Abstract
The processing of spatial and mnemonic information is believed to depend on hippocampal theta oscillations (5–12 Hz). However, in rats both the power and the frequency of the theta rhythm are modulated by locomotor activity, which is a major confounding factor when estimating its cognitive correlates. Previous studies have suggested that hippocampal theta oscillations support decision-making processes. In this study, we investigated to what extent spatial decision making modulates hippocampal theta oscillations when controlling for variations in locomotion speed. We recorded local field potentials from the CA1 region of rats while animals had to choose one arm to enter for reward (goal) in a four-arm radial maze. We observed prominent theta oscillations during the decision-making period of the task, which occurred in the center of the maze before animals deliberately ran through an arm toward goal location. In speed-controlled analyses, theta power and frequency were higher during the decision period when compared to either an intertrial delay period (also at the maze center), or to the period of running toward goal location. In addition, theta activity was higher during decision periods preceding correct choices than during decision periods preceding incorrect choices. Altogether, our data support a cognitive function for the hippocampal theta rhythm in spatial decision making.
Keywords: LFP, oscillations, speed, spatial choice, locomotion, radial maze
INTRODUCTION
During decision making (DM), animals have to integrate sensorial and mnemonic information, accumulating evidence until a final choice is made (Schall, 2001; Gold and Shadlen, 2007; Kepecs et al., 2008; Rangel et al., 2008; Wimmer and Shohany, 2011; van der Meer et al., 2012). As the hippocampus is involved in contextual coding (Smith and Mizumori, 2006), relational memory (Eichenbaum, 2004), spatial working memory (Jarrard, 1993), and trajectory planning (Johnson and Redish, 2007; Pfeiffer and Foster, 2013), many believe that the hippocampus plays a critical role in deliberative DM (Johnson et al., 2007; Buckner, 2010; Pennartz et al., 2011; Wimmer and Shohany, 2011; Penner and Mizumori, 2012). In accordance, it has recently been shown that hippocampal-lesioned rats have impaired decision accuracy in a double Y-maze task (Bett et al., 2012), and that the ablation of NMDA transmission in the dorsal hippocampus of mice is associated with lower choice performance in a radial maze task (Bannerman et al., 2012).
Hippocampal networks produce a myriad of rhythms that depend on cognitive and behavioral states (Timo-Iaria et al., 1970; Silva and Arnolds, 1978; Buzsaki et al., 1983; Buzsaki, 2006). Over the last decade, several researchers have proposed that network oscillations are involved in cognitive processes (Leung, 1998; Buzsaki and Draguhn, 2004; Fries, 2005; Fell and Axmacher, 2011). Among them, hippocampal theta oscillations (5–12 Hz) have been linked to learning (Seager et al., 2002; McNaughton et al., 2006; Benchenane et al., 2010), place coding (O'Keefe and Recce, 1993; Jensen and Lisman, 2000; Buzsaki, 2005), spatial memory (Winson, 1978; Jones and Wilson, 2005), and item–context associations (Tort et al., 2009). In particular, the loss of theta rhythm owing to lesions of the medial septum is associated with impaired performance in spatial memory tasks (Winson, 1978; McNaughton et al., 2006; Shirvalkar et al., 2010). Hippocampal theta oscillations might thus be involved in the cognitive processing required for DM. Consistent with this possibility, the amplitude of hippocampal theta oscillations is highest when rats traverse the decision point in a T-maze task (DeCoteau et al., 2007; Tort et al., 2008; Womelsdorf et al., 2010b).
On the other hand, the rodent hippocampus is well known to produce prominent theta oscillations during voluntary behaviors independently of cognitive demands (Vanderwolf, 1969; Buzsaki, 2002). For instance, correlations between the locomotion speed and the amplitude or frequency of theta oscillations have been reported (Shin et al., 2001; Hinman et al., 2011; Ledberg and Robbe, 2011; Wells et al., 2013). Although speed is not the sole determinant of theta power (Montgomery et al., 2009), studies linking hippocampal theta oscillations and cognitive function are often entangled with locomotor activity. For example, in T-maze tasks the speed is highest at the same decision point where theta is highest (DeCoteau et al., 2007; Tort et al., 2008), making it difficult to disambiguate the putative effects of cognition and locomotion.
Thus, although previous studies have associated theta activity to hippocampal functions (Buzsaki, 2002,2005; Wang, 2010; Buzsaki and Moser, 2013), few have attempted to dissociate cognitive and behavioral variables (but see Montgomery et al., 2009). Recently, Schmidt et al. (2013) reported higher theta power in the dorsal hippocampus while rats ran to goal location (“decision epoch”) compared to when animals ran back to the start area (“control epoch”) in a spatial choice task. Interestingly, the authors also reported decoupling of theta amplitude and locomotion speed while animals approached the goal area. These results suggest a cognitive role for theta oscillations during spatial DM. However, in this study the “decision epoch” lumped together epochs before and after animals had actually decided which arm to enter; in addition, as in T-maze tasks, the DM process is assumed to occur while animals are running. It remains to be demonstrated whether the DM process modulates hippocampal theta oscillations per se, before animals start to deliberately move toward a goal.
To investigate this possibility, we set out to record local field potentials (LFPs) from the dorsal CA1 of rats during a spatial choice task in a radial four-arm maze. On each trial, animals started on the central area of the maze and had to choose one arm to enter for reward. DM occurred before arm running, during a period of limited mobility. This paradigm allowed us to test whether DM modulates hippocampal theta oscillations before animals move volitionally to goal location.
METHODS
Animal care and surgery procedures complied with the National Institute of Health guidelines, and were approved by the Ethics Committee for Animal Experimentation of the Edmond and Lily Safra International Institute of Neuroscience of Natal (permit 02/2007). Five adult male Wistar rats (age, 3–6 months; 250–350 g) were kept on a 12-h light/dark schedule (lights on at 06:00), housed individually with free access to water and limited access to food, so as to maintain ∼85% of the body weight reached by Wistar rats fed ad libitum.
Four-Arm Radial Maze
We used a modified version of the eight-arm radial maze (Olton et al., 1978; Floresco et al., 1997). The maze was a black four-arm maze elevated 50 cm from the floor; the arms were 50 cm long, 10 cm wide, and 10 cm tall (Fig. 1A). The maze was wiped with 70% of alcohol solution before each trial block to remove odors. Reward was delivered in round plastic bowls at the end of each arm. The recording room and maze walls displayed distal and proximal geometrical cues, respectively. Animals were individually habituated to the experimental procedure for 5 days prior to experiments.
Fig 1.
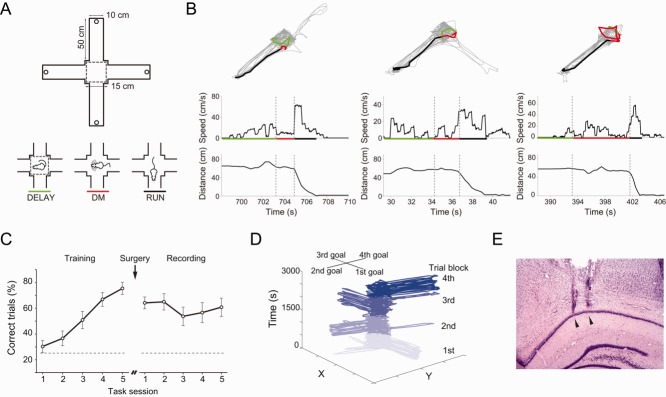
Experimental design. A: (Top) Schematic representation of the four-arm maze. Dashed lines mark the central area of the maze where rats stay during intertrial intervals; each trial starts after the removal of the central barriers. Circles at the end of each arm denote reward positions; only one position was rewarded per block of 10 trials (METHODS section). (Bottom) Schematic representation of the intertrial interval (DELAY), DM, and RUN periods. B: (Top) Thin gray lines show locomotion trajectories during three representative 10-trial blocks. Thick lines represent the trajectories for DELAY (green), DM (red), and RUN (black) during a single trial. (Bottom) Locomotion speed and distance to reward location for the single trial highlighted in the top panels. Horizontal segments indicate DELAY, DM, and RUN periods colored as above. C: Task performance before (“Training”) and after (“Recording”) surgical implantation of electrodes. D: Locomotion trajectory of a trained rat across four 10-trial blocks within one recording session. Reward location changes from arm to arm at every block in a clockwise manner. Trials from different blocks are represented by different shades of blue. E: Representative cresyl-stained brain section showing glial tracks corresponding to electrodes implanted in the dorsal CA1 region; arrowheads indicate electrode tips. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Spatial Choice Task
In a daily experimental session, rats executed four blocks of 10 trials of a spatial choice task in the four-arm radial maze. Animals had to choose one among the four arms to obtain reward. Only one arm was rewarded in each block of trials, and on the following block the rewarded arm was shifted according to a clockwise sequential order. Animals rested for 30 min after the execution of a 10-trial block in a plastic cage (height, 35 cm; width, 35 cm; and length, 50 cm) placed aside of the maze. Within a trial block, plastic opaque barriers were used to restrain the rats into the central area of the maze during the intertrial period. In each trial, the barriers were removed and the rats had to choose one arm to enter. When a correct choice was made, a chocolate cereal pellet was delivered at the end of the arm. Incorrect choices were not rewarded. The animals then returned to the central area, where they remained restricted by the barriers for a 60-s delay period until the next trial. The beginning of the DM period was defined as the timestamp when the central barriers were removed; the end of the DM period was considered as the timestamp in which the distance between the animal and the end of the chosen arm started to decrease monotonically (Fig. 1B). The running (RUN) period was defined as the time interval between the end of the DM period and the moment when the animal reached the end of the arm. The intertrial (DELAY) period was defined as the 5-s interval preceding the removal of the central barriers, that is, the 5 s before the beginning of the DM period. Rats were trained to obtain 75% of correct performance before being implanted with multielectrode arrays (Fig. 1C).
Surgical Implantation of Electrodes
The animals were surgically implanted with 4 × 8 multielectrode arrays (Teflon-coated tungsten microwires; diameter, 35–50 µm; interelectrode spacing, 300 µm; impedance, ∼0.5 MΩ at 1 KHz) targeting the pyramidal layer of the right CA1 region of the dorsal hippocampus (AP, −3.6 and ML: +1.6 from Bregma; DV: 2.4 from the pial surface; Paxinos and Watson, 1998) under ketamine and xylazine anesthesia (100 and 8 mg/kg, respectively). Spiking activity was used to guide array implantation. Ground and reference were provided by a silver wire soldered to a stainless steel screw positioned in the frontal bone, 3 mm in front of Bregma (Romcy-Pereira and Pavlides, 2004). Animals were allowed to recover for 7–10 days after surgery before electrophysiological recordings.
Electrophysiological and Behavioral Recording
We obtained continuous electrophysiological and video recordings of freely moving animals during the execution of the spatial choice task. A total of 1,520 trials were recorded for more than 38 sessions (rats 1–5 executed 9, 5, 7, 10, and 7 experimental sessions, respectively). Experiments began daily at 11:00 with lights on. Electrophysiological recordings were performed using a multichannel acquisition processor (MAP, Plexon, Dallas, TX). LFPs were preamplified (1,000×), filtered between 0.7 and 300 Hz, and sampled at 1 KHz. Behavior was recorded with a digital video camera (30 frames per second) and spatial position was tracked by an automated system that synchronized behavioral and neural data (Cineplex, Plexon, Dallas, TX). Spatial position was defined as the center of the animals' body in each frame. The spatial occupancy plots (Figs. 2A,B) represent the time spent in spatial bins of 1 cm × 1 cm. To measure quadrant preference, we first divided the central area of the maze into four quadrants (inset, Fig. 2C), and quantified the time spent within each quadrant during the last 10 s preceding the removal of the barriers. The quadrant occupancy ratio was then calculated as the time spent in the quadrant associated to the rewarded arm divided by the sum of the time spent in the other quadrants.
Fig 2.
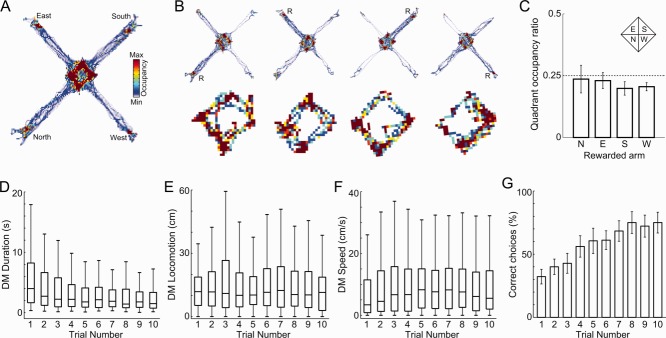
Behavioral characteristics in the spatial choice task. A: Spatial occupancy for a representative session. B: (Top row) Occupancy in each of the 10-trial blocks in the same session; “R” indicates reward location. (Bottom row) Zoomed in view of spatial occupancy of the central part of the maze during the last 10 s of the intertrial interval. C: Group result of quadrant occupancy ratio, defined as the time spent in the quadrant associated with the rewarded arm divided by the time spent in the other quadrants (see inset for quadrant boundaries). Notice that animals have no preference for the quadrant associated with the rewarded arm during the intertrial interval (P > 0.05, Student's t-test for each occupation ratio against 0.25). D–F: Distribution of DM duration (D), distance traveled (E), and speed (F) during DM across trials. G: Percentage of correct choices on each trial (mean ± SEM over animals). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To select subsets of trials with similar locomotion speed (Figs. 4 and 6A–C), we discarded trials with highest and/or lowest speeds (Supporting Information Fig. S1). To measure theta power and frequency as a function of speed (Figs. 5 and 6D), trials were grouped according to nonoverlapping speed bins separated by 5 cm/s.
Fig 4.
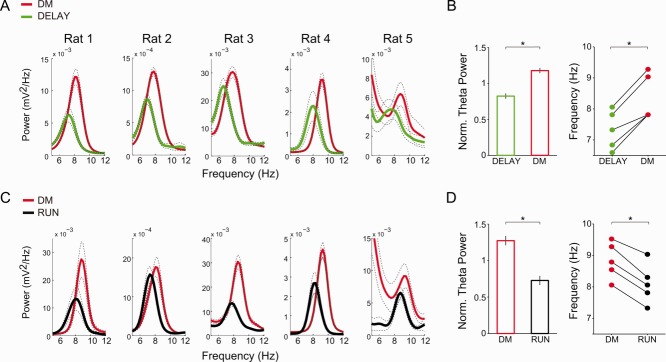
DM has higher theta power and frequency than delay and running periods in speed-matched conditions. A: Power spectra in speed-matched trials during DELAY (green) and DM (red) for each animal (mean ± SEM over trials). For speed distributions, see Supporting Information Figure S1. B: (Left) Normalized theta power in DM and DELAY (mean ± SEM, n = 5). (Right) Theta peak frequency for the power spectra in A. C,D: The same as above but for DM and RUN periods (*P < 0.05, Student's t-test). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Fig 6.
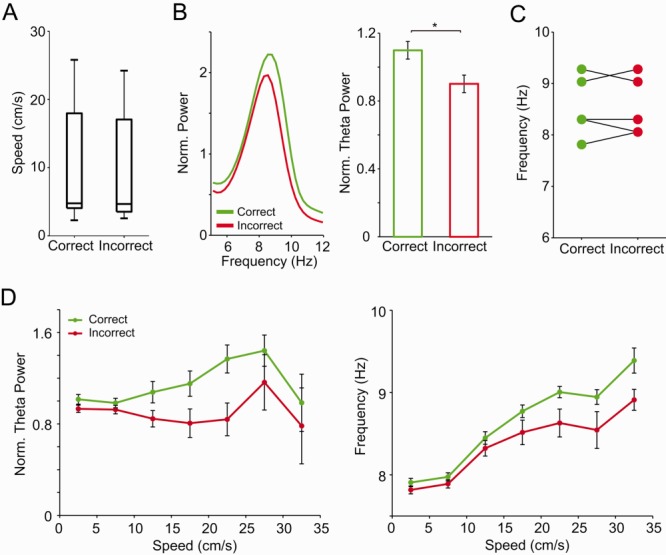
Correct choices have strongest theta power during DM. A: Speed distribution in subsets of trials used in panels B and C. B: (Left) Mean-normalized power spectra during DM previous to correct (green) and incorrect (red) choices. (Right) Normalized DM theta power (mean ± SEM across rats; *P < 0.05, Student's t-test). C: Theta peak frequency during DM in correct and incorrect trials for each animal. D: Normalized theta power (left) and theta peak frequency (right) as a function of speed during DM that preceded correct (green) or incorrect (red) choices. Data points represent mean ± SEM over trials across rats. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Fig 5.
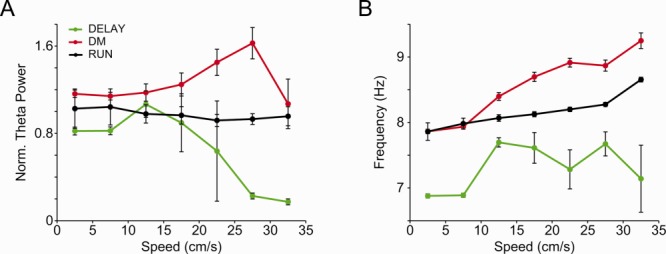
Highest theta power during DM occurs for a wide range of speeds. A,B: Normalized theta power (A) and theta peak frequency (B) as a function of speed during DM (red), RUN (black), and DELAY (green) periods (mean ± SEM over trials across rats). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Histology
After the recording sessions, rats were overdosed with pentobarbital (100 mg/kg) and perfused with saline, followed by 4% paraformaldehyde solution. Brains were removed and stored in 4% paraformaldehyde/20% sucrose for 24 h, then frozen, and sectioned in a cryostat (Micron). Coronal sections (50 µm) were thaw-mounted over glass slides and stained with cresyl violet for inspecting the anatomical location of the implants. The final positions of the electrode tips were estimated using light microscopy.
Spectral Analysis
Spectral analyses were performed using custom-made and built-in MATLAB routines (MathWorks, Natick, MA). Power spectra were estimated by the Welch periodogram method using the “pwelch” function from the Signal Processing Toolbox (50% overlapping Hamming windows with a length of 1 s). DM or RUN periods shorter than 1 s and longer than 10 s were discarded (Supporting Information Fig. S2). For each animal, only the electrode with highest ripple band power (150–250 Hz) was considered for further analyses; similar results were obtained when considering the mean over all electrodes for each animal (data not shown; Belchior et al., 2012). We averaged power spectra for DELAY, DM, and RUN periods across trials. Theta power was obtained by the mean power in the theta band (5–12 Hz). Theta peak frequency was considered the frequency associated with the local power maximum in the theta band.
The time–frequency decomposition shown in Figure 3A was obtained using complex Morlet wavelets with central frequencies ranging from 0.5 to 20 Hz in 0.5-Hz steps. The instantaneous energy of each frequency was obtained by the absolute value of the transform. In Figure 3B, we first computed the mean percentage of energy in the theta band across trials and then averaged across animals.
Fig 3.
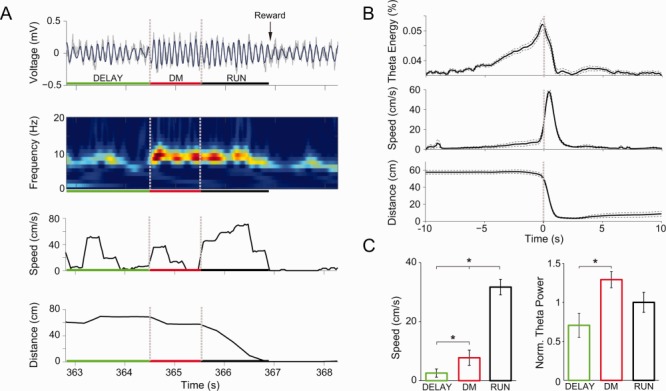
Theta power increases during DM. A: (First panel) Hippocampal LFP (gray) recorded during a representative trial. Vertical dashed lines mark the moment when barriers were removed and when the animal started to move toward reward location. Horizontal green, red, and black segments indicate intertrial interval (DELAY), DM, and running (RUN) periods, respectively. Notice emergence of robust theta oscillations (5–12 Hz, blue) at the beginning of DM. (Second panel) Wavelet spectrogram showing increased theta energy during DM and RUN. (Third panel) Locomotion speed for the same representative trial. (Fourth panel) Distance from the animal to reward location. B: Group results for percentage energy in the theta band (top), locomotion speed (middle), and distance to reward location (bottom). Solid and dashed lines represent mean ± SEM, respectively (n = 5 animals). Only correct trials were taken into account. C: Mean locomotion speed (left) and normalized theta power (right) during DELAY, DM, and RUN periods. Error bars represent SEM (*P < 0.01, one-way ANOVA followed by Tukey's post hoc test; n = 5 animals). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Power normalization
Since animals had different levels of baseline power (Figs. 4A,C), theta power values were normalized before computing group results. To that end, we divided power values of each animal by a normalization factor. The normalization factor was defined as the mean theta power over the groups under comparison. For instance, in Figure 3C we normalized theta power in DELAY, DM, and RUN periods by their mean theta power (i.e., normalized DM power = DM power/[DELAY power + DM power + RUN power]/3, and similarly for RUN and DELAY). The same normalization was applied when comparing theta power across trials (Supporting Information Fig. 3A), as a function of performance (Supporting Information Fig. 3B), in speed-matched conditions (Figs. 6), and between correct and incorrect choices (Fig. 6). Notice that the average of normalized power values equals 1 in each case.
Statistical Analysis
Means were compared by Student's t-test, or one- or two-way ANOVA followed by Tukey–Kramer post hoc test, as indicated in the text. We used an alpha level of 0.05 to denote statistical significance.
RESULTS
Behavioral Performance
We recorded LFP from the CA1 region of the dorsal hippocampus of five freely moving rats executing a spatial choice task in a four-arm radial maze (Figs. 1A,E). On each trial, the removal of central plastic barriers allowed animals to choose one of the arms to enter for reward; the rewarded arm changed after blocks of 10 trials (METHODS section). Animals increased task performance from 30 ± 0.1% of correct trials at the first training session to achieve performance criterion of 75 ± 0.1% before surgery (Fig. 1C, left). After surgery, task performance was stable across multiple sessions, ranging between 51 and 63%, with mean performance of 56 ± 1% correct choices (Fig. 1C, right). Figure 1D shows the displacement of an animal during a recording session, with trajectories of each of the 10-trial block represented by different shades of blue. Notice that trajectories shifted clockwise among trial blocks after the change in reward location.
As expected by task design, animals spent most of time in the central part of the maze, followed by the reward locations at the end of the arms (for a representative example, see Fig. 2A). When analyzing individual blocks of trials, we found that animals exhibited similar occupation of the central area, and higher occupation of the rewarded arms (Fig. 2B, top row). Further analyses showed that animals did not wait closer to the rewarded arms during intertrial delay periods (Fig. 2B, bottom row, and Fig. 2C). Thus, the future choice of animals cannot be predicted by spatial position during the delay period. Within a 10-trial block, the duration of the DM period slightly decreased from the first to the last trial (Fig. 2D, P < 0.05, one-way ANOVA followed by Tukey's post hoc test), whereas variations in locomotion and speed during DM did not reach statistical significance (Figs. 2E,F). Choice performance consistently increased across trials (Fig. 2G, P < 0.05, one-way ANOVA followed by Tukey's post hoc test), indicating that animals learned new reward locations within a trial block.
Increased Theta Oscillations during the DM Period
We next analyzed CA1 LFPs triggered by the end of the DM period, which is defined as when animals started to move consistently (i.e., without stopping or changing direction) toward the end of an arm. We observed strong 5–12 Hz theta oscillations during DM, noticeable both in the raw signal and by time–frequency wavelet analysis (for a representative trial, see Fig. 3A). In the group level, we found that the amplitude of CA1 theta oscillations increased seconds before major changes in locomotion speed and spatial position, which were typical of the RUN period of the task when animals deliberatively approached reward location (compare top and bottom panels in Fig. 3B).
We then computed average locomotion speed and theta power in the different periods of the task (DELAY, DM, and RUN). As shown in the left panel of Figure 3C, mean speed was significantly higher during RUN than DM and DELAY, and also significantly higher during DM than DELAY (*P < 0.05, one-way ANOVA followed by Tukey's post hoc test). Mean theta power was significantly higher during DM than DELAY (*P < 0.05, one-way ANOVA followed by Tukey's post hoc test), and not statistically different from RUN (Fig. 3C, right panel). In all, these results show that DM is associated with the emergence of prominent theta oscillations.
Highest Theta Activity during DM when Controlling for Locomotion Speed
Hippocampal theta oscillations have been shown to depend on locomotion speed (DeCoteau et al., 2007; Hinman et al., 2011,2013). We next computed mean power spectra of CA1 LFPs in subsets of trials matched for similar speed in the different task periods (Supporting Information Fig. S1). As shown in Figure 4, we found that theta power and theta peak frequency were highest in DM compared to DELAY or RUN under speed-matched conditions in all animals (P < 0.05, Student's t-test). In addition, when plotting mean theta power and mean theta peak frequency for each task period as a function of speed, we observed that theta activity was highest during the DM period in a wide range of speeds (Fig. 5, P < 0.05, two-way ANOVA followed by Tukey's post hoc test). These results, therefore, show that the changes in locomotion speed cannot account for increased theta activity during DM.
Theta Oscillations During DM and Choice Performance
The above-mentioned results indicate that variations in theta oscillations are not solely explained by locomotor activity, but may reflect cognitive processing during DM. We next investigated whether theta activity during DM is related to choice outcomes. As animals improved performance across trials (Fig. 2G), we started by simply comparing DM theta power within trial blocks. We found that theta power was not statistically different among trials (Supporting Information Fig. S3A), nor were there statistical differences in DM theta power as a function of task performance (Supporting Information Fig. S3B). Notice that these analyses, however, do not take variations in locomotor activity into account.
We next compared theta activity during DM preceding correct and incorrect choices in subsets of trials matched for locomotion speed (Fig. 6A). We found that DM theta power, but not peak frequency, was significantly higher in correct than incorrect trials in speed-matched conditions (Figs. 6B,C, P < 0.01, Student's t-test). However, plotting mean theta activity as a function of speed revealed higher theta peak frequency in correct trials for the highest speeds (Fig. 6D, P < 0.05, two-way ANOVA followed by Tukey's post hoc test). In all, these results are consistent with a role for theta oscillations in successful DM.
DISCUSSION
In this study, we investigated the influence of spatial DM on hippocampal theta oscillations. We recorded CA1 LFPs while rats performed a spatial choice task in a four-arm radial maze; on each trial, animals started at the center of the maze and had to choose one arm to enter for reward. We found prominent theta oscillations during the DM period of the task in the maze center, before animals deliberately ran to goal location. In addition, in subsets of trials matched for locomotion speed, theta power and theta peak frequency were higher during the DM period than during the intertrial and running periods of the task. Finally, we found higher theta activity during DM periods associated with correct choices. Altogether, our results support a cognitive role for hippocampal theta oscillations in spatial DM.
A critical feature of spatial DM is the integration of current contextual information with the retrieval of past associations stored in memory (Platt, 2002; Gold and Shadlen, 2007; Rangel et al., 2008; Wimmer and Shohany, 2011), two functions that may depend on the hippocampal formation (Buzsaki, 2006). Our four-arm maze is a modified version of an eight-arm radial maze, which measures both the reference and the working memory components of spatial memory (Olton et al., 1977; Becker et al., 1980). The increase in performance within trial blocks along with the performance decrease between consecutive blocks (i.e., compare trial 10 and 1 in Fig. 2G) suggests that animals adopt a successful shift in strategy within a trial block. It is worth noting that the radial-arm maze task is hippocampal dependent when there is a delay period between consecutive trials (Becker et al., 1980; Floresco et al., 1997), which was the case in our experiments.
The link between the emergence of LFP oscillations of different frequencies in the rat hippocampus and specific behavioral states has been extensively described (Silva and Arnolds, 1978; Buzsaki et al., 1983; Leung, 1998; Buzsaki, 2006). In particular, theta oscillations consistently appear whenever animals exhibit active exploratory behaviors such as locomotion (Vanderwolf, 1969; Buzsaki, 2002). Importantly, prominent theta oscillations resurge during REM sleep (Timo-Iaria et al., 1970); as REM sleep is believed to play a role in cognitive functions such as memory consolidation (Louie and Wilson 2001; Ribeiro et al., 2002; Ulloor and Datta, 2005; Diekelmann and Born, 2010), it has often been suggested that theta oscillations may be involved in some of the functions executed by the hippocampus (Winson, 1993; Buzsaki, 2002). During waking states, though, behavioral variables have been major confound factors when trying to link theta activity to cognitive function. Although the previous studies have reported that decision points in spatial maze tasks are associated with increases in hippocampal theta power (DeCoteau et al., 2007; Johnson and Redish, 2007; Tort et al., 2008; Montgomery et al., 2009; Schmidt et al., 2013), as well as greater theta coherence between the hippocampus and the other brain regions (Jones and Wilson, 2005; DeCoteau et al., 2007; Tort et al., 2008; Benchenane et al., 2010; Womelsdorf et al., 2010a), in these tasks animals usually traverse at high speeds the arm in which DM is assumed to occur, making it difficult to dissociate behavioral from cognitive influences.
Our results add to others (Montgomery et al., 2009; Schmidt et al., 2013) in providing evidence that theta oscillations are related to the computations occurring in the hippocampus during spatial DM. By applying multiple regression analysis to hippocampal LFPs recorded from rats performing a modified T-maze task (an eight-shaped maze), Montgomery et al. (2009) have previously shown that maze region (e.g., decision arm vs. return arm) better accounts for variations in theta power than locomotion speed. Recently, Schmidt et al. (2013) showed that the correlation between locomotion speed and theta power in the dorsal hippocampus disappears when rats run toward goal location. However, it should be noted that in these tasks the decision process (i.e., the choice of arm) is believed to occur while animals are running. In our task, animals had to decide while in the center of the maze, before major changes in running speed and distance to reward location (Fig. 3B). We were thus able to confirm that the increase in hippocampal theta power during the DM process cannot be accounted for by variations in locomotion speed. In addition, the finding that theta power was greatest during decision periods prior to correct choices constitutes further evidence for a cognitive role of theta oscillations.
Recent results suggest that hippocampal activity also supports memory-guided decisions in humans. A functional neuroimaging study has shown that hippocampal activity increases when subjects retrieve goal location in a virtual maze (Viard et al., 2011). In temporal areas, theta oscillations are modulated by contextual and spatial decision tasks, and also correlate with memory retrieval (Kahana et al., 1999; Guitart-Masip et al., 2013). In addition, theta power was reported to increase in parieto-temporal areas when subjects correctly recognize previously presented items (Osipova et al., 2006). The capacity to forecast the successful mnemonic retrieval of word lists based on hippocampal theta oscillations recorded during encoding also suggests that theta oscillations may reflect cognitive processes in the hippocampus (Kahana et al., 1999; Sederberg et al., 2003). Additionally, there is a functional overlap between memory retrieval and future thinking, which is associated with the activity of hippocampal networks (Hassabis et al., 2007; Schacter et al., 2007). In all, our results showing the emergence of strong theta activity in the hippocampus when memory-guided spatial decisions are required suggest that theta oscillations support the retrieval of rewarded choices. However, as it is also the case for other LFP rhythms (Kay et al., 2009), it remains to be determined whether theta oscillations play a mechanistic role in cognition, or are only epiphenomena of the neuronal computations carried out by the hippocampus. In addition, it also remains to be determined whether the hippocampus per se engages, or is engaged by, other brain regions involved in DM such as the prefrontal cortex (Siapas et al., 2005, Guitart-Masip et al., 2013).
Acknowledgments
The authors thank H. Eichenbaum and R. H. Silva for valuable inputs; R. Pavao, S. A. Mota-Rolim, N. A. Lemos, and F. C. Miranda for help with recordings and analysis; and A. Ragoni and G. Filho for technical support.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary
REFERENCES
- Bannerman DM, Bus T, Taylor A, Sanderson DJ, Schwarz I, Jensen V, Hvalby Ø, Rawlins JNP, Seeburg PH, Sprengel R. Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion. Nat Neurosci. 2012;15:1153–1159. doi: 10.1038/nn.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Walker JA, Olton DS. Neuroanatomical bases of spatial memory. Brain Res. 1980;200:307–320. doi: 10.1016/0006-8993(80)90922-1. [DOI] [PubMed] [Google Scholar]
- Belchior H, Lopes-dos-Santos V, Miranda FC, Tort ABL, Ribeiro S. 2012. pp. 289–221. . Retrieval-related increase in theta oscillations and coordinated neuronal activity in the hippocampus during spatial decision-making. Society for Neuroscience Annual Meeting, San Diego; Abstract.
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Bett D, Allison E, Murdoch LH, Kaefer K, Wood ER, Dudchenko PA. The neural substrates of deliberative decision making: Contrasting effects of hippocampus lesions on performance and vicarious trial-and-error behavior in a spatial memory task and a visual discrimination task. Front Behav Neurosci. 2012;6:70. doi: 10.3389/fnbeh.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: Insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 2010;61:27–48, C1–C8. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the Brain. Oxford NY: Oxford University Press Inc; 2006. [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Thorn C, Gibson DJ, Courtemanche R, Mitra P, Kubota Y, Graybiel AM. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc Natl Acad Sci USA. 2007;104:5644–5649. doi: 10.1073/pnas.0700818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12:105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M, Barnes GR, Horner A, Bauer M, Dolan RJ, Duzel E. Synchronization of medial temporal lobe and prefrontal rhythms in human decision making. J Neurosci. 2013;33:442–451. doi: 10.1523/JNEUROSCI.2573-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA. 2007;104:1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman JR, Penley SC, Long LL, Escabí Ma, Chrobak JJ. Septotemporal variation in dynamics of theta: Speed and habituation. J Neurophysiol. 2011;105:2675–2686. doi: 10.1152/jn.00837.2010. [DOI] [PubMed] [Google Scholar]
- Hinman JR, Penley SC, Escabí Ma, Chrobak JJ. Ketamine disrupts theta synchrony across the septotemporal axis of the CA1 region of hippocampus. J Neurophysiol. 2013;109:570–579. doi: 10.1152/jn.00561.2012. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Position reconstruction from an ensemble of hippocampal place cells: Contribution of theta phase coding. J Neurophysiol. 2000;83:2602–2609. doi: 10.1152/jn.2000.83.5.2602. [DOI] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, van der Meer MA, Redish AD. Integrating hippocampus and striatum in decision-making. Curr Opin Neurobiol. 2007;17:692–697. doi: 10.1016/j.conb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Wilson M. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Brea J, Martin C, Rojas-Libano D, Kopell N. Olfactory oscillations: The what, how and what for. Trends Neurosci. 2009;32:207–214. doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Zariwala Ha, Mainen ZF. Neural correlates, computation and behavioural impact of decision confidence. Nature. 2008;455:227–231. doi: 10.1038/nature07200. [DOI] [PubMed] [Google Scholar]
- Ledberg A, Robbe D. Locomotion-related oscillatory body movements at 6–12 Hz modulate the hippocampal theta rhythm. PLoS One. 2011;6:e27575. doi: 10.1371/journal.pone.0027575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS. Generation of theta and gamma rhythms in the hippocampus. Neurosci Biobehav Rev. 1998;22:275–290. doi: 10.1016/s0149-7634(97)00014-6. [DOI] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Ruan M, Woodnorth M. Restoring theta-like rhythmicity in rats restores initial learning in the morris water maze. Hippocampus. 2006;16:1102–1110. doi: 10.1002/hipo.20235. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Betancur MI, Buzsaki G. Behavior-dependent coordination of multiple theta dipoles in the hippocampus. J Neurosci. 2009;29:1381–1394. doi: 10.1523/JNEUROSCI.4339-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Recce M. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Olton DS, Collison C, Werz MANN. Spatial memory and radial arm maze performance of rats. Learn Motiv. 1977;8:289–314. [Google Scholar]
- Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Res. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- Osipova D, Takashima A, Oostenveld R, Fernández G, Maris E, Jensen O. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci. 2006;26:7523–7531. doi: 10.1523/JNEUROSCI.1948-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. New York: Academic Press; 1998. [Google Scholar]
- Pennartz CMA, Ito R, Verschure PFMJ, Battaglia FP, Robbins TW. The hippocampal–striatal axis in learning, prediction and goal-directed behavior. Trends Neurosci. 2011;34:548–559. doi: 10.1016/j.tins.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Penner MR, Mizumori SJY. Neural systems analysis of decision making during goal-directed navigation. Prog Neurobiol. 2012;96:96–135. doi: 10.1016/j.pneurobio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML. Neural correlates of decisions. Curr Opin Neurobiol. 2002;12:141–148. doi: 10.1016/s0959-4388(02)00302-1. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression. J Neurosci. 2002;22:10914–10923. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romcy-Pereira R, Pavlides C. Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur J Neurosci. 2004;20:3453–3462. doi: 10.1111/j.1460-9568.2004.03808.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neural basis of deciding, choosing and acting. Nat Rev Neurosci. 2001;2:33–42. doi: 10.1038/35049054. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Hinman JR, Jacobson TK, Szkudlarek E, Argraves M, Escabi Ma. Markus EJ. Dissociation between dorsal and ventral hippocampal theta oscillations during decision-making. J Neurosci. 2013;33:6212–6224. doi: 10.1523/JNEUROSCI.2915-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seager Ma. ohnson LD, Chabot ES, Asaka Y, Berry SD. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proc Natl Acad Sci USA. 2002;99:1616–1620. doi: 10.1073/pnas.032662099. , J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Talnov A, Matsumoto G, Brankack J. Hippocampal theta rhythm and running speed: A reconsideration using within-single trial analysis. Neurocomputing. 2001;40:1567–1574. [Google Scholar]
- Shirvalkar PR, Rapp PR, Shapiro ML. Bidirectional changes to hippocampal theta-gamma comodulation predict memory for recent spatial episodes. Proc Natl Acad Sci USA. 2010;107:7054–7059. doi: 10.1073/pnas.0911184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Silva FHL, Arnolds DEAT. Physiology of the hippocampus and related structures. Ann Rev Physiol. 1978;40:185–216. doi: 10.1146/annurev.ph.40.030178.001153. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJY. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006;729:716–729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Timo-Iaria C, Negrão N, Schmidek WR, Hoshino K, Lobato de Menezes CE, Leme da Rocha T. Phases and states of sleep in the rat. Physiol Behav. 1970;5:1057–1062. doi: 10.1016/0031-9384(70)90162-9. [DOI] [PubMed] [Google Scholar]
- Tort ABL, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci USA. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort ABL, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci USA. 2009;106:20942–209427. doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloor J, Datta S. Spatio-temporal activation of cyclic AMP response element-binding protein, activity-regulated cytoskeletal-associated protein and brain-derived nerve growth factor: A mechanism for pontine-wave generator activation dependent two-way active-avoidance memory processing in the rat. J Neurochem. 2005;95:418–428. doi: 10.1111/j.1471-4159.2005.03378.x. [DOI] [PubMed] [Google Scholar]
- Van der Meer M, Kurth-Nelson Z, Redish AD. Information processing in decision-making systems. Neuroscientist. 2012;18:342–359. doi: 10.1177/1073858411435128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroncephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Viard A, Doeller CF, Hartley T, Bird CM, Burgess N. Anterior hippocampus and goal-directed spatial decision making. J Neurosci. 2011;31:4613–4621. doi: 10.1523/JNEUROSCI.4640-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CE, Amos DP, Jeewajee A, Douchamps V, Rodgers J, O'Keefe J, Burgess N, Lever C. Novelty and anxiolytic drugs dissociate two components of hippocampal theta in behaving rats. J Neurosci. 2013;33:8650–8667. doi: 10.1523/JNEUROSCI.5040-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Shohany D. Decision Making, Affect and Learning: Attention and Performance. Oxford NY: Oxford University Press; 2011. The striatum and beyond: Contributions of the hippocampus to decision making; pp. 281–309. [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Winson J. The biology and function of rapid eye movement sleep. Curr Opin Neurobiol. 1993;3:243–248. doi: 10.1016/0959-4388(93)90217-m. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci USA. 2010a;107:5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Vinck M, Leung LS, Everling S. Selective theta-synchronization of choice-relevant information subserves goal-directed behavior. Front Hum Neurosci. 2010b;4:210. doi: 10.3389/fnhum.2010.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary


