Abstract
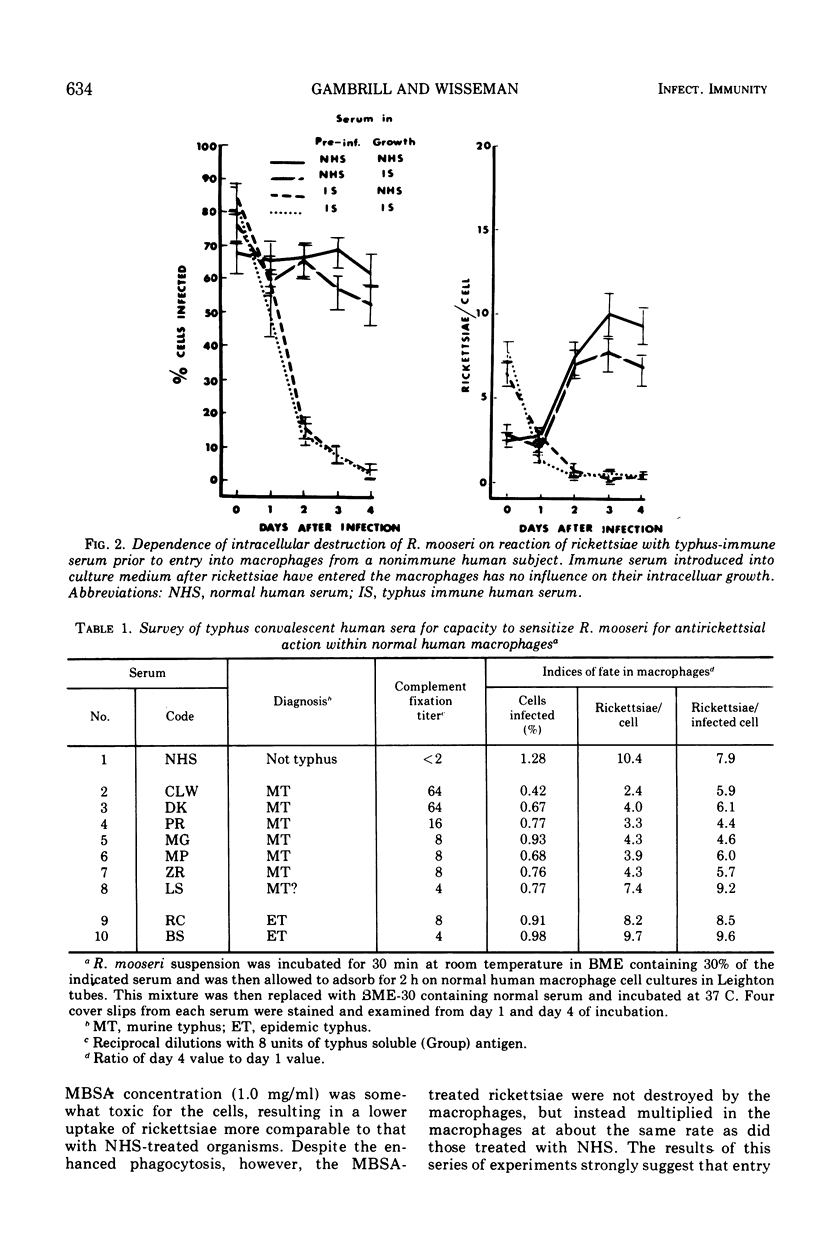
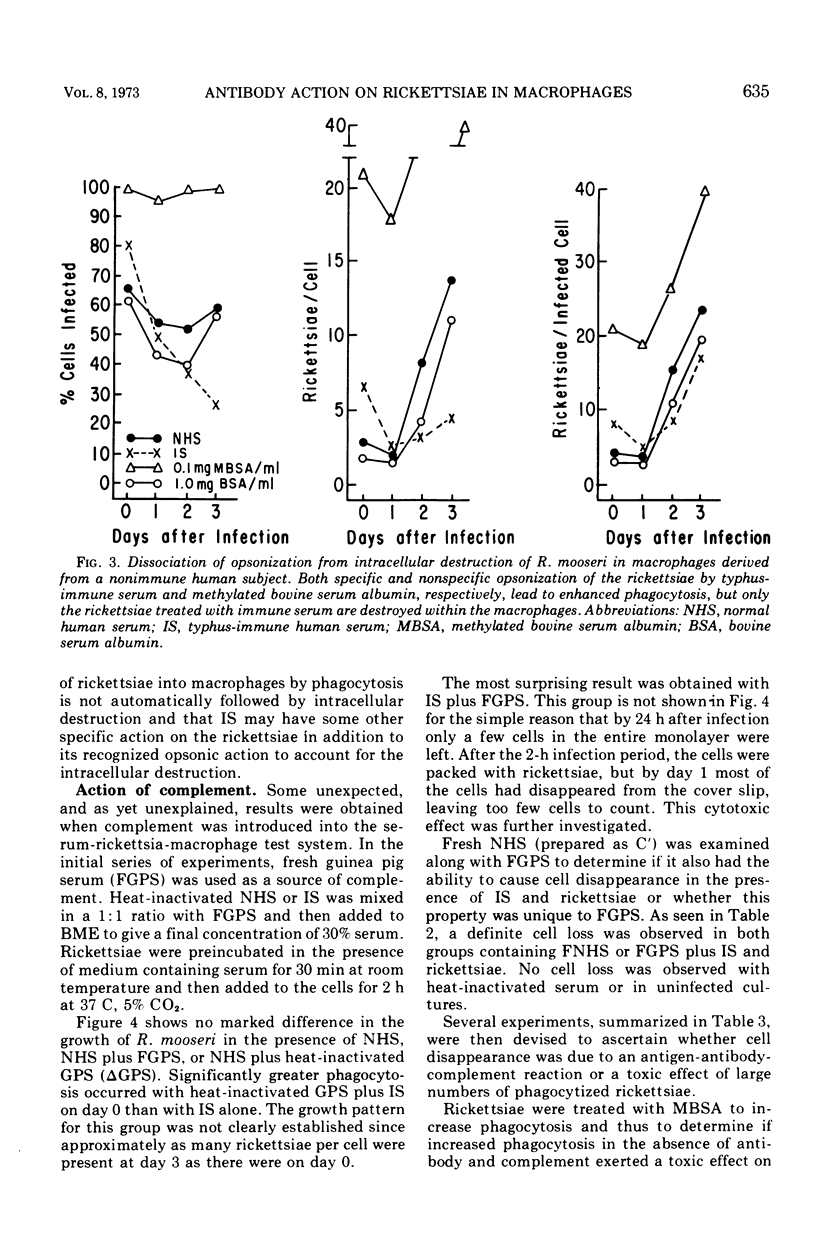
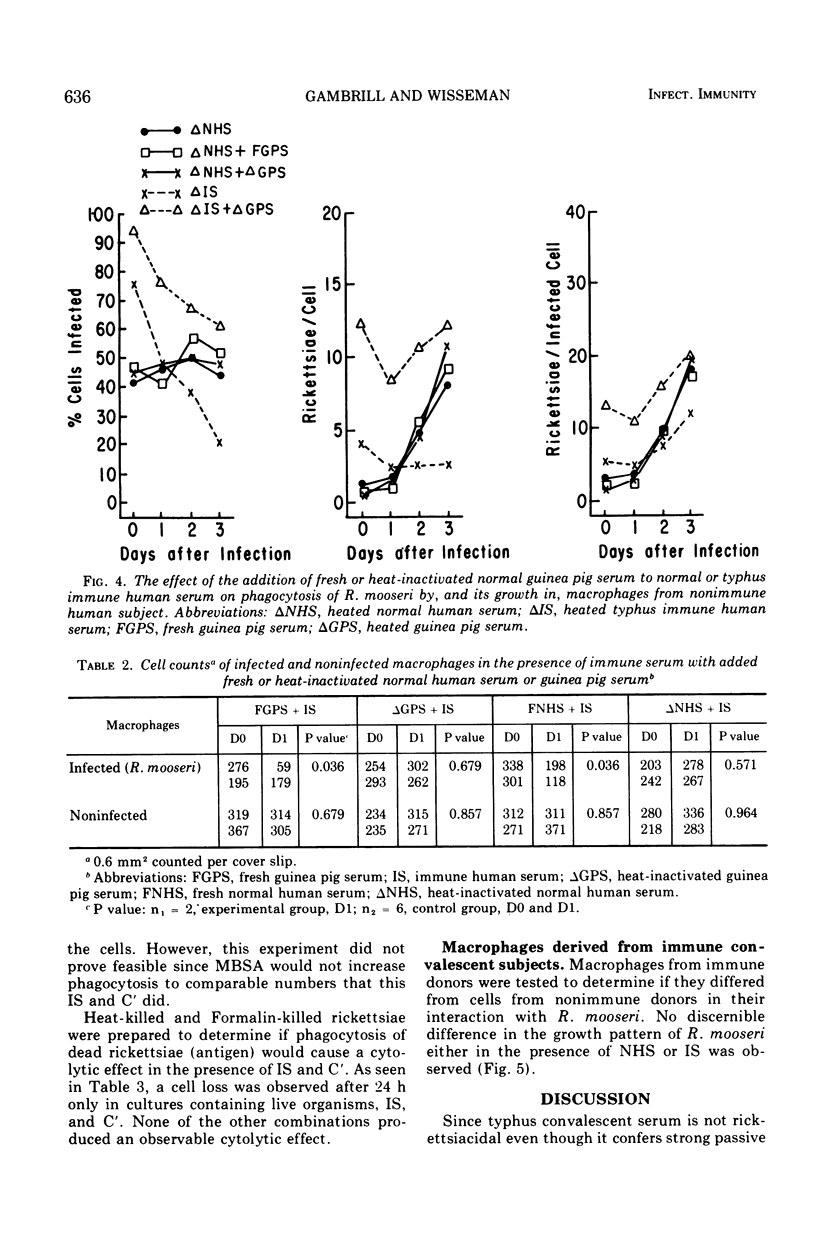
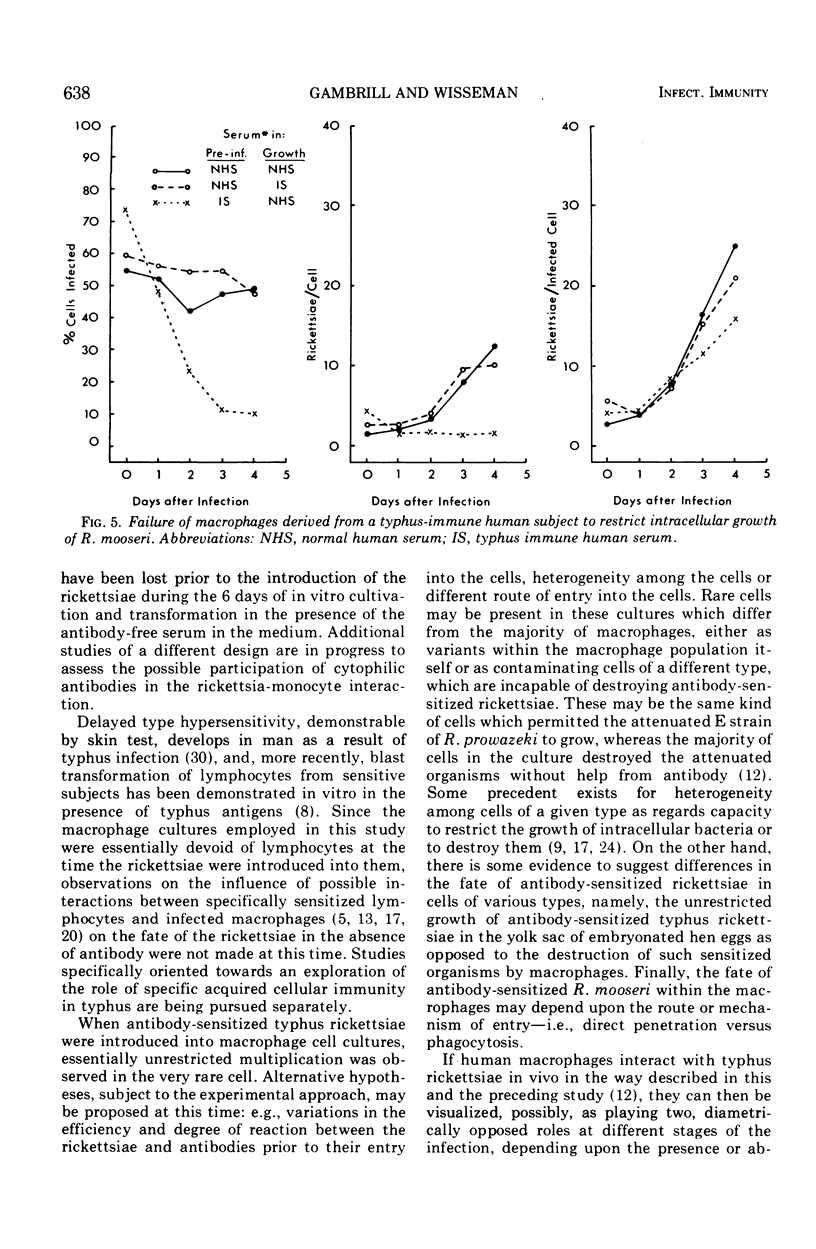
Preincubation of Rickettsia mooseri with human typhus convalescent serum, which is not rickettsiacidal but which confers passive protection to animals, opsonizes the rickettsiae for enhanced phagocytosis by monocyte-derived human macrophages in cell culture and renders them susceptible to destruction within the macrophages. Nonspecific opsonization by preincubation of the rickettsia with methylated bovine serum albumin enhances phagocytosis, but the rickettsiae are not prepared for intracellular destruction. Instead, they grow within the macrophages and eventually destroy these cells. Thus, immune serum and macrophages, neither of which is capable of killing these rickettsiae alone, act in concert to destroy the virulent organisms. In this system, immune serum appears to exert two distinct, possibly dissociable, actions on the rickettsiae: enhancement of phagocytosis and preparation for intracellular destruction. Complement is not required for this action but, when present with immune serum, markedly enhances phagocytosis of the rickettsiae, often leading to rapid destruction of the macrophage.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. V. CYTOPHILIC ANTIBODY IN GUINEA-PIGS WITH DELAYED-TYPE HYPERSENSITIVITY. Immunology. 1964 Jul;7:474–483. [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Contribution of humoral and cellular factors to the resistance to experimental infection by Pseudomonas aeruginosa in mice. I. Interaction between immunoglobulins, heat-labile serum factors, and phagocytic cells in the killing of bacteria. Infect Immun. 1971 Oct;4(4):462–467. doi: 10.1128/iai.4.4.462-467.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVANAUGH D. C., RANDALL R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959 Oct;83:348–363. [PubMed] [Google Scholar]
- COHN Z. A., BOZEMAN F. M., CAMPBELL J. M., HUMPHRIES J. W., SAWYER T. K. Study on growth of Rickettsia. V. Penetration of Rickettsia tsutsugamushi into mammalian cells in vitro. J Exp Med. 1959 Mar 1;109(3):271–292. doi: 10.1084/jem.109.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. A., Salsbury A. J., Willoughby D. A. Some scanning electron-microscope observations on stimulated lymphocytes. J Pathol. 1971 Jun;104(2):115–118. doi: 10.1002/path.1711040205. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. The structure and function of monocytes and macrophages. Adv Immunol. 1968;9:163–214. doi: 10.1016/s0065-2776(08)60443-5. [DOI] [PubMed] [Google Scholar]
- Coonrod J. D., Shepard C. C. Lymphocyte transformation in rickettsioses. J Immunol. 1971 Jan;106(1):209–216. [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. I. Multiplication of typhus rickettsiae in human macrophage cell cultures in the nonimmune system: influence of virulence of rickettsial strains and of chloramphenicol. Infect Immun. 1973 Oct;8(4):519–527. doi: 10.1128/iai.8.4.519-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. H., Otto V., Gupta R. K. Lymphocyte-mediated cellular immunity in histoplasmosis. Infect Immun. 1971 Nov;4(5):605–610. doi: 10.1128/iai.4.5.605-610.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Klemperer M. R., Alper C. A., Rosen F. S. The enhancement of bacterial phagocytosis by serum. The role of complement components and two cofactors. J Exp Med. 1969 Jun 1;129(6):1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas W. E., Gurner B. W., Nelson D. S., Coombs R. R. Passive sensitization of tissue cells. 1. Passive sensitization of macrophages by guinea-pig cytophilic antibody. Int Arch Allergy Appl Immunol. 1965;28(1):87–104. [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T. The mediator of cellular immunity. IV. Cooperation between lymphocytes and mononuclear phagocytes. Cell Immunol. 1971 Aug;2(4):317–325. doi: 10.1016/0008-8749(71)90066-9. [DOI] [PubMed] [Google Scholar]
- Morelli R., Rosenberg L. T. The role of complement in the phagocytosis of Candida albicans by mouse peripheral blood leukocytes. J Immunol. 1971 Aug;107(2):476–480. [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLESCIA O. J., BRAUN W., PALCZUK N. C. PRODUCTION OF ANTIBODIES TO DENATURED DEOXYRIBONUCLEIC ACID (DNA). Proc Natl Acad Sci U S A. 1964 Aug;52:279–285. doi: 10.1073/pnas.52.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston D. J., Elberg S. S. Serum-mediated immune cellular responses to Brucella melitensis REV 1. II. Restriction of Brucella by immune sera and macrophages. J Reticuloendothel Soc. 1969 Apr;6(2):109–139. [PubMed] [Google Scholar]
- Rowley D. Phagocytosis and immunity. The carrier state and cellular immunity. Experientia. 1966 Jan 15;22(1):9–13. [PubMed] [Google Scholar]
- Tizard I. R. Macrophage cytophilic antibody in mice. Differentiation between antigen adherence due to these antibodies and opsoni adherence. Int Arch Allergy Appl Immunol. 1969;36(4):332–346. [PubMed] [Google Scholar]
- VOLKMAN A., GOWANS J. L. THE ORIGIN OF MACROPHAGES FROM BONE MARROW IN THE RAT. Br J Exp Pathol. 1965 Feb;46:62–70. [PMC free article] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr Interaction of Rickettsiae and phagocytic host cells. II. Chemotactic action of typhus Rickettsiae on human polymorphonuclear leukocytes in vitro. J Immunol. 1961 Oct;87:468–471. [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, TABOR H. INTERACTION OF RICKETTSIAE AND PHAGOCYTIC HOST CELLS. IV. EARLY CELLULAR RESPONSE OF MAN TO TYPHUS RICKETTSIAE AS REVEALED BY THE SKIN WINDOW TECHNIQUE, WITH OBSERVATIONS ON IN VIVO PHAGOCYTOSIS. J Immunol. 1964 Nov;93:816–825. [PubMed] [Google Scholar]
- Wisseman C. L., Jr, el Batawi Y., Wood W. H., Jr, Noriega A. R. Gross and microscopic skin reactions to killed typhus Rickettsiae in human beings. J Immunol. 1967 Jan;98(1):194–209. [PubMed] [Google Scholar]



