Abstract
A sensitive liquid chromatography tandem mass spectrometry (LC-MS/MS) assay was developed and validated to facilitate the assessment of clinical pharmacokinetics of dolutegravir (DTG) in plasma samples. This work describes an assay system requiring only a 20 μL aliquot of human plasma that is subjected to a simple acetronitrile protein precipitation containing a stably labeled isotope of DTG used as an internal standard. Chromatography was performed on an XBridge C18, 2.1 × 50 mm, reversed phase analytical column, using a 60:40 acetonitrile/water mobile phase containing 0.1 % formic acid. Detection of the analyte and internal standard was achieved by ESI positive ionization tandem mass spectrometry. The precursor/product transitions (m/z) monitored were 420.1/136.0 and 428.1/283.1 for DTG and DTG-IS, respectively. The dynamic range of this assay extends from 5 to 10,000 ng/mL, with a mean coefficient of determination (r, mean ± SD) of 0.9996 ± 0.0003. The mean precision values for calibration standards ranged from 0.7 to 4.1 %, while accuracy values were 98.3 to 102.0 %. Validation results demonstrated high accuracy (≤ 6.5 % deviation) and high precision (≤ 9.1 % CV) for the quality control samples. This assay system provides an accurate, precise, and sensitive method for DTG quantitation and was successfully applied to clinical research samples as part of a phase I/II pediatric clinical trial.
Keywords: HIV, integrase inhibitor, mass spectrometry, human, dolutegravir
1. Introduction
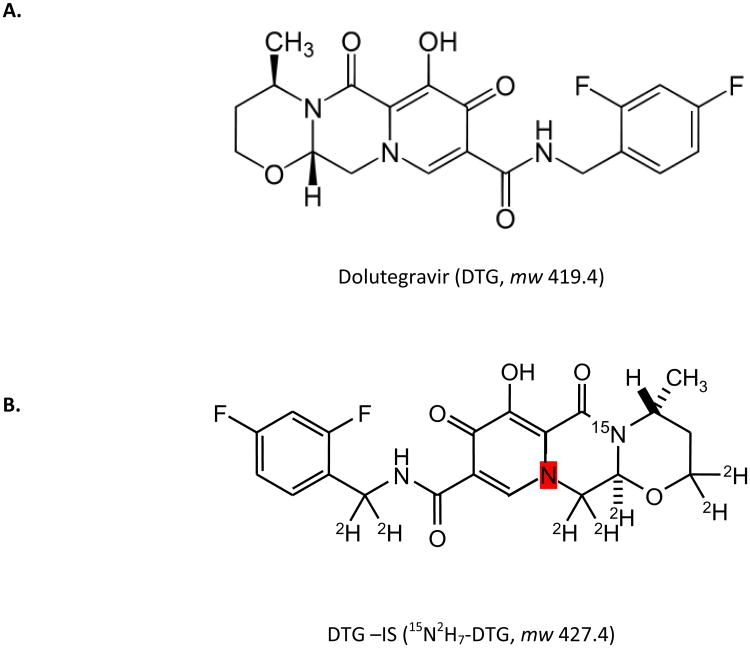
Dolutegravir (DTG, S/GSK-1349572, Figure 1) is a newly developed human immunodeficiency virus (HIV) integrase inhibitor from ViiV Healthcare (Research Triangle Park, NC, USA). DTG is an integrase strand transfer inhibitor (INSTI) that does not require ritonavir for cytochrome P450 3A4 inhibition, and preferentially blocks the strand transfer step of integration of the viral genome into the host cell's DNA [1], which is a two-step process mediated by the viral integrase enzyme. Like the other approved INSTIs raltegravir (RAL) and elvitegravir (EVG), DTG inhibits the binding of the integrase-viral DNA complex to host cell DNA by chelating Mg2+ ions in the active site [2]. Once integration is blocked, HIV-1 can no longer replicate, and the viral replication cycle is interrupted.
Figure 1.
The chemical structures of (A.) DTG and (B.) DTG-IS.
In phase II trials, DTG has been shown to be highly effective at rapidly decreasing viral burden, with a concomitant increase in CD4+ cell count, in treatment-naïve patients receiving 10, 25 or 50 mg once-daily along with a nucleoside reverse transcriptase inhibitor (NRTI) background [3]. Moreover, when the DTG dosing groups were compared to the 600 mg efavirenz (EFV) dose group, the response was more rapid for all DTG groups, Phase III studies in treatment-naïve subjects demonstrate that DTG has sustained antiviral activity comparable to standard of care in combination with dual NRTIs [4,5]. DTG was also shown to be superior to RAL as part of a combination regimen in treatment-experienced, integrase inhibitor-naïve subjects [6].In addition, DTG has been shown to retain in vitro activity against a large variety of viral phenotypes no longer susceptible to RAL [7]. This translates into in clinical data demonstrating DTG's activity in subjects with resistance to RAL [8].
DTG has been well tolerated in Phase III studies with a low incidence of discontinuation due to adverse events [4,5,6] The most common adverse events of moderate to severe intensity in these trials were insomnia and headache. Additional studies to investigate the metabolism and disposition of DTG indicate the primary route of metabolism is glucuronidation via UDP-glucuronosyl transferase 1A1 (UGT1A1), without induction or inhibition of cytochrome P450 enzymes [9,10]. Since DTG will be administered as part of a multi-drug regimen, the lack of significant interactions with other antiretroviral agents is clinically advantageous.
INSTIs are the newest class of antiretrovirals (ARVs) demonstrating potent anti-HIV activity. With DTG retaining activity in a variety of INSTI resistant phenotypes, having an excellent safety and tolerability profile, and predictable pharmacokinetic (PK) profile with low to moderate inter-subject variability, it has the potential for treatment-experienced patients, but also holds promise to become a first-line antiretroviral agent, and will likely become a commonly used component of antiviral regimens. Therefore, to help further our understanding of DTG plasma pharmacokinetics, we have developed an assay for DTG using a simple protein precipitation extraction and liquid chromatography tandem mass spectrometry (LC-MS/MS) detection in order to measure plasma drug concentrations. The general aspects of the assay have been briefly described in previously published DTG pharmacokinetic analyses [4,11,12,13,14]. This work describes the validation of a slight adaptation of those assays for a method that widens the dynamic range of the assay (5 to 10,000 ng/mL) to encompass the range of most clinical samples analyzed.
2. Experimental
2.1. Reagents
All solvents, including HPLC-grade methanol (MeOH), isopropanol (IPA), acetonitrile (AcN), and dimethyl sulfoxide (DMSO), were obtained from Fisher Scientific (Pittsburg, PA). Formic acid (88%) was also obtained from Fisher Scientific (Pittsburg, PA). 0.5M ethylenediamine tetraacetic acid (EDTA) solution (pH = 8) was obtained from Promega via Fisher Scientific (Pittsburg, PA). GlaxoSmithKline (Research Triangle Park, NC) provided both DTG sodium salt and a stably labeled isotope of DTG ([15N2H7]-GSK1349572) which served as the internal standard (DTG-IS). Multiple lots of K2 and K3EDTA treated plasma was obtained from Biological Specialties Corporation (Colmar, PA). Water was purified on-site using a Milli-Q Advantage A-10 Water purification system from Millipore Corporation (Billerica, MA) with a Q-Guard 0.22 μm point-of-use filter and LC-PAK to produce ultrapure water with minimal trace organic contamination.
2.2. Instrumentation and Software
A Shimadzu Integrated UFLC-XR system (consisting of a CMB20A controller, 2 LC20ADXR pumps, a GDU20A3 degasser, a SIL20ACXR cooled autosampler, and a CTO20AC column oven) coupled with an AB Sciex Linear Ion Trap Quadrupole 5500 (AB Sciex Instruments, Foster City, CA) were utilized for the separation and detection of DTG and DTG-IS. Both the UFLC and mass spectrometer were controlled remotely using Analyst software v. 1.5.1 (AB Sciex, Foster City, CA). Chromatographic integrations and data calculations were performed with the MultiQuant module v. 2.0.2 that comes as an addition to Analyst. All statistical calculations were performed using Microsoft Excel capabilities.
2.3. HPLC separation and MS-MS detection
All samples were subjected to chromatographic separation using a Shimadzu integrated UFLC-XR system with an XBridge C18 analytical column (3.5 μm, 50 × 2.1 mm, Waters Corp., Milford, MA). Chromatographic analyses were performed isocratically at 30°C and at a flow rate of 0.475 mL/min, with an overall run time of 1.5 min. The mobile phase was composed of 0.1 % formic acid in H2O:0.1 % formic acid in AcN (60:40, v/v). Samples are maintained at 15°C in the autosampler with a 5 μL aliquot of each sample being injected onto the column. The needle was washed before sample aspiration with a 0.1 % formic acid solution in IPA:MeOH (20:80, v/v), to help minimize carryover between injections. DTG and DTG-IS eluted from the column at approximately 0.75 min, and were detected using multi reaction monitoring (MRM). The API 5500 instrument was used in positive TurbolonSpray mode and the following transitions for protonated products [M+H]+ were monitored and acquired: m/z DTG, 420.1→136.0; m/z DTG-IS 428.1→283.1. Settings for the individual mass spectrometer parameters are listed in Table 1. Traces correlating to the above transitions were integrated using the MultiQuant software and concentration values were obtained using DTG to DTG-IS peak area ratio.
Table 1. Summary of MS/MS Parameters Optimized for DTG Detection.
| MS Settings | ||
|---|---|---|
| General | TEM | 550 |
| GS1 | 60 | |
| GS2 | 60 | |
| CUR | 25 | |
| CAD | HIGH | |
| DP | 186 | |
| EP | 10 | |
|
|
||
| DTG Specific | CE | 75 |
| CXP | 12 | |
|
|
||
| DTG-IS Specific | CE | 41 |
| CXP | 24 | |
2.4. Stock solutions, working solutions, plasma calibration and control samples
A master stock solution of DTG was prepared in DMSO at a concentration of 1 mg/mL This solution was diluted in 1:1-AcN:water to make a series of eight calibration curve working stock solutions at 0.1, 0.2, 1, 2, 10, 20, 100, and 200 μg/mL, and four quality control (QC) working stock solutions at 0.1, 2, 40 and 400 μg/mL. A separate weighing of DTG was used to verify concentration but only one solution was used for preparation of calibration and QC working stock solutions. A stock solution of DTG-IS was also prepared in DMSO at a concentration of 1 mg/mL and subsequently diluted to 10 μg/mL in AcN. Calibration curves were prepared in plasma for each run by adding 5 μL of the appropriate level of calibration working stock to 95 μL of freshly thawed K2 or K3EDTA plasma in microcentrifuge tubes, which was then thoroughly mixed. The resulting concentrations in plasma were 5, 10, 50, 100, 500, 1000, 5000, and 10,000 ng/mL due to the 1:20 dilution that occurs when preparing a standard curve in plasma on a routine basis.
Plasma QC samples were prepared by the addition of the appropriate level of QC working stock to plasma resulting in final concentrations of 5, 15, 450, 9000, and 10,000 ng/mL. Aliquots of 100 μL were prepared and stored at -80°C prior to use. All master stocks and working stocks were stored at 4°C.
2.5. Sample preparation
Extraction of DTG from 20 μL aliquots of calibration standards, QC samples and unknown (patient) plasma was achieved using a simple protein precipitation in 120 μL of AcN spiked with internal standard at a concentration of 10 ng/mL Following a mixing step on the orbital shaker (@ 1500 rpm, 2 min), precipitate was pelleted by centrifugation for 5 min at 5000 rpm (2655 × g). A subsequent dilution step was performed by adding a 20 μL aliquot of supernatant to 120 μL of 1 mg/mL EDTA (pH 8) in 0.1% formic acid and mixing well before injection onto the HPLC-MS/MS for analysis.
2.6. Analytical Method Validation
The validation of this method was based on the FDA Bioanalytical Method Validation publication [15].
2.6.1. Accuracy and Precision
Each validation run included one blank plasma sample, one zero sample (blank plasma with DTG-IS only), and eight calibration standards at 5, 10, 50, 100, 500, 1000, 5000 and 10,000 ng/mL In addition, replicate analysis (n=6) of QC samples were used for precision and accuracy determinations over five separate days. Five different concentrations were chosen to encompass the lower limit of quantitation (LLOQ, 5 ng/mL), upper limit of quantitation (ULQ, 10,000 ng/mL) and fill out the range of the calibration curve using a low (15 ng/mL), mid (450 ng/mL), high (9000 ng/mL) concentration. Precision was calculated as the coefficient of variation (%CV) within a single run (intra-assay) and across all assay dates (inter-assay), and accuracy as the percentage of deviation between the nominal and calculated concentration. Peak area ratios were calculated as detector response of analyte versus detector response of the IS for the standard curve, QC, and unknown samples. The resulting standard curve data is fit to a weighted linear regression of 1/concentration2. The standard curve parameters are then used to determine the QC and unknown sample concentrations from the peak area ratios already obtained for these samples. The results of these runs determine the inter-day and intra-day precision and accuracy values. Acceptance criteria were such that calibration curves had to have an r ≥0.98 and the back-calculated values of standards used to created calibration curves were required to be within ± 15% of the nominal concentration, except at the LLOQ where 20% was accepted. Standards could be excluded if they did not meet these criteria, but sequential standards could not be excluded. Concentrations of LLOQ, ULQ, and other QC samples were determined from calibration curves created with each run and at least four out of six at each level had to be within 15% of the nominal value.
2.6.2. K2 and K3EDTA plasma equivalence
To demonstrate the equivalence of K2 and K3EDTA plasma, QCs were also prepared in K2EDTA at 15, 450, and 9000 ng/mL and frozen at -80°C. The K2EDTA QCs were extracted and quantitated against a standard curve prepared in K3EDTA plasma and conversely K3EDTA QCs were extracted and quantitated against a standard curve prepared in K2EDTA plasma. The mean, standard deviation, precision and accuracy were calculated from the replicates for one run (n=3).
2.6.3. Sample dilution
The ability to accurately dilute a sample that has a concentration above the ULQ was examined by spiking blank plasma sample at 30,000 ng/mL, which is three times the highest calibrator. Blank plasma once spiked was frozen at -80°C for a few days and then processed using triplicate sets of three dilution ratios (1/5, 1/10, and 1/20) to investigate dilution integrity, as well as partial volume precision and accuracy.
2.6.4. Matrix effects, recovery, and selectivity
To evaluate recovery, matrix effects, and process efficiency of this assay system, three sets of samples were prepared and evaluated. These concepts were investigated by comparing DTG concentrations obtained from neat analyte solutions spiked with representative low, mid, and high concentrations (10, 100, and 1000 ng/mL, respectively), extracted plasma samples spiked with analyte post-extraction, and pre-extraction spiked plasma samples, across six independent plasma lots.
DTG assay selectivity was tested by extracting DTG in the presence of possible multiple concomitant medications at concentrations near the maximum plasma concentration that could be expected for each drug. Other antiretrovirals used in the treatment of HIV, along with a couple of antiviral medications were investigated for possible interference with the DTG assay system. These included 8 nucleoside/nucleotide reverse transcriptase inhibitors (didanosine, stavudine, lamivudine, zalcitabine, zidovudine, abacavir, tenofovir and emtricitabine), 3 non-nucleoside reverse transcriptase inhibitors (nevirapine, efavirenz, and etravirine), 8 protease inhibitors (amprenavir, indinavir, darunavir, saquinavir, atazanavir, lopinavir, ritonavir, and nelfinavir), a CCR5 receptor antagonist (maraviroc), a second integrase inhibitor (RAL), and 2 antiviral drugs (acyclovir and ganciclovir). Plasma containing other concomitant medications was spiked at the low DTG QC concentration (15 ng/mL) and compared to control samples containing DTG only. Assays were extracted in triplicate and run sequentially to verify that the secondary drug would not carryover and interfere with subsequent injections.
2.7. Analyte stability
Stability studies performed for dolutegravir included examinations of stock solutions, short and long term plasma storage, freeze-thaw investigations in plasma, and post-preparative performance in injection matrix.
2.7.1. Stock solution stability
Stability of DTG stock solution, prepared at 1 mg/mL in DMSO, was determined when stored at 4°C for up to 13 months. Previously prepared DTG master stock solution was compared to freshly prepared stock solution by diluting both stocks by 1/1000 with 50% AcN, which were then further diluted 1/1000 in the 1 mg/mL EDTA solution in 0.1 % formic acid injection solvent, before replicate (n=5) injections were made. Peak areas were used for comparison and the percentage difference was calculated between the old and new master stock solutions.
2.7.2. Plasma stability
Plasma samples containing DTG are stored at -80°C once received by our laboratory. In order to be consistent with patient samples, aliquots of each QC level (low, mid, and high) were prepared and stored at -80°C. Aliquots were then subjected to three rounds of freezing and thawing, where the thawing component was comprised of sitting at room temperature for approximately five hours and then returned to the freezer overnight for a minimum of twelve hours. This was repeated for a total of three freeze-thaw cycles. Samples were then run in triplicate, and compared to freshly thawed QC samples used as a control. Furthermore, after QC samples were prepared, a few aliquots were maintained at -80°C for more extended periods of time to monitor long-term plasma stability. Aliquots were removed at 3 month, 6 month and 15 month intervals analyzed in triplicate in comparison to newly prepared QC samples of the same concentrations which served as controls.
2.7.3. Post-preparative stability
The ability to re-inject samples following a weekend in the autosampler was investigated by extracting and running a full validation assay on a Friday afternoon. The unused volume remained in the autosampler over the weekend and all samples were re-analyzed on the following Monday in order to simulate equipment failure. The resulting calibration curves and sample values were calculated within each run and cross-checked between runs (the calibration curve from run 1 was used to calculate sample values from run 2). This experimental design permitted us to verify the integrity of results obtained after sample sat over a weekend for both a full run and re-analysis of partially obtained data if equipment failed and sample volumes were inadequate for re-injection (i.e., some data were obtained on a Friday and the remaining data acquired on the following Monday).
3. Results and Discussion
3.1. Chromatography, detection, and quantitation of DTG
Initial optimization of the triple quadrupole mass spectrometer for the detection of an analyte begins with optimizing for the parent compound, in this case DTG, in the single-quadrupole mode. This step of optimization revealed a large peak at [M+H]+ for DTG at m/z 420.1, which correlated with the expected molecular ion mass of DTG. Five main product ions were found for DTG at m/z 277.1, 127.0, 136.0, 101.0 and 107.0 and mass spectrometer conditions were initially optimized for detection of DTG (Table 1) and its three strongest product ions. Plasma samples were then spiked with DTG, extracted and reconstitution volume and product ions were optimized further. Ultimately, only a single channel each for DTG and DTG-IS (m/z 420.1→136.0) and (m/z 428.1→283.1) was chosen and used for quantitation (Figure 2).
Figure 2.
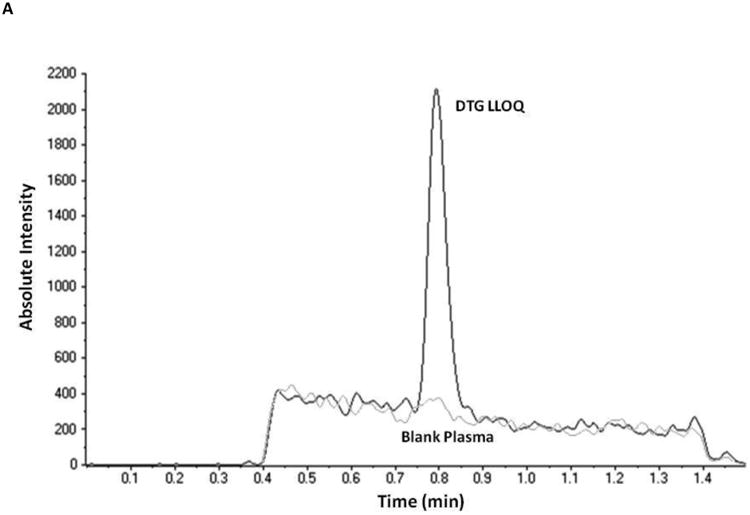
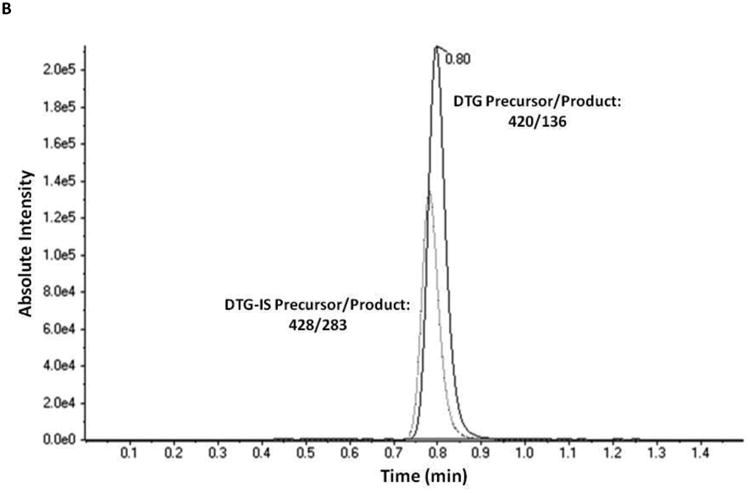
Representative chromatograms obtained by applying the present method to: A.) a double blank extracted EDTA-plasma sample overlaid with the lowest standard (5 ng/mL); B.) a mid-range quality control sample (450 ng/mL).
Under the conditions set forth, the separation of DTG and DTG-IS from human plasma components was successfully achieved (Figure 2). No interfering peaks were detected across the six independent lots of human plasma, utilized during assay development and validation, as seen in extracted blank samples. Furthermore, when a plasma sample spiked with a high concentration of DTG (9000 ng/mL final concentration) was extracted without DTG-IS, no peak was seen in the IS channel. Examination of the single blank plasma samples containing DTG-IS but no DTG, revealed no significant interferences with DTG (<20% of the LLOQ peak area). DTG maintained its linearity from 5 to 10,000 ng/mL with minimal loss of signal intensity at the upper end of the curve. The correlation coefficient for the five validation runs was 0.9996 ± 0.0002 (r, mean ± SD).
3.2. Assay validation
Validation runs were performed on five different days. Each validation run contained blank and ‘zero’ control samples, a full 8-point curve, plus six replicates each of LLOQ, low, mid, high, and ULQ quality control standards (5, 15, 450, 9000 and 10,000 ng/mL, respectively). Overall inter-day precision values calculated from the back-calculated concentrations for the calibration curve standards ranged from 0.7 to 4.1%, while accuracy ranged from 98.3 to 102.0% (Table 2A). Concentrations determined for the LLOQ, low, mid, high and ULQ quality control standards (six replicates per validation run) were utilized to calculate the intra-day precision and accuracy values. The mean intra-day precision values for the five validation assays ranged from 0.5 to 12.1%, while the mean accuracy values were 90.2 to 110.3% (Table 2B). The quality of the standard curves was evaluated by the mean correlation coefficient (r = 0.9996 ± 0.0003) and the reproducibility of the slope and intercept; mean slope was 0.0046 ± 0.0002 and intercept values ranged from 0.0024 to 0.0073.
Table 2. A. Inter-Assay Accuracy and Precision of DTG in Human Plasma.
| DTG Interassay Statistics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nominal conc. | 5 | 10 | 50 | 100 | 500 | 1000 | 5000 | 10000 | slope | r |
|
|
||||||||||
| mean | 5.00 | 10.02 | 51.02 | 100.90 | 505.36 | 988.10 | 4936.10 | 9830.82 | 0.0046 | 0.9996 |
| SD | 0.10 | 0.41 | 0.54 | 2.37 | 12.96 | 17.91 | 33.96 | 175.73 | 0.0002 | 0.0003 |
| %CV | 2.0 | 4.1 | 1.1 | 2.3 | 2.6 | 1.8 | 0.7 | 18.0 | 4.2 | 0.0 |
| %dev | 0.0 | 0.2 | 2.0 | 0.9 | 1.1 | -1.2 | -1.3 | -1.7 | ||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
|
| ||||||||||
| nominal conc. | 5 | 15 | 450 | 9000 | 10000 | |||||
| mean | 5.27 | 14.54 | 427.62 | 8412.55 | 10115.99 | |||||
| SD | 0.48 | 0.89 | 16.60 | 273.21 | 441.14 | |||||
| %CV | 9.1 | 6.1 | 3.9 | 3.4 | 4.4 | |||||
| %dev | 5.4 | -3.1 | -5.0 | -6.5 | 1.2 | |||||
| n | 30 | 29 | 30 | 28 | 30 | |||||
| B. Intra-Assay Accuracy and Precision of DTG in Human Plasma | |||||
|---|---|---|---|---|---|
|
| |||||
| DTG Intraassay Statistics | |||||
| nominal conc. | 5 | 15 | 450 | 9000 | 10000 |
| mean | 4.72 – 5.52 | 13.88 – 15.34 | 411.65 – 439.13 | 8122.25 – 8774.57 | 9723.93 – 10666.60 |
| SD | 0.264 – 0.640 | 0.288 – 1.230 | 6.618 – 21.064 | 46.301 – 153.833 | 135.241 – 518.114 |
| %CV | 4.8 to 12.1 | 2.0 to 8.0 | 1.5 to 5.0 | 0.5 to 1.8 | 1.3 to 5.1 |
| %dev | -5.7 to 10.3 | -7.4 to 2.3 | -8.5 to -2.4 | -9.8 to -2.5 | -2.8 to 6.7 |
| n | 5 | 5 | 5 | 5 | 5 |
To demonstrate equivalence of K2 and K3EDTA, intra-day precision and accuracy were obtained for three replicates at three QC concentrations, low (15 ng/mL), mid (450 ng/mL), and high (9000 ng/mL). The mean, standard deviation, precision and accuracy were calculated from the replicates for one run (n=3). Equivalence data for K2EDTA controls analyzed against a K3EDTA curve and K3EDTA controls analyzed against a K2EDTA curve are shown in Table 3. The precision of the non-matrix matched low, mid, and high quality controls prepared in K2EDTA ranged from 0.7 to 7.2%, while the accuracies ranged from -8.3 to -3.6%. The precision of the non-matrix matched low, mid, and high quality controls prepared in K3EDTA ranged from 1.6 to 4.5%, while the accuracies ranged from 2.4 to 5.6%. Although the K3EDTA samples seemed to have slightly higher concentrations than their K2EDTA counterparts, all levels showed <10% difference from their matrix matched QCs and were considered equivalent.
Table 3. K2 and K3EDTA Plasma Equivalence and Dilution Accuracy.
| DTG in Plasma Matrix | |||||||
|---|---|---|---|---|---|---|---|
| EDTA Equivalence | K2EDTA | K3EDTA | |||||
|
| |||||||
| Low | Mid | High | Low | Mid | High | ||
|
| |||||||
| 15 | 450 | 9000 | theoretical conc. | 15 | 450 | 9000 | |
| 14.10 | 412.67 | 8676.90 | mean | 15.83 | 460.87 | 9236.90 | |
| 1.02 | 22.75 | 59.93 | SD | 0.31 | 20.72 | 149.29 | |
| 7.2 | 5.5 | 0.7 | %CV | 1.9 | 4.5 | 1.6 | |
| -6.0 | -8.3 | -3.6 | %dev | 5.6 | 2.4 | 2.6 | |
| -9.2 | -3.5 | -3.3 | %diff* | 9.0 | 3.5 | 3.3 | |
|
| |||||||
| Dilutions of Ultra High QC | K2EDTA | K3EDTA | |||||
|
| |||||||
| 1/5 dil | 1/10 dil | 1/20 dil | 1/5 dil | 1/10 dil | 1/20 dil | ||
|
| |||||||
| 6000 | 3000 | 1500 | theoretical conc. | 6000 | 3000 | 1500 | |
| 5477.57 | 3004.63 | 1459.60 | mean | 5615.77 | 2863.67 | 1457.73 | |
| 173.29 | 24.99 | 17.13 | SD | 184.31 | 58.19 | 45.08 | |
| 3.2 | 0.8 | 1.2 | %CV | 3.3 | 2.0 | 3.1 | |
| -8.7 | 0.2 | -2.7 | %dev | -6.4 | -4.5 | -2.8 | |
%difference as compared to matrix matched QCs, determined by the following equation: (mean unmatched – mean matched)/[((mean unmatched + mean matched)/2)*100].
The ability to use partial volumes or dilution of samples that are above the upper limit of quantitation was determined by testing three dilutions (1/5, 1/10, and 1/20) of a 30,000 ng/mL sample prepared in either K2 or K3EDTA. Mean accuracy values ranged from 91.3 to 100.2%, and precision values ranged from 0.8 to 3.3% across dilutions and plasma types (Table 3). The results from this experiment demonstrates the ability to freeze and store a sample at a high concentration, accurately quantitate it after thawing and dilution, and that dilutions up to 1/20 are acceptable for quantitation of samples and should cover the range of systemic peak concentrations measured in patient samples.
Another important aspect of the DTG assay validation involved determining analyte plasma stability under three distinct conditions; freeze-thaw stability, long term storage stability and short term post-preparative stability in the injection matrix. All patient plasma samples containing DTG are stored at -80°C along with aliquots of each QC level after preparation. A comparison was made between aliquots that were subjected to three rounds of freezing and thawing and freshly thawed QC samples used as a control, run in triplicate. The thawing component was also used as an indicator of short-term room temperature stability since the aliquots were maintained at room temperature for approximately five hours before being returned to the freezer overnight. As seen in Table 4, all test samples were within 5% of the matching control QC level. Therefore, DTG is considered to be stable through three freeze-thaw cycles when stored at -80° C and for at least 5 hours at room temperature.
Table 4. Stability of DTG Under Various Conditions.
| DTG in Plasma Matrix | |||||||
|---|---|---|---|---|---|---|---|
| 3 F/T Cycles | Control Samples | Test Samples | |||||
|
| |||||||
| Low | Mid | High | Low | Mid | High | ||
|
| |||||||
| 15 | 450 | 9000 | theoretical conc. | 15 | 450 | 9000 | |
| 13.5 | 411.3 | 8201.5 | mean | 12.8 | 405.1 | 8070.6 | |
| 0.5 | 12.1 | 179.1 | SD | 0.2 | 2.5 | 229.0 | |
| 3.3 | 2.9 | 2.2 | %CV | 1.2 | 0.6 | 2.8 | |
| -10.2 | -8.6 | -8.9 | %dev | -14.4 | -10.0 | -10.3 | |
| %diff* | -4.7 | -1.5 | -1.6 | ||||
|
|
|||||||
| Long Term (15 months) | Control Samples | Test Samples | |||||
|
| |||||||
| Low | Mid | High | Low | Mid | High | ||
|
| |||||||
| 15 | 450 | 9000 | theoretical conc. | 15 | 450 | 9000 | |
| 14.9 | 453.9 | 9185.7 | mean | 14.3 | 428.2 | 8482.4 | |
| 0.7 | 13.5 | 101.1 | SD | 0.4 | 11.9 | 39.0 | |
| 4.4 | 3.0 | 1.1 | %CV | 2.7 | 2.8 | 0.5 | |
| -0.7 | 0.9 | 2.1 | %dev | -4.9 | -4.8 | -5.8 | |
| %diff* | -4.3 | -5.7 | -7.7 | ||||
|
| |||||||
| DTG in Injection Matrix | |||||||
|
|
|||||||
| 3 Days in Autosampler 15°C | Control Samples | Test Samples | |||||
|
| |||||||
| Low | Mid | High | Low | Mid | High | ||
|
| |||||||
| 15 | 450 | 9000 | theoretical conc. | 15 | 450 | 9000 | |
| 16.2 | 411.7 | 8262.1 | mean | 15.7 | 402.3 | 8027.2 | |
| 2.4 | 11.5 | 116.9 | SD | 2.5 | 8.4 | 131.7 | |
| %diff* | -3.4 | 0.1 | -0.4 | ||||
|
| |||||||
| DTG Stock in DMSO | |||||||
|
|
|||||||
| 4°C for 13 months | Old Master Stock | New Master Stock | |||||
|
| |||||||
| 2017641 | mean area | 2028447 | |||||
| 56465 | SD | 64842 | |||||
| 2.8 | %CV | 3.2 | |||||
| %diff | 0.5 | ||||||
%difference as compared control samples, determined by the following equation: (mean test – mean control)/[((mean test + mean control)/2)*100].
Long-term DTG stability in plasma was determined using the three QC levels (15 ng/mL, 450 ng/mL, and 9000 ng/mL) when stored at -80°C for an extended period of time. Triplicate QC samples were analyzed at various periods after preparation with newly prepared QCs and DTG concentrations were compared between the stability test and new control samples. For the longest storage period evaluated the deviation from the actual concentration ranged from -5.8 to 2.1%, and only a slight decrease was observed (approximately 5%) over this time frame (Table 4). We concluded that DTG is stable in human plasma stored at -80°C for a period of at least 15 months.
Injection matrix stability was determined by re-injection of samples including a full calibration curve, six each of the LQC, MQC, and HQC samples after uninjected volume remained at 15°C in the autosampler for at least three nights (to simulate an instrument failure over a weekend). Curves were generated on both Friday and Monday. The resulting curves were compared to each other and the curve from the original run was used to calculate the QC standards. The quality of the calibration curves generated after reinjection of samples three days apart resulted in similar slopes (-2.6% difference), intercepts and correlation coefficients, indicating that the curves generated from reinjection of samples after sitting for three days in the autosampler results in nearly identical calibration curves. The difference between the determined mean values for LQC, MQC, and HQC samples when analyzed independently after three days in the autosampler ranged from -3.1 to -2.3% (data not shown). Therefore, if enough sample volume exists to re-analyze an entire analytic run, the results are equivocal between same day analysis and analysis after three days at 15°C. However, if sample volume is a limiting factor and samples are divided so that results are acquired several days apart, the results varied from -3.4 to 0.6% as seen in Table 4, indicating that acquisition of partial sample sets three days apart is also acceptable.
Lastly, long-term DTG stability in stock solutions prepared at 1 mg/mL in DMSO was determined by replicate injections (n=5) of two separate stock solutions stored at 4°C and prepared 13 months apart. Previously prepared DTG master stock solution was compared to the freshly prepared stock solution after equivalent dilution steps into injection matrix. The peak areas were compared and showed only a 0.5% difference between the solutions (Table 4), confirming DTG stability under these conditions for a period of at least 13 months.
3.2. Matrix effects and recovery
Matrix effects in mass spectrometry are defined as direct or indirect alteration or interference in response due to the presence of unintended analytes or other interfering substances in the sample. Six independent lots of plasma were used for these experiments and the summarized data is presented in Table 5. Recovery was calculated by comparing the mean concentrations of pre-extraction spiked samples to plasma matched post-extraction spiked samples. Across the concentration range studied, DTG recovery ranged from 96.5 to 99.3%. Overall matrix effects were calculated by comparing the mean concentrations of post-extraction spiked samples to unextracted analyte spikes. Matrix effects on final DTG concentrations were determined to be minimal with <2% difference between spiked solutions when extracted plasma components were or were not present in the solution. Process efficiency represents the combined effects of extraction recovery and matrix influence on analyte concentration and was calculated by comparing the mean concentrations of pre-extraction spiked samples to unextracted analyte spikes. Across the concentration range studied, the extraction process efficiency for DTG ranged from 95.2 to 99.2%.
Table 5. DTG Recovery and Plasma Matrix Effects.
| DTG/DTG-IS | |||
|---|---|---|---|
| Low | Mid | High | |
|
|
|||
| Matrix Effect | 98.7% | 99.0% | 99.9% |
| Recovery | 96.5% | 98.1% | 99.3% |
| Process Efficiency | 95.2% | 97.2% | 99.2% |
|
|
|||
| Unextracted Analytes | Post-Extraction Spike | Pre-Extraction Spike | |
|
|
|||
| Matrix Effect Slope Precision (n=6 EDTA lots) | 1.1% | 2.2% | 2.5% |
DTG and DTG-IS peak areas were also analyzed independently (versus peak area ratios, which were used in results described above to calculate concentrations). Comparisons were made between the three sets of samples using mean peak areas as well as peak area precision. Evaluation of DTG and DTG-IS peak areas independently indicate some signal enhancement in the presence of extracted matrix components. However, the relative and absolute matrix effects are negligible and the assay is considered valid since any effect on DTG is compensated for by a matching effect on DTG-IS (peak area ratios and precision are consistent, <5%). As a final check for matrix effects, slopes were obtained by fitting a line through the three concentration points for each lot of plasma (replicate injections for unextracted samples). Comparison of the slopes and observation of the variability (%CV) between the six independent lots of plasma confirm the absence of any significant matrix effect on quantitation with all three sets having a CV of <2.5% as seen in Table 5.
Since patients taking DTG will usually be administered other antiretroviral drugs concomitantly, this assay was performed in the presence of commonly used antiviral drugs mentioned previously (see Assay Validation section 2.6.4) and listed individually in Table 6, to asses any possible interferences with regard to DTG quantitation. Plasma spiked at the low QC concentration had other antivirals and antiretrovirals added at a higher concentration (5000 ng/mL), in order to maximize the potential for interference. Plasma was extracted from control samples (containing DTG only) and compared to test samples containing DTG plus other possible concomitantly administered medications. All assays were run in triplicate and the results are presented in Table 6. None of the tested drugs were found to interfere with the performance of this assay system since the mean % of LQC when compared to control samples was ≤9.0%.
Table 6. DTG Assay Selectivity in the Presence of Concomitant Medications.
| DTG | |
|---|---|
|
| |
| Drug Name | Mean % of LQC |
| didanosine (ddI) | 103.7 |
| stavudine (d4T) | 103.0 |
| lamivudine (3TC) | 109.0 |
| zalcitibine (ddC) | 102.7 |
| zidovudine (AZT) | 101.2 |
| abacavir (ABV) | 104.2 |
| tenofovir (TFV) | 99.3 |
| emtricitabine (FTC) | 100.7 |
| nevirapine (NVP | 100.2 |
| efavirenz (EFV) | 102.7 |
| etravirine (ETV) | 104.0 |
| amprenavir (APV) | 97.3 |
| indinavir (IDV) | 102.5 |
| darunavir (DRV) | 94.5 |
| saquinavir (SQV) | 104.7 |
| atazanavir (ATV) | 108.2 |
| lopinavir (LPV) | 99.0 |
| ritonavir (RTV) | 105.0 |
| nelfinavir (NFV) | 99.0 |
| maraviroc (MVC) | 102.2 |
| raltegravir (RAL) | 102.5 |
| acyclovir (ACV) | 106.7 |
| ganciclovir (GCV) | 101.0 |
3.4. Application of the analytical method
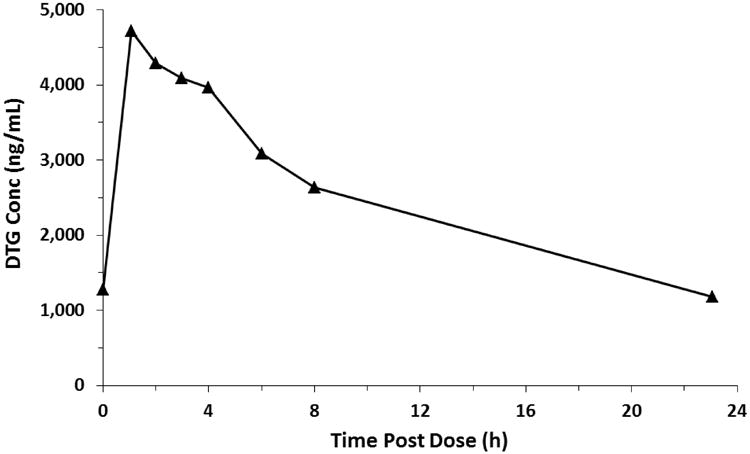
Recently, as part of an ongoing phase I/II clinical trial, 350 plasma samples from HIV-infected adolescents and children receiving a daily dose of approximately 1 mg/kg were analyzed to determine DTG concentrations using the method described herein. Steady-state, intensive pharmacokinetic samples were taken at 0, 1, 2, 3, 4, 6, 8, and 24 hours post-dose. Measurable DTG concentrations ranged from 5.6 to 15407.5 ng/mL with a mean ± SD AUC24 and Cmax were 52 ± 22 mgxh/L and 3.8 ± 1.5 mg/L, respectively. A representative pharmacokinetic curve from a single patient from this study is shown in Figure 3.
Figure 3. A representative steady-state DTG concentration-time profile.
4. Conclusion
DTG is the newest drug in the latest antiretroviral INSTI class under development for the treatment of HIV-1, however no full HPLC-MS/MS assay or complete assay validation has been reported for this drug. The work described herein details the validation of a method for the measurement of dolutegravir in plasma that possesses a wide dynamic range to encompass the range of peak concentrations in adult, adolescent, and juvenile patients receiving targeted doses of dolutegravir. As a result of the increased dynamic range of this assay, fewer samples will have to be diluted and re-analyzed, therefore decreasing turn-around time for patient samples. The current assay has been successfully validated and used to measure plasma DTG concentrations in clinical samples and should be easily transferred, with minimal change, and implemented in other laboratories performing plasma sample analysis using similar equipment.
Highlights.
We validated the first LC-MS/MS assay for dolutegravir quantitation in human plasma
The assay is accurate, and precise, and sensitive with a broad calibration range
The assay was successfully applied to 350 clinical samples in a phase I/II trial
Acknowledgments
Supported in part by: UM1 AI068632 from the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) of the National Institute of Allergy and Infectious Diseases (NIAID) and ViiV Healthcare.
Abbreviations in this manuscript
- AcN
acetonitrile
- ARVs
antiretrovirals
- AUC
area under the concentration-time curve
- CV
coefficient of variation
- DMSO
dimethyl sulfoxide
- DTG
dolutegravir
- EDTA
ethylenediamine tetraacetic acid
- EVG
elvitegravir
- EFV
efavirenz
- HIV
human immunodeficiency virus
- INSTI
integrase strand transfer inhibitor
- IPA
isopropanol
- IS
internal standard
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- LLOQ
lower limit of quantitation
- MeOH
methanol
- MRM
multi reaction monitoring
- NRTI
nucleoside reverse transcriptase inhibitor
- PK
pharmacokinetic
- QC
quality control
- RAL
raltegravir
- UGT1A1
UDP-glucuronosyl transferase 1A1
- ULQ
upper limit of quantitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pommier Y, Johnson A, Marchland C. Nat Rev Drug Discov. 2005;4:236. doi: 10.1038/nrd1660. [DOI] [PubMed] [Google Scholar]
- 2.Katlama C, Murphy R. Opin Investig Drugs. 2012;21:523. doi: 10.1517/13543784.2012.661713. [DOI] [PubMed] [Google Scholar]
- 3.van LUnzen J, Maggiolo F, Arribas R, Rakhmanova A, Yeni P, Young B, Rockstroh J, Almond S, Song I, Brothers C, Min S. Lancet Infec Dis. 2012;12:111. doi: 10.1016/S1473-3099(11)70290-0. [DOI] [PubMed] [Google Scholar]
- 4.Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, Bloch M, Podzamczer D, Pokrovsky V, Pulido F, Almond S, Margolis D, Brennan C, Min S. Lancet. 2013;381:735. doi: 10.1016/S0140-6736(12)61853-4. [DOI] [PubMed] [Google Scholar]
- 5.Walmsley S, et al. Dolutegravir (DTG; S/GSK1349572) + abacavir/lamivudine once daily statistically superior to tenofovir/emtricitabine/efavirenz: 48-week results - SINGLE (ING114467); 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco. 2012. abstract H-556b. [Google Scholar]
- 6.Cahn P, Pozniak AL, Mingrone H, Khuldyakov A, Brites C, Andrade-Villanueva JF, Richmond G, Buendia CB, Fourie J, Ramgopal M, Hagins D, Felizarta F, Madruga J, Reuter T, Newman T, Small CB, Lombaard J, Grinsztejn B, Dorey D, Underwood M, Griffith S, Min S. on behalf of the extended SAILING Study Team. Lancet. 2013 Jul 2; doi: 10.1016/S0140-6736(13)61221-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Underwood R, Johns B, Sato A, Martin J, Deeks S, Fujiwara T. J Acquir Immune Defic Syndr. 2012;61:297. doi: 10.1097/QAI.0b013e31826bfd02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eron JJ, Clotet B, Durant J, Katlama C, Kumar P, Lazzarin A, Poizot-Martin I, Richmond G, Soriano V, Ait-Khaled M, Fujiwara T, Huang J, Min S, Vavro C, Yeo J for the VIKING Study Group. J Infect Dis. 2013;207:740. doi: 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reese MJ, Savina PM, Generaux GT, Tracy H, Humphreys JE, Kanaoka E, Webster LO, Harmon KA, Clarke JD, Polli JW. Drug Metab Dispos. 2013;41:353. doi: 10.1124/dmd.112.048918. [DOI] [PubMed] [Google Scholar]
- 10.Castellino S, Moss L, Wagner D, Borland J, Song I, Shuguang C, Lou Y, Min SS, Goljer I, Culp A, Piscitelli SC, Savina PM. Antimicrob Agents Chemother. 2013 May; doi: 10.1128/AAC.00292-13. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song I, Borland J, Chen S, Lou Y, Peppercorn A, Wajima T, Min S, Piscitelli SC. Br J Clin Pharmacol. 2011;72:103. doi: 10.1111/j.1365-2125.2011.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min S, Song I, Borland J, Chen S, Lou Y, Fujiwara T, Piscitelli SC. Antimicrob Agents Chemother. 2010;54:254. doi: 10.1128/AAC.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel P, Song I, Borland J, Patel A, Lou Y, Chen S, Wajima T, Peppercorn A, Min SS, Piscitelli SC. J Antimicrob Chemother. 2011;66:1567. doi: 10.1093/jac/dkr139. [DOI] [PubMed] [Google Scholar]
- 14.Song I, Borland J, Min S, Lou Y, Chen S, Patel `P, Wajima T, Piscitelli SC. Antimicrob Agents Chemother. 2011;66:3517. doi: 10.1128/AAC.00073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. Bioanalytical Method Validation. Rockville MD: US Department of Health and Human Services, CDER and CVM; May, 2001. Guidance for Industry. [Google Scholar]