hepatocellular carcinoma
- IPA
Ingenuity Pathway Analysis
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- DEN
N-nitrosodiethylamine
- PC
principal component
- PCNA
proliferating cell nuclear antigen
- VEGFA
vascular endothelial growth factor A
Footnotes
The views expressed in this paper do not necessarily represent those of the U.S. Food and Drug Administration.
References
- 1.Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20:2362–2368. doi: 10.1158/1055-9965.EPI-11-0643. [DOI] [PubMed] [Google Scholar]
- 2.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999-through 2008. CA Cancer J Clin. 2012;62:118–128. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 4.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma. Consider the population. J Clin Gastroenterol. 2013;47:S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Association for Cancer Research. AACR Cancer Progress Report 2013. Clin Cancer Res. 2013;19:S1–S86. [Google Scholar]
- 6.Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–527. doi: 10.1053/j.gastro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umar A, Dunn BK, Greenwald P. Future directions in cancer prevention. Nat Rev Cancer. 2012;12:835–848. doi: 10.1038/nrc3397. [DOI] [PubMed] [Google Scholar]
- 8.Steward WP, Brown K. Cancer chemoprevention: a rapidly evolving field. Br J Cancer. 2013;109:1–7. doi: 10.1038/bjc.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuroiwa-Trzmielina J, de Conti A, Scolastici C, Pereira D, Horst MA, Purgatto E, Ong TP, Moreno FS. Chemoprevention of rat hepatocarcinogenesis with histone deacetylase inhibitors: Efficacy of tributyrin, a butyric acid prodrug. Int J Cancer. 2009;124:2520–2527. doi: 10.1002/ijc.24212. [DOI] [PubMed] [Google Scholar]
- 10.Chagas CE, Bassoli BK, de Souza CA, Deminice R, Jordão AA, Júnior, Paiva SA, Dagli ML, Ong TP, Moreno FS. Folic acid supplementation during early hepatocarcinogenesis: cellular and molecular effects. Int J Cancer. 2011;129:2073–2082. doi: 10.1002/ijc.25886. [DOI] [PubMed] [Google Scholar]
- 11.de Conti A, Kuroiwa-Trzmielina J, Horst MA, Bassolli BK, Chagas CEA, Purgatto E, Cavalher FP, Camargo AA, Jordão AA, Jr, Vannucchi H, Scolastici C, Ong TP, Moreno FS. Chemopreventive effects of the dietary deacetylase inhibitor tributyrin alone or in combination with vitamin A during the promotion phase of rat hepatocarcinogenesis. J Nutr Biochem. 2012;23:860–866. doi: 10.1016/j.jnutbio.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 12.de Conti A, Tryndyak V, Koturbash I, Heidor R, Kuroiwa-Trzmielina J, Ong TP, Beland FA, Moreno FS, Pogribny IP. The chemopreventive activity of the butyric acid prodrug tributyrin in experimental rat hepatocarcinogenesis is associated with p53 acetylation and activation of the p53 apoptotic signaling pathway. Carcinogenesis. 2013;34:1900–1906. doi: 10.1093/carcin/bgt124. [DOI] [PubMed] [Google Scholar]
- 13.Semple-Roberts E, Hayes MA, Armstrong D, Becker RA, Racz WJ, Farber E. Alternative methods of selecting rat hepatocellular nodules resistant to 2-acetylaminofluorene. Int J Cancer. 1987;40:643–645. doi: 10.1002/ijc.2910400512. [DOI] [PubMed] [Google Scholar]
- 14.O’Broin S, Kelleher B. Microbiological assay of microtitre plates of folate in serum and red cells. J Clin Pathol. 1992;45:344–347. doi: 10.1136/jcp.45.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su E, Zhang NN, Ho PC. Determination of tributyrin and its metabolite butyrate in Wistar rats plasma samples by gaseous chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:2217–2222. doi: 10.1002/rcm.1607. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen L, Fifis T, Malcontenti-Wilson C, Chan LS, Costa PN, Nikfarjam M, Muralidharan V, Christophi C. Spatial morphological and molecular differences within solid tumors may contribute to the failure of vascular disruptive agent treatments. BMC Cancer. 2012;12:522. doi: 10.1186/1471-2407-12-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong TP, Heidor R, de Conti A, Dagli ML, Moreno FS. Farnesol and geraniol chemopreventive activities during the initial phases of hepatocarcinogenesis involve similar actions on cell proliferation and DNA damage, but distinct actions on apoptosis, plasma cholesterol and HMGCoA reductase. Carcinogenesis. 2005;27:1194–1203. doi: 10.1093/carcin/bgi291. [DOI] [PubMed] [Google Scholar]
- 18.Stinchcombe S, Buchmann A, Bock KW, Schwarz M. Inhibition of apoptosis during 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated tumor promotion in rat liver. Carcinogenesis. 1995;16:1271–1275. doi: 10.1093/carcin/16.6.1271. [DOI] [PubMed] [Google Scholar]
- 19.Fang H, Harris SC, Su Z, Chen M, Qian F, Shi L, Perkins R, Tong W. ArrayTrack: an FDA and public genomic tool. Methods Mol Biol. 2009;563:379–398. doi: 10.1007/978-1-60761-175-2_20. [DOI] [PubMed] [Google Scholar]
- 20.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bannasch P, Haertel T, Su Q. Significance of hepatic preneoplasia in risk identification and early detection of neoplasia. Toxicol Pathol. 2003;31:134–139. doi: 10.1080/01926230390173923. [DOI] [PubMed] [Google Scholar]
- 22.Pitot HC. Adventures in hepatocarcinogenesis. Annu Rev Pathol. 2007;2:1–29. doi: 10.1146/annurev.pathol.2.010506.092027. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Sharma RA, Harris AL, Dalgleish AG, Steward WP, O’Byrne KJ. Angiogenesis as a biomarker and target in cancer prevention. Lancet. 2011;2:726–732. doi: 10.1016/S1470-2045(01)00586-1. [DOI] [PubMed] [Google Scholar]
- 25.Yang ZF, Poon RT. Vascular changes in hepatocellular carcinoma. Anat Rec (Hoboken) 2008;291:721–734. doi: 10.1002/ar.20668. [DOI] [PubMed] [Google Scholar]
- 26.Kaseb AO, Hanbali A, Cotant M, Hassan MM, Wollner I, Philip PA. Vascular endothelial growth factor in the management of hepatocellular carcinoma: a review of literature. Cancer. 2009;115:4895–4906. doi: 10.1002/cncr.24537. [DOI] [PubMed] [Google Scholar]
- 27.Folkman J, Watson C, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Xu GL, Jia WD, Wang ZH, Li JS, Ma JL, Ge YS, Xie SX, Yu JH. Expression and correlation of hypoxia-inducible factor-1α, vascular endothelial growth factor and microvessel density in experimental rat hepatocarcinogenesis. J Int Med Res. 2009;37:417–425. doi: 10.1177/147323000903700217. [DOI] [PubMed] [Google Scholar]
- 29.Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061–1065. doi: 10.5858/2000-124-1061-IEOVEG. [DOI] [PubMed] [Google Scholar]
- 30.Nascimento C, Caroli-Bottino A, Paschoal J, Pannain VL. Vascular immunohistochemical markers: contributions to hepatocellular nodule diagnosis in explanted livers. Transplant Proc. 2009;41:4211–4213. doi: 10.1016/j.transproceed.2009.09.068. [DOI] [PubMed] [Google Scholar]
- 31.Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 32.Boström K, Zebboudj AF, Yao Y, Lin TS, Torres A. Matrix GLA protein stimulates VEGF expression through increased transforming growth factor-β1 activity in endothelial cells. J Biol Chem. 2004;279:52904–52913. doi: 10.1074/jbc.M406868200. [DOI] [PubMed] [Google Scholar]
- 33.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrinαvβ3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12:177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 35.Eto N, Miyagishi M, Inagi R, Fujita T, Nangaku M. Mitogen-activated protein 3 kinase 6 mediates angiogenic and tumorigenic effects via vascular endothelial growth factor expression. Am J Pathol. 2009;174:1553–1563. doi: 10.2353/ajpath.2009.080190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcovic A, MacKenzie KL, Lock RB. Induction of vascular endothelial growth factor secretion by childhood acute lymphoblastic leukemia cells via the FLT-3 signaling pathway. Mol Cancer Ther. 2011;11:183–193. doi: 10.1158/1535-7163.MCT-11-0503. [DOI] [PubMed] [Google Scholar]
- 37.Bianchi-Smiraglia A, Paesante S, Bakin AV. Integrin β5 contributes to the tumorigenic potential of breast cancer cells through the Src-FAK and MEK-ERK signaling pathways. Oncogene. 2013;32:3049–3058. doi: 10.1038/onc.2012.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagnato A, Spinella F. Emerging role of endothelin-1 in tumor angiogenesis. Trends Endocrinol Metab. 2003;14:44–50. doi: 10.1016/s1043-2760(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 39.Knowles J, Loizidou M, Taylor I. Endothein-1 and angiogenesis in cancer. Curr Vasc Pharmacol. 2005;3:309–314. doi: 10.2174/157016105774329462. [DOI] [PubMed] [Google Scholar]
- 40.Kather JN, Kroll J. Rho guanine exchange factors in blood vessels: fine-tuners of angiogenesis and vascular function. Exp Cell Res. 2013;319:1289–1297. doi: 10.1016/j.yexcr.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Bishayee A, Darvesh AS. Angiogenesis in hepatocellular carcinoma: a potential target for chemoprevention and therapy. Curr Cancer Drug Targets. 2012;12:1095–1118. [PubMed] [Google Scholar]
- 42.Gaythier A, Ho M. Role of sorafenib in the treatment of advanced hepatocellular carcinoma: an update. Hepatol Res. 2013;43:147–154. doi: 10.1111/j.1872-034X.2012.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ranieri G, Gadaleta-Caldarola G, Goffredo V, Patruno R, Mangia A, Rizzo A, Sciorsci RL, Gadaleta CD. Sorafenib (BAY 43-9006) in hepatocellular carcinoma patients: from discovery to clinical development. Curr Med Chem. 2012;19:938–944. doi: 10.2174/092986712799320736. [DOI] [PubMed] [Google Scholar]
- 44.Di Marco V, De Vita F, Koskinas J, Semela D, Toniutto P, Verslype C. Sorafenib: from literature to clinical practice. Ann Oncol. 2013;24:ii30–ii37. doi: 10.1093/annonc/mdt055. [DOI] [PubMed] [Google Scholar]
- 45.Albini A, Tosetti F, Li VW, Noonan DM, Li WW. Cancer prevention by targeting angiogenesis. Nature Rev Clin Oncol. 2012;9:498–509. doi: 10.1038/nrclinonc.2012.120. [DOI] [PubMed] [Google Scholar]
- 46.Pez F, Lopez A, Kim M, Wands JR, Fromentel CC, Merle P. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.07.001. (in press). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.