Abstract
The pathophysiology of Autism Spectrum Disorder (ASD) is not yet known; however, studies suggest that dysfunction of the immune system affects many children with ASD. Increasing evidence points to dysfunction of the innate immune system including activation of microglia and perivascular macrophages, increases in inflammatory cytokines/chemokines in brain tissue and CSF, and abnormal peripheral monocyte cell function. Dendritic cells are major players in innate immunity and have important functions in the phagocytosis of pathogens or debris, antigen presentation, activation of naïve T cells, induction of tolerance and cytokine/chemokine production. In this study, we assessed circulating frequencies of myeloid dendritic cells (defined as Lin-1−BDCA1+CD11c+ and Lin-1−BDCA3+CD123−) and plasmacytoid dendritic cells (Lin-1− BDCA2+CD123+ or Lin-1−BDCA4+ CD11c−) in 57 children with ASD, and 29 typically developing controls of the same age, all of who were enrolled as part of the Autism Phenome Project (APP). The frequencies of dendritic cells and associations with behavioral assessment and MRI measurements of amygdala volume were compared in the same participants. The frequencies of myeloid dendritic cells were significantly increased in children with ASD compared to typically developing controls (p < 0.03). Elevated frequencies of myeloid dendritic cells were positively associated with abnormal right and left amygdala enlargement, severity of gastrointestinal symptoms and increased repetitive behaviors. The frequencies of plasmacytoid dendritic cells were also associated with amygdala volumes as well as developmental regression in children with ASD. Dendritic cells play key roles in modulating immune responses and differences in frequencies or functions of these cells may result in immune dysfunction in children with ASD. These data further implicate innate immune cells in the complex pathophysiology of ASD.
Keywords: Autism, dendritic cells, repetitive behaviors, amygdala volume, innate immunity
Introduction
Autism spectrum disorder (ASD) appears early in childhood and is characterized by core features of impaired social interaction, deficits in communication and restricted repetitive behaviors and interests (APA 2000). Once thought of as a rare disorder the prevalence rates for ASD are now considered to be approximately 1% of all children (MMWR 2009). The etiology and pathophysiology of ASD largely remain a mystery but are likely to involve complex interactions between genetic, epigenetic and environmental factors. Rapidly accumulating evidence highlights a role for dysfunctional immune function in many children with ASD (Onore et al., 2012). Many candidate genes linked with ASD have influence over immune responses while immune dysfunction in the brain and periphery have been reported (Abrahams and Geschwind, 2010; Ashwood et al., 2011a; Vargas et al., 2005). Furthermore, models of maternal immune activation cast light on how aberrant immune responses during critical periods of development can cause changes in neurodevelopment that lead to altered behaviors resembling those of core autistic features (Patterson et al., 2009).
Several lines of evidence point to ongoing and prominent activation of innate immune cells within the brain, such as microglia and perivascular macrophages (Vargas et al., 2005; Morgan et al., 2010; Voineagu et al., 2011). Further studies have shown increased activation of monocytes in the periphery following stimulation with Toll-like receptor ligands, including changes in gene expression, increased HLA-DR cell surface expression and the release of pro-inflammatory cytokines interleukin (IL)-1β and IL-6 (Enstrom et al., 2010, Jyonouchi et al., 2001). Moreover, circulating levels of cytokines exhibit a profile reminiscent of innate immune cell activation with increased IL-1β, IL-6, IL-12p40, tumour necrosis factor (TNF)-α and CCL-2 production (Ashwood et al., 2011a, b). Both the circulating levels of these pro-inflammatory cytokines and the degree of monocyte activation were associated with more impaired behaviors in children with ASD (reviewed in Onore et al., 2012). Furthermore, activation of innate immune cells leads to increases in oxygen free radicals and an increase in oxidative stress, a process that is increased in ASD (Rose et al., 2011). Together, these data point to an important role for innate immune cell dysfunction in modulating behaviors and neuroinflammation observed in ASD.
Dendritic cells serve a central role in many immune functions (Steinman, 2007). They are highly phagocytic and express many innate pattern-recognition receptors that capture pathogen-associated molecular pattern molecules (PAMPs) on microbes or damage-associated molecular pattern molecules (DAMPs) of endogenous tissues. Upon binding of these ligands/antigens, dendritic cells undergo maturation steps that increase mobility for migration, express chemokine receptors for homing to lymphoid organs, produce chemokines to recruit other immune cells, up regulate MHC class II molecules and co-stimulatory molecules for priming of naïve T cells or stimulation of effector T cells and secrete large quantities of cytokines that polarize or modulate neighboring immune cells (Banchereau and Steinman, 1998; Banchereau et al., 2000; Ueno et al., 2007). Dendritic cells also play an important role in inducing both central and peripheral tolerance. Dendritic cells in the periphery continuously capture and present low dose non-immunogenic antigens to T cells with limited or absent co-stimulation to maintain tolerance either by deletion, the induction of unresponsiveness (anergy) or generation of adaptive T regulatory cells (Steinman, 2007). The biology of dendritic cells is complex but they represent a critical link between innate and adaptive immune responses. Two dendritic cell subsets, myeloid dendritic cells and plasmacytoid dendritic cells have been described in human blood and differences in these populations have been observed in a number of autoimmune conditions (Jego et al., 2003; Pashenkov et al., 2001; Richez et al., 2009). Although, previous studies have examined a variety of innate immune cell effectors in ASD, including monocytes and NK cells, the essential role dendritic cells play in controlling many immune processes make these cells particularly interesting targets for study. To our knowledge, this is the first study to examine differences in frequencies of circulating dendritic cells in young children with ASD and typically developing controls of the same age.
Through the UC Davis MIND Institute Autism Phenome Project, we evaluated frequencies of circulating dendritic cells in very young children with ASD and age-matched typically developing controls. We also evaluated whether levels of circulating dendritic cells are associated with brain volume measurements and/or the core symptoms of ASD. Specifically, we evaluated the association between dendritic cells and amygdala volume. Abnormal amygdala structure and function has been widely reported in neuropathological and imaging studies of individuals with ASD (Schumann and Nordahl, 2011). However, the mechanism underlying abnormal amygdala enlargement in ASD remains unknown. As association with circulating dendritic cells may provide a clue to the pathophysiology of abnormal amygdala growth in ASD.
Methods
Participants and behavioral assessments
Eighty-six study participants aged between 2-3 years of age were recruited through the UC Davis M.I.N.D. Institute as part of the Autism Phenome Project (APP). Participants consisted of 57 children with ASD (mean age 2.92 ± 0.05 years; minimum-maximum range 2.08 - 3.75 years; 48 males) and 29 typically developing (TD) controls children (mean age 2.96 ± 0.06 years; range 2.17 – 3.58; 19 males). The study protocol was approved by the Institutional Review Board for the UC Davis School of Medicine, and parents or guardians of each subject provided written informed consent for their child to participate.
All diagnostic assessments were conducted or directly observed by trained, licensed clinical psychologists who specialize in ASD and had been trained according to research standards for these tools. Inclusion criteria for a diagnosis of ASD were based on the NIH Collaborative Programs of Excellence in Autism network. These involved: (1) meeting either the Autism Diagnostic Observation Schedule – Generic (ADOS-G) cut-off score for autistic disorder or PDD, (2) or meeting the Autism Diagnostic Interview – Revised (ADI-R) cutoff score for autistic disorder and scoring within two points of this cutoff on the other measure i.e. within 2 points on ADI-R or 2 points on ADOS, (3) combined with clinical judgment. Of the 57 children with ASD, 49 met the cut-offs for autistic disorder and 8 met the cut-offs for PDD-NOS. The ADI-R also collects information about the onset of symptoms. 26 participants demonstrated an early onset pattern, in which signs of autism were present in the first year of life, while 31 participants were reported by parents to have a regressive pattern of onset, in which early development appeared typical, followed by a loss of social-communication skills in the second year of life. All participants were administered the Mullen Scales of Early Development (MSEL) and a developmental quotient (DQ) was calculated based on of the ratio between the average of mental age equivalent scores and chronological age multiplied by 100. Three quotients were calculated: verbal, nonverbal, and overall. The TD children were screened and included after assessment with the Social Communication Questionnaire (excluded if scores > 11) (SCQ - Lifetime Edition) ruled out ASD risk and the MSEL revealed developmental scores within two standard deviations of the mean for performance quotient and verbal quotient subscales. Exclusion criteria for TD controls included a diagnosis of mental retardation or specific language impairment, or any known developmental, neurological, or behavioral problems. Further exclusion criteria for all subjects consisted of the presence of Fragile X or other serious neurological, psychiatric or known medical conditions including autoimmune disease and inflammatory bowel diseases/celiac disease. Further inclusion criteria for all children, both TD controls and children with ASD, included being native English speakers, ambulatory, and with no suspected vision or hearing problems.
All subjects were screened via parental interview for current and past physical illness. The ASD and TD children had similar vaccination histories. No differences were noted for time from last vaccine in the two groups. Children with known endocrine, cardiovascular, pulmonary, liver, kidney disease, or current fever were excluded from enrollment in the study. Additional exclusionary criteria were limited to those with physical contraindications to MRI.
In addition to the diagnostic measures, all children were assessed using the Vineland Adaptive Behavior Scales (VABS), a parent report measure for adaptive behaviors and the Repetitive Behavior Scale-Revised (RBSR), a parent report questionnaire for measuring repetitive behavior in children. Parent report of frequent or always gastrointestinal symptoms of irregular bowel habits (i.e. constipation and/or diarrhea) was also collected to determine if there were associations with dendritic cell frequencies.
Flow Cytometry
Peripheral blood was drawn from the participants into sodium citrate (ACD) treated vacutainers (BD Bioscience; San Jose, CA) on the last day after behavioral assessments were performed. No participant presented with a cold, fever or other common illness. If such a condition occurred, the blood draw was delayed until the child’s health status was stable for 48 hours. Peripheral blood mononuclear cells (PBMC) were separated from the whole blood by centrifugation over Histopaque-1077 Hybri-Max lymphocyte separation medium (Sigma; St. Louis, MO) before washing once in Hanks Balanced Salt Solution (HBSS; VWR; Brisbane, CA) and twice more in FACS buffer (PBS, 1% fetal bovine serum albumin (VWR, USA) and 0.1 % sodium azide (Sigma). The number of viable PBMC was determined by Trypan Blue exclusion (Sigma) and PBMC concentrations were adjusted to 1 × 106 cells/ml in FACS buffer prior to staining with the following monoclonal antibodies: fluorescein isothiocyanate (FITC)-conjugated mouse anti-human Lineage-1 cocktail (Lin-1) containing CD3, CD14, CD16, CD19, CD20, and CD56 ; phycoerythrin (PE)-conjugated mouse anti-human BDCA3 (CD303), BDCA4 (CD304); phycoerythrin (PE)-Cy5-conjugated mouse anti-human CD11c, CD123; and allophycocyanin (APC)-conjugated mouse anti-human BDCA1 (CD1c), BDCA2 (CD303) (all antibodies were from BD Biosciences, CA, USA). For the purpose of this study, myeloid dendritic cells were defined as Lin-1−BDCA1+CD11c+ (mDC1) or Lin-1−BDCA3+CD123− (mDC2) and plasmacytoid dendritic cells as Lin-1−BDCA2+CD123+ (pDC1) or Lin-1−BDCA4+ CD11c− (pDC2) according to classifications established by the Nomenclature Committee of the International Union of Immunological Societies (Ziegler-Heitbrock et al., 2010). Cells were incubated at 4°C for 30 minutes before being spun down and washed with FACS buffer.
Cell surface marker expression was analyzed using a LSR II flow cytometer (BD Immunocytometry Systems). Singly stained compensation controls and isotype mAb controls (BD Biosciences) were run for each participant. Compensation for each marker was calculated using anti-mouse antibody coated compensation beads (BD Biosciences), IgG isotype controls and FACS Diva Software (BD Biosciences). The data acquired were analyzed with FlowJo software (Treestar Inc; Ashland, OR). In brief, dendritic cells were gated using forward scatter and side scatter parameter as an indication of cell size and granularity in order to exclude non-cellular debris and discriminated from other cells by the exclusion of Lin-1 staining.
General Imaging Procedures
All scanning was performed during natural, nocturnal sleep (Nordahl et al., 2008). Earplugs and/or headphones were used to attenuate the MRI gradient sounds. Each child was monitored carefully for the duration of the scan. Because scans were acquired during natural sleep, there was no motion artifact. If the child moved or woke up during the scan, the imaging session was halted.
Imaging Protocol
All scans were acquired at the UC Davis Imaging Research Center on a 3T Siemens Trio (TIM) whole-body MRI system (Siemens Medical Solutions, Erlangen, Germany) using an 8-channel head coil. For each participant, a three-dimensional T1-weighted MPRAGE scan (TR 2170 ms; TE 4.86 ms; matrix 256 × 256; 1 mm isotropic voxels) was obtained. A T2-weighted scan was also obtained for clinical evaluation when possible (i.e. when the child remained asleep). All MRPAGE and available T2 scans were reviewed by a pediatric neuroradiologist and screened for significant, unexpected clinical findings.
Volumetric Measurements
Image preprocessing included removing non-brain tissue using FMRIB’s Brain Extraction Tool (BET) (Smith, 2002) and removing inhomogeneity using the Non-parametric Non-uniform intensity Normalization (N3) method (Sled et al., 1998) (http://www.bic.mni.mcgill.ca/software/N3). Total cerebral volume was determined using a template-based automated procedure that removed brainstem and cerebellum (Nordahl 2011, 2012).
The amygdala was manually defined using Analyze 10.0 (Robb et al., 1989) based on an anatomically defined protocol (Schumann et al., 2004; Nordahl et al., 2012). Images were first re-sampled to 0.5 mm isotropic voxels and aligned along the long axis of the hippocampus. Boundaries were traced in the coronal plane in a caudal to rostral direction. Axial views were used to aid in defining the boundary between the amygdala and the putamen, and sagittal views were used to aid in determining the rostral extent of the amygdala. Two blinded raters performed all manual tracings and had an intraclass correlation coefficient of .93 for the left amygdala and .96 for the right amygdala.
Statistical Analysis
All of the statistical analyses were conducted in SAS statistical software (version 9.3). Some of the models were run independently in R (version 2.13.0) to verify the model results and to obtain graphical displays of model diagnostics. We performed regression analyses to evaluate the degree to which diagnosis (ASD vs. TD) was significantly associated with dendritic cell subsets. To assess the effect of diagnosis, we controlled for age, sex and two interaction terms (Age * Diagnosis; Sex * Diagnosis). To minimize over-fitting the model, we used the Akaike Information Criterion (AIC) to evaluate the tradeoffs between model fit and model complexity. Although sex and the interaction effects were significant in some of the models, a relatively small increase in the AIC values suggested that age was the most important control variable. In addition, to ensure that some of the brain structures such as the amygdala had independent effects beyond overall brain volume, we added total brain volume as a covariate for models that include brain structure variables.
The regression models were fitted using the SAS procedure for generalized linear models (Proc Genmod). To evaluate the robustness of the findings, a number of model distributions and link functions were considered (e.g., gamma, logit, normal). For example, we split the continuous distributions into two parts using the median as the cut point, and then fit logistic regression models. For the various models, we reviewed the diagnostic plots to evaluate if the model residuals are normally distributed and were constant across the levels of the predicted values. In addition, we looked at leverage statistics to determine the impact of outliers on the model results. We determined that an “ordinary least squares” linear regression model was the most appropriate. For the outcome or dependent variable, this model assumes a continuous (and relatively normal) distribution. To elucidate the relative effect sizes for the independent variables, we standardized them by setting the mean to zero and the standard deviation to one. For all of the models, we found parameter estimates or coefficients that indicate the strength of the relationship between the dependent and independent variables, after holding constant control variables. Probability values less than 0.05 were considered statistically significant. Unadjusted p values are presented (Rothman, 1990).
Results
After controlling for age and sex, the frequency of the myeloid dendritic cell population mDC1 was significantly increased in children with ASD (mean ± SEM (%); 4.6 ± 0.41%) compared with the TD controls (3.7 ± 0.38%, p = 0.03, Figure 1, Table 1). This represented an approximate 25% increase in mDC1 in the ASD group. In addition, the frequency of mDC2 myeloid dendritic cells was increased, by approximately 40% in children with ASD (3.49 ± 0.29%) compared with TD controls (2.54 ± 0.30, p = 0.01, Figure 1, Table 1). The frequency of plasmacytoid dendritic cell populations was not statistically significantly different between ASD and TD controls (Table 1). However, there was an approximate 25% increase in frequency of BDCA2+ labeled cells within the Lin-1− gate (denoted Lin-1−BDCA2+) in children with ASD (6.92 ± 0.45) compared with TD controls (5.52 ± 0.43, p = 0.05, Table 1).
Figure 1. Frequencies of blood myeloid dendritic cells in children with ASD.
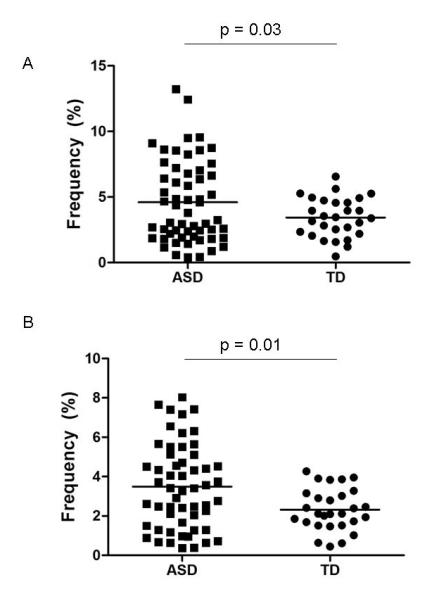
Mean levels (black horizontal bar) of (A) Lin-1−BDCA1+CD11c+ and (B) Lin-1−BDCA3+CD123− are significantly increased in young children with ASD in comparison with typically developing controls.
Table 1. Frequencies of blood dendritic cells.
The frequencies of blood dendritic cells in young children with ASD aged 2-4 years of age compared with typically developing children of the same age. Data is expressed as mean plus or minus SEM. Models were adjusted for age, sex and diagnosis of the participants,
| Autism Spectrum Disorder | Typically Developing controls | |
|---|---|---|
|
|
||
| Cell population | Mean ± SEM (%) | Mean ± SEM (%) |
| Myeloid dendritic cells | ||
| Lin-1−BDCA1+ | 6.47 ± 0.52* | 5.12 ± 0.51 |
| Lin-1−BDCA1+CD11c+ | 4.6 ± 0.41* | 3.70 ± 0.38 |
| Lin-1−BDCA3+ | 5.81 ± 0.43 | 5.10 ± 0.49 |
| Lin-1−BDCA3+CD123− | 3.49 ± 0.29* | 2.54 ± 0.30 |
| Plasmacytoid dendritic cells | ||
| Lin-1−BDCA2+ | 6.92 ± 0.45* | 5.52 ± 0.43 |
| Lin-1−BDCA2+CD123+ | 5.47 ± 0.42 | 4.49 ± 0.42 |
| Lin-1−BDCA4+ | 3.56 ± 0.24 | 3.33 ± 0.3 |
| Lin-1−BDCA4+CD11c− | 3.13 ± 0.23 | 3.04 ± 0.27 |
p < 0.05 was considered significant.
We then examined whether there were associations between the frequencies of circulating dendritic cells with clinical, behavioral and brain imaging variables. A positive association was observed between mDC1 frequencies and measures of left amygdala volume (estimated effect size = 3.56, p = 0.004, Figure 2) and right amygdala volume (effect size = 2.76, p = 0.02, Figure 2) in children with ASD after adjusting for age and sex, and using overall brain volume as a covariate. Similarly, there was a positive association between mDC2 cells and right amygdala volume (estimated effect size = 1.78, p = 0.05). The frequencies of pDC1 plasmacytoid dendritic cells were also positively associated with left (estimate effect size = 2.42, p = 0.02) and right amygdala volumes (estimate effect size = 2.28, p = 0.03). In TD controls, mDC1 frequencies were associated with left amygdala volume (estimated effect size = 0.76, p = 0.02) and right amygdala volume (effect size = 0.93, p = 0.003); however, no associations were observed between mDC2, pDC1 or pDC2 frequencies and amygdala volumes in TD controls. In children with ASD positive associations were observed between the mDC1 frequencies and scores on the RBSR (effect size = 2.49; p = 0.02) and repetitive behaviors as measured on ADOS (effect size = 0.43; p = 0.02), such that, as the mDC1 frequencies increased, repetitive behaviors became more pronounced. Myeloid dendritic cells were also associated with the severity of gastrointestinal symptoms, a common problem in children with ASD, such that increased frequency of mDC2 was positively associated with increased frequency of symptoms of constipation (effect size 1.85, p = 0.002). There was also a trend for increased mDC1cells to be associated with constipation in ASD children but this did not reach statistical significance (effect size 1.69, p = 0.06). Based on the ADI-R assessment, we also examined whether there was an association between dendritic cell frequencies and the onset of behavioral symptoms in terms of regression of behaviors after a period of reaching developmental milestones. The frequencies of plasmacytoid cells pDC1 and pDC2 (effect size 0.35 and 0.59 respectively, p < 0.02), but not the myeloid dendritic cells, were associated with regression. In TD children composite total scores for adaptive behaviors, as assessed by VABS, were negatively associated with mDC1, mDC2 and pDC2 dendritic cell subsets (effect size −0.96, −1.94 and −0.70 respectively, p < 0.05), suggesting that as dendritic cell numbers increased there was a decrease in adaptive behavior skills.
Figure 2. The frequencies of blood myeloid dendritic cells in children with ASD are associated with amygdala volume.
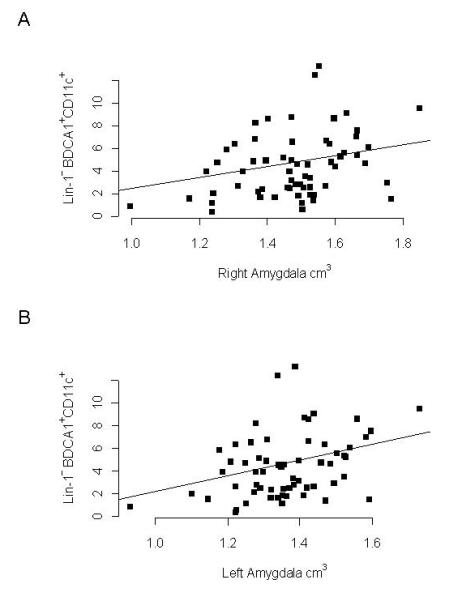
Positive correlations were observed for (A) Lin-1−BDCA1+CD11c+ myeloid dendritic cells and left amygdala volume (r = 0.37, p = 0.003) and (B) right amygdala volume (r = 0.33, p = 0.012) as determined by MRI analysis. Amygdala volume measures were controlled for with total brain volumes and age and sex of the participant.
Discussion
For the first time, we show that frequencies of circulating Lin-1−BDCA1+CD11c+ and Lin-1−BDCA3+CD123−, two populations of blood myeloid dendritic cells, are significantly increased in young children with ASD compared with typically developing children of the same age. These observations are consistent with previous reports of increased numbers and enhanced activation of other myeloid cells including monocytes, perivascular macrophages and microglia cells in ASD (Enstrom et al., 2010; Morgan et al, 2010; Sweeten et al., 2003; Vargas et al., 2005). In addition, we observed significant associations between the frequencies of myeloid dendritic cells and larger right and left amygdala volumes, higher repetitive behavior scores and more frequent symptoms of constipation in children with ASD. The plasmacytoid dendritic cells were also associated with increased left and right amygdala volume and associated with developmental regression and the later onset of ASD behaviors. In addition, in typically developing children dendritic cell frequencies were associated with poorer adaptive behavior skills. These data suggest that dendritic cells may interact with factors that influence the ontogenesis of the amygdala. The amygdala has been repeatedly demonstrated to be of pathological importance in autism although a link between immune processes and the abnormal brain structure have not yet been evaluated. Functionally, the amygdala has been implicated in alterations of core social abilities in autism and in common co-morbid symptoms such as anxiety. Interestingly, there is substantial evidence that the amygdala also normally modulates gastrointestinal activity in the face of stressful situations.
Our current findings are consistent with previous reports of a dysregulation of the innate immune system in ASD (Onore et al., 2012). An increase in activated microglia and perivascular macrophages has been observed in brain specimens from individuals with ASD over a wide range of ages (4-45 years of age) (Morgan et al., 2010; Vargas et al., 2005; Voineagu et al., 2011). These findings were associated with increased inflammatory cytokine and chemokine production, including IL-1β, IL-6, IL-12p40, TNFα and CCL-2 in postmortem brain tissue and in cerebral spinal fluid (CSF) (Morgan et al., 2010; Vargas et al., 2005). Recent transcriptome studies in brains of individuals with ASD implicate abnormalities in gene networks related to innate immunity including the function of microglia, macrophages and dendritic cells (Garbett et al., 2008; Voineagu et al., 2011). The profile of cytokines present in the periphery reflects findings in the brain, with children with ASD exhibiting increased plasma levels of predominantly myeloid cell derived cytokines GM-CSF, IL-1β, IL-6, and IL-12p40 (Ashwood et al., 2011a). Many of these cytokines, in particular GM-CSF, are known to be important in dendritic cell maturation. In addition, activated T cells expressing CD137 on their cell surface are also known to drive dendritic cell development and have been shown to be increased in children with ASD (Ashwood et al., 2011c). Thus, in children with autism the balance of cytokines and activated cells may predispose to increases in dendritic cell frequencies. In turn, myeloid dendritic cells are major producers of IL-1β, IL-6, TNFα and IL-12 and likely contribute to the cytokine profiles described in the periphery. Taken together these findings suggest that in children with ASD, an environment exists that promotes the generation and maturation of dendritic cells and that these cells may contribute to a peripheral inflammatory profile.
Many genetic studies have shown that of the candidate genes or variations in copy number variants in individuals with ASD many have important roles in modulating the immune response (Abrahams and Geschwind, 2008; Careaga et al., 2010). These include genes encoding for proteins within the signaling pathways that regulate the function of myeloid cells such as MET, a pleiotropic receptor tyrosine kinase. For example, MET signaling induces a tolerogenic phenotype in dendritic cells without affecting their antigen presenting capabilities (Okunishi et al., 2005; Rutella et al., 2006). We recently showed that the MET ‘C’ variant predisposes to innate immune cell activation and the loss of self-tolerance (Heuer et al., 2011). Individuals with a functional polymorphism in the promoter for MET, namely rs1858830 ‘C’ allele, carry a 2.25 fold increased relative risk for autism (Campbell et al., 2006). In addition, the MET “C” allele polymorphism was shown to be associated with gastrointestinal symptoms in ASD (Campbell et al., 2009). In the current study we found associations between myeloid dendritic cells and the severity of constipation symptoms in ASD. Taken together these data suggest that genetic mechanisms in ASD may impact important controlling processes relevant to innate immune function including the function of dendritic cells that may be important in neuronal and mucosal development. We are pursuing further studies to look at the function of dendritic cells in ASD.
Blood dendritic cells are most likely cells that are in transit. Upon entry into tissues of the body, they become fully mature, which includes up-regulating activation markers such as CD83 that are not expressed on blood dendritic cells, and where they are more likely to form close cell-to cell contacts with other immune cells (Ziegler-Heitbrock et al., 2010). Dendritic cells have been observed in the CSF, meninges, choroid plexus and perivascular spaces in the healthy central nervous system (CNS) of humans and rodents (Hanly and Petito, 1998; Matysak and Perry, 1996; Pashenkov et al., 2001; Serot et al., 1997) where they play a putative role in immune surveillance. In healthy tissue, the number of dendritic cells in the CNS is low but can dramatically increase during infection or in autoimmune diseases such as multiple sclerosis (MS) (Greter et al., 2005; Kivisakk et al., 2004; Panshenkov et al., 2001; Pashenkov et al., 2002). To determine the function of dendritic cells in the human CNS during such conditions is very challenging. But, as in other tissue, they may serve a pathological role by activating naïve T cells, presenting autoantigen to infiltrating activated T cells, contributing to the inflammatory milieu by production of inflammatory cytokines and the production of reactive oxygen species. The release of cytokines such as TNFα from dendritic cells may also reduce mitochondrial function that ahs recently been associated with ASD (Onore et al., 2012). In brain specimens isolated from the asocial BTBR T+tf/J mouse strain, increased numbers of dendritic cells were observed compared with the more social B6 mice (Heo et al., 2011). These observations are interesting and further studies need to be pursued to determine the relevance of increases in dendritic cells and brain volumes in the asocial BTBR compared with social B6 mice.
Structural MRI studies have provided evidence for abnormal amygdala development in autism. In young children with autism, ages 2-5 years, there is substantial evidence for abnormal amygdala enlargement relative to typically developing controls (Mosconi et al., 2009; Nordahl et al., 2012; Schumann et al., 2009; Sparks et al., 2002). This enlargement appears to persist, into middle childhood (Schumann et al., 2004), but may diminish in adolescence and adulthood (Haznedar et al., 2000; Palmen et al., 2005; Schumann et al., 2004). In young children, two studies have reported correlations between amygdala volume and social and communications scores on the ADI-R (Munson et al., 2006; Schumann et al., 2009). Previous studies have also shown that amygdala volumes in typically developed subjects correlate with the number and complexity of social contact (Bickart et al., 2010). In ASD, Munson et al (2006) found that enlarged right amygdala volume at age 3-4 years was predictive of poorer social and communication abilities at age 6 (Munson et al., 2006). Interestingly, while there appears to be precocious growth of the amygdala in a sizable subset of young children with autism ( Nordahl et al 2012) the only quantitative histological study of the amygdala in the postmortem brain actually demonstrated a lower number of neurons (Schumann and Amaral, 2006). This raises the prospect of a degenerative process taking place in the amygdala of adults with autism spectrum disorder.
To our knowledge, no previous study has evaluated the relationship between abnormal amygdala growth and parameters of immune function in children with autsim. Our current study showed that both myeloid and plasmacytoid cell populatiosn were associated with amygdala volumes in children with ASD. Considering the ability of dendritic cells to manufacturer and secrete large quantities of growth factors and neuropoeitic cytokines such as IL-6, it is not inconceivable that any increase in dendritic cell frequency or altered activation could alter the growth of amygdala. Why this might be more selective for the amygdala is currently unclear. Moreover, dendritic cells may have a number of effects peripherally that could alter changes in brain growth and function, such as; interaction with peripheral nerves that innervate lymph nodes or the gastrointestinal tract, the release of cytokines, activation of naïve self-reactive T cells that target CNS tissue, activation of anergic T cells and/or failure to induce/promote tolerance via negative signals to regulatory T cells. Recent data indicates that the amygdala may also be privy to the degree/extent of peripheral immune activation and its activity is increased in response to immune challenges such as lipopolysaccharide (Prager et al 2012). Perhaps some portion of the amygdala enlargement is activity dependent due to immune challenges during the prenatal period or early infancy. In addition, hyperactivity of the amygdala can have modulatory effects on gastrointestinal activity (Myers and Greenwood-Van Meerveld, 2009) through monosynaptic gut-related neurons in the dorsal motor nucleus of the vagus and nucleus of the solitary tract (Zhang et al 2003). Our data are intriguing in establishing an association between dendritic cells, amygdala growth and gastrointestinal symptoms. More research is warranted to determine which of these factors are driving and which are driven.
In conclusion, dendritic cells play many important roles in directing and controlling immune responses. Data from the current study shows that there are increased circulating frequencies of blood myeloid dendritic cells in young children with ASD. These increased frequencies were associated with increased amygdala volumes and increased repetitive behaviors. These are new and novel findings that require further investigation to determine dendritic cell function in the context of early neurodevelopment both in typically developing children and children with ASD. Although we find increased frequencies of myeloid dendritic cells in children between the ages of 2 and 4 years of age, nothing is known about possible fluctuations in the frequencies before diagnosis of ASD or throughout development. The results from this study warrant further follow-up in a replication study to measure dendritic cell frequencies longitudinally and determine whether dendritic cells are dysfunctional in ASD. Such future studies may help elucidate the physiological role of dendritic cells during typical neurodevelopment and may help towards our understanding of the initiation and progression of neurodevelopmental disorders such as ASD.
Highlights.
Increased frequencies of myeloid dendritic cells observed in children with autism spectrum disorder were associated with larger amygdala volumes and more impaired behaviors
Acknowledgements
This study was funded by the National Institute of Neurological Disorders and Stroke (R21HD065269), National Institutes of Mental Health (1R01MH089626, U24MH081810) Autism Speaks Foundation (7567), the Jane Botsford Johnson Foundation, National Alliance for Research on Schizophrenia and Depression, and, the Peter Emch Foundation, and the UC Davis MIND Institute. The commitment of the families who took part in these studies, at both the M.I.N.D Institute and as part of the APP study, is gratefully acknowledged. We would like to thank Amanda Enstrom, Charity Onore, Milo Careaga, the staff of both the UC Davis M.I.N.D. Institute and the APP study for their technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures The authors report no conflicts of interest for this study.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th edition American Psychiatric Association Publishing Inc; Washington DC, USA: 2000. [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011a;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011b;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain Behav Immun. 2011c;25(5):840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nature neuroscience. 2010;14(2):163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, Elia M, Schneider C, Melmed R, Sacco R, Persico AM, Levitt P. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103:16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, Buie TM, Winter H, Bauman M, Sutcliffe JS, Perrin JM, Levitt P. Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics. 2009;123:1018–1024. doi: 10.1542/peds.2008-0819. [DOI] [PubMed] [Google Scholar]
- Careaga M, Van de Water J, Ashwood P. Immune dysfunction in autism: a pathway to treatment. Neurotherapeutics. 2010;7:283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, Persico AM. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol Dis. 2008;30(3):303–311. doi: 10.1016/j.nbd.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Hanly A, Petito CK. HLA-DR-positive dendritic cells of the normal human choroid plexus: a potential reservoir of HIV in the central nervous system. Hum. Pathol. 1998;29:88–93. doi: 10.1016/s0046-8177(98)90395-1. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum M..S, Wei, T.C., Hof PR, Cartwright C, Bienstock CA, Hollander E. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonace imaging. American Journal of Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- Heo Y, Zhang Y, Gao D, Miller VM, Lawrence DA. Aberrant immune responses in a mouse with behavioral disorders. PLoS One. 2011;6(7):e20912. doi: 10.1371/journal.pone.0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer L, Braunschweig D, Ashwood P, Van de Water J, Campbell DB. Association of a MET genetic variant with autism-associated maternal autoantibodies to fetal brain proteins and cytokine expression. Translational Psychiatry. 2011;1:e48. doi: 10.1038/tp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–34. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Kivisakk P, Mahad DJ, Callahan MK, Sikora K, Trebst C, Tucky B, Wujek J, Ravid R, Staugaitis SM, Lassmann H, Ransohoff RM. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann. Neurol. 2004;55:627–638. doi: 10.1002/ana.20049. [DOI] [PubMed] [Google Scholar]
- Lanteaume L, Khalfa S, Régis J, Marquis P, Chauvel P, Bartolomei F. Emotion induction after direct intracerebral stimulations of human amygdala. Cerebral Cortex. 2007;17(6):1307–1313. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
- Matyszak MK, Perry VH. The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience. 1996;74:599–608. doi: 10.1016/0306-4522(96)00160-1. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Role of anxiety in the pathophysiology of irritable bowel syndrome: importance of the amygdala. Front Neurosci. 2009;3:47. doi: 10.3389/neuro.21.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MMWR Prevalence of Autism Spectrum Disorders --- Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill. Summ. 2009;58:1–20. [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66(5):509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, Shaw D, Artru A, Dager SR. Amygdalar volume and behavioral development in autism. Arch Gen Psychiatry. 2006;63(6):686–693. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Simon TJ, Zierhut C, Solomon M, Rogers SJ, Amaral DG. Brief report: methods for acquiring structural MRI data in very young children with autism without the use of sedation. J Autism Dev Disord. 2008;38(8):1581–1590. doi: 10.1007/s10803-007-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, Simon TJ, Rogers S, Ozonoff S, Amaral DG. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci U S A. 2011;108(50):20195–20200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Scholz R, Yang X, Buonocore MH, Simon T, Rogers S, Amaral DG. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Arch Gen Psychiatry. 2012;69(1):53–61. doi: 10.1001/archgenpsychiatry.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunishi K, Dohi M, Nakagome K, Tanaka R, Mizuno S, Matsumoto K, Miyazaki J, Nakamura T, Yamamoto K. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol. 2005;175:4745–4753. doi: 10.4049/jimmunol.175.7.4745. [DOI] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26(3):383–92. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen SJ, Hulshoff Pol HE, Kemner C, Schnack HG, Durston S, Lahuis BE, Kahn RS, Van Engeland H. Increased gray-matter volume in medication-naive high-functioning children with autism spectrum disorder. Psychol Med. 2005;35(4):561–570. doi: 10.1017/s0033291704003496. [DOI] [PubMed] [Google Scholar]
- Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124:480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- Pashenkov M, Teleshova N, Kouwenhoven M, Smirnova T, Jin YP, Kostulas V, Huang YM, Pinegin B, Boiko A, Link H. Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. J. Neuroimmunoil. 2002;122:106–116. doi: 10.1016/s0165-5728(01)00451-9. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behavioural brain research. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Prager G, Hadamitzky M, Engler A, Doenlen R, Wirth T, Pacheco-López G, Krügel U, Schedlowski M, Engler H. Amygdaloid Signature of Peripheral Immune Activation by Bacterial Lipopolysaccharide or Staphylococcal Enterotoxin B. J Neuroimmune Pharmacol. 2012 doi: 10.1007/s11481-012-9373-0. (in press) [DOI] [PubMed] [Google Scholar]
- Richez C, Schaeverbeke T, Dumoulin C, Dehais J, Moreau JF, Blanco P. Myeloid dendritic cells correlate with clinical response whereas plasmacytoid dendritic cells impact autoantibody development in rheumatoid arthritis patients treated with infliximab. Arthritis Res Ther. 2009;11(3):R100. doi: 10.1186/ar2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13(6):433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Rose S, Melnyk S, Trusty TA, Pavliv O, Seidel L, Jingyun Li J, Nick T, James SJ. Intracellular and Extracellular Redox Status and Free Radical Generation in Primary Immune Cells from Children with Autism. Autism Research and Treatment Article ID 986519. 2012 doi: 10.1155/2012/986519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutella S, Bonanno G, Procoli A, Mariotti A, de Ritis DG, Curti A, Danese S, Pessina G, Pandolfi S, Natoni F, Di Febo A, Scambia G, Manfredini R, Salati S, Ferrari S, Pierelli L, Leone G, Lemoli RM. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood. 2006;108:218–227. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26(29):7674–9. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66(10):942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Nordahl CW. Bridging the gap between MRI and postmortem research in autism. Brain Res. 2011;1380:175–86. doi: 10.1016/j.brainres.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serot JM, Foliguet B, Bene MC, Faure GC. Ultrastructural and immunohistological evidence for dendritic-like cells within human choroid plexus epithelium. Neuroreport 8, 1995– 1998. Neuroimmunol. 1997;122:106–116. doi: 10.1097/00001756-199705260-00039. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain sructural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13(10):1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- Sweeten TL, Posey DJ, McDougle CJ. High blood monocyte counts and neopterin levels in children with autistic disorder. Am J Psychiatry. 2003;160:1691–1693. doi: 10.1176/appi.ajp.160.9.1691. [DOI] [PubMed] [Google Scholar]
- Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–42. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cui J, Tan Z, Jiang C, Fogel R. The central nucleus of the amygdala modulates gut-related neurons in the dorsal vagal complex in rats. J Physiol. 2003;553(Pt 3):1005–1018. doi: 10.1113/jphysiol.2003.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]


