Abstract
The objective of this study was to assess the mechanisms responsible for the experimentally observed nonlinear addition of forces produced by voluntary contractions during superimposed electrical stimulation of the same muscle. A model of action potential interaction predicts increased motor unit firing rates during superimposed stimulation. The resulting effects on force production reproduce experimental results, confirming that motor unit force saturation contributes to nonlinear force addition. The model further predicts that the voluntary EMG will be reduced by stimulation, due to collision block and phase resetting of motor unit action potentials. Both effects have implications for the design of FES neuroprosthesis systems.
I. Introduction
Electrical nerve stimulation is widely used as a therapeutic or restorative intervention, e.g., cutaneous stimulation to ameliorate pain or Functional Electrical Stimulation (FES) to restore arm and hand function in cases of tetraplegia following spinal cord injury. However, there has been no systematic analysis of the expected patterns of simultaneous action potential trains created by electrical nerve stimulation during ongoing physiological activity. Depending on the interaction between the internal and external activation sources, different physiological effects will be evoked by the stimulation at the output. We examined the interaction between voluntary motor activity and peripheral motor axon stimulation on a muscle’s force and EMG generation. This is particularly relevant to the design of neuroprostheses for stroke survivors, since they often have weakness that might be reduced by electrical stimulation of muscle, but they also have ongoing physiologically generated muscle excitation patterns that are activated by residual voluntary effort and by elevated levels of tone and reflexes. Thus, the ability of superimposed stimulation to generate additional force may be limited, as observed experimentally [1], [2]. The total force is less than the sum of the forces measured separately.
II. Methods
A. Modeling the interaction between voluntary and stimulated action potential trains
The strength of a muscle contraction produced by either a voluntary or electrical stimulation source is modulated by processes of recruitment and firing rate modulation, and in the case where there is simultaneous excitation, i.e., the same axon is excited by both sources at different locations, the resulting action potentials will interact as illustrated in Figure 1. Action potentials from the motor neuron are conducted orthodromically to the muscle fibers. The electrical stimulus excites the axon at some point between the motor neuron and the muscle, and produces pairs of action potentials that travel in both the orthodromic and antidromic directions. The interactions between the voluntarily and electrically generated action potentials in single axons depend on their relative timing and the conduction time between the two sources.
Fig. 1.

Interaction between voluntarily and electrically elicited action potentials on a single motor axon.
Assume that a voluntary action potential is elicited at time tv, and an electrical stimulus elicits a pair of action potentials after an amount of ts. since the most recent stimulus pulse. The conduction time between the two sources is tc, (distance divided by velocity), and the conduction time from the stimulating electrode to the muscle is designated as tp.
The voluntarily elicited orthodromic action potential will collide with the electrically elicited antidromic action potential if the time between the generation of the two action potentials is less than the conduction time between the neuron and the electrical stimulation source, i.e. if either ts < tv + tc or tv < ts + tc. The mutual annihilation at the point of collision of the two action potentials will prevent the voluntary action potential from reaching the muscle and the electrically generated antidromic action potential from reaching the motor neuron. However, the paired electrically generated orthodromic action potential will reach the muscle, effectively replacing the voluntary action potential but arriving at the muscle shifted in time (either earlier or later) by an amount equal to the difference in the two firing times, ts – tv.
If the voluntary action potential travels past the stimulation site prior to the time of stimulation (tv < ts – tc) it will continue to the muscle. In contrast, if the antidromic stimulated action potential arrives at the neuron before it fires (ts < tv – tc), we assume that it will reset the firing time of the motor neuron, since antidromic action potentials have been reported to eliminate pre-existing post-synaptic potentials [3]. Resetting essentially delays the next voluntary action potential by an amount equal to the voluntary firing period.
To illustrate the effects of these different conditions on the overall rate of action potentials arriving at the motor end plate, we calculated the rate observed at the muscle as a function of the stimulation period (1/frequency). This relationship between muscle firing period and the firing periods of the two action potential sources is illustrated in Fig. 2. In this example, the voluntary period was fixed at 0.05 s (20 Hz), and the stimulus period was varied over a range of periods both longer and shorter than the voluntary firing period. Each point in Fig. 2 represents the muscle’s average firing period was calculated over a 3 s period of constant activity.
Fig. 2.
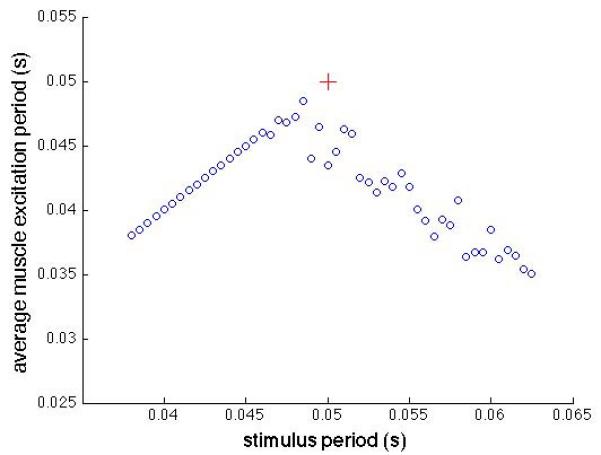
Average muscle firing period as a function of stimulus period. The voluntary firing rate had an average period of 0.05 s with a 0.1 coefficient of variation. The red  symbol marks the point where the two firing periods would be equal.
symbol marks the point where the two firing periods would be equal.
Simultaneous excitation decreases the average firing period, i.e. increases the average frequency, regardless of whether the stimulus period is higher or lower than the voluntary period. Either decreasing or increasing the stimulus period results in an average decrease in muscle firing period. The variance in muscle firing rate decreases substantially when the stimulus period is less than the voluntary period (0.05 s), due to synchronous resetting of the motor neurons.
B. Effects of interaction on muscle force and EMG
We chose to investigate voluntary and stimulated force and EMG production in the human first dorsal interosseus because of the availability of previously developed data and models of voluntary recruitment and rate modulation, and EMG-force relationships. We chose the ‘onion-skin’ model of voluntary motor unit control published by De Luca and Contessa [4], and the muscle force and EMG generation models developed by Fuglevand [5] and subsequently adapted by Zhou and Rymer [6].
The details of the model are well described in the above papers and will not be repeated in this abstract. The overall structure is a set of 120 motor units, with contraction time constants spanning from 0.09 s to 0.03 s, and peak twitch forces spanning from 1 to 100 (arbitrary force units) respectively. A single scalar input to the pool modulates both recruitment and voluntary firing rates with full recruitment of all motor units at 67% of maximal excitation [4]. The coefficient of variation of the voluntary firing period (1/rate) was fixed at 0.05 for the force and EMG interactions described below. We assumed conduction times of 0.005 s and 0.001 s for tc and tp respectively as these are comparable to action potentials traveling 50 cm and 10 cm respectively. The voluntary and stimulated contraction levels were chosen as percentages of the maximal voluntary force, vFmax in 10% increments ranging from 0% to 50%. We modulated stimulation force by varying the number of recruited motor units at a fixed stimulus frequency (20 or 35 Hz). The stimulated motor units were chosen randomly employing a uniform probability distribution in the results reported here, reflecting experimental studies that support a random recruitment pattern with surface stimulation electrodes [7] [1].
We simulated combined voluntary and stimulated contractions beginning with an isolated voluntary contraction for the first 1.5 seconds, followed by voluntary with superimposed stimulation during the final 1.5 seconds of each 3 s trial, as illustrated in Fig. 3.
Figure 3.
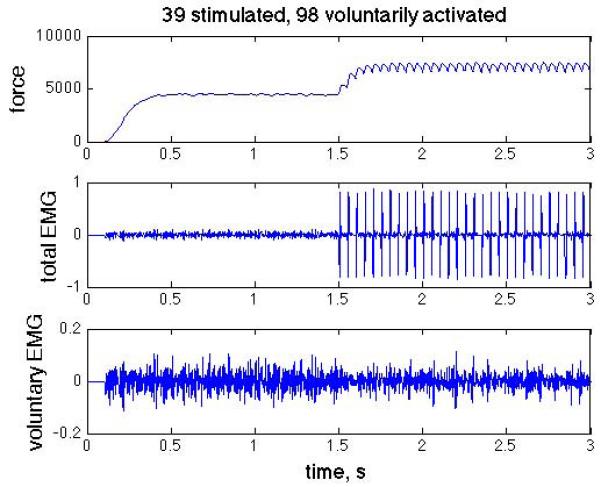
Simulated force (top), total EMG including M-waves (middle), and the voluntary component of the EMG (bottom), with M-waves removed. Step in voluntary activation at t = 0s and step in stimulated activation at t = 1.5s.
Single motor unit forces were calculated by summing the force impulses elicited by each action potential, scaled nonlinearly by the preceding action potential interval. Total force was calculated as the linear sum of all the active motor unit forces. Single motor unit EMGs were described by 0.012 s second order impulses [6], and were scaled (arbitrary units) in proportion to motor unit twitch force. The total composite EMG was calculated as linear sum of the individual motor unit action potentials, whether they were generated voluntarily or by stimulation (the M-waves are evident after the onset of stimulation in Fig. 3). Since the source of each action potential was known, we also calculated the M-waves separately and subtracted them from the total to get the net voluntary component of the EMG.
III. Results
Fig. 4 shows the firing rates and added forces of motor units during a 40% vFmax voluntary contraction and a superimposed 40% vFmax stimulated (20 Hz) contraction. While average action potential frequencies at the muscle are increased by stimulation, increments in force due to stimulation are limited by the relative values of the two firing frequencies and the motor unit’s operating point on the force-frequency relationship. Motor units with low voluntary recruitment thresholds (low motor unit numbers) have low fusion frequencies, and even though the stimulation increases firing rate by almost 50%, there is essentially no added force. The increased muscle firing frequency decreases with motor unit number until the voluntary rate equals the stimulation rate, and then remains fixed at the stimulation rate. For the specific example shown here, the simulation does not produce noticeable additional force until the middle of the motor unit range, i.e., about half of the stimulated motor units do not produce any additional force due to the stimulation. This is due to two compounding factors – the low threshold motor units are already firing at or near their fusion frequency, and these motor units are smaller than the high threshold units.
Fig. 4.
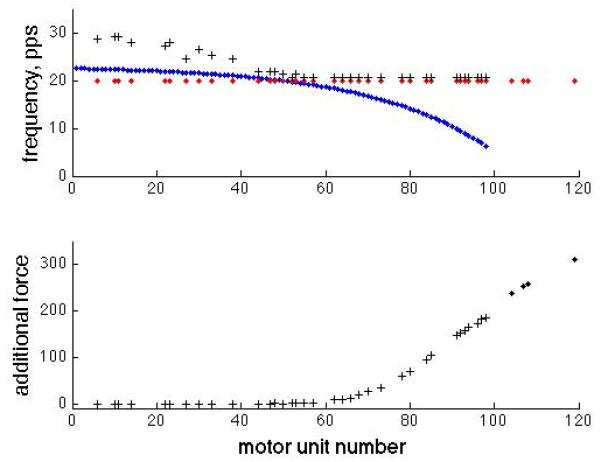
Left – Firing frequencies for both voluntarily activated and stimulated motor units as a function of motor unit number, i.e. voluntary recruitment rank order. This is for the same simulation as Fig. 3. The blue dots show motor neuron voluntary mean firing frequencies, the red dots show stimulation frequency, and the black + symbols show the average muscle unit firing frequency. Right – The increments in motor unit forces due to stimulation for each stimulated motor unit. Plus symbols indicate motor units that are activated both voluntarily and by stimulation.
All combinations of voluntary and stimulated contractions ranging from 0% to 50% maximal voluntary force in increments of 10% were simulated. Voluntary force was calculated as the average force over the one-second period prior to the stimulation onset, and the total force was calculated as the average over the last second of superimposed stimulation. The difference between these two averages was the increment due to stimulation. The simulations were repeated six times, with a different set of randomly selected stimulated motor units, and the results were averaged.
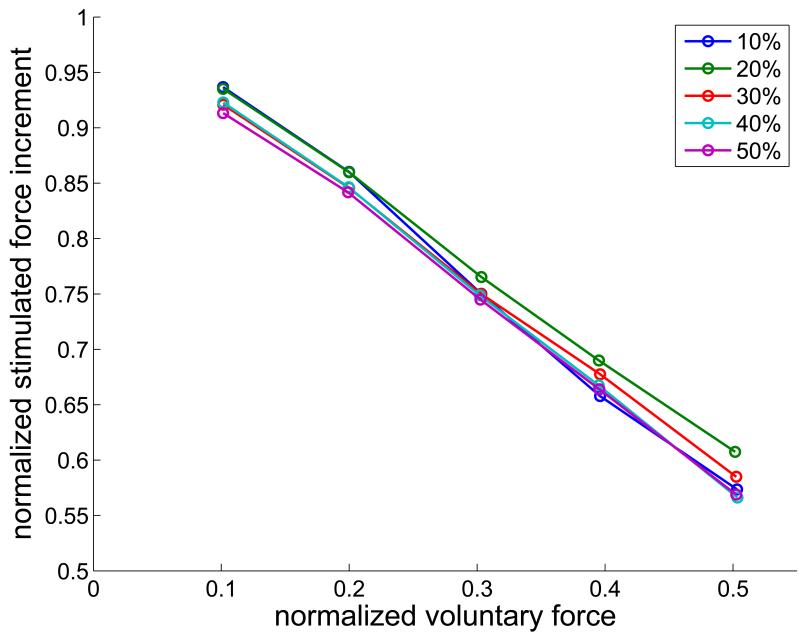
Superimposing stimulation always increased the total force, but the size of the increment decreased as the voluntary force increased (Fig. 5 Top). For a fixed level of stimulation, the increment in force produced by the superimposed stimulation decreased as a straight-line function of the voluntary force, with a slope that was proportional to the voluntary force. Thus, when the stimulated force increment is normalized to the stimulated force in the absence of any voluntary contraction, the dependencies on voluntary force superimpose. The dependence on voluntary force is substantial. At 20Hz stimulation rate, the ability of stimulation to augment the voluntary force is reduced by about 40% at half maximal voluntary force.
Fig. 5.
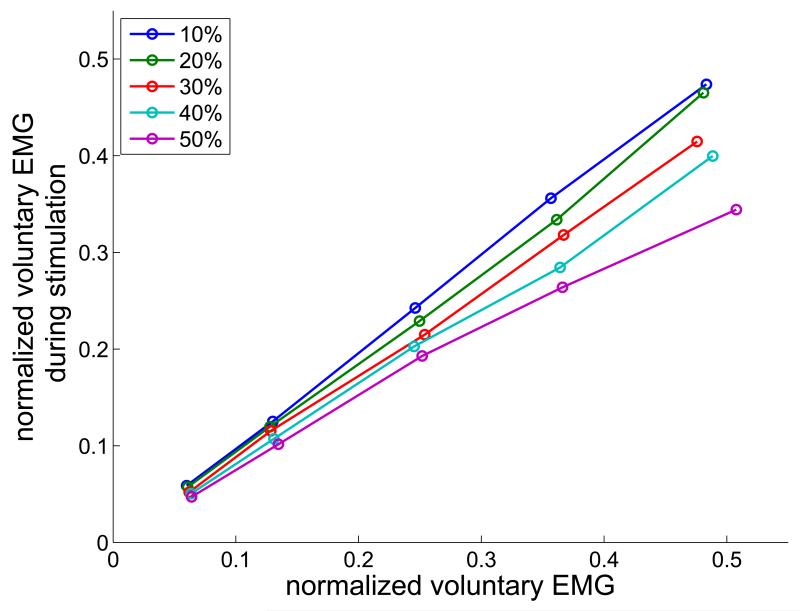
Top – Dependence of the stimulated force increment on the normalized voluntary force, for stimulated force levels of 10% – 50%, as indicated in the legend. Bottom – Normalized voluntary EMG component during stimulation as a function of the normalized voluntary EMG in the absence of stimulation. EMG was quantified as the average absolute value over the same 1 s time periods as force.
Simultaneous stimulation also reduced the voluntary component of the EMG, as can be seen in Fig. 3 after stimulation onset. As the level of stimulation increases from 20% to 50% vFmax, the slope of the dependence of the normalized EMG on voluntary contraction level decreases, as seen in the bottom of Fig. 5. At the maximal levels studied (50% voluntary and 50% stimulated forces) the voluntary EMG was reduced by approximately 1/3. Two mechanisms contribute to the reduction in voluntary EMG: the direct collision block of some voluntary action potentials, and phase resetting of motor neurons, which directly reduces the frequency of voluntary action potentials arriving at the muscle.
IV. Discussion
Voluntary and electrically stimulated contractions have been shown to add nonlinearly in experiments in able-bodied individuals as well as in stroke survivors [1, 2]. This is not surprising, given that the two excitation sources activate some of the same motor units, and individual motor unit forces saturate at high stimulus rates. The contributions of the current report include a model for the interaction of the two action potential trains, the quantitative demonstration of one potential mechanism for the observed nonlinear addition, and the prediction of a decrease in amplitude of the voluntary EMG, which has not been reported previously.
The reduction in stimulated force increment as voluntary force increases implies that there is a decreasing benefit of adding stimulation to increase force in a stroke neuroprosthesis. Since increased voluntary force typically results in unwanted co-activation synergies [8, 9], a reasonable approach would be to limit voluntary contractions and increase muscle force instead by selective stimulation of targeted muscles [10]. Stimulation may be able to be controlled by EMG recorded from low-level voluntary contractions, following removal of M-waves [11]. Our prediction of decreased voluntary EMG with stimulation is modest, especially at low voluntary force levels, supporting the feasibility of this approach. We have not found strong direct evidence of motor neuron phase resetting by antidromic action potentials. If phase resetting does not occur, the reduction in voluntary EMG will not be as dramatic as observed here.
The power of the model lies in its ability to assess quantitatively the sensitivity of the results to modeling assumptions, such as motor neuron pool recruitment and rate modulation models, and as a tool in the design of neuro-rehabilitation interventions. For instance, we are currently investigating the choice of stimulation patterns and parameters, particularly recruitment order and stimulation frequency, on force enhancement in moderately to severely impaired stroke survivors.
Acknowledgment
We gratefully acknowledge the contributions of Julie Murphy and Danny Young who implemented a dynamic simulation of voluntary and stimulated action potentials in a single axon, which helped formulate our approach. We also gratefully acknowledge helpful discussions with Andrew Fuglevand, Zev Rymer, Carlo De Luca, and Paola Contessa during the development and implementation of the model.
Footnotes
Research supported in part by the U.S. National Institutes of Health, National Institute for Child Health and Development under Grant R21HD05256 and American Heart Association Grant 11PRE6600000.
References
- [1].Perumal R, Wexler AS, Kesar TM, Jancosko A, Laufer Y, Binder-Macleod SA. A phenomenological model that predicts forces generated when electrical stimulation is superimposed on submaximal volitional contractions. Journal of applied physiology. 2010 Jun;108:1595–604. doi: 10.1152/japplphysiol.01231.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Makowski NS, Knutson J, Chae J, Crago PE. The interaction of FES with voluntary effort in chronic stroke; 39th Neural Interfaces Conference; Long Beach, CA. 2010.p. 62. [Google Scholar]
- [3].Brock LG, Coombs JS, Eccles JC. Intracellular recording from antidromically activated motoneurones. The Journal of Physiology. 1953 Dec 29;122:429–61. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].De Luca CJ, Contessa P. Hierarchical control of motor units in voluntary contractions. J Neurophysiol. 2012 Jan 1;107:178–195. doi: 10.1152/jn.00961.2010. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fuglevand AJ, Winter DA, Patla AE. Models of Recruitment and Rate Coding Organization in Motor-Unit Pools. J Neurophysiol. 1993;70:2470–2488. doi: 10.1152/jn.1993.70.6.2470. [DOI] [PubMed] [Google Scholar]
- [6].Zhou P, Suresh NL, Rymer WZ. Model based sensitivity analysis of EMG-force relation with respect to motor unit properties: applications to muscle paresis in stroke. Ann Biomed Eng. 2007 Sep;35:1521–31. doi: 10.1007/s10439-007-9329-3. [DOI] [PubMed] [Google Scholar]
- [7].Knaflitz M, Merletti R, De Luca CJ. Inference of motor unit recruitment order in voluntary and electrically elicited contractions. Journal of Applied Physiology. 1990 Apr 1;68:1657–1667. doi: 10.1152/jappl.1990.68.4.1657. 1990. [DOI] [PubMed] [Google Scholar]
- [8].Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001 Feb;24:273–83. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [9].Miller LC, Dewald JPA. Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke. Clin Neurophysiol. 2012 doi: 10.1016/j.clinph.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Makowski NS, Knutson J, Chae J, Crago PE. Neuromuscular electrical stimulation to augment reach and hand opening after stroke; 33rd Annual International Conference of the IEEE EMBS; Boston, MA. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yeom H, Chang y.-H. Autogenic EMG-controlled functional electrical stimulation for ankle dorsiflexion control. J Neurosci Methods. 2010;193:118–125. doi: 10.1016/j.jneumeth.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]