Abstract
Importance of the field
The incidence of malignant melanoma is increasing throughout the world and is currently rising faster than any other cancer in men and second only to lung cancer in women. Current strategies focused on systemic therapy for treatment of have shown no effect on survival. Therefore there is a pressing need for developing novel targeted therapeutics.
Areas covered in this review
Our goal is to provide an overview regarding targeting CXCR1/2 in malignant melanoma, the rationale behind these approaches and the future perspective.
What the reader will gain
This review illustrates our current understanding of CXCR1/2 receptor in melanoma progression and metastasis. We describe approaches that are being developed to block CXCR1/2 activation, including low-molecular-weight antagonists, modified chemokines and antibodies directed against ligands and receptors.
Take home message
The chemokine receptors CXCR1 and CXCR2 and their ligands play an important role in the pathogenesis of malignant melanoma. Recent reports demonstrated that CXCR1 is constitutively expressed in all melanoma cases irrespective of stage and grade, however, CXCR2 expression was restricted to aggressive melanoma tumors,. Furthermore, modulation of CXCR1/2 expression and/or activity has been shown to regulate malignant melanoma growth, angiogenesis and metastasis, suggesting CXCR1/2 targeting as a novel therapeutic approach for malignant melanoma.
Keywords: chemokine receptors, CXCR1, CXCR2, melanoma, targeted therapeutics
1. Introduction
Chemokines are a family of small proteins (8 – 11 kDa) that are divided into four groups according to the number and spacing of the first two cysteine residue in their amino-terminal end (C, CC, CXC, CX3C) [1,2]. They represent a large family of polypeptide signaling molecules, originally characterized by their ability to promote the directed chemotaxis of leukocytes, and are known to play important roles in inflammation and cancer [3]. Out of these chemokines, CXC chemokines can be further sub divided into two groups on the basis of presence or absence of an ELR motif (glutamic acid, leucine and arginine) which precedes the first cysteine residues in the protein. These CXC chemokines have been implicated in the initiation and amplification of inflammatory diseases [4–7]. CXC chemokines are known to bind to G-protein-coupled receptors (GPCR) mainly CXCR1 and CXCR2 which plays an important role in cancer progression and metastasis [8–10]. There are 15 human CXC chemokines (CXCL1 – 16), but their function extends beyond leukocyte chemotaxis as the cognate receptors CXCR1 and CXCR2 are expressed on many different cell types. Over the last decade, the understanding of the function of these receptors has increased exponentially [3,8,11], leading to the discovery of potent and selective antagonists [11,12], neutralizing monoclonal antibodies to these receptors and their ligands [4,5,13], the availability of CXCR2 knockout mice [6,14–16] and the identification of polymorphisms and genetic mutations [17–20]. Recent reports provide compelling evidence that CXCR1/2 play an important role in tumor progression and metastasis and several pharmaceutical companies have identified potent CXCR1/2 antagonists, and neutralizing antibodies that are now being tested in clinical trials for inflammatory disease. In this review, we will provide an overview and future implications regarding targeting CXCR1/2 in malignant melanoma.
2. CXCR1/2 and its ligands in malignant melanoma
Although chemokines were first known as chemoattractants for leucocytes, it has been recognized that many cell types express chemokines and chemokine receptors. Interactions between chemokines and their receptors play an important part in regulating various steps of tumor development, including tumor growth, progression, and metastasis (Figure 1). In the case of melanoma, several reports strongly support the proposition that tumor cells take advantage of this chemokine– chemokine receptor interaction either to stimulate the immune response, or to induce tumor angiogenesis and tumor growth, which alters the tumor microenvironment and facilitates metastasis to secondary site [8,10,21].
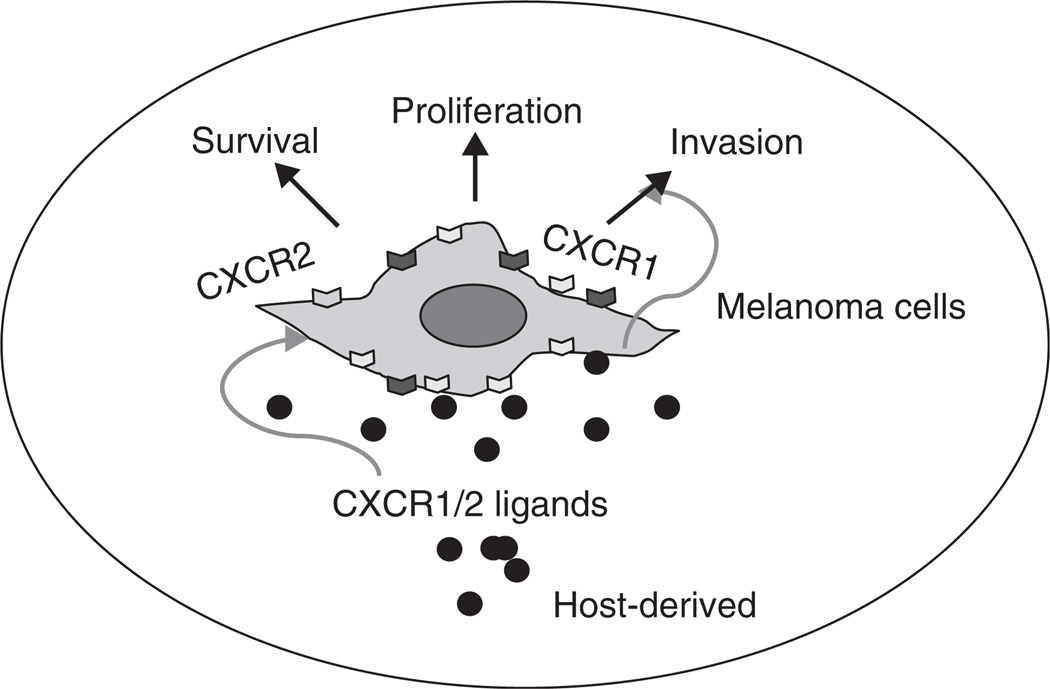
Figure 1.
CXCR1- and CXCR2-dependent regulation of phenotypes associated with malignant melanoma progression and metastasis.
Melanoma arises from melanocytes and manifests mainly on the skin and is the sixth most common cancer in the United States. According to the American Cancer Society there will be about 68,720 new cases and 8650 deaths due to melanoma during 2009 [22]. The chance of developing melanoma increases with age, but it affects all age groups and is one of the most common cancers in young adults. Most often, melanomas progress through an initial radial growth phase, or in situ melanoma, to a more aggressive vertical growth phase that exhibits growth in the mesenchyme and in the epithelium [23]. Melanoma tissues and cell lines derived from them have been shown to express a variety of chemokines, including CXCL8 and its receptors CXCR1 and CXCR2 [23,24]. CXCL8 alone and with its receptors can induce angiogenesis and influence migration and invasion of tumor cells along with metastasis in melanoma [23–29]. In this review, we provide an update on targeting CXCL8 and its receptors in melanoma progression and metastasis.
3. CXCR1/2 and their ligands in melanoma tumor progression and metastasis
Tumor progression is a chain of cellular and molecular events that occur gradually during the development of neoplasia. CXCL8 was the first chemokine reported to induce melanoma cell chemotactic migration [30] and can act in an autocrine/paracrine fashion to influence the process of melanoma progression by activating CXCR1 and CXCR2 (Figure 1) [26,30]. The expression of CXCL8 along with its receptors CXCR1 and CXCR2 have been shown to correlate positively with melanoma progression [31,32]. The overexpression of CXCR1 and CXCR2 in melanoma cells is associated with aggressive phenotypes of melanoma cells based on their enhanced proliferation, migration and tumor growth in mice [23,25,26]. Knockdown of the receptors or the use of antagonists or neutralizing antibodies against them affects melanoma cell proliferation migration and tumor growth, strongly indicating the involvement of these receptors in melanoma progression [33]. CXCR2 knockout mice exhibited significant inhibition of human melanoma tumor growth [25]. Furthermore, UVB which stimulates the production of CXCL8 in turn enhances the migration of metastatic melanoma cells in vitro [34,35]. Altogether, these data suggest an important role for CXCL8 and its receptors in melanoma progression.
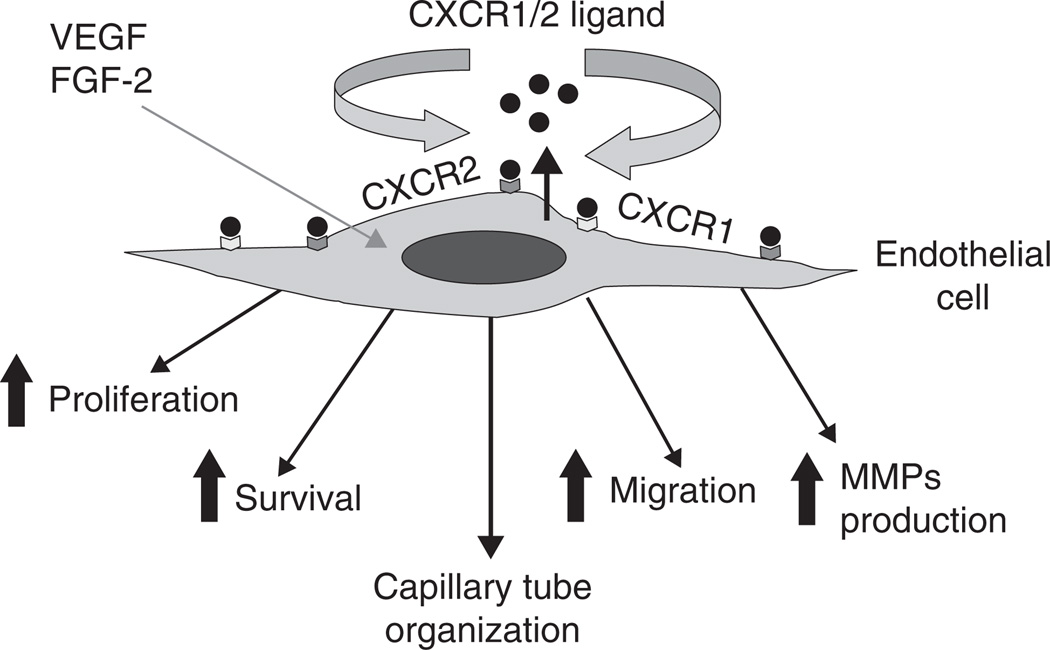
CXCL8 and its receptors can affect tumor growth not only directly but also indirectly by promoting angiogenesis and the ability of CXCL8 to elicit angiogenic activity depends on the expression of its receptors by endothelial cells. Recent studies indicate that CXCR1 is highly and CXCR2 is moderately expressed on human microvascular endothelial cells (HMEC), whereas HUVEC show low levels of CXCR1 and CXCR2 expression [36]. Neutralizing antibodies to CXCR1 and CXCR2 abrogated CXCL8-induced migration of endothelial cells, indicating that these two receptors are critical for the CXCL8 angiogenic response (Figure 2) [37,38]. Of these two high-affinity receptors for CXCL8, the importance of CXCR2 in mediating chemokine-induced angiogenesis was demonstrated to be fundamental to CXCL8-induced neovascularization [13,38,39]. CXCL8 stimulates both endothelial proliferation and capillary tube formation in vitro in a dose-dependent manner, and both of these effects can be blocked by monoclonal antibodies to CXCL8 [40,41]. Recent studies have also highlighted the importance of CXCR1 and CXCR2 in angiogenesis (Figure 2) [23,25–27]. In addition, it has been reported that there is a direct correlation between high levels of CXCL8 and tumor angiogenesis, progression and metastasis in xenograft models of human melanoma [42,43].
Figure 2.
Autocrine and paracrine signaling and role of CXCR1 and CXCR2-dependent signaling in regulation of angiogenic phenotype.
FGF: Fibroblast growth factor.
CXCL8 exerts its angiogenic activity by upregulating MMP-2 and MMP-9 in tumor and endothelial cells [37,42,44]. Degradation of the extracellular matrix by MMPs is required for endothelial cell migration, organization, and, hence, angiogenesis [45]. It has been demonstrated that CXCL8 directly enhances endothelial cell proliferation, survival and MMP expression in CXCR1- and CXCR2-expressing endothelial cells, indicating that CXCL8 is an important player in the process of angiogenesis [40].
Cell proliferation, angiogenesis and migration (invasion) are important components of the metastatic process and CXCL8 and its receptors have been implicated inmelanoma progression through several mechanisms, including the promotion of tumor cell growth and migration [30,46]. Our previous study has demonstrated a correlation between CXCL8 expression and metastatic behavior in human melanoma cells in nude mice. Additionally, in a nude mouse model, induction of UV-induced melanoma cell tumorigenesis and metastasis correlated with CXCL8 mRNA and protein expression [47]. It has also been shown that the metastatic variant of melanoma cells expressed higher levels of CXCL8 protein as compared with the non-metastatic variant [47,48]. Elevated serum levels of CXCL8 in patients with metastatic melanoma and hepatocellular carcinoma have also been reported to correlate with tumor burden and poor prognosis [49,50]. Expression of CXCL8 inmetastatic and invasive lesions may result in an increase in the serum CXCL8 concentration and may be of prognostic use and serve as a determinant of melanoma growth and metastasis [33,49,50]. In vivo murine studies showed that CXCR2 plays a major role in melanoma metastasis to the lung and a recent study from our laboratory suggested that human melanoma xenografts onto CXCR2 knockout nude mice exhibited inhibition of lung metastasis. This was accompanied by a reduction in tumor cell proliferation, angiogenesis and reduced inflammation [25]. CXCL8 in tumor specimens from different stages of melanoma is differentially expressed in radial growth phase (RGP; melanoma-in-situ) and vertical growth phase (VGP; invasive) primary malignant melanoma and subcutaneous, muscle and lymph node metastases. While the RGP tumors did not show any staining for CXCL8, 50% of the VGP tumors were positive and showed a heterogeneous pattern of staining. Interestingly, an intense CXCL8 immunoreactivity was observed in the metastatic lesions from skin, muscle and lymph node [23]. These data suggest an association between the expression of CXCL8 and metastasis in human cutaneous melanoma. Additionally, a concomitant upregulation of one of two putative CXCL8 receptors has been reported in human melanoma specimens [23]. Analysis of CXCR1 in human melanoma specimens from different Clark levels demonstrated that it is expressed ubiquitously in all Clark levels. In contrast, CXCR2 is expressed predominantly by higher grade melanoma tumors and metastases, suggesting an association between expression of CXCL8 and CXCR2 with vessel density in advanced lesions and metastases [23]. More specifically, the effect of CXCL8 can be mediated by CXCR1 and CXCR2, with CXCR1 being a selective receptor for CXCL8 [24]. Overall, the aberrant expression of CXCL8 and its receptors may be a common feature of melanoma. From the functional significance of its receptors, we can contemplate that the expression of CXCL8 and its receptors (CXCR1/CXCR2) play a key role in deciding the fate of developing melanoma tumors and their ability to metastasize to certain preferred organ sites. Given their important role, they are potential targets for therapy against human melanoma.
4. Putative signaling pathways involved in CXCR1 and CXCR2-dependent modulation of cellular phenotypes
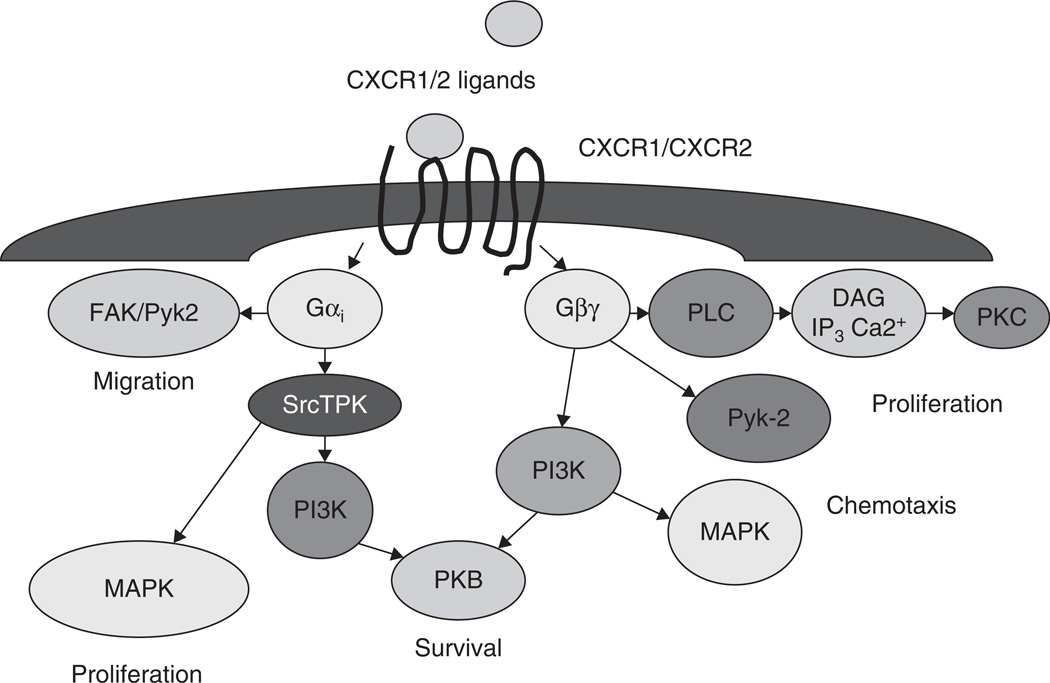
Most of the studies with neutrophils and transfected cell lines have demonstrated that CXCR1 and CXCR2 undergo receptor phosphorylation, internalization, calcium mobilization, actin polymerization, enzyme release, chemotaxis and a weak respiratory burst upon activation by CXCL8 [51–57]. Mechanisms regulating CXCR1 and CXCR2 activation and downstream signaling events following activation with CXCL8 in malignant melanoma are not known. Both CXCR1 and CXCR2 show a similar preference for G protein family members [58]. However, recent reports suggest that the two receptors not only possess distinct ligand binding properties but also could transduce different post-receptor signals [56,59]. In spite of the apparent redundancy, CXCR1 and CXCR2markedly differ in their capacity to activate signal transduction pathways. CXCR1 stimulates phospholipase D activation and the formation of superoxide by NADPH oxidase, whereas CXCR2 does not trigger either response [60–62]. Since CXCR1 and CXCR2 have a similar affinity for CXCL8 and bind with the same selectivity to G proteins, additional receptor-specific signal transducing mechanism(s) are assumed. Despite similar affinities for CXCL8 and similar receptor numbers of CXCR1 and CXCR2, neutrophil chemotaxis is primarily mediated by CXCR1 [63,64] suggesting diverse roles for CXCR1 and CXCR2. Previous reports using CXCL8 have shown that CXCR2, compared with CXCR1, internalizes more rapidly and recovers more slowly [55,56,59]. These differences in receptor trafficking which is mediated by β-arrestins, appear to regulate CXCR1 and CXCR2 activation during neutrophil recruitment and activation [55,56,59]. Nevertheless the molecular basis of such receptor-specific signal transduction has not yet been determined in malignant melanoma. The initial events in chemokine- induced signal transduction determine the outcome of the response and must take place in proximity of the receptor. The scheme, which is shown in Figure 3 and discussed in this paragraph, is not meant to be complete, but to present the most evident effectors in chemokine receptor signaling. Activation of the receptor by a chemokine ligand induces the exchange in the Gα-subunit from the GDP- to the GTP-bound state dissociating the α-subunit from the β and γ from G-protein subunits. These subunits activate phospholipase (PL) Cβ1 and Cβ2, followed by hydrolysis of PIP2 which leads to formation of inositol triphosphate (IP3) and diacylglycerol (DAG) with a subsequent increase in intracellular Ca2+ mobilization [61]. Although chemokine receptors lack tyrosine kinase activity, they can stimulate the phosphorylation of cytoskeleton proteins, p130 Cas and paxillin [65] and induce activation of the related adhesion focal tyrosine kinases (FAK) (also known as Pyk2 and CAKβ) [66], MAPK (ERK1/2, p38 and c-jun kinase) [66], PI3K [66] and Janus kinase 2 [67,68]. p44/42 kinases, also termed extracellular signal-regulated kinases (ERK1 and ERK2) are important mediators of growth and other signals [69,70]. Because most of the G protein coupled receptors can activate a variety of effector pathways via various G protein subunits, considerable heterogeneity exists in signaling pathways leading to ERK1/2 phosphorylation and subsequent activation of transcription factors [61]. Recent data suggests that signals dependent on G proteins are mediated through Rho and Rac pathways which result in actin polymerization, reconstitution of adhesion molecules and other cellular components leading to cell migration [52,71,72]. CXCL8-mediated signal transduction is more complex especially if one takes into account the cross-regulatory mechanism(s) of the integrated network [61,73]. The analysis of signaling events further downstream from receptor activation is more complicated because of the potential contributions from various pathways since many signaling pathways are shared by different receptor systems.
Figure 3.
CXCR1/2 receptor signaling in regulation of malignant cell phenotypes.
DAG: Diacylglycerol; FAK: Focal adhesion kinase; IP3: Inositol triphosphate; PKB: Protein kinase B; PLC: Phospholipase C; Pyk-2: Proline-rich tyrosine kinase 2; srcTPK: Src family tyrosine protein kinase.
5. Modulation of CXCR1/2 expression and/or activity for therapeutic intervention in malignant melanoma
Various strategies have been employed to modulate the expression of CXCR1/2 which includes low-molecular-weight antagonists, antibodies, siRNA and inhibitory peptides. Here we describe some of these approaches and the data obtained using preclinical models and discuss future perspectives. It has been shown by various groups that CXCL8 is constitutively expressed in malignant melanoma where it functions in an autocrine/paracrine fashion and acts as an invasive and angiogenic factor [31,33,74,75]. The multiple functions that are attributed to CXCR1/2 and their ligand emphasize the possibility of targeting them for cancer therapy.
Antibodies against CXCL8 and other chemokines have shown promising effects against melanoma. Humanized antibodies to CXCL8 have also been shown to inhibit tumor growth, angiogenesis and metastasis in case of melanoma [76,77]. But the positive response of neutralizing antibodies against chemokines other than CXCL8 suggests that melanoma may utilize different chemokine ligand to support its growth. It has also been reported that 17 beta-estradiol, progesterone and dihydrotestosterone suppresses the growth of melanoma by inhibiting CXCL8 production in a receptor dependent manner [79]. Earlier studies have demonstrated that neutralizing antibodies to CXCR1 and CXCR2 inhibit melanoma cell proliferation and its invasive potential. It has also been reported that 17 beta-estradiol, progesterone, and dihydrotestosterone suppress the growth of melanoma by inhibiting CXCL8 production in a receptor dependent manner [79]. All these above evidence emphasizes targeting CXCL8 receptors rather than CXCL8 alone.
Several antagonists for CXCR1/CXCR2 receptors are also under consideration for melanoma therapy. Low-molecular-weight inhibitors with affinity for CXCR1 such as repertaxin or with affinity for CXCR2 such as SB-225002 or SB-332235 have been used against inflammatory diseases [12,80,81]. A recent study, have shown potential of the CXCR1/2 specific inhibitors, SCH-479833 and SCH-527123 in inhibiting human melanoma growth by decreasing tumor cell proliferation, survival and invasion [27]. Histological and histochemical analyses showed significant (p < 0.05) decreases in tumor cell proliferation and microvessel density in tumors. A significant increase in melanoma cell apoptosis was also in SCH-479833 or SCH-527123-treated animals as compared to controls [27]. Similarly, SCH-527123 has also been shown to inhibit neutrophil recruitment and inflammatory responses in an animal model [82].
6. CXCR1/2 targeting and chemotherapy
Current strategies focused on systemic therapy for treatment of metastatic melanoma have shown no effect on survival. Different therapeutic approaches have been evaluated including chemotherapy and biological therapy, either as single agents or in combination. Systemic chemotherapy is still considered the mainstay of treatment for stage IV melanoma and is used largely with palliative intent [83]. Systemic chemotherapy with dacarbazine (DTIC) is the standard clinical treatment for malignant melanoma but response rates are very low (10 – 20%) [83,84] with only limited effect on survival [83,85]. Recent reports suggest that expression of CXCR2 ligands and activation of CXCR2-dependent pathways might provide survival signals for therapy-resistant tumor cells [86–88]. An increase in the expression of CXCR1 and CXCR2 and their ligands in response to chemotherapy in various cancers have been observed [84,86,87,89,90]. An increase in the level of CXCL8, and CXCL1 after chemotherapy suggests that this pathway may be used as an escape mechanism leading to drug resistance. Treatment of malignant melanoma cells with Dacarbazine transcriptionally upregulates CXCL8 expression, which might render them resistant to the cytotoxic effect of drugs [84]. These reports suggest that inhibition of CXCR1/CXCR2 signaling might improve the efficacy of systemic chemotherapy against malignant melanoma progression and metastasis.
7. Expert opinion
The accumulated evidence from the experimental studies points toward a critical role for CXCR1 and CXCR2 and their ligands in melanoma progression and metastasis. The expression of these receptors in melanoma indicates the potential use as a biomarker of relative tumor aggressiveness. Despite decades of research, therapy for early-stage melanoma is surgery with a minor benefit noted with adjuvant therapy; however, there is no effective treatment for advanced disease. This clearly indicates the pressing need for novel and effective therapeutic measures for restricting melanoma tumor growth and metastasis. Blocking CXCR1/CXCR2 signaling using novel therapeutic strategies such as low-molecular-weight antagonists or neutralizing antibodies can inhibit malignant melanoma growth, progression and metastasis. In addition, inhibition of CXCR1/CXCR2 signaling can be targeted to improve the efficacy of systemic chemotherapy against malignant melanoma.
Article highlights.
The expression of CXCR1 and CXCR2 and its ligands in melanoma correlate positively with disease progression.
CXCR1 and CXCR2 receptors and their ligands modulate melanoma growth, angiogenesis and metastasis.
Targeting CXCR1 and CXCR2 using specific low-molecular-weight inhibitors decreased human melanoma growth and invasion, which gives us hope for utilizing these antagonists for future melanoma therapy.
The aberrant expression and proven role of CXCR1 and CXCR2 receptors and their ligands in melanoma progression and metastasis demonstrates their potential as biomarkers of tumor aggressiveness.
This box summarises key points contained in the article.
Acknowledgments
Declaration of interest
This paper was sponsored by the National Institute of Health.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Murphy PM, Baggiolini M, Charo IF, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 2.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 3. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. • An interesting recent review with updates on chemokineas and chemokines receptors in tumor growth and metastasis.
- 4.Belperio JA, Keane MP, Burdick MD, et al. CXCR2/CXCR2 ligand biology during lung transplant ischemia-reperfusion injury. J Immunol. 2005;175:6931–6939. doi: 10.4049/jimmunol.175.10.6931. [DOI] [PubMed] [Google Scholar]
- 5.Londhe VA, Belperio JA, Keane MP, et al. CXCR2/CXCR2 ligand biological axis impairs alveologenesis during dsRNA-induced lung inflammation in mice. Pediatr Res. 2005;58:919–926. doi: 10.1203/01.PDR.0000181377.78061.3E. [DOI] [PubMed] [Google Scholar]
- 6.Strieter RM, Burdick MD, Gomperts BN, et al. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2003;284:L566–L577. doi: 10.1152/ajplung.00233.2002. [DOI] [PubMed] [Google Scholar]
- 8. Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev. 2007;26:453–467. doi: 10.1007/s10555-007-9068-9. • This paper highlights the roles of chemokines in tumor angiogenesis and metastasis.
- 9.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 10.Richmond A, Yang J, Su Y. The good and the bad of chemokines/chemokine receptors in melanoma. Pigment Cell Melanoma Res. 2009;22:175–186. doi: 10.1111/j.1755-148X.2009.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman RW, Phillips JE, Hipkin RW, et al. CXCR2 antagonists for the treatment of pulmonary disease. Pharmacol Ther. 2009;121:55–68. doi: 10.1016/j.pharmthera.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Bertini R, Allegretti M, Bizzarri C, et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci USA. 2004;101:11791–11796. doi: 10.1073/pnas.0402090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belperio JA, Keane MP, Arenberg DA, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 14.Del Rio L, Bennouna S, Salinas J, Denkers EY. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J Immunol. 2001;167:6503–6509. doi: 10.4049/jimmunol.167.11.6503. [DOI] [PubMed] [Google Scholar]
- 15.Johnston RA, Mizgerd JP, Shore SA. CXCR2 is essential for maximal neutrophil recruitment and methacholine responsiveness after ozone exposure. Am J Physiol Lung Cell Mol Physiol. 2005;288:L61–L67. doi: 10.1152/ajplung.00101.2004. [DOI] [PubMed] [Google Scholar]
- 16.Hallgren J, Jones TG, Abonia JP, et al. Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc Natl Acad Sci USA. 2007;104:20478–20483. doi: 10.1073/pnas.0709651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stemmler S, Arinir U, Klein W, et al. Association of interleukin-8 receptor alpha polymorphisms with chronic obstructive pulmonary disease and asthma. Genes Immun. 2005;6:225–230. doi: 10.1038/sj.gene.6364181. [DOI] [PubMed] [Google Scholar]
- 18.Donahue TR, Hines OJ. CXCR2 and RET single nucleotide polymorphisms in pancreatic cancer. World J Surg. 2009;33:710–715. doi: 10.1007/s00268-008-9826-z. [DOI] [PubMed] [Google Scholar]
- 19.Kamali-Sarvestani E, Aliparasti MR, Atefi S. Association of interleukin-8 (IL-8 or CXCL8) -251T/A and CXCR2 +1208C/T gene polymorphisms with breast cancer. Neoplasma. 2007;54:484–489. [PubMed] [Google Scholar]
- 20.Matheson MC, Ellis JA, Raven J, et al. Association of IL8, CXCR2 and TNF-alpha polymorphisms and airway disease. J Hum Genet. 2006;51:196–203. doi: 10.1007/s10038-005-0344-7. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Zaja-Milatovic S, Thu YM, et al. Molecular determinants of melanoma malignancy: selecting targets for improved efficacy of chemotherapy. Mol Cancer Ther. 2009;8:636–647. doi: 10.1158/1535-7163.MCT-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 23. Varney ML, Johansson SL, Singh RK. Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol. 2006;125:209–216. doi: 10.1309/VPL5-R3JR-7F1D-6V03. • This paper highlights the relevance of CXCL8 and its receptors in malignant melanoma.
- 24.Singh RK, Varney ML, Bucana CD, Johansson SL. Expression of interleukin-8 in primary and metastatic malignant melanoma of the skin. Melanoma Res. 1999;9:383–387. doi: 10.1097/00008390-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 25. Singh S, Varney M, Singh RK. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res. 2009;69:411–415. doi: 10.1158/0008-5472.CAN-08-3378. •• An important study of CXCR2 knockout mice defining the host regulation of melanoma progression.
- 26. Singh S, Nannuru KC, Sadanandam A, et al. CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. Br J Cancer. 2009;100:1638–1646. doi: 10.1038/sj.bjc.6605055. •• An interesting recent study defining the fuctional role of CXCR1 and CXCR2 in melanoma progression.
- 27. Singh S, Sadanandam A, Nannuru KC, et al. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin Cancer Res. 2009;15:2380–2386. doi: 10.1158/1078-0432.CCR-08-2387. •• An interesting recent study defining the fuctional role of CXCR1 and CXCR2 in melanoma progression.
- 28.Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67:12–18. doi: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]
- 29.Ramjeesingh R, Leung R, Siu CH. Interleukin-8 secreted by endothelial cells induces chemotaxis of melanoma cells through the chemokine receptor CXCR1. FASEB J. 2003;17(10):1292–1294. doi: 10.1096/fj.02-0560fje. [DOI] [PubMed] [Google Scholar]
- 30.Wang JM, Taraboletti G, Matsushima K, et al. Induction of haptotactic migration of melanoma cells by neutrophil activating protein/interleukin-8. Biochem Biophys Res Commun. 1990;169:165–170. doi: 10.1016/0006-291x(90)91449-3. [DOI] [PubMed] [Google Scholar]
- 31.Singh RK, Gutman M, Radinsky R, et al. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–3247. [PubMed] [Google Scholar]
- 32.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 33.Varney ML, Li A, Dave BJ, et al. Expression of CXCR1 and CXCR2 receptors in malignant melanoma with different metastatic potential and their role in interleukin-8 (CXCL8)-mediated modulation of metastatic phenotype. Clin Exp Metastasis. 2003;20:723–731. doi: 10.1023/b:clin.0000006814.48627.bd. [DOI] [PubMed] [Google Scholar]
- 34.Gebhardt C, Averbeck M, Viertel A, et al. Ultraviolet-B irradiation enhances melanoma cell motility via induction of autocrine interleukin 8 secretion. Exp Dermatol. 2007;16:636–643. doi: 10.1111/j.1600-0625.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 35.Singh J, Reddy BS. Molecular markers in chemoprevention of colon cancer. Inhibition of expression of ras-p21 and p53 by sulindac during azoxymethane-induced colon carcinogenesis. Ann NY Acad Sci. 1995;768:205–209. doi: 10.1111/j.1749-6632.1995.tb12123.x. [DOI] [PubMed] [Google Scholar]
- 36.Salcedo R, Resau JH, Halverson D, et al. Differential expression and responsiveness of chemokine receptors (CXCR1-3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 2000;14:2055–2064. doi: 10.1096/fj.99-0963com. [DOI] [PubMed] [Google Scholar]
- 37.Li A, Varney ML, Valasek J, et al. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8:63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 38.Addison CL, Daniel TO, Burdick MD, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 39.Keane MP, Belperio JA, Xue YY, et al. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J Immunol. 2004;172:2853–2860. doi: 10.4049/jimmunol.172.5.2853. [DOI] [PubMed] [Google Scholar]
- 40.Li A, Dubey S, Varney ML, et al. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 41.Shono T, Ono M, Izumi H, et al. Involvement of the transcription factor NF-κB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol Cell Biol. 1996;16:4231–4239. doi: 10.1128/mcb.16.8.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luca M, Huang S, Gershenwald JE, et al. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol. 1997;151:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 43.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 44.Inoue K, Slaton JW, Eve BY, et al. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000;6:2104–2119. [PubMed] [Google Scholar]
- 45.McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today. 2000;6:149–156. doi: 10.1016/s1357-4310(00)01686-5. [DOI] [PubMed] [Google Scholar]
- 46.Norgauer J, Metzner B, Schraufstatter I. Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J Immunol. 1996;156:1132–1137. [PubMed] [Google Scholar]
- 47.Singh RK, Gutman M, Reich R, Bar-Eli M. Ultraviolet B irradiation promotes tumorigenic and metastatic properties in primary cutaneous melanoma via induction of interleukin 8. Cancer Res. 1995;55:3669–3674. [PubMed] [Google Scholar]
- 48.Herlyn M. Human melanoma: development and progression. Cancer Metastasis Rev. 1990;9:101–112. doi: 10.1007/BF00046337. [DOI] [PubMed] [Google Scholar]
- 49.Ueda T, Shimada E, Urakawa T. Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol. 1994;29:423–429. doi: 10.1007/BF02361238. [DOI] [PubMed] [Google Scholar]
- 50.Scheibenbogen C, Mohler T, Haefele J, et al. Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res. 1995;5:179–181. doi: 10.1097/00008390-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norgauer J, Krutmann J, Dobos GJ, et al. Actin polymerization, calcium-transients, and phospholipid metabolism in human neutrophils after stimulation with interleukin-8 and N-formyl peptide. J Invest Dermatol. 1994;102:310–314. doi: 10.1111/1523-1747.ep12371788. [DOI] [PubMed] [Google Scholar]
- 53.Richardson RM, Ali H, Tomhave ED, et al. Cross-desensitization of chemoattractant receptors occurs at multiple levels. Evidence for a role for inhibition of phospholipase C activity. J Biol Chem. 1995;270:27829–27833. doi: 10.1074/jbc.270.46.27829. [DOI] [PubMed] [Google Scholar]
- 54.Richardson RM, Dubose RA, Ali H, et al. Regulation of human interleukin-8 receptor A: identification of a phosphorylation site involved in modulating receptor functions. Biochemistry. 1995;34:14193–14201. doi: 10.1021/bi00043a025. [DOI] [PubMed] [Google Scholar]
- 55.Richardson RM, Ali H, Pridgen BC, et al. Multiple signaling pathways of human interleukin-8 receptor A. Independent regulation by phosphorylation. J Biol Chem. 1998;273:10690–10695. doi: 10.1074/jbc.273.17.10690. [DOI] [PubMed] [Google Scholar]
- 56.Feniger-Barish R, Ran M, Zaslaver A, Ben Baruch A. Differential modes of regulation of CXC chemokine-induced internalization and recycling of human CXCR1 and CXCR2. Cytokine. 1999;11:996–1009. doi: 10.1006/cyto.1999.0510. [DOI] [PubMed] [Google Scholar]
- 57.Mueller SG, White JR, Schraw WP, et al. Ligand-induced desensitization of the human CXC chemokine receptor-2 is modulated by multiple serine residues in the carboxyl-terminal domain of the receptor. J Biol Chem. 1997;272:8207–8214. doi: 10.1074/jbc.272.13.8207. [DOI] [PubMed] [Google Scholar]
- 58.Wu D, Larosa GJ, Simon MI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 59.Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904–2911. doi: 10.4049/jimmunol.170.6.2904. [DOI] [PubMed] [Google Scholar]
- 60.Jones SA, Moser B, Thelen M. A comparison of post-receptor signal transduction events in Jurkat cells transfected with either IL-8R1 or IL-8R2. Chemokine mediated activation of p42/p44 MAP-kinase (ERK-2) FEBS Lett. 1995;364:211–214. doi: 10.1016/0014-5793(95)00397-r. [DOI] [PubMed] [Google Scholar]
- 61.Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129–134. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- 62.Jones SA, Dewald B, Clark-Lewis I, Baggiolini M. Chemokine antagonists that discriminate between interleukin-8 receptors. Selective blockers of CXCR2. J Biol Chem. 1997;272:16166–16169. doi: 10.1074/jbc.272.26.16166. [DOI] [PubMed] [Google Scholar]
- 63.Chuntharapai A, Kim KJ. Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J Immunol. 1995;155:2587–2594. [PubMed] [Google Scholar]
- 64.Quan JM, Martin TR, Rosenberg GB, et al. Antibodies against the N-terminus of IL-8 receptor A inhibit neutrophil chemotaxis. Biochem Biophys Res Commun. 1996;219:405–411. doi: 10.1006/bbrc.1996.0246. [DOI] [PubMed] [Google Scholar]
- 65.Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1alpha and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: a potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–3658. [PubMed] [Google Scholar]
- 66.Ganju RK, Brubaker SA, Meyer J, et al. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 67.Mellado M, Rodriguez-Frade JM, Aragay A, et al. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J Immunol. 1998;161:805–813. [PubMed] [Google Scholar]
- 68.Takami M, Terry V, Petruzzelli L. Signaling pathways involved in IL-8-dependent activation of adhesion through Mac-1. J Immunol. 2002;168:4559–4566. doi: 10.4049/jimmunol.168.9.4559. [DOI] [PubMed] [Google Scholar]
- 69.Gutkind JS. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 70.Gutkind JS. Cell growth control by G protein-coupled receptors: from signal transduction to signal integration. Oncogene. 1998;17:1331–1342. doi: 10.1038/sj.onc.1202186. [DOI] [PubMed] [Google Scholar]
- 71.Schraufstatter IU, Chung J, Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1094–L1103. doi: 10.1152/ajplung.2001.280.6.L1094. [DOI] [PubMed] [Google Scholar]
- 72.Wang D, Sai J, Carter G, et al. PAK1 kinase is required for CXCL1-induced chemotaxis. Biochemistry. 2002;41:7100–7107. doi: 10.1021/bi025902m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jordan JD, Landau EM, Iyengar R. Signaling networks: the origins of cellular multitasking. Cell. 2000;103:193–200. doi: 10.1016/s0092-8674(00)00112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schadendorf D, Moller A, Algermissen B, et al. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151:2667–2675. [PubMed] [Google Scholar]
- 75.Singh RK, Varney ML. Regulation of interleukin 8 expression in human malignant melanoma cells. Cancer Res. 1998;58:1532–1537. [PubMed] [Google Scholar]
- 76.Villares GJ, Zigler M, Wang H, et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008;68:9078–9086. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melnikova VO, Bar-Eli M. Bioimmunotherapy for melanoma using fully human antibodies targeting MCAM/MUC18 and IL-8. Pigment Cell Res. 2006;19:395–405. doi: 10.1111/j.1600-0749.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- 78.Fujisawa N, Hayashi S, Miller EJ. A synthetic peptide inhibitor for alpha-chemokines inhibits the tumour growth and pulmonary metastasis of human melanoma cells in nude mice. Melanoma Res. 1999;9:105–114. doi: 10.1097/00008390-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 79.Bendrik C, Dabrosin C. Estradiol increases IL-8 secretion of normal human breast tissue and breast cancer in vivo. J Immunol. 2009;182:371–378. doi: 10.4049/jimmunol.182.1.371. [DOI] [PubMed] [Google Scholar]
- 80.White JR, Lee JM, Young PR, et al. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095–10098. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 81.Thatcher TH, McHugh NA, Egan RW, et al. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L322–L328. doi: 10.1152/ajplung.00039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chapman RW, Minnicozzi M, Celly CS, et al. A novel, orally active CXCR1/2 receptor antagonist, Sch527123, inhibits neutrophil recruitment, mucus production, and goblet cell hyperplasia in animal models of pulmonary inflammation. J Pharmacol Exp Ther. 2007;322:486–493. doi: 10.1124/jpet.106.119040. [DOI] [PubMed] [Google Scholar]
- 83.Becker JC, Kampgen E, Brocker E. Classical chemotherapy for metastatic melanoma. Clin Exp Dermatol. 2000;25:503–508. doi: 10.1046/j.1365-2230.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- 84.Lev DC, Ruiz M, Mills L, et al. Dacarbazine causes transcriptional up-regulation of interleukin 8 and vascular endothelial growth factor in melanoma cells: a possible escape mechanism from chemotherapy. Mol Cancer Ther. 2003;2:753–763. [PubMed] [Google Scholar]
- 85.Tawbi HA, Kirkwood JM. Management of metastatic melanoma. Semin Oncol. 2007;34:532–545. doi: 10.1053/j.seminoncol.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 86.De Larco JE, Wuertz BRK, Manivel JC, Furcht LT. Progression and enhancement of metastatic potential after exposure of tumor cells to chemotherapeutic agents. Cancer Res. 2001;61:2857–2861. [PubMed] [Google Scholar]
- 87.De Larco JE, Wuertz BRK, Rosner KA, et al. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol. 2001;158:639–646. doi: 10.1016/S0002-9440(10)64005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Acosta JC, Gil J. A role for CXCR2 in senescence, but what about in cancer? Cancer Res. 2009;69:2167–2170. doi: 10.1158/0008-5472.CAN-08-3772. [DOI] [PubMed] [Google Scholar]
- 89.Maxwell PJ, Gallagher R, Seaton A, et al. HIF-1 and NF-κB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007;26:7333–7345. doi: 10.1038/sj.onc.1210536. [DOI] [PubMed] [Google Scholar]
- 90.Uslu R, Sanli UA, Dikmen Y, et al. Predictive value of serum interleukin-8 levels in ovarian cancer patients treated with paclitaxel-containing regimens. Int J Gynecol Cancer. 2005;15:240–245. doi: 10.1111/j.1525-1438.2005.15210.x. [DOI] [PubMed] [Google Scholar]