Abstract
Background
Early revascularization (ERV) is beneficial in the management of CS complicating myocardial infarction. The severity of CS varies widely, and identification of independent risk factors for outcome is needed. The effect of ERV on mortality in different risk strata is also unknown. We created a severity scoring system for cardiogenic shock (CS) and used it to examine the potential benefit of ERV in different risk strata using data from the SHOCK Trial and Registry.
Methods
Data from 1217 patients (294 from the randomized trial and 923 from the registry) with CS due to pump failure were included in a Stage 1 severity scoring system using clinical variables. A Stage 2 scoring system was developed using data from 872 patients who had invasive hemodynamic measurements. The outcome was in-hospital mortality at 30 days.
Results
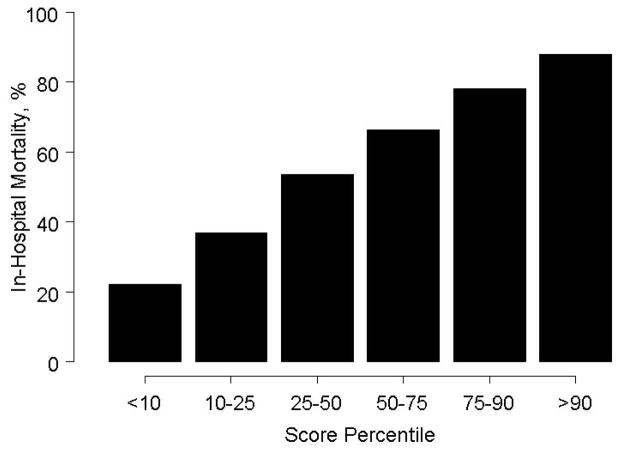
In-hospital mortality at 30 days was 57%. Multivariable modeling identified eight risk factors (Stage 1): age, shock on admission, clinical evidence of end-organ hypoperfusion, anoxic brain damage, systolic BP, prior CABG, non-inferior MI, and creatinine≥1.9 mg/dl (c-statistic=0.74). Mortality ranged from 22–88% by score category. ERV benefit was greatest in moderate-to-high-risk patients (p=0.02). The Stage 2 model based on patients with pulmonary artery catheterization included age, end-organ hypoperfusion, anoxic brain damage, stroke work and LVEF<28% (c-statistic=0.76). In this cohort the effect of ERV did not vary by risk stratum.
Conclusions
Simple clinical predictors provide good discrimination of mortality risk in CS complicating MI. ERV is associated with improved survival across a broad range of risk strata.
Keywords: cardiogenic shock, myocardial infarction, risk assessment
INTRODUCTION
Cardiogenic shock (CS) complicates 5–8% of all cases of acute myocardial infarction (AMI) and is a common cause of death in patients hospitalized with MI1–7 An estimated 120,000–140,000 patients in North America and Europe develop CS annually. Early revascularization (within 18 hours of shock diagnosis) has a large, favorable impact on one-year survival.8 However, not all patients receive early revascularization1 and there remains wide variability in mortality rates from CS with and without early revascularization and use of other support measures, from less than 30 to over 80%.7,9–15 Previous studies have identified patient, MI and treatment determinants of outcome7,10,14–16 Published risk models have primarily examined populations undergoing percutaneous coronary intervention (PCI) or participating in fibrinolytic trials.7,12,17 A robust risk model applicable to a broader group of shock patients would be useful not only for discussion of benefits and risks of therapeutic options with patients and families, but also for health care policies on resource utilization and in risk adjustment for outcome reporting from quality improvement programs. Due to the challenging nature of randomized trials in this critically ill population, a severity scoring system can also be used to control for patient differences in observational studies of new therapies. If a randomized trial is undertaken, the risk strata can facilitate patient selection.
We developed a simple severity score to estimate in-hospital mortality for patients with AMI complicated by CS (AMI-CS) using data from the SHOCK trial and registry, using measures available at the time of shock diagnosis, as well as a set arising from more invasive or time-consuming diagnostic assessment. The association between early revascularization and outcome was evaluated across the range of severity scores.
METHODS
Study Design
The NHLBI-funded SHOCK (SHould we emergently revascularize Occluded coronaries for Cardiogenic shocK?) trial randomized 302 patients within 12 hours of diagnosis of CS complicating MI to either early revascularization (ERV) or initial medical stabilization (IMS; including delayed revascularization at if clinically appropriate) at 30 international sites between 1993 and 1998.18 Trial eligibility required pulmonary artery (PA) catheterization. A concurrent registry included 1189 non-randomized patients with suspected CS from 1993–1997. In this analysis, ERV for all patients was defined as PCI or CABG performed within 18 hours of CS diagnosis. Patients who underwent late revascularization (>18 hours after CS diagnosis) were combined with patients who did not undergo revascularization, in accordance with the original study protocol. All centers obtained Institutional Review Board approval and written informed consent was obtained from each trial patient or a surrogate as permitted by local laws.
Patient Sample
All SHOCK Trial and Registry patients with CS due to predominant left or right ventricular failure were included in Stage 1 analysis to identify risk factors that can be assessed early after the diagnosis of CS– a total of 1217 patients (294 from the randomized trial and 923 from the registry). Patients with shock due to mechanical causes were excluded. For Stage 2 analysis, 872 patients who underwent PA catheterization close to the time of shock diagnosis and prior to revascularization (if applicable) were included.
Definitions
End-organ hypoperfusion, a trial inclusion criterion, was defined as urine output <30 cc/hour or cool extremities. Anoxic brain damage was declared according to local physician judgment regarding unconscious patients, e.g., post-arrest. Sites were instructed to take into consideration hemodynamic course, duration and effectiveness of resuscitative efforts, and concomitant medications that could affect mental status.
Statistical Methods
The outcome measure for this report is defined as in-hospital mortality within 30 days. Stage 1 (“Clinical”) logistic regression modeling included 32 candidate predictor variables that were available early after shock diagnosis for all patients and included patient demographics, heart rate, blood pressure (BP), medical history, shock timing and baseline laboratory values. Stage 2 (“Hemodynamic”) logistic regression modeling used measurements obtained from PA catheterization as well as left ventricular ejection fraction (LVEF) obtained by echocardiogram or left ventriculogram. Significant Stage 2 hemodynamic predictors were then added to the final clinical model and all entries were evaluated for significance. A p-value ≤0.05 was considered significant. Cox proportional hazards regression was used to obtain a severity-adjusted relative risk estimate for the effect of ERV.
Multiple imputation using 10 imputed datasets was used to derive a complete set of covariate values for all clinical variables for all patients, and to derive complete covariate values for all Stage 2 variables for the 872 patients who underwent PA catheterization. Data completeness was >90% for the majority of variables within Stage 1, with the exception of weight, creatinine, history of peripheral vascular disease and smoking. The c-statistic is presented to assess goodness of fit of the logistic regression models. Nonlinear relationships between outcome and continuous predictors were explored by fitting upper and lower quartile indicator variables. Interactions between significant main effects and age and sex, and a specific hypothesized interaction between systolic BP and intra-aortic balloon counterpulsation (IABP), were examined. The scoring system was derived using point assignments on the log odds ratio scale.
RESULTS
There were 697 deaths among 1217 patients (57% in-hospital mortality within 30 days). The cohort was (mean±SD) 67.7±11.7 years old and 64% male; 38% had prior MI and shock was diagnosed on admission in 21% (Table 1). Anoxic brain damage and end-organ hypoperfusion were strongly associated with mortality (c-statistic=0.56), and these two variables plus age rendered a c-statistic of 0.68. The full Stage I “Clinical” multivariable model (c-statistic=0.74) included eight risk factors (Table 2A) including older age, the presence of shock on admission and end-organ hypoperfusion, anoxic brain damage, lower supported systolic BP, prior CABG, non-inferior MI, and serum creatinine ≥1.9 mg/dl (75th percentile) (all p<0.02). Creatinine clearance ≤33 ml/min was interchangeable with creatinine ≥1.9 mg/dl (odds ratio 1.59, p=.013). Weight <190 lb as well as lower diastolic BP were independent risk factors for mortality when hypoperfusion and anoxic brain damage were omitted from modeling. No interactions between the Stage I predictors and age or sex were significant. An interaction between systolic BP and IABP was not an independent predictor, indicating higher systolic BP was protective regardless of IABP support.
Table 1.
Patient characteristics (N=1217) by vital status (in-hospital mortality within 30 days) – Stage 1 (Clinical - no invasive hemodynamics).
| Characteristic | Mean± SE or % | p-value | |
|---|---|---|---|
| Dead | Alive | ||
| N* | 697 | 520 | |
| Age, years | 70.3±0.2 | 64.2±0.3 | <.001 |
| Male | 63.0% | 65.8% | .002 |
| White, non-hispanic | 81.4% | 80.8% | .741 |
| MI to Shock, hours | 15.3±1.1 | 18.5±1.5 | .082 |
| MI to Shock <2 hr | 29.9% | 25.2% | .090 |
| Shock on Admission | 26.0% | 14.0% | <.001 |
| Prior MI | 42.3% | 32.1% | <.001 |
| History of Hypertension | 52.6% | 48.6% | .171 |
| Diabetes | 35.1% | 29.5% | .043 |
| Congestive heart failure | 18.8% | 13.3% | .014 |
| Prior CABG | 10.9% | 6.4% | .008 |
| Prior PCI | 5.9% | 8.0% | .176 |
| Current Smoker | 49.0% | 54.8% | .060 |
| Peripheral Vascular Disease | 21.41 | 14.8% | .023 |
| Other Severe systemic illness | 7.0% | 4.0% | <.001 |
| Anterior MI | 58.9% | 54.6% | .151 |
| Inferior MI (non-Anterior) | 33.5% | 39.2% | .056 |
| Absence of ST elevation and | 16.0% | 13.7% | .274 |
| LBBB | |||
| Weight, lb. | 163.6±1.7 | 172.4±1.9 | <.001 |
| Anoxic brain damage | 4.2% | 0.4% | .001 |
| End-organ hypoperfusion | 97.5% | 88.6% | <.001 |
| Systolic BP near shock, mmHg† | 84.6±0.9 | 92.9±0.9 | <.001 |
| Lowest Systolic BP, mmHg | 68.3±0.7 | 70.3±0.8 | .056 |
| Diastolic BP near shock, mmHg† | 49.8±0.7 | 55.7±0.7 | <.001 |
| Heart Rate near shock, bpm | 97.0±1.0 | 95.7±1.1 | .389 |
| Left ventricular ejection fraction,%† | 29.0±0.9 | 32.9±0.7 | .002 |
| Initial serum creatinine, mg/dl | 1.84±0.07 | 1.56±0.07 | .007 |
| Creatinine ≥1.9 mg/dl | 31.9% | 19.6% | .0001 |
| Creatinine Clearance, ml/min‡ | 73.8±6.3 | 95.6±9.5 | .043 |
| Creatinine Clearance ≤30, ml/min‡ | 36.1% | 24.1% | <.001 |
| Creatine kinase (xULN) | 16.4±0.8 | 19.8±1.8 | .118 |
| Pulmonary Edema on X-ray | 58.1% | 65.4% | .032 |
N represents total number of observations after imputation (see Methods)
on support measures, including vasopressors, inotropes±IABP; Peak BP is reported as systolic BP, i.e., for patients on IABP support, augmented diastolic pressure is reported as systolic BP; LVEF, when measured by echocardiogram or left ventriculogram close to shock diagnosis, was obtained without IABP support in 74%.
creatinine clearance estimated by C=((140-age)/creatinine)*(weight/72) for males and C*0.85 for females, with age in years, creatinine in mg/dl, and weight in kg
BP=blood pressure; CABG=coronary artery bypass grafting; LBBB=left bundle branch block, MI=myocardial infarction, PTCA=percutaneous transluminal coronary angioplasty; ULN=upper limit of normal.
Table 2A.
STAGE 1 (Clinical): Logistic Regression Multivariable Model without Invasive Hemodynamics (N=1217)
| Variable | Estimate | Std. Error | Odds Ratio | p-value |
|---|---|---|---|---|
| Age | 0.047 | .006 | 1.27 per 5 yr increase | <.001 |
| Anoxic Brain Damage | 3.069 | .799 | 21.52 | .0001 |
| End-Organ Hypoperfusion | 1.425 | .333 | 4.16 | <.001 |
| Shock on Admission | 0.654 | .179 | 1.92 | .0003 |
| Prior CABG | 0.694 | .235 | 2.00 | .0032 |
| Non-Inferior MI* | 0.327 | .137 | 1.39 | .0172 |
| Creatinine ≥1.9 mg/dl | 0.516 | .162 | 1.68 | .0016 |
| Systolic BP | −0.018 | .003 | 1.09 per 5 mmHg decrease | <.001 |
Defined as MI locations except for those with inferior but no anterior involvement
on support measures, including vasopressors, inotropes±IABP. Obtained prior to or without IABP support in 76%.
Table 2B displays the scoring system based on the Stage I model. Mortality increases with severity score category (Figure 1), up to 88% in the upper decile.
Table 2B.
STAGE 1 (Clinical): Scoring System without Invasive Hemodynamics*
| Variable | Points |
|---|---|
| Anoxic Brain | 30 |
| Damage | |
| Shock on Admission | 6 |
| Non-Inferior MI | 3 |
| Age, years‡ | |
| ≤45 | 0 |
| 46.50 | 2 |
| 51.55 | 5 |
| 56.60 | 7 |
| 61.65 | 10 |
| 66.70 | 12 |
| 71.75 | 15 |
| 76.80 | 17 |
| 81.85 | 20 |
| 86.90 | 22 |
| >90 | 25 |
| Hypoperfusion | 14 |
| Prior CABG | 7 |
| Creatinine ≥1.9 mg/dl | 5 |
| Systolic BP, mmHg† | |
| ≤55 | 12 |
| 56–60 | 11 |
| 61–65 | 10 |
| 66–70 | 9 |
| 71–75 | 8 |
| 76–80 | 7 |
| 81–85 | 6 |
| 86–90 | 5 |
| 91–95 | 4 |
| 96–100 | 3 |
| 101–105 | 2 |
| 105–110 | 1 |
| >110 | 0 |
When LVEF is added to this system, non-inferior MI has 0 points, and LVEF has 10 points if ≤15%, 7 points if 16–25%, 5 points if 26–35%, 2 points if 36–45%, and 0 points if >45%.
on support measures, including vasopressors, inotropes±IABP. Obtained prior to or without IABP support in 76%.
When dichotomized to assess elderly risk, patients ≥75 years assigned 9 points and patients <75 years assigned zero points.
CABG=coronary artery bypass graft surgery
Figure 1.
Risk Factor Profile by Stage I Clinical Severity Score Category. Y-axis represents percentage of total sample.
Because LVEF is often measured by echocardiography close to the time of shock presentation, a multivariable model including LVEF (available for 51% of all patients, imputed for the remainder) was also developed using the entire cohort (odds ratio 0.88 per 5% absolute increase in LVEF, p=0.001). This multivariable model (c=0.75) included all clinical model predictors except for inferior (non-anterior) MI location (p=0.30).
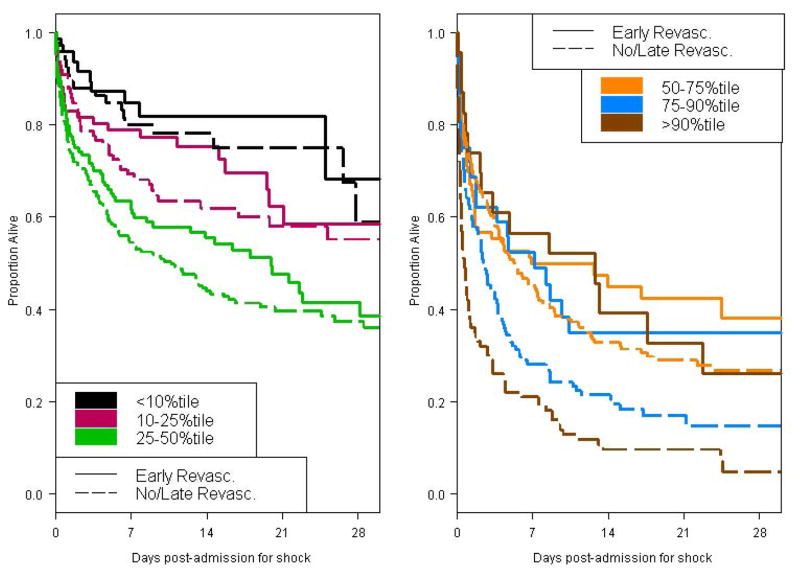
The mean clinical severity scores±SE for the early revascularization and no/late revascularization groups were 33.4±0.5 and 37.1±0.4, respectively. ERV conferred a significant benefit overall (adjusted odds ratio 0.57, 95% confidence interval 0.44 to 0.75; relative risk 0.71, both p<0.001). We also examined whether early revascularization benefit depended on severity score category, fit as a linear predictor. Greatest benefit of early ERV was in patients with severity scores above the 25th percentile (risk stratum by treatment interaction p=0.022). Of note, patients in the first severity quartile were less likely to have had shock on admission (5% vs. 26%), and consequently more likely to have received thrombolytic therapy for the index MI prior to shock diagnosis (50% vs. 36%). Actual and model-based mortality rates by treatment group demonstrated very good model fit (Table 2C).
Table 2C.
In-hospital mortality within 30 days by Stage 1 (Clinical) Severity Score Category and Revascularization Status*.
| Observed Mortality, % | Model-Predicted Mortality, % | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Risk Category† | Percentile | N | % ERV | All | Early Revasc. | No or Late Revasc. | Early Revasc. | No or Late Revasc. |
| <24 | <10 | 116 | 41 | 23 | 20 | 25 | 26 | 26 |
| 24 to <30 | 10–25 | 175 | 42 | 38 | 35 | 40 | 35 | 40 |
| 30 to <37 | 25–50 | 326 | 28 | 54 | 47 | 59 | 44 | 57 |
| 37 to <43 | 50–75 | 263 | 23 | 67 | 57 | 71 | 55 | 72 |
| 43 to <48 | 75–90 | 147 | 13 | 78 | 62 | 82 | 65 | 83 |
| ≥48 | >90 | 131 | 11 | 88 | 66 | 92 | 73 | 91 |
P=0.022 for interaction of risk category and treatment
Use of the Stage 1 system with LVEF instead of non-inferior MI yields risk categories approximately 4 points higher to correspond to percentile groupings shown.
Timing of revascularization was not available for 59 patients
ERV=Early Revascularization (=18 hours following shock diagnosis); Revasc.=Revascularization
The Stage 2 model was developed using the 872 patients who underwent PA catheterization. There were 435 deaths (50% in-hospital mortality ≤30 days). Mean±SD age was 66.5±11.4 years and 64% were male; 34% had prior MI (Table 3); 3% had isolated RV shock which was not associated with mortality. The Stage 2 “Hemodynamic” multivariable model (c-statistic=0.75, Tables 4A-4B) included five predictors (all p≤0.002): three clinical (age, end-organ hypoperfusion and anoxic brain damage), and two hemodynamic parameters (stroke work and LVEF<28%).
Table 3.
Characteristics of Patient Cohort with Invasive Hemodynamic Measurements (N=872) by vital status (in-hospital mortality within 30 days) – Stage 2
| Characteristic | Mean±SE or % | p-value | |
|---|---|---|---|
| Dead | Alive | ||
| N | 435 | 437 | |
| Age, years | 69.2±0.2 | 63.8±0.3 | <.001 |
| Age ≥ 75 years | 30.1% | 17.6% | <.001 |
| Male | 61.6% | 65.9% | .188 |
| White, non-Hispanic | 19.2% | 19.7% | .850 |
| MI to Shock, hours | 16.4±1.3 | 19.3±1.6 | .154 |
| Shock on Admission | 21.9% | 14.0% | .004 |
| Prior MI | 38.9% | 29.2% | .003 |
| History of Hypertension | 52.8% | 48.8% | .246 |
| Diabetes | 36.6% | 28.9% | .018 |
| Prior CABG | 11.0% | 7.0% | .040 |
| Prior PCI | 7.0% | 7.6% | .735 |
| Weight, lb | 162.3±2.1 | 173.0±2.1 | <.001 |
| Systolic BP near shock, mmHg* | 89.7±1.1 | 94.7±1.0 | <.001 |
| Heart rate near shock, bpm* | 101.5±1.2 | 97.0±1.2 | .006 |
| Anoxic brain damage | 3.7% | 0.5% | .005 |
| End-organ hypoperfusion | 97.1% | 88.1% | <.001 |
| Initial serum creatinine, mg/dl | 1.82±0.08 | 1.57±0.08 | .049 |
| Creatinine <1.9 mg/dl | 30.2% | 19.9% | .005 |
| Left ventricular ejection fraction, % | 26.9±0.9 | 33.1±0.7 | <.001 |
| Cardiac index, L/min/m2 | 1.78±0.04 | 2.09±0.04 | <.001 |
| PCWP, mmHg | 24.9±0.4 | 22.9±0.4 | <.001 |
| Stroke work, g/m† | 18.5±0.7 | 27.5±0.9 | <.001 |
| Stroke work index, g/m/m2† | 10.0±0.4 | 14.3±0.4 | <.001 |
| Systemic vascular resistance, dyne sec/cm5 | 1426±40 | 1294±32 | .023 |
| Mean right atrial pressure, mmHg | 15.2±0.4 | 15.2±0.5 | .993 |
| Cardiac power index, watt/m2† | 0.26±0.01 | 0.33±0.01 | <.001 |
BP=blood pressure; CABG=coronary artery bypass grafting; MI=myocardial infarction, PTCA=percutaneous transluminal coronary angioplasty
on support measures, including vasopressors, inotropes±IABP;72% of values reported without IABP support
Stroke work index = (MAP-PCWP)*0.0136*(CO/HR)*1000/body surface area, where MAP=mean arterial pressure, mmHg; PCWP=pulmonary capillary wedge pressure, mmHg; CO=cardiac output, L/min; HR=heart rate
Cardiac power index=MAP*cardiac index/451
Table 4A.
STAGE 2: Multivariable Model‡ (N=872) based on Cohort with Invasive Hemodynamic Measurements
| Variable | Estimate | Std. Error | Odds Ratio | p-value |
|---|---|---|---|---|
| Stroke Work, g/m*† | −.0358 | .0069 | 0.84 per 5 units | <.001 |
| LVEF <28%* | 0.7880 | .1924 | 2.20 | <.001 |
| Age, years | .0413 | .0072 | 1.23 per 5 yr | <.001 |
| Anoxic Brain Damage | 2.4902 | .8168 | 12.1 | .002 |
| End Organ Hypoperfusion | 1.3667 | .3889 | 3.92 | <.001 |
on support measures
Stroke work=(MAP-PCWP)*0.0136*(CO/HR)*1000, where MAP=mean arterial pressure in mmHg, PCWP=pulmonary capillary wedge pressure in mmHg, CO=cardiac output, L/min, and HR=heart rate, bpm
Secondary analysis demonstrated that vasopressor score was also an independent predictor (p<0.001; odds ratio 2.02 per vasopressor used during CS hospitalization)
Table 4B.
STAGE 2: Scoring System for Invasive Hemodynamic Cohort
| Variable | Points |
|---|---|
| Anoxic Brain Damage | 25 |
| LVEF <28%* | 8 |
| Age, yr | |
| ≤45 | 0 |
| 46.51 | 2 |
| 51.56 | 4 |
| 56.61 | 6 |
| 61.66 | 8 |
| 66.71 | 10 |
| 71.76 | 12 |
| 76.81 | 14 |
| 81.86 | 16 |
| 86.91 | 18 |
| >90 | 20 |
| End Organ Hypoperfusion | 14 |
| Stroke Work, g/m*† | |
| ≤5 | 18 |
| 6–15 | 15 |
| 16–25 | 12 |
| 26–35 | 9 |
| 36–45 | 6 |
| 46–55 | 3 |
| >55 | 0 |
on support measures
Stroke work definition is in Table 4A
Table 5 displays outcome by Stage 2 severity score category. The mean±SE Stage 2 severity score for those undergoing early vs. no or late revascularization was 43.7±0.6 and 45.2±0.4, respectively. ERV was associated with lower mortality (adjusted odds ratio 0.62, 95% confidence interval 0.45–0.85, p=0.003, c-statistic=0.75; relative risk 0.80, 95% confidence interval 0.64–0.99, p=0.04). There was no significant interaction between revascularization status and severity score quantile (p=0.35), indicating that ERV provides benefit at all levels of severity amongst patients who undergo PA catheterization.
Table 5.
STAGE 2: In-hospital mortality within 30 days for Invasive Hemodynamic Cohort (N=825) by Severity Score Category and Revascularization Status*
| Observed Mortality, % | Model-Predicted Mortality, % | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Risk Category | Percentile | Severity Score | % ERV | All | Early Revasc. | No or Late Revasc. | Early Revasc. | No or Late Revasc. |
| <25 | <10 | 18.0±0.7 | 41 | 15 | 5 | 21 | 9 | 19 |
| 25 to <32 | 10–25 | 28.2±0.2 | 49 | 24 | 18 | 31 | 18 | 30 |
| 32 to <48 | 25–50 | 34.6±0.1 | 38 | 41 | 35 | 45 | 32 | 45 |
| 38 to <45 | 50–75 | 40.8±0.2 | 32 | 57 | 52 | 60 | 50 | 61 |
| 45 to <49 | 75–90 | 46.3±0.1 | 39 | 70 | 63 | 76 | 68 | 75 |
| ≥49 | >90 | 53.3±0.6 | 33 | 85 | 84 | 86 | 82 | 85 |
No significant interaction of risk category and treatment (p=0.35): ERV vs. No/Late Revascularization Relative Risk=0.80 (95% CI 0.64 to 0.99); Odds Ratio=0.62 (95% CI 0.45 to 0.85)
Timing of revascularization was not available for 47 patients
ERV=Early Revascularization (≤18 hours following shock diagnosis
We also examined whether the benefit of ERV across Stage 2 severity score category depended on age (<75 vs. ≥75 years), and found no interaction (p=0.47).
DISCUSSION
Cardiogenic shock complicating MI is associated with a wide range of mortality rates that can be estimated using readily available clinical characteristics and hemodynamic measurements. Predictors of mortality in this analysis had partial agreement with those reported in large registries (NRMI and GRACE). 9,10 The SHOCK models were derived from a clinical trial and related registry devoted to cardiogenic shock and therefore include more extensive information, including hemodynamic data not available in the other studies, in part due to the requirement of PA catheterization to assess trial eligibility. This may have led to differing multivariable findings, especially in the Stage 2 model. Clinical selection for PCI in NRMI and GRACE may have affected relationships between patient characteristics and mortality in these registries. For example, age was a predictor of mortality in GRACE and SHOCK but not NRMI, likely relating to selection for revascularization. Diabetes was a predictor of mortality in NRMI and GRACE, but not an independent risk factor in SHOCK.
Shock on admission, which was associated with more severe hemodynamic derangement, was an independent predictor of mortality in SHOCK when right heart catheterization hemodynamics were not considered but, for somewhat unclear reasons, was protective in the GRACE multivariate analysis.17 One possible explanation for this difference is that the GRACE registry included mechanical complications, which are associated with high mortality and often develop somewhat later after MI.
Despite their very strong association with outcome19, invasive hemodynamic measurements obtained from PA catheterization surprisingly offered minimal improvement in predictive value when included in the model (c-statistic=0.76 compared to the Stage I model c-statistic=0.74). This may be due to lower overall risk in the PA catheterization cohort as evidenced by its lower mortality rate.
Anoxic brain injury was a particularly powerful predictor of adverse outcome in this dataset. However, early after arrest it can be difficult to assess whether there is irreversible injury. The diagnosis of anoxic brain injury at SHOCK centers was a clinical determination made locally. Because neither formal neurologic testing nor brain imaging was required for this diagnosis, assessed within 12 hours following shock onset, the scoring system can be readily applied to patients diagnosed with CS.
Remarkably, systemic hypoperfusion was also a very strong independent predictor of mortality in this cohort with suspected cardiogenic shock, even when hemodynamics and EF were considered. This was defined as the presence of cool extremities or urine output <30cc/hr, and would be expected to be reproducible in practice, allowing application of the scoring system.
A major strength of this analysis is that inclusion of Registry patients allows the entire spectrum of patients with CS due to pump failure complicating MI to be represented, including patients who met a randomized trial exclusion criterion such as anoxic braidamage, prior severe systemic illness, or dilated cardiomyopathy or did not meet the randomized trial hypoperfusion inclusion criterion. Importantly, we found no significant interactions between the risk factors identified and age or sex, which indicates that the severity scoring system is applicable to patients of all ages and both sexes.
A secondary aim was to assess the benefit of ERV across severity levels. Our findings should influence physicians to consider ERV in a broader group of patients with AMI-CS. Amongst all those presenting with shock, we found that ERV conferred benefit in Stage 1 cohort patients with scores above the first quartile of severity. This is an important finding because it negates concerns of futility of ERV in high risk patients. Amongst the lower risk patients, the attenuation of ERV benefit in our cohort may be due in part to the higher rate of thrombolytic use as a form of reperfusion therapy (50% vs. 36%). In addition, in the PA catheterization cohort, we found that the benefit of ERV at all levels of severity did not depend on age (<75 vs. ≥75 years). This finding supports the ACC/AHA class IIa recommendation of ERV in older patients.
Our findings have policy and reporting implications. We identified several variables that are important predictors of mortality but not included in typical procedural databases and therefore cannot be included in the risk adjustment model which is used for public reporting of procedural mortality rates, and which is known to affect practice. 20,21 These include the presence of anoxic brain damage, vasopressor requirement (Table 4A), systolic BP on support and stroke work. Our results suggest that this information (and other unmeasured variables, as suggested by the c-statistic of 0.76) might help adjust outcomes for case mix among patients undergoing PCI or CABG for AMI-CS.
Research on patients with CS complicating AMI is challenging due to the wide range of observed mortality rates and subsequent difficulty in making appropriate assumptions for the control event rate in the design of a trial. Our scoring system should facilitate trial design by providing refined estimates of outcome for select patient subgroups. For example, if those planning a trial of a new therapy for CS to be applied after ERV specified inclusion criteria of LVEF<28% and the presence of end-organ hypoperfusion, with expected mean age of 72 years and mean stroke work of 20 g/m, then the predicted mortality rate is 68%. This estimate could then be used in to develop sample size calculations for the target patient population.
This analysis has several limitations. The patients were diagnosed with shock and treated between 1993 and 1999, and the management of their condition may not be completely representative of current approaches. However, since that period there has been no major advance in the therapy for CS beyond ERV, and outcomes have changed little22. Another limitation is that non-randomized clinical selection of registry patients for revascularization may have altered the apparent benefit of ERV in some risk strata. In addition, the test of interaction between ERV and severity score category in the Stage 2 model may be underpowered to detect a smaller benefit of ERV in the highest decile of severity. Finally, our model has not been validated using an independent dataset.
In conclusion, we report a scoring system for in-hospital mortality risk in patients with AMI-CS and have shown that ERV improves outcome across nearly all risk strata in patients with pump failure. This scoring system can be used for risk assessment when counseling patients and families, in health care policy resource utilization decisions, in quality improvement systems, and design of trials to test novel therapies.
Figure 2.
Thirty-day mortality by Stage I clinical severity score quantile and revascularization status. Early revascularization is defined as ≤18 hours of shock diagnosis. Estimates based on one imputation dataset.
Acknowledgments
This research was supported by grants from the National Heart, Lung, and Blood Institute, R01 HL50020 and R01 HL49970
We gratefully acknowledge the SHOCK Trial study coordinators and study subjects for these data.
Footnotes
No Disclosures
Clinical Trials Registration #NCT00000552
Severity Score Calculator (fall 2010):
http://www.med.nyu.edu/medicine/cardiology/about_us/cardiogenic-shock-survival-predictor.html
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berger PB, Holmes DR, Jr, Stebbins AL, et al. Impact of an aggressive invasive catheterization and revascularization strategy on mortality in patients with cardiogenic shock in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO-I) trial. An observational study. Circulation. 1997;96(1):122–7. doi: 10.1161/01.cir.96.1.122. [DOI] [PubMed] [Google Scholar]
- 2.Fox KA, Anderson FA, Jr, Dabbous OH, et al. Intervention in acute coronary syndromes: do patients undergo intervention on the basis of their risk characteristics? The Global Registry of Acute Coronary Events (GRACE) Heart. 2007;93(2):177–82. doi: 10.1136/hrt.2005.084830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg RJ, Samad NA, Yarzebski J, et al. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med. 1999;340(15):1162–8. doi: 10.1056/NEJM199904153401504. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn LA. The treatment of cardiogenic shock. I. The nature of cardiogenic shock. Am Heart J. 1967;74(4):578–81. doi: 10.1016/0002-8703(67)90019-1. [DOI] [PubMed] [Google Scholar]
- 5.Lindholm MG, Kober L, Boesgaard S, et al. Cardiogenic shock complicating acute myocardial infarction; prognostic impact of early and late shock development. Eur Heart J. 2003;24(3):258–65. doi: 10.1016/s0195-668x(02)00429-3. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RH, Sadiq I, Goldberg RJ, et al. Effectiveness of primary percutaneous coronary intervention compared with that of thrombolytic therapy in elderly patients with acute myocardial infarction. Am Heart J. 2004;147(2):253–9. doi: 10.1016/j.ahj.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Zeymer U, Vogt A, Zahn R, et al. Predictors of in-hospital mortality in 1333 patients with acute myocardial infarction complicated by cardiogenic shock treated with primary percutaneous coronary intervention (PCI); Results of the primary PCI registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK) Eur Heart J. 2004;25(4):322–8. doi: 10.1016/j.ehj.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006;295(21):2511–5. doi: 10.1001/jama.295.21.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babaev A, Frederick PD, Pasta DJ, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294(4):448–54. doi: 10.1001/jama.294.4.448. [DOI] [PubMed] [Google Scholar]
- 10.Dauerman HL, Goldberg RJ, White K, et al. Revascularization, stenting, and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol. 2002;90(8):838–42. doi: 10.1016/s0002-9149(02)02704-2. [DOI] [PubMed] [Google Scholar]
- 11.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341(9):625–34. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 12.Klein LW, Shaw RE, Krone RJ, et al. Mortality after emergent percutaneous coronary intervention in cardiogenic shock secondary to acute myocardial infarction and usefulness of a mortality prediction model. Am J Cardiol. 2005;96(1):35–41. doi: 10.1016/j.amjcard.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 13.Ohman EM, Nanas J, Stomel RJ, et al. Thrombolysis and counterpulsation to improve survival in myocardial infarction complicated by hypotension and suspected cardiogenic shock or heart failure: results of the TACTICS Trial. J Thromb Thrombolysis. 2005;19(1):33–9. doi: 10.1007/s11239-005-0938-0. [DOI] [PubMed] [Google Scholar]
- 14.Sutton AG, Finn P, Hall JA, et al. Predictors of outcome after percutaneous treatment for cardiogenic shock. Heart. 2005;91(3):339–44. doi: 10.1136/hrt.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26(13):1276–83. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 16.Iakobishvili Z, Behar S, Boyko V, et al. Does current treatment of cardiogenic shock complicating the acute coronary syndromes comply with guidelines? Am Heart J. 2005;149(1):98–103. doi: 10.1016/j.ahj.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Hasdai D, Holmes DR, Jr, Califf RM, et al. Cardiogenic shock complicating acute myocardial infarction: predictors of death. GUSTO Investigators. Global Utilization of Streptokinase and Tissue-Plasminogen Activator for Occluded Coronary Arteries. Am Heart J. 1999;138(1 Pt 1):21–31. doi: 10.1016/s0002-8703(99)70241-3. [DOI] [PubMed] [Google Scholar]
- 18.Hochman JS, Sleeper LA, Godfrey E, et al. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK: an international randomized trial of emergency PTCA/CABG-trial design. The SHOCK Trial Study Group. Am Heart J. 1999;137(2):313–21. doi: 10.1053/hj.1999.v137.95352. [DOI] [PubMed] [Google Scholar]
- 19.Fincke R, Hochman JS, Lowe AM, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004;44(2):340–8. doi: 10.1016/j.jacc.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 20.Apolito RA, Greenberg MA, Menegus MA, et al. Impact of the New York State Cardiac Surgery and Percutaneous Coronary Intervention Reporting System on the management of patients with acute myocardial infarction complicated by cardiogenic shock. Am Heart J. 2008;155(2):267–73. doi: 10.1016/j.ahj.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Narins CR, Dozier AM, Ling FS, et al. The influence of public reporting of outcome data on medical decision making by physicians. Arch Intern Med. 2005;165(1):83–7. doi: 10.1001/archinte.165.1.83. [DOI] [PubMed] [Google Scholar]
- 22.French JK, Mahaffey KW, Hellkamp AS, et al. Mortality after cardiogenic shock remains high in ST-elevation myocardial infarction treated with primary PCI: insights from APEX AMI. Eur Heart J. 2008;29(Suppl I):447. [Google Scholar]