Abstract
It is widely assumed that distraction reduces pain. Similarly, it is assumed that pain distracts from concurrent, unrelated cognitive processing, reducing performance on difficult tasks. Taken together, these assumptions suggest pain processing and cognitive function engage an overlapping set of domain-general, capacity-limited mental resources. However, experimental tests of this proposal have yielded mixed results, leading to alternative proposals that challenge the common model of a bidirectional relationship between concurrent pain and task performance. We tested these contrasting positions using a novel concurrent pain and executive working memory paradigm. Both task difficulty and nociceptive stimulus intensity were individually calibrated for each participant. Participants reported less pain during the working memory task than a visually matched control condition. Conversely, increasing levels of heat incrementally reduced task performance. Path analyses showed that variations in pain completely mediated this effect, and that even within a given heat level, trial-by-trial fluctuations in pain predicted decrements in performance. In sum, these findings argue that overlapping cognitive resources play a role in both pain processing and executive working memory. Future studies could use this paradigm to understand more precisely which components of executive function or other cognitive resources contribute to the experience of pain.
Keywords: pain, attention, cognition, n-back, mediation, distraction
It is commonly assumed that distraction reduces pain. Also common is the assumption that pain captures attention, reducing performance on difficult mental tasks [25]. Taken together, these assumptions imply a tradeoff between the experience of pain and goal-directed task performance [42]. When tradeoffs are observed between two concurrently performed tasks, it may be inferred that the tasks overlap in the mental resources they engage, and that the processing capacity of these resources is limited [50]. Applying this logic to the tradeoff between performance and pain suggests the same executive resources that are believed to support goal-directed mental functioning may also play a role in the experience of pain.
Extensive research has tested the common wisdom assumption that pain engages domain-general cognitive resources in non-human animals [21; 31; 32; 10; 9; 4], chronic pain patients [28; 52; 18; 68; 30; 74; 19; 51; 60], and healthy volunteers exposed to transient noxious stimuli [53; 78; 27; 1; 6; 14; 17; 57; 61; 20; 33–35; 58; 64; 69–71; 77; 55; 73; 2; 40; 63; 62; 12; 39; 56]. We can distinguish these studies according to the explicit hypothesis tested (Fig. 1):
Fig. 1.
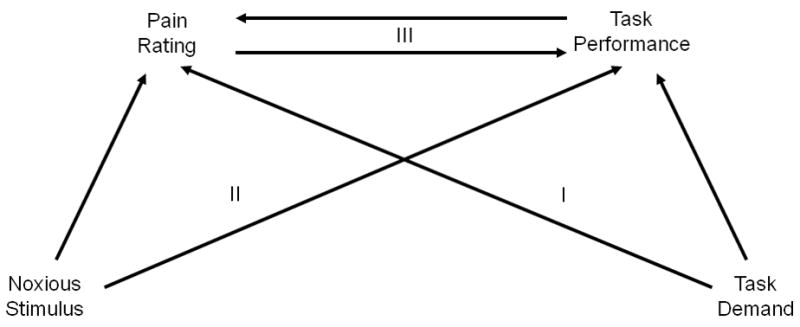
Conceptual model of the relationship between pain and performance. Three general hypotheses can be tested to evaluate this model: I. Pain ratings or other indices of pain experience are reduced by unrelated, concurrent, cognitive demand; II. cognitive performance is reduced by concurrent pain; III. A negative relationship exists between trial-by-trial fluctuations of performance and pain, even within a given heat level.
pain ratings or other indices of pain experience are reduced by unrelated, concurrent, cognitive demand;
cognitive performance is reduced by concurrent pain.
Research in which healthy humans are exposed to transient pain balances the experimental control afforded by animal studies and the applicability and specificity possible in research with chronic pain patients (Table 1).
Table 1.
Summary of literature since 2000 examining the relationship between experimentally-induced pain and concurrent, unrelated task demand in healthy adults.
| Study | Number of participants | Pain induction method | Cognitive task | Did noxious stimulation disrupt performance?a | Did task demand reduce pain?a | Did worse performance correspond to greater pain?a,b |
|---|---|---|---|---|---|---|
| Bantick et al. [1] | 8 | Heat | Counting Stroop | NR | Yes | NR |
| Bingel et al. [2] | 16 | Laser | n-Back | Yes | Yes | NR |
| Brooks et al. [6] | 11,18c | Pressure | Global motion discrimination | NR | NR | NR |
| Buhle and Wager (present study) | 24 | Heat | 3-Back | Yes | Yes | Yes |
| Coen et al. [12] | 12 | Esophageal pressure | 1-Back | No | Yes | NR |
| Crombez et al. [14] | 67 | Electrical nerve stimulation | Tone discrimination | Yes | NA | NA |
| Dick et al. [16] | 16 | Pressure | Auditory oddball | No | NA | NA |
| Compatibility | No | NA | NA | |||
| Dowman [20] | 28 | Electrical nerve stimulation | Subtraction | NA | Yes | NA |
| Frankenstein et al. [27] | 10 | Cold pressor | Word generation | NA | Yes | NA |
| Hoffman et al. [33] | 8 | Heat | Virtual reality game | NA | Yes | NA |
| Hoffman et al. [34] | 39 | Heat | Virtual reality game | NA | Yes | NA |
| Houlihan et al. [35] | 20 | Cold pressor | Sternberg | Mixed | No | NR |
| Kobor et al. [39] | 16 | Capsaicin and pinprick | Mental rotation | No | Yes | NR |
| Lautenbacher et al. [40] | 20 | Heat and electrical | Counting | NR | Yes | NR |
| Petrovic et al. [54] | 7 | Cold pressor | Maze | No | Yes | NR |
| Pud and Sapir [56] | 60 | Heat | Auditory discrimination | No | Mixed | NR |
| Raudenbush et al. (exp 1) [57] | 30 | Cold pressor | Video gamesd | NA | Yese | NA |
| Raudenbush et al. (exp 2) [57] | 27 | Cold pressor | Video gamesd | NA | Yese | NA |
| Remy et al. [58] | 12 | Heat | Word generation | NA | Yes | NA |
| Roelofs et al. [59] | 60 | Cold pressor | Tone discrimination | NA | No | NA |
| Schlereth et al. [62] | 10 | Laser | Subtraction | NA | Yes | NR |
| Seminowicz and Davis et al. [63] | 23 | Electrical nerve stimulation | Multi-source interference | No | No | NR |
| Seminowicz and Davis et al. [64] | 13e | Electrical nerve stimulation | Counting Stroop | No | Yes | NR |
| Emotional distraction Stroop | No | No | NR | |||
| Seminowicz et al. [65] | 16 | Electrical nerve stimulation | Counting Stroop | Nof | NA | NA |
| Emotional distraction Stroop | No | NA | NA | |||
| Terkelsen et al. [70] | 26 | Electrical nerve stimulation | Addition | NA | Yes | NA |
| Valet et al. [71] | 7 | Heat | Stroop | NR | Yes | NA |
| Van Damme et al. [72] | 99 | Cold pressor | Tone detection | NA | No | NA |
| Veldhuijzen et al. (exp 1) [74] | 16 | Cold pressor | Visual search (1) | No | NA | NA |
| Veldhuijzen et al. (exp 2) [74] | 12 | Cold pressor | Visual search (2) | No | Yes | NR |
| Wiech et al. (behavioral) [78] | 11 | Capsaicin and heat | Rapid serial visual processing | NR | Yes | NR |
| Wiech et al. (fMRI) [78] | 15 | Capsaicin and heat | Rapid serial visual processing | No | NA | NA |
| Yamasaki et al. [79] | 11 | Electrical | Addition | NR | Yes | NR |
| Memorization | NR | Yes | NR |
”NA” indicates “Not applicable”, meaning the necessary conditions were not included in the experimental design. “NR” indicates “Not reported”, meaning the necessary conditions were included in the experimental design, but the relevant analysis was not presented.
Within level of nociceptive input or task demand.
The effect of noxious stimulation on performance could be assessed for only 11 of the 18 participants, while the effect of task demand on pain could be assessed for entire sample.
Although several video game conditions were used, it is not clear that pain ratings were reduced in all conditions.
Personal communication with David Seminowicz, August 6, 2009.
While no performance difference was evident at the group level, additional analyses revealed two distinct subgroups: performance decreased in 7 of 16 participants, but improved in the remaining 9. Although pain did not produce significant effects on task-related brain responses, the group that showed performance improvement with pain also showed decreased pain-related activity, suggesting that pain may facilitate increased task focus in at least some participants.
Looking at these studies in total, the results are surprising. While many found that participants reported less pain when task demand was greater [53; 78; 27; 1; 57; 61; 20; 33; 34; 69; 70; 55; 73; 2; 40; 63; 12; 39; 56], a large number found no effect of increased task demand [35; 58; 71; 55; 63; 62].
Furthermore, only a few studies have reported a decline in cognitive performance as a function of pain [14; 35; 2], while most have found no effect [53; 17; 35; 64; 77; 16; 55; 73; 63; 62; 12; 39]. This paucity of supportive findings has given rise to alternative proposals that task demand does not reduce concurrent pain [45; 48], that pain does not reduce concurrent performance [73], and that pain and goal-directed cognitive performance can occur simultaneously without meaningful interaction [62; 63]. All of these proposals challenge the common model of a bidirectional relationship between pain and goal-directed cognitive performance.
Alternatively, conceptual and methodological factors may account for the lack of support for the shared resources model found in the current literature [15; 23]. To discriminate among these competing models, we designed a paradigm to examine the relationship between pain and performance that accounted for several potentially confounding factors. Previous studies of the relationship between pain and cognitive demand have restricted their hypotheses to the level of experimental condition. However, the shared processes model would further predict a negative relationship between trial-by-trial fluctuations of performance and pain, even within a given heat level (pathway III in Fig. 1). A second goal of the current research was to test this prediction using a multilevel mediation framework. These analyses allowed us to further ask whether pain is a mediator of the heat level-performance relationship, which would suggest that conscious access to pain processing is an indicator of resource utilization.
Methods
Design
We designed a novel paradigm combining three levels of transient thermal pain with a 3-back executive working memory task. We chose the n-back paradigm [38] because of the high demand it places on central executive resources [65; 37]. To ensure the 3-back was sufficiently challenging for each participant, we calibrated difficulty prior to the main experiment by adaptively adjusting the interval between probes. The allocation of executive resources in a given task reflects both task difficulty and contextual factors such as motivation [42; 44]. To increase motivation, participants were told that they could earn bonus money for good 3-back performance.
We compared pain in this demanding executive working memory condition to pain in the context of passively viewing a continuous letter mask, a baseline condition requiring minimal executive processes. In order to assure sufficiently high nociceptive input, we calibrated heat levels for each participant prior to the main task. We excluded participants who during this calibration procedure did not give pain ratings that corresponded reliably with temperature or for whom we could not safely induce a high level of pain. For those individuals who remained in the study heat stimulation was only applied to the three most reliable skin sites out of eight initially tested. These procedures helped to substantially reduce within-participant variation. Finally, to obtain sensitive pain and performance measurements during the main experiment, participants rated each stimulus immediately after it occurred on a continuous rating scale, and 3-back responses were considered within a signal detection framework [67; 79].
All procedures were approved by the Columbia University Morningside Institutional Review Board.
Participants
Thirty participants began the experiment but five completed the calibration procedure with results that prohibited their continuation in the experiment: two participants were insufficiently sensitive to the maximum permitted temperature (48°C), while 3 participants were insufficiently reliable in their ratings across sites (R2 less than .5, as described below). One additional participant began the main experimental task but could not complete it on account of intolerable pain. Twenty-four right-handed volunteers (mean age: 25.0 years, range: 18.2 years to 43.5 years; 15 female) completed the experiment in its entirety and were included in analyses. All participants had normal or corrected-to-normal vision and were free of neurological and psychiatric illness. Compensation was given at a rate of $12 per hour. Participants were told they could earn up to $10 in bonus compensation for fast and accurate performance to enhance motivation, but in fact this additional $10 was given to everyone, regardless of performance. Most participants completed the entire experimental session in 2 hours to 2.5 hours, resulting in total payment of $34 to $40.
3-back task
At the beginning of each trial, an on-screen message stated whether the current trial would require performance of the 3-back task or passive viewing of the serial letter mask. In the 3-back task, participants indicated whether each letter presented in a pseudorandom sequence was the same or different from the letter exactly three positions prior. The letters were presented foveally for 840 ms, subtending approximately 0.7° visual angle vertically and 0.4° visual angle horizontally. Subsequent to the first three letters of a sequence, approximately 30% of letters were targets. Immediately after each probe letter a serial letter mask began. As described in greater detail below, for each participant a calibration procedure was conducted prior to the main experiment to determine a unique mask duration (mean: 698 ms, range: 104 ms to 1404 ms). Each letter in the serial letter mask was displayed for 26 ms. Participants pressed the “1” and “2” keys of the numeric keypad on a standard keyboard to indicate responses of “same” or “not the same”. Responses could be made any time during the presentation of the letter or the subsequent mask. The mapping of the keys was randomized across participants.
Rating Scale
During both the nociceptive calibration procedure and the main task (described in greater detail below), ratings were made on a visual analogue scale anchored with numbers from 0 to 8 and the following verbal descriptors: 0 was “no sensation”; 1 was “non-painful warmth”; 2 was “just painful”; 5 was “moderate pain”; 8 was “the maximum level of pain you are willing to experience here today”. Although pain intensity and unpleasantness can be dissociated with specific instructions (for example, [29]), they are often highly correlated under normative conditions [11]. This scale was designed to integrate the two in a single intuitive rating. Although 8 was the largest number depicted on the scale, if the pain induced by a stimulation was greater than the maximum a participant was willing to tolerate in the experimental session, he or she was asked to rate the pain with a number reflecting how much greater the pain was than a level 8, up to a maximum of 10.
Procedure
The experimental session consisted of three distinct parts: nociceptive calibration, task difficulty calibration, and the main experimental task.
Nociceptive Calibration
Nociceptive calibration involved 24 trials in which participants rated the pain induced by thermal stimulation (10°C/s ramp up, 7 s at target temperature, 10°C/s ramp down) applied using a 16 mm TSA-II Neurosensory Analyzer (Medoc Ltd., Chapel Hill, NC). Ratings were given verbally, and participants were told they were free to give non-integer ratings. Trials proceeded in a fixed order through 8 different candidate skin sites on the participants left forearm. On each trial after the initial three stimulations, an adaptive procedure was used to predict temperatures corresponding to pain ratings of 2, 5, and 8 (henceforth referred to as low, medium, and high). First, a linear regression model was fit with Temperature as the independent variable and Pain as the dependent variable. On the basis of this regression, trials were identified for which the absolute value of the residual was greater than the median of the absolute values of the residuals of all trials. A second regression was then performed in which Pain values for these trials were replaced with predicted values from the first regression. The low, medium, and high heat level temperatures predicted by this second model were used to determine the temperature applied on the subsequent trial. A fixed, counterbalanced order, chosen to minimize predictive power, ensured one application each of a predicted low, medium and high temperature at each of the eight locations. Thus, the order of low, medium, and high trials was always the same, but the actual temperatures applied varied across trials and participants. If the predicted temperature for the heat level to be applied on a given trial was greater than the maximum permitted temperature of 48°C, 48°C was used instead. Participants were not told how the temperatures were determined or what they were. Following completion of the calibration trials, participants were excluded from further participation if the ratings they provided did not reliably correspond to the applied temperatures (R2 less than .5) or if the maximum permitted temperature of 48°C did not induce sufficient pain (estimated pain rating less than 6.5).
For 6 out of the 24 participants included in this analysis, the temperature estimated to correspond to a pain rating of 8 was greater than the maximum permitted temperature of 48°C (max = 50.5°C, mean = 49.1°C, SD = .8°C). For these participants, 48°C was used as their final high heat level temperature in place of their estimated level 8. For all participants, the final heat level temperatures determined at the end of the calibration procedure were used for the duration of the experimental session (low: mean = 41.4, SD = 2.0; medium: mean = 44.5, SD = 1.4; high: mean = 47.4, SD = .9).
Task difficulty calibration
The second part of the experimental session was intended to familiarize participants with the 3-back task and to calibrate its difficulty. Following written and verbal instruction, participants practiced the task in a short block of trials. Accuracy was indicated with a positive or negative sound immediately after each response. Participants were required to repeat this practice block if low performance suggested a lack of understanding, and were allowed to choose to repeat the practice block as many times as they wished. The calibration block consisted of 160 letter stimuli. Initial mask duration was 1000 ms. Prior to letter stimulus number 26, target sensitivity over the previous 15 stimuli was assessed with the nonparametric signal detection measure A [79], which provides a measure of performance accuracy independent of response bias (the tendency to report “yes” or “no” systematically). If sensitivity was higher than the targeted level of A = .75, mask duration was reduced by 200 ms * (A-.75) * 4, while sensitivity equal or lower than A = .75 lead to an increase of 200 ms * (A-.75) * −4/3. Additional adjustments were made every 15 stimuli until all 160 stimuli were complete, yielding ten adjustments for each participant.
Main task: Pain judgment and 3-back dual task
The third part of the experimental session consisted of 36 trials lasting about 50 s each (Fig. 2). Before each trial, the experimenter placed the thermode on one of the 3 skin sites identified as reliable during the nociceptive calibration. When ready, the participant pressed a key to begin the trial. An on-screen message indicated whether the current trial would require performance of the 3-back task (Working Memory Load trial) or passive viewing of the serial letter mask (No Load trial). On Working Memory Load trials, participants were cued to perform the 3-back task for the next 39 s of the trial. On No Load trials, they were cued to maintain fixation on a continuous serial letter mask for the 39 s trial. Each participant performed 18 trials of each type over the course of the experiment; the assignment of task condition on each trial was randomized. On both Working Memory Load and No Load trials, after 13 s a tone indicated to the participant that noxious heat would be delivered. Heat onset began after a 26 s delay. The heat lasted for approximately 13 s, (2.1 s ramp up, 8.8 s target temperature, 2.1 s ramp down). Ramp rates ranged from 4.2°C/s to 10°C/s, depending on the target temperature. Unbeknownst to participants, only the temperatures determined at the end of the nociceptive calibration to correspond to the low, medium, and high heat levels were applied during the main task. In total, each participant performed 6 Working Memory Load and 6 No Load trials at each of the 3 heat levels.
Fig. 2.
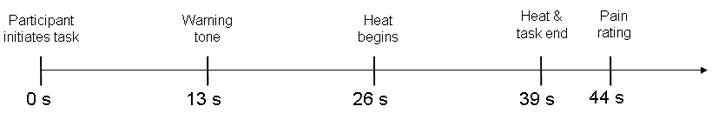
Timeline of single trial.
After 39 s, the temperature returned to baseline. On Working Memory Load trials the 3-back task ended at this point. For both Working Memory Load and No Load trials, the remaining portion of the trial was identical. After an additional 5 s of the serial letter mask, an onscreen rating bar appeared, along with the cue “how painful?” Participants were instructed to use the mouse to rate the pain they experienced during the heat stimulus by clicking anywhere on the rating bar that appeared on the screen, using the same anchors and following the same instructions as during the nociceptive calibration. After the rating was made following each trial, the experimenter then moved the thermode to the next skin site, after which the participant could begin the next trial whenever she was ready. To ensure the ratings given during the main experimental session were consistent with those given during the calibration procedure, participants were given an opportunity to practice using the onscreen rating bar with feedback in a training procedure prior to the main experimental task.
Unique letter and trial sequences were created for every participant and every trial with scripts written in MATLAB (version 7.5.0.342). Pseudorandom sequences were determined using the Mersenne Twister number generation algorithm (Matsumoto & Nishimura, 1998), with constraints to avoid long strings of identical letters and trial types.
Mediation analyses
A mediation framework was used to assess the hypothesis that trial-by-trial fluctuations in pain would negatively correlate with task performance. A test for mediation indicates whether a covariance between two variables (x and y) can be explained by a third variable (m). A significant mediator is one whose inclusion as an intermediate variable in a path model of the effects of x on y significantly affects the slope of the x – y relationship; that is, the difference (c – c′) is statistically significant. More formally, the mediation test can be captured in a system of three equations:
where y, x, and m are n (Participant) by t (Trials) data vectors containing the outcome (either y1, Performance, or y2, Pain), the predictor (x1,2, Heat Level), and data from a candidate mediating variable (either m1, Pain, or m2, Performance). e y, e m, and e′y vectors denote residual error for the outcome and mediator controlling for x and the outcome controlling for x and m, respectively. The a path is the estimated linear change in m per x (the slope of the Heat Level-Performance or Heat Level-Pain relationship). The b path is the slope of the mediator-outcome relationship controlling for x (Pain-Performance, or Performance-Pain, controlling for Heat Level). The c and c′ paths are as described above. Statistical tests on a and b path coefficients assess the significance of each relationship. In addition, a statistical test of (c – c′) can be performed by testing the significance of the product of the path coefficients ab. We tested the significance of ab using the accelerated, bias-corrected bootstrap test [26] with 10,000 bootstrap samples to test each of the a, b, and ab path coefficients. Since the hypotheses contained explicit predictions of the direction of the relationships between variables (all negative except for the relationship between Heat Level and Pain), all tests were one-tailed.
Results
To test the hypothesis that task demand would reduce pain, we analyzed the data in a linear mixed effects model with Participant as a random-effects predictor, Task Demand (Working Memory Load or No Load) as a fixed-effects predictor, Heat Level as a continuous, fixed-effects predictor (low, medium, high), and Pain as the dependent variable (Fig. 3). A main effect of Heat Level indicated that higher levels of heat led to greater Pain, F(1, 768) = 281.82, MSE = 2934.89, p < .001, while a main effect of Task Demand indicated that greater demand led to lower Pain, F (1, 768) = 48.3, MSE = 166.73, p < .001. A main effect of Participant indicated that average Pain was different across individuals, F(23, 768) = 3.07, MSE = 10.61, p < .005. An interaction of Heat Level and Task Demand indicated that greater Task Demand reduced Pain by different amounts depending on the level of heat, F(1, 768) = 12.64, MSE = 17.45, p < .005. Post-hoc comparisons using Tukey s honestly significant difference procedure confirmed that task demand reduced pain ratings at each Heat Level (p < .05, corrected). A Participant × Task Demand interaction indicated that the magnitude of task-induced reduction in Pain varied across individuals, F(23, 768) = 3.45, MSE = 3.45, p < .05, and a Participant × Heat Level interaction indicated additional individual variability in the amount of Pain reported across the three levels of heat, F(23, 768) = 10.41, MSE = 7.54, p < .001.
Fig. 3.
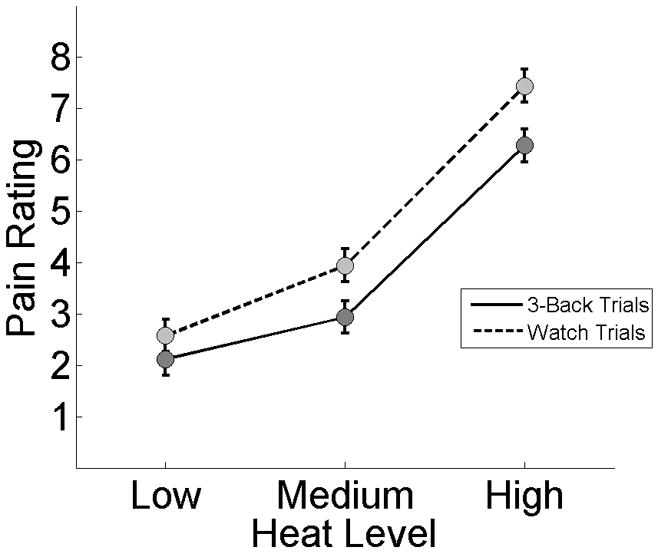
The effect of task demand on pain. Error bars reflect within-subject standard error computed using pooled variance from the Participant × Performance and Participant × Performance × Heat Level interactions [47].
A second mixed effects model tested the hypothesis that higher heat levels would reduce task performance (Fig. 4). Heat Level was entered as a continuous, fixed-effects predictor and Participant was entered as a random-effects predictor. The dependent measure was Performance, assessed with the nonparametric measure of target sensitivity A. Only Working Memory Load trials were included in this analysis, as no performance data were available for the No Load trials. A main effect of Heat Level indicated that higher levels of heat led to lower Performance, F(1, 378) = 9.24, MSE = .130, p < .01, while a main effect of Participant indicated that Performance varied across individuals, F(23, 378) = 5.51, MSE = .157, p < .001. Post-hoc comparisons using Tukey s honestly significant difference procedure indicated worse Performance at the high versus low level of heat, but no difference between the medium level of heat and the other two (p < .05, corrected).
Fig. 4.
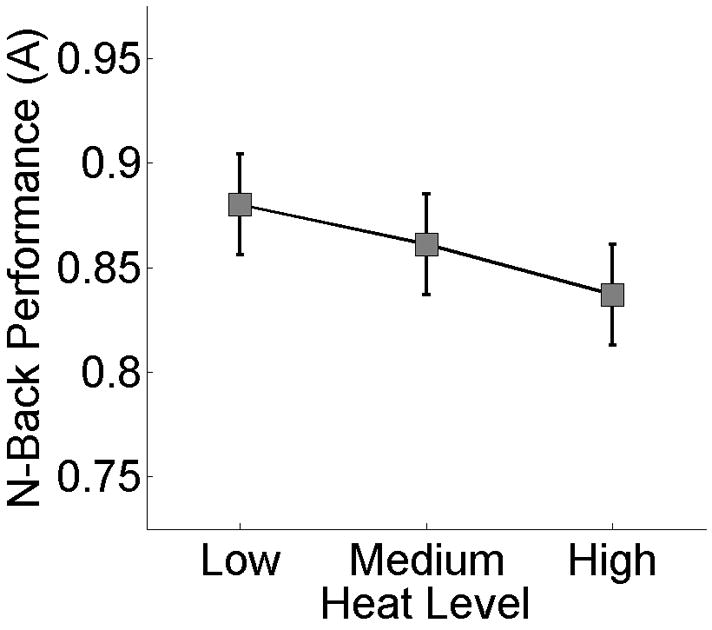
The effect of heat level on performance. Error bars reflect within-subject standard error of the Participant × Heat Level interaction. The mean within-subject standard deviations of A were .14, .14, and .16 for low, medium, and high levels of heat, respectively.
In order to test the relationships among heat level, pain, and task performance, we conducted two multilevel mediation analyses. The results of these are summarized in Figure 6. The first analysis assessed the hypothesis that trial-by-trial fluctuations in pain mediated the relationship between heat level and performance. We found that Pain fully mediated the relationship between Heat Level and Performance (ab= −.03, Z = −3.91, p < .001). In addition to the significant mediation (ab) effect, there was a significant, positive effect of Heat Level on Pain (a = 2.07, Z = 4.22, p < .001), and a negative effect of Pain on Performance, controlling for Heat Level (b = −.02, Z = −3.52, p < .001). Although there was a strong positive relationship between Heat Level and Performance (c = −.02, Z = −3.35, p < .001), after controlling for Pain, this relationship was no longer significant (c′ = .02, ns), indicating that Pain was a complete mediator.
Fig. 6.
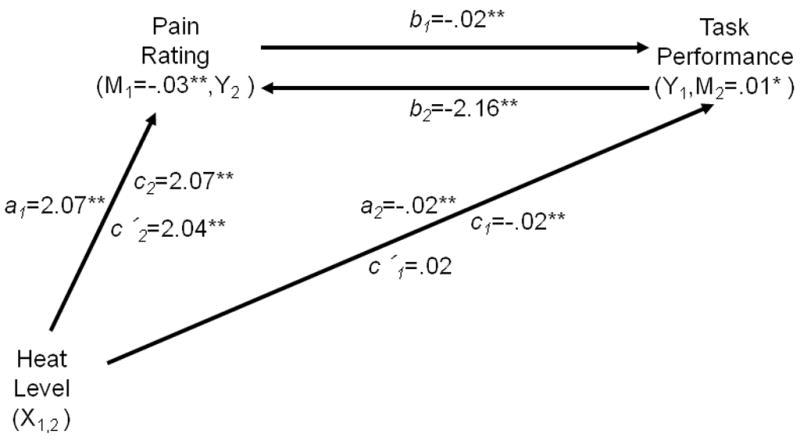
Summary of mediation results for Working Memory Load trials. A first mediation analysis assessed whether Pain (M1) mediated the relationship between Heat level (X1) and Performance (Y1): a1: the relationship between Heat Level and Pain; b1: the relationship between Pain and Performance, controlling for Heat Level; c1: the observed relationship between Heat level and Performance; c′1: the relationship between Heat Level and Performance, controlling for a1 and b1. A second mediation analysis assessed whether trial-by-trial fluctuations in Performance (M2) mediated the relationship between Heat Level (X2) and Pain (Y2): a2: the relationship between Heat Level and Performance; b2: the relationship between Performance and Pain, controlling for Heat Level; c2: the observed relationship between Heat Level and Pain; c′2: the relationship between Heat Level and Pain, controlling for a2 and b2. * p< .05. **p< .001.
A second mediation analysis assessed the complementary hypothesis that trial-by-trial fluctuations in performance mediated the relationship between heat level and pain. We found that Performance partially mediated the relationship between Heat Level and Pain (ab = .01, Z = 1.77, p < .05). In addition to the significant mediation (ab) effect, there was a significant, negative effect of Heat Level on Performance (a = −.02, Z = −3.36, p < .001), and a negative effect of Performance on Pain, controlling for Heat Level (b = −2.16, Z = −3.61 p < .001). After controlling for Performance, a direct relationship remained between Heat Level and Pain (c = 2.07, Z = 4.20, p < .001; c′ = 2.04, Z = 4.13, p < .001).
Discussion
Previous research has typically assumed a bidirectional relationship between pain and task performance [42], implying both engage an overlapping set of domain-general, capacity-limited cognitive resources. Yet experimental evidence has been equivocal, leading to alternative proposals [45; 48; 73; 63; 62]. We sought to distinguish between these competing views using a novel paradigm designed to place continuous demand on executive processes and sensitive, trial-level analyses. Participants reported less pain during a difficult 3-back working memory task than a visually matched control condition. Conversely, increasing levels of heat incrementally reduced task performance, and trial-by-trial pain reports predicted performance within a given heat level. Using a mediation framework, we found that accounting for these trial-by-trial pain reports fully explained the relationship between heat and performance. In a separate mediation analysis, we also found that trial-by-trial performance in the task partially explained pain reports. Taken together, these findings suggest that the processes that contribute positively to both pain and executive working memory performance share capacity-limited resources [42]. Furthermore, resource allocation varies from one process to the other over time, so that observed variation in each predicts effects on the other. That is, better performance on a given trial predicts lower pain, and higher pain predicts worse performance. Though our mediation analyses are consistent with the notion that each causally influences the other, follow-up experiments that independently manipulate both pain and task performance experimentally are needed to solidify causal inferences.
A shared resources model of pain and cognitive performance is consistent with several neuroimaging meta-analyses that found reliable pain-related activity in lateral and anterior prefrontal cortex (PFC) [54; 59]. These regions have been strongly implicated in diverse executive processes [76], and activity in them has been shown to increase parametrically with demand in an number of executive tasks [5; 13; 36; 66; 22; 75]. However, the few previous studies that have directly examined the effects on PFC activity of incremental changes in pain have found activation with painful stimulation, but not incremental changes of activity that tracked increases in stimulus intensity or reported pain [3; 7]. One possible explanation for this difference is that PFC activity may not provide a sensitive index of resource limitation across levels of pain. For example, PFC may be activated under both weak and strong noxious stimulation, but for different reasons. At low levels of stimulation, PFC might be recruited to reappraise pain or allocate attention elsewhere, consistent with previous studies that suggest a pain-regulatory role [81; 82; 80; 46; 70]. Conversely, at high levels of stimulation, greater PFC activity might reflect increased generation of pain-related cognitions or allocation of attention towards pain. This account predicts that the PFC-pain relationship may be moderated by the intensity of noxious stimulation: it should be negatively correlated with pain at low stimulus intensity and positively correlated with pain at high stimulus intensity. Other explanations that need to be tested are also possible, including: (a) BOLD activity may show a ceiling effect, because PFC is strongly engaged by even weak noxious stimuli; (b) as pain increases, individuals may shift toward alternate coping strategies that do not recruit lateral PFC; and (c), PFC may be recruited to resolve ambiguity and enhance discrimination under weak stimulation, but to regulate pain during intense stimulation. Interestingly, these latter two alternatives imply moderation effects opposite to our initial explanation above, yielding divergent empirical predictions.
The affirmative findings of the present research again raise the question of why some studies have observed interference between pain and cognitive performance while others did not (see Table 1). Comparing these studies suggests several technical and conceptual factors may be critical to observing this relationship, including the type and intensity of task demand and the degree of temporal overlap between task and pain processing (see also discussions in [23; 63]). Specifically, we posit that the task must substantially and continuously demand executive resources. While we did not test this hypothesis in the current research, several aspects of the experimental design reflect this assumption. First, we chose a task that places heavy demands on executive working memory and that has been well characterized both theoretically and neurally. N-back performance requires both the continuous updating of representations in working memory and response selection [76]. An earlier study similarly found that concurrent n-back performance reduced pain [2]. However, consistent with the view that executive demand is critical for a task to interfere with pain processing, another study that used the Sternberg task, a working memory task that places relatively little demand on executive function, found no reduction in pain during task performance [35]. Interference tasks and other Stroop-like tasks also engage executive processes [49], but current results from interference tasks are mixed: pain reduction was reported with a standard Stroop task [70] and with numeric Stroop task variants [1; 63], but not when the challenging Multisource Interference Task was used [62]. Several other studies found a task-related reduction in pain using paradigms that are less well characterized in the literature, including maze performance [53], visual search [73], arithmetic [78; 61; 20; 69], word generation [27; 57], video and virtual reality games [33; 34; 56], mental rotation [39], detection and discrimination [6; 14; 58; 71; 55], and rapid serial visual presentation tasks [77]. Because it is less clear which component processes these tasks require, at present these findings cannot be used assess the hypothesis that executive demand is critical. Furthermore, tasks which should place minimal demand on executive resources have yielded mixed results: while an emotional distraction counting Stroop task variant [8] had no effect on pain [63], a simple 1-back task successfully reduced pain [12]. Future research will need to explicitly compare different types of demand within a single experiment in order to provide a rigorous test of the role of executive demand in pain reduction.
A second choice was to calibrate task difficulty. Our goal was twofold. First, we sought to ensure the task would yield a measure sufficiently sensitive to detect a deleterious influence of pain on performance for each participant. If the task is not sufficiently demanding, then painful stimulation may only transiently and subtly interrupt task performance. Modest decrements in performance will only be detectable if participants are performing near capacity and performance measures are sensitive. Second, we sought to ensure that successful performance of the task would require a profound commitment of executive resources [23]. Even tasks that engage executive function may not interfere with pain if they do not place a heavy demand on information processing.
A third choice we made was to motivate participants with a monetary reward for good performance. Our intention was to ensure the greatest possible dedication of resources to the task. Unmotivated participants might perform at a lower level sufficiently below their ability, leaving idle resources available for concomitant pain processing [42]. However, given the possibility that reward processing may interact with pain [43], future research should confirm that motivated performance of a demanding task can reduce pain regardless of reward context.
Even if pain and task processing engage overlapping executive resources, if this engagement does not overlap in time, participants would be able to switch attention back and forth between pain and cognitive demand, allowing both to be fully processed [24; 72; 73]. Thus, a fourth choice we made was to combine continuous thermal pain with a speeded n-back task that placed relatively continuous demand on executive working memory. In contrast, when Seminowicz and Davis [62] combined relatively continuous electrical pain with the relatively brief and interspersed processing demands of the Multisource Interference Task, no reductions in pain or performance were observed.
Future studies could directly test the hypotheses we offered to explain the inconsistent previous findings. For example, the criticality of executive demand could be examined by directly comparing the pain reduction incurred by executive working memory tasks such as the n-back with working memory tasks which only involve storage, such as the Sternberg task (for more on this distinction, see [76]). Future research could also examine whether different types of executive function, such as perceptual attention demand and executive working memory, influence pain differently [41]. A third important goal for future research would be to clarify the role of pain duration. We hypothesize that brief shock or contact heat will cause intermittent and minor disruption of task performance on response selection, such as a Stroop task, but more profound disruption of tasks that require temporal continuity, such as a difficult n-back. Furthermore, brief noxious stimuli with rapid onsets may capture attention even in the context of a challenging cognitive task.
In sum, these findings support the view that subjective pain and executive working memory performance engage overlapping, capacity-limited cognitive resources. Furthermore, reciprocal variation in pain and performance within a given heat level suggests these limited resources are dynamically allocated between the two processes. Future studies could use the paradigm and analyses we present here to more precisely identify which cognitive resources participate in pain processing and to illuminate the specific roles they play.
Fig. 5.
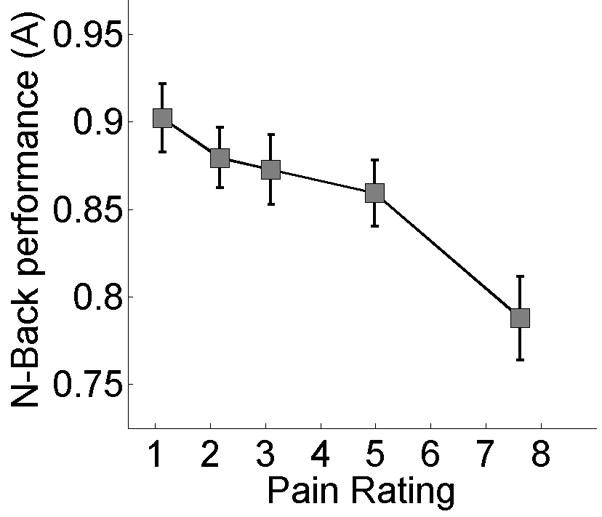
The relationship between pain and performance. For visualization, performance data were binned into quintiles based on pain ratings.
Acknowledgments
We would like to thank Jennifer Silvers for her assistance in designing this study and preparing this manuscript, Kate Hard for her assistance recruiting and screening participants, Lauren Atlas for technical assistance, and two anonymous reviewers for their insightful comments. Author contributions: Design: J.B. and T.D.W. Data collection: J.B. Analysis: J.B. and T.D.W. Writing: J.B. and T.D.W. MATLAB code implementing mediation analyses is freely available at http://www.columbia.edu/cu/psychology/tor/. This research was made possible with the support of grant funding from NSF 0631637 and NIH MH076136, awarded to T.D.W. We have no relevant conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125(Pt 2):310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- 2.Bingel U, Rose M, Glascher J, Buchel C. fMRI reveals how pain modulates visual object processing in the ventral visual stream. Neuron. 2007;55(1):157–167. doi: 10.1016/j.neuron.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125(Pt 6):1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- 4.Boyette-Davis JA, Thompson CD, Fuchs PN. Alterations in attentional mechanisms in response to acute inflammatory pain and morphine administration. Neuroscience. 2008;151(2):558–563. doi: 10.1016/j.neuroscience.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 6.Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15(2):293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- 7.Buchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study. J Neurosci. 2002;22(3):970–976. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhle J, Wager TD, Smith EE. Using the Stroop task to study emotional regulation. In: Hassin R, Ochsner KN, Trope Y, editors. Self control in society, mind, and brain. New York: Oxford University Press; in press. [Google Scholar]
- 9.Bushnell MC, Duncan GH, Dubner R, He LF. Activity of trigeminothalamic neurons in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. J Neurophysiol. 1984;52(1):170–187. doi: 10.1152/jn.1984.52.1.170. [DOI] [PubMed] [Google Scholar]
- 10.Casey KL, Morrow TJ. Nocifensive responses to cutaneous thermal stimuli in the cat: stimulus-response profiles, latencies, and afferent activity. J Neurophysiol. 1983;50(6):1497–1515. doi: 10.1152/jn.1983.50.6.1497. [DOI] [PubMed] [Google Scholar]
- 11.Chapman CR, Nakamura Y, Donaldson GW, Jacobson RC, Bradshaw DH, Flores L, Chapman CN. Sensory and affective dimensions of phasic pain are indistinguishable in the self-report and psychophysiology of normal laboratory subjects. J Pain. 2001;2(5):279–294. doi: 10.1054/jpai.2001.25529. [DOI] [PubMed] [Google Scholar]
- 12.Coen SJ, Aziz Q, Yaguez L, Brammer M, Williams SC, Gregory LJ. Effects of attention on visceral stimulus intensity encoding in the male human brain. Gastroenterology. 2008;135(6):2065–2074. 2074, e2061. doi: 10.1053/j.gastro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 14.Crombez G, Eccleston C, Van den Broeck A, Van Houdenhove B, Goubert L. The effects of catastrophic thinking about pain on attentional interference by pain: no mediation of negative affectivity in healthy volunteers and in patients with low back pain. Pain Res Manag. 2002;7(1):31–39. doi: 10.1155/2002/576792. [DOI] [PubMed] [Google Scholar]
- 15.Devine DP, Spanos NP. Effectiveness of maximally different cognitive strategies and expectancy in attenuation of reported pain. J Pers Soc Psychol. 1990;58(4):672–678. doi: 10.1037//0022-3514.58.4.672. [DOI] [PubMed] [Google Scholar]
- 16.Dick BD, Connolly JF, Houlihan ME, McGrath PJ, Finley GA, Stroink G, Clark AJ. Effects of experimentally induced pain on mismatch negativity. Journal of Psychophysiology. 2006;20(1):21–31. [Google Scholar]
- 17.Dick BD, Connolly JF, McGrath PJ, Finley GA, Stroink G, Houlihan ME, Clark AJ. The disruptive effect of chronic pain on mismatch negativity. Clin Neurophysiol. 2003;114(8):1497–1506. doi: 10.1016/s1388-2457(03)00133-0. [DOI] [PubMed] [Google Scholar]
- 18.Dick BD, Eccleston C, Crombez G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum. 2002;47(6):639–644. doi: 10.1002/art.10800. [DOI] [PubMed] [Google Scholar]
- 19.Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesth Analg. 2007;104(5):1223–1229. doi: 10.1213/01.ane.0000263280.49786.f5. [DOI] [PubMed] [Google Scholar]
- 20.Dowman R. Distraction produces an increase in pain-evoked anterior cingulate activity. Psychophysiology. 2004;41(4):613–624. doi: 10.1111/1469-8986.00186.x. [DOI] [PubMed] [Google Scholar]
- 21.Dubner R, Hoffman DS, Hayes RL. Neuronal activity in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. III. Task-related responses and their functional role. J Neurophysiol. 1981;46(3):444–464. doi: 10.1152/jn.1981.46.3.444. [DOI] [PubMed] [Google Scholar]
- 22.Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, Watts R, Ulug AM, Casey BJ. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. Neuroimage. 2003;20(4):2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Eccleston C. The attentional control of pain: methodological and theoretical concerns. Pain. 1995;63(1):3–10. doi: 10.1016/0304-3959(95)00093-8. [DOI] [PubMed] [Google Scholar]
- 24.Eccleston C. Chronic pain and distraction: an experimental investigation into the role of sustained and shifting attention in the processing of chronic persistent pain. Behav Res Ther. 1995;33(4):391–405. doi: 10.1016/0005-7967(94)00057-q. [DOI] [PubMed] [Google Scholar]
- 25.Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125(3):356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 26.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Vol. 57. New York: Chapmann & Hall; 1993. [Google Scholar]
- 27.Frankenstein UN, Richter W, McIntyre MC, Remy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage. 2001;14(4):827–836. doi: 10.1006/nimg.2001.0883. [DOI] [PubMed] [Google Scholar]
- 28.Glass JM, Park DC. Cognitive dysfunction in fibromyalgia. Curr Rheumatol Rep. 2001;3(2):123–127. doi: 10.1007/s11926-001-0007-4. [DOI] [PubMed] [Google Scholar]
- 29.Gracely RH, Dubner R, McGrath PA. Narcotic analgesia: fentanyl reduces the intensity but not the unpleasantness of painful tooth pulp sensations. Science. 1979;203(4386):1261–1263. doi: 10.1126/science.424753. [DOI] [PubMed] [Google Scholar]
- 30.Harman K, Ruyak P. Working through the pain: a controlled study of the impact of persistent pain on performing a computer task. Clin J Pain. 2005;21(3):216–222. doi: 10.1097/00002508-200505000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Hayes RL, Dubner R, Hoffman DS. Neuronal activity in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. II. Behavioral modulation of responses to thermal and mechanical stimuli. J Neurophysiol. 1981;46(3):428–443. doi: 10.1152/jn.1981.46.3.428. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman DS, Dubner R, Hayes RL, Medlin TP. Neuronal activity in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. I. Responses to innocuous and noxious thermal stimuli. J Neurophysiol. 1981;46(3):409–427. doi: 10.1152/jn.1981.46.3.409. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman HG, Richards TL, Coda B, Bills AR, Blough D, Richards AL, Sharar SR. Modulation of thermal pain-related brain activity with virtual reality: evidence from fMRI. Neuroreport. 2004;15(8):1245–1248. doi: 10.1097/01.wnr.0000127826.73576.91. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman HG, Sharar SR, Coda B, Everett JJ, Ciol M, Richards T, Patterson DR. Manipulating presence influences the magnitude of virtual reality analgesia. Pain. 2004;111(1–2):162–168. doi: 10.1016/j.pain.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Houlihan ME, McGrath PJ, Connolly JF, Stroink G, Allen Finley G, Dick B, Phi TT. Assessing the effect of pain on demands for attentional resources using ERPs. Int J Psychophysiol. 2004;51(2):181–187. doi: 10.1016/j.ijpsycho.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal Working Memory Load Affects Regional Brain Activation as Measured by PET. J Cogn Neurosci. 1997;9(4) doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- 37.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 38.Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55(4):352–358. doi: 10.1037/h0043688. [DOI] [PubMed] [Google Scholar]
- 39.Kobor I, Gal V, Vidnyanszky Z. Attentional modulation of perceived pain intensity in capsaicin-induced secondary hyperalgesia. Exp Brain Res. 2009;195(3):467–472. doi: 10.1007/s00221-009-1799-0. [DOI] [PubMed] [Google Scholar]
- 40.Lautenbacher S, Prager M, Rollman GB. Pain additivity, diffuse noxious inhibitory controls, and attention: a functional measurement analysis. Somatosens Mot Res. 2007;24(4):189–201. doi: 10.1080/08990220701637638. [DOI] [PubMed] [Google Scholar]
- 41.Lavie N. Distracted and confused?: selective attention under load. Trends Cogn Sci. 2005;9(2):75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Legrain V, Damme SV, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain. 2009;144(3):230–232. doi: 10.1016/j.pain.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9(4):314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 44.Leotti LA, Wager TD. Motivational influences on response inhibition measures. Journal of Experimental Psychology: Human Perception and Performance. doi: 10.1037/a0016802. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leventhal H. I know distraction works even though it doesn’t! Health Psychol. 1992;11(4):208–209. doi: 10.1037/h0090350. [DOI] [PubMed] [Google Scholar]
- 46.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(Pt 5):1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 47.Masson MEJ, Loftus GR. Using confidence intervals for graphically based data interpretation. Can J Exp Psychol. 2003;57(3):203–220. doi: 10.1037/h0087426. [DOI] [PubMed] [Google Scholar]
- 48.McCaul KD, Monson N, Maki RH. Does distraction reduce pain-produced distress among college students? Health Psychol. 1992;11(4):210–217. doi: 10.1037//0278-6133.11.4.210. [DOI] [PubMed] [Google Scholar]
- 49.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 50.Norman DA, Bobrow DG. On Data-limited and Resource-limited Processes. Cognitive Psychology. 1975;7(1):44–64. [Google Scholar]
- 51.Oosterman JM, de Vries K, Dijkerman HC, de Haan EH, Scherder EJ. Exploring the relationship between cognition and self-reported pain in residents of homes for the elderly. Int Psychogeriatr. 2008:1–7. doi: 10.1017/S1041610208007941. [DOI] [PubMed] [Google Scholar]
- 52.Park DC, Glass JM, Minear M, Crofford LJ. Cognitive function in fibromyalgia patients. Arthritis Rheum. 2001;44(9):2125–2133. doi: 10.1002/1529-0131(200109)44:9<2125::AID-ART365>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 53.Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85(1–2):19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- 54.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin. 2000;30(5):263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 55.Pud D, Sapir S. The effects of noxious heat, auditory stimulation, a cognitive task, and time on task on pain perception and performance accuracy in healthy volunteers: a new experimental model. Pain. 2006;120(1–2):155–160. doi: 10.1016/j.pain.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 56.Raudenbush B, Koon J, Cessna T, McCombs K. Effects of playing video games on pain response during a cold pressor task. Percept Mot Skills. 2009;108(2):439–448. doi: 10.2466/PMS.108.2.439-448. [DOI] [PubMed] [Google Scholar]
- 57.Remy F, Frankenstein UN, Mincic A, Tomanek B, Stroman PW. Pain modulates cerebral activity during cognitive performance. Neuroimage. 2003;19(3):655–664. doi: 10.1016/s1053-8119(03)00146-0. [DOI] [PubMed] [Google Scholar]
- 58.Roelofs J, Peters ML, van der Zijden M, Vlaeyen JW. Does fear of pain moderate the effects of sensory focusing and distraction on cold pressor pain in pain-free individuals? J Pain. 2004;5(5):250–256. doi: 10.1016/j.jpain.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009;45(3):810–823. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 60.Scherder EJ, Eggermont L, Plooij B, Oudshoorn J, Vuijk PJ, Pickering G, Lautenbacher S, Achterberg W, Oosterman J. Relationship between chronic pain and cognition in cognitively intact older persons and in patients with Alzheimer’s disease. The need to control for mood. Gerontology. 2008;54(1):50–58. doi: 10.1159/000113216. [DOI] [PubMed] [Google Scholar]
- 61.Schlereth T, Baumgartner U, Magerl W, Stoeter P, Treede RD. Left-hemisphere dominance in early nociceptive processing in the human parasylvian cortex. Neuroimage. 2003;20(1):441–454. doi: 10.1016/s1053-8119(03)00345-8. [DOI] [PubMed] [Google Scholar]
- 62.Seminowicz DA, Davis KD. Interactions of pain intensity and cognitive load: the brain stays on task. Cereb Cortex. 2007;17(6):1412–1422. doi: 10.1093/cercor/bhl052. [DOI] [PubMed] [Google Scholar]
- 63.Seminowicz DA, Davis KD. A re-examination of pain-cognition interactions: implications for neuroimaging. Pain. 2007;130(1–2):8–13. doi: 10.1016/j.pain.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 64.Seminowicz DA, Mikulis DJ, Davis KD. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 2004;112(1–2):48–58. doi: 10.1016/j.pain.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 65.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 66.Szameitat AJ, Schubert T, Muller K, Von Cramon DY. Localization of executive functions in dual-task performance with fMRI. J Cogn Neurosci. 2002;14(8):1184–1199. doi: 10.1162/089892902760807195. [DOI] [PubMed] [Google Scholar]
- 67.Tanner WP, Jr, Swets JA. A decision-making theory of visual detection. Psychol Rev. 1954;61(6):401–409. doi: 10.1037/h0058700. [DOI] [PubMed] [Google Scholar]
- 68.Tassain V, Attal N, Fletcher D, Brasseur L, Degieux P, Chauvin M, Bouhassira D. Long term effects of oral sustained release morphine on neuropsychological performance in patients with chronic non-cancer pain. Pain. 2003;104(1–2):389–400. doi: 10.1016/s0304-3959(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 69.Terkelsen AJ, Andersen OK, Molgaard H, Hansen J, Jensen TS. Mental stress inhibits pain perception and heart rate variability but not a nociceptive withdrawal reflex. Acta Physiol Scand. 2004;180(4):405–414. doi: 10.1111/j.1365-201X.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 70.Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004;109(3):399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 71.Van Damme S, Crombez G, Eccleston C, Goubert L. Impaired disengagement from threatening cues of impending pain in a crossmodal cueing paradigm. Eur J Pain. 2004;8(3):227–236. doi: 10.1016/j.ejpain.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Veldhuijzen DS. Pain and attention: a discussion of two studies. J Pain. 2006;7(1):31. doi: 10.1016/j.jpain.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 73.Veldhuijzen DS, Kenemans JL, de Bruin CM, Olivier B, Volkerts ER. Pain and attention: attentional disruption or distraction? J Pain. 2006;7(1):11–20. doi: 10.1016/j.jpain.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Veldhuijzen DS, van Wijck AJ, Wille F, Verster JC, Kenemans JL, Kalkman CJ, Olivier B, Volkerts ER. Effect of chronic nonmalignant pain on highway driving performance. Pain. 2006;122(1–2):28–35. doi: 10.1016/j.pain.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 75.Veltman DJ, Rombouts SA, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage. 2003;18(2):247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 76.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 77.Wiech K, Seymour B, Kalisch R, Stephan KE, Koltzenburg M, Driver J, Dolan RJ. Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage. 2005;27(1):59–69. doi: 10.1016/j.neuroimage.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 78.Yamasaki H, Kakigi R, Watanabe S, Hoshiyama M. Effects of distraction on pain-related somatosensory evoked magnetic fields and potentials following painful electrical stimulation. Brain Res Cogn Brain Res. 2000;9(2):165–175. doi: 10.1016/s0926-6410(99)00056-7. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Mueller ST. A note on ROC analysis and non-parametric estimate of sensitivity. Psychometrika. 2005;70(1):145–154. [Google Scholar]
- 80.Zhang S, Tang JS, Yuan B, Jia H. Inhibitory effects of electrical stimulation of ventrolateral orbital cortex on the rat jaw-opening reflex. Brain Res. 1998;813(2):359–366. doi: 10.1016/s0006-8993(98)01050-6. [DOI] [PubMed] [Google Scholar]
- 81.Zhang S, Tang JS, Yuan B, Jia H. Involvement of the frontal ventrolateral orbital cortex in descending inhibition of nociception mediated by the periaqueductal gray in rats. Neurosci Lett. 1997;224(2):142–146. doi: 10.1016/s0304-3940(97)13478-4. [DOI] [PubMed] [Google Scholar]
- 82.Zhang YQ, Tang JS, Yuan B, Jia H. Inhibitory effects of electrically evoked activation of ventrolateral orbital cortex on the tail-flick reflex are mediated by periaqueductal gray in rats. Pain. 1997;72(1–2):127–135. doi: 10.1016/s0304-3959(97)00025-0. [DOI] [PubMed] [Google Scholar]


