Abstract
Group-living folivorous primates can experience competition for food, and feeding competition has also been documented for solitarily foraging gummivorous and omnivorous primates. However, little is known about the types and consequences of feeding competition in solitary folivorous foragers. We conducted this study in the spiny forest of Berenty Reserve, southern Madagascar, to characterize the competitive regime of the nocturnal solitarily foraging white-footed sportive lemur (Lepilemur leucopus), a species that lives in dispersed pairs. We analyzed 1,213 hr of behavioral observations recorded simultaneously for the male and female of each of seven social units and recorded seasonal changes in food availability over a complete annual cycle. Lepilemur leucopus exhibited low selectivity in its dietary choice and mainly included the most abundant plant species in its diet. Contrary to our predictions, we did not find evidence for increased rates of contest (i.e., displacement from food trees) or scramble competition (i.e., shared use of food patches) during the lean season, neither within nor between social units. Instead, conflict rates were low throughout the year, and, during these observations, any feeding stress may have been more related to food quality than quantity. The resource defense hypotheses may not explain pair-living in this species as there was no indication that males defend food resources for their female pair-partners. The observed lack of feeding competition may indicate that a cryptic anti-predator strategy is a better predictor of spatial avoidance of pair-partners than conflict over food. While anti-predator benefits of crypsis may explain, at least partly, female-female avoidance, studies on the relationship between territory size/quality and reproductive success are required to understand whether feeding competition reduces the potential for female association in L. leucopus.
Keywords: feeding competition, resource defense, seasonality, spatial avoidance, predation, crypsis
INTRODUCTION
Folivorous primates have traditionally been assumed to experience little to no feeding competition as leaves are apparently an abundant and evenly dispersed food resource [Isbell, 1991]. However, recent studies indicated that at least some folivores experience food limitation [Borries et al., 2008; Harris et al., 2010; Koenig et al., 1998]. By exploiting high-quality, patchily distributed, temporally variable food resources, they may experience within-group scramble competition [Snaith & Chapman, 2005] as well as within- and between-group contest competition [Koenig, 2002]. Studies that take place when preferred foods are abundant may not find evidence for food limitation and feeding competition, whereas longitudinal studies on effects of reductions in main food resources may provide valuable insights into the selective pressures that diet places on folivorous primates [Harris et al., 2010].
Previous studies of Malagasy primates (Lemuriformes) revealed that feeding competition does not occur only in group-living species, but also among solitary foragers. For example, within-group scramble and contest competition as well as female feeding dominance were demonstrated for gummivorous Phaner pallescens [Schülke, 2003]. Similarly, resource distribution and resulting competitive regimes have been shown to determine distribution and association patterns of solitary omnivorous Microcebus berthae and M. murinus [Dammhahn & Kappeler, 2009]. Competition for food in solitarily foraging folivorous primates has not been studied in detail yet, however.
Sportive lemurs (genus Lepilemur) are strictly folivorous and nocturnal. As with other congeners, white-footed sportive lemurs (Lepilemur leucopus) have evolved adaptations to a folivorous diet despite small body size (<1 kg), including prolonged resting bouts, small night ranges, an enlarged caecum and caecotrophy [Hladik & Charles-Dominique, 1974]. Known predators of sportive lemurs are fossas (Cryptoprocta ferox), long-eared owls (Asio madagascariensis), Madagascar boas (Acrantophis sp.) and Harrier hawks [Polyboroides radiatus; Fichtel, 2007; Goodman et al., 1993; Rasoloarison et al., 1995; Schülke & Ostner, 2001; Sussman, 1999]. Sportive lemurs live in dispersed pairs, which are characterized by spatial overlap between one adult male and female but low cohesion between pair partners [Dröscher & Kappeler, 2013; Hilgartner et al., 2012; Méndez-Cárdenas & Zimmermann, 2009; Schülke & Kappeler, 2003; Zinner et al., 2003]. In L. leucopus, pair-partners show signs of active avoidance, and home range overlap among neighboring females is minimal [Dröscher & Kappeler, 2013].
If males defend resources that are important to females, instead of defending females directly, resource defense can explain the evolution of pair-living [Emlen & Oring, 1977; van Schaik & Dunbar, 1990; Wrangham, 1979]. Under this scenario, female reproductive success is limited by male resource holding potential [Parker, 1974], whereas male reproductive success is limited by female choice of mates with variable resource access [Balmford et al., 1992]. Pairs evolve under this scenario whenever males are unable to defend territories that can support more than one female [Hilgartner et al., 2012; Lukas & Clutton-Brock, 2013]. However, to evaluate this hypothesis, quantitative data on resource use and competition are required.
Competition for food may explain female-female avoidance [Lukas & Clutton-Brock, 2013] as well as avoidance of pair-partners. For example, pair partners in P. pallescens reduce feeding competition by avoiding competitors in time instead of space, as they rely on relatively rare gum trees [Schülke, 2003]. However, solitary foraging seems to characterize almost all nocturnal primates irrespective of their diet, suggesting that factors other than feeding competition may promote this type of social organization [Schülke, 2003]. More studies on ranging behavior, resource use and competitive regimes are therefore indicated to further our understanding of the factors that promote intra- and intersexual avoidance in solitary foragers.
The main aims of the present study were to investigate the types and consequences of feeding competition between and within social units of white-footed sportive lemurs. In particular, we predicted contest competition (i.e., displacement from food trees) as well as scramble competition (i.e., food patches shared by individuals) to increase in intensity during the pronounced lean season characterizing southern Madagascar. Alternatively, based on the fact that L. leucopus is folivorous and leaves can be expected to be relatively abundant, feeding stress could be more related to food quality than quantity. In this case, we predicted scramble as well as contest competition to be rare. In addition, we explored whether female-female avoidance as well as avoidance between pair partners is a consequence of feeding competition. Our second aim was to evaluate the importance of resource defense as a male mating strategy. In this case, we predicted that males of neighboring social units would engage in conflict over resources. In the absence of more precise measures of territory quality, we assume that differences in territory quality are related to territory size.
METHODS
Study Site
We conducted our study at Berenty (S 25.00°, E 46.30°), an approximately 200 km2 private ecotourism reserve. Hot and wet summers characterize Berenty's semi-arid climate (November to April), which alternate with cold dry and winters [May to October; Jolly et al., 2006]. We observed animals in a spiny forest fragment of about 5 ha (HAH Reserve Forestière parcel 1), which is connected to gallery forest on one side via a transitional forest and a further 40 ha spiny forest fragment on the other side [Norscia & Palagi, 2008]. We recorded minimum and maximum temperatures on a daily basis as well as the amount of precipitation after each rainfall. Seasonality in temperature and rainfall was pronounced during our study. High daytime temperatures with monthly averages of up to 35°C characterized the wet season, while monthly average nighttime temperatures fell to 15°C during the dry season. While precipitation amounted to 480 mm during the wet season, we recorded only 64 mm (or 12% of the annual rainfall) during the dry season. Between 1984 and 2000 annual rainfall at Berenty ranged between 265 and 894 mm, with an average annual rainfall of about 545 mm [Jolly et al., 2002], which corresponds to the 544 mm recorded during our study.
Animal Capture
To allow continuous focal observations on known individuals, we captured 20 individuals of L. leucopus and equipped them with radio-tracking transmitters. We anesthetized animals with 0.4 ml Ketanest (100 mg/ml) in their day-time resting sites, using a blow pipe and 1 ml air-pressured narcotic syringe projectiles (Telinject, Germany). While they were anesthetized, we fitted the animals with radio transmitters (TW-3 button-cell tags, Biotrack, UK). The assembled transmitter packs weighed 20 g and were fastened around the neck of the animals using a coated brass loop that also functioned as antenna. We kept the animals in animal transport boxes until they were fully recovered and released them at their capture site in the evening. The same individuals later reused sleeping trees where they were captured. We fitted adult as well as subadult individuals with radio-collars. We differentiated adult individuals from subadults by their larger degree of tooth wear and body mass. We did not radio-collar juvenile animals (<1 year-old) because radio-collars exceeded 4% of their body mass. We removed all radio-collars after the end of the study. The research adhered to the American Society of Primatologists (ASP) principles for the ethical treatment of non-human primates and was approved by the Commission Tripartite CAFF (Madagascar).
Behavioral Observations
Five out of seven social units consisted of pairs, whereas in the remaining cases an adult male was associated with two adult females each (social unit 1 and 3; Appendix A). However, these females had exclusive ranges as they were regularly seen within the range of the associated adult male, but never within the range of the other adult female. No behavioral observations could be conducted on these females because they were not equipped with radio-collars. For more detailed information on the identification of the social units within the study population see Dröscher and Kappeler [2013]. For the present study, we considered only focal individuals that were adult and belonged to social units for which both the male and the female were radio-collared (N = 16 individuals). We collected behavioral and locational data between October 2011 and October 2012 for a total of 1,530 hr.
We divided the study period into four seasons: early wet (November to January), late wet (February to April), early dry (May to July) and late dry season (August to October). We followed each radio-collared animal for up to two full nights during each season with the help of a TR-4 receiver and a RA-14K antenna (Telonics, USA). We started continuous focal animal observations when an animal left its sleeping site at dusk and continued them until it returned to its daytime resting tree at dawn. During continuous focal observations, we tagged all trees the focal animal visited with biodegradable tape in a continuous manner to mark the spatial locations of the animal within its home range. After each full-night follow, we determined the position of the tagged tree with reference to a 10 × 10 m study grid system. We used this method instead of GPS tracking to achieve more precise measurements of spatial locations.
During each feeding bout of a focal animal, we recorded species and types of food eaten along with duration of feeding on that particular food item. A feeding bout started when an individual started to introduce food items into its mouth and ended when an animal stopped inserting food items for more than 15 sec. To measure contest competition, we recorded agonistic interactions over food resources. We defined all interactions that were either aggressive (chase, charge, bite, and grab) or submissive (flee, be displaced, or jump away) as agonistic [sensu Pereira and Kappeler, 1997]. We defined agonistic interactions as displacements from food patches when the displacing animal foraged in the food patch from which it displaced another individual. To measure scramble competition, we investigated the number of food patches that were used by different individuals, either simultaneously or consecutively.
Food Availability
We collected phenology data between November 2011 and October 2012. We included as many plant species as possible in the phenology transects, as we did not know at the beginning of the study which species would be consumed by the sportive lemurs. Initially, we tagged 430 individual trees, shrubs and lianas belonging to 105 species along three line transects of 250 m each crossing home ranges of all study animals. We tagged between one and 13 individual plants per species according to their abundance in the forest. We monitored trees twice a month, whereas we monitored shrubs and lianas on a monthly basis. For all plants, we recorded the abundance of young, mature and old leaves as well as fruits and flowers by estimating their crown coverage visually, based on what a full tree would look like, using the following scale: 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%).
We collected information on the local tree community using the point-quarter method [Ganzhorn, 2003]. We selected points (N = 127) every 20 m with reference to the study grid system covering the entire study area. In each quarter, we measured the distance from the point to the nearest tree with a DBH of >10 cm and recorded the species identity along with the DBH of the respective tree. To infer species abundance of lianas we used the plot method. We counted all trees with a DBH of >10 cm carrying lianas, whereby a single tree could carry several liana species. We recorded species identity and abundance of lianas within 10 randomly selected plots of 10 × 10 m.
Data Analyses
We calculated tree density (individuals/ha) as 10,000/(mean point-to-tree distance)2. We calculated the relative density of a tree species (%) as (number of individuals of a species/total number of individuals) × 100. Finally, we calculated the density of a tree species as (relative density of a tree species/100) × tree density. To characterize seasonal changes in food availability, we multiplied the density of each food species with its average DBH and the corresponding average abundance of food items recorded during bimonthly phenology surveys, yielding our food availability scores.
We restricted our analyses to behavioral observations where pair-partners (i.e., adult male and female individuals that shared a common range) were followed simultaneously during full night observations by ID and a second trained observer (N = 52 simultaneous observation nights; Appendix A). We subsampled locational data at 5-min intervals for analyses of ranging behavior. We computed nightly average distance between individuals of a pair to examine seasonal changes in inter-individual cohesiveness. We used nearest-neighbor analysis as implemented in the Animal Movement extension for ArcView [Hooge & Eichenlaub, 1997] to test for spatial randomness of identified food patches within the territories of the seven social units. We defined food patches as single feeding trees in which animals were observed eating. While R values (obtained by nearest-neighbor analyses) of 1 indicate a random distribution, R values of <1 and >1 indicate a tendency towards a clumped or a uniform distribution, respectively. Significant deviations from the null-hypothesis of complete spatial randomness were tested using Z scores based on Randomization Null Hypothesis computation.
We calculated the sizes of home ranges and core areas from fixed kernel range utilization distributions [Worton, 1989] using ad hoc smoothing [Silverman, 1986]. We delineated core areas using a time-maximizing function derived from kernel analyses [Vander Wal & Rodgers, 2012]. To quantify space-use sharing by pairs, we calculated the utilization distribution overlap index [UDOI; Fieberg & Kochanny, 2005] of nightly core areas of simultaneously followed individuals. The UDOI takes on values of 0 for two ranges that do not overlap and equals 1 if both utility distributions are uniformly distributed and overlap 100% [Fieberg & Kochanny, 2005]. We delineated core areas and calculated the utilization distribution overlap indices in R [R Core Development Team, 2012] using the code provide by Vander Wal & Rodgers [2012] and the package “adehabitatHR” [Calenge, 2006], respectively. We did not correct for spatial autocorrelation, as kernel densities do not require serial independence of observations [De Solla et al., 1999]. However, we based our home range estimates on a time interval (i.e., 5 min) that is biologically meaningful, as it allows individuals to traverse their home range at maximum speed [Rooney et al., 1998].
We calculated seasonal influence on scramble competition (i.e., food resource sharing), sociality (i.e., average distance between pair partners and rates of agonistic interactions), and space-use sharing (i.e., as calculated by the UDOI of core areas) for seven pairs, using multilevel modeling (MLM) for a one-way repeated measures design [Field et al., 2012]. The advantage of this method over traditional ANOVA is its robustness against violations of sphericity. We averaged nightly values for each season and dyad (N = 28 based on four seasons and seven dyads). Similarly, we analyzed seasonal influences on the activity budgets for the same 14 individuals (N = 56 based on four seasons and 14 individuals). We based estimates of activity budgets (resting, feeding, traveling, other) on the time the animals were in sight.
We analyzed the data using the function “lme” of the R package “nlme” [Pinheiro et al., 2013]. For each variable of interest (food resource sharing, average distance between pair partners, rates of agonistic interactions, space-use sharing, time spent resting, time spent feeding, and time spent traveling), we specified a separate model. We used season as predictor variable and the respective variable of interest as outcome variable. We set social unit or individual, respectively, as a random factor within the variable season. We fitted the models using maximum likelihood. Based on visual inspection of histograms and q-q plots, residuals did not deviate from a normal distribution. To test whether season had an overall effect on our variables of interest, we compared the full model to a model in which the predictor was absent, using a likelihood ratio test. To investigate the influence of seasonality on overall rates of agonistic interactions, we used non-parametric statistics (i.e., Friedman's ANOVA), as the data were not normally distributed. We carried out statistical analyses using the software R. We considered an alpha level of P ≤ 0.05 as statistically significant.
RESULTS
Availability and Distribution of Food Resources
We could reliably identify food species and food item consumed during a total of 337 hr of feeding observations (total feeding time 349 hr) of focal animals (1,213 total observation hours). The animals ate mainly leaves (mean ± SD = 90.1 ± 3.01%, N = 16); however, they also included flowers (mean ± SD = 4.4 ± 3.1%), fruits (mean ± SD = 0.6 ± 1.0%) and shoots of non-leafy lianas (mean ± SD = 2.5 ± 3.3%) in their diet. In total, we identified food items belonging to 32 species of trees and 16 species of lianas. We recorded a total of 69 tree and liana species at the study site and the animals concentrated their feeding effort on the most abundant species, as usage intensity was highest for the most common tree (i.e., Alluaudia procera) and liana species (i.e., Metaporana parvifolia; Table1). A total of 63% of feeding time was devoted to the 5 most abundant tree and 5 most abundant liana species. Nearest-neighbor analyses of individual food patches of Alluaudia procera and Metaporana parvifolia, the two main food species of L. leucopus, produced R values ranging between 0.58 and 1.91 (N = 14). Although a tendency to clumping occurred in four cases (R ≤ 0.84, P ≤ 0.05), the main food resources of L. leucopus generally exhibited a random or even spatial distribution.
TABLE I.
Tree and Liana Species at the Study Site Ranked According to Their Density (Individuals/ha) and Their Usage Intensity (% Feeding Time Pooled Across All Observations and Individuals)
| Scientific name | Vernacular name | Growth form | Density | % |
|---|---|---|---|---|
| Alluaudia procera | Fantsiolotra | T | 369 | 29.76 |
| Commiphora humbertii | Daro siky | T | 147 | 2.00 |
| Alantsilodendron alluaudianum | Avoha | T | 85 | 7.26 |
| Gyrocarpus americanus | Sirosiro | T | 79 | 0.23 |
| Commiphora sp. 2 | Daro tandroka | T | 60 | 0.65 |
| Commiphora aprevalii | Daro be | T | 55 | 3.28 |
| Salvadora angustifolia | Sasavy | T | 36 | 4.00 |
| Alluaudia ascendens | Sogno | T | 28 | 0.28 |
| Euphorbia laro | Famata | T | 28 | 0.00 |
| Commiphora orbicularis | Daro mena | T | 21 | 1.53 |
| Margaritaria sp. | Malamamay | T | 21 | 1.31 |
| Euphorbia sp. 1 | Famantamboay | T | 21 | 0.00 |
| Grewia sp. 1 | T | 15 | 1.81 | |
| Maerua filiformis | Solety | T | 13 | 1.59 |
| Grewia grevei | Tabinala | T | 11 | 3.20 |
| Rhigozum madagascariensis | Hazontaha | T | 9 | 0.06 |
| Fernandoa madagscariensis | Somontsoy | T | 9 | 0.01 |
| Albizia sp. | T | 6 | 0.94 | |
| Tarenna sp. | T | 6 | 0.01 | |
| Strychnos decussata | Relefo | T | 6 | 0.00 |
| Bauhinia grandidieri | Marovambaka | T | 4 | 2.73 |
| Boscia longifolia | Somangipaky | T | 4 | 0.90 |
| Maerua nuda | Solety | T | 4 | 0.90 |
| Stereospermum nematocarpum | Hiligne | T | 4 | 0.39 |
| Androya decaryi | Hazombolala | T | 4 | 0.34 |
| Grewia sp. 2 | Tabarike | T | 4 | 0.16 |
| Cedrelopsis grevei | Katrafay | T | 4 | 0.00 |
| Chadsia sp. | Remote | T | 4 | 0.00 |
| Tetrapterocarpon sp. | Vaovy | T | 2 | 0.70 |
| Commiphora sp. 1 | Daro fengoka | T | 2 | 0.06 |
| Alluaudia humbertii | Sognombarika | T | 2 | 0.03 |
| Humbertiella decaryi | Hazombatango | T | 2 | 0.01 |
| Adansonia za | Za | T | 2 | 0.00 |
| Alluaudia demosa | T | 2 | 0.00 | |
| Ehretia sp. | T | 2 | 0.00 | |
| Euphorbia sp. 2 | Famata mainty | T | 2 | 0.00 |
| Mundulea sp. | T | 2 | 0.00 | |
| Unidentified species 1 | T | 2 | 0.00 | |
| Canthium sp. | T | <2 | 0.84 | |
| Commiphora simplicifolia | Daro sengatse | T | <2 | 0.49 |
| Olax sp. | T | <2 | 0.31 | |
| Rothmania sp. | Tainoro | T | <2 | 0.10 |
| Albizia tulearensis | T | <2 | 0.01 | |
| Metaporana parvifolia | L | 680 | 17.14 | |
| Leptadenia sp. | L | 470 | 2.83 | |
| Cynanchum sp. | Try | L | 470 | 0.99 |
| Seyrigia gracilis | L | 320 | 0.01 | |
| Hippocratea angustipetalata | Vahipindy | L | 150 | 2.02 |
| Asparagus sp. | L | 120 | 0.00 | |
| Kalanchoe beauverdii | L | 80 | 0.00 | |
| Hildebrandtia valo | L | 70 | 0.10 | |
| Plectaneia hildebrantii | L | 60 | 1.19 | |
| Combretum sp. | L | 60 | 0.00 | |
| Paederia sp. | Tamboro | L | 40 | 8.28 |
| Diascorea fandra | L | 40 | 0.00 | |
| Menabea venenata | Fiofio | L | 30 | 0.06 |
| Folotsia grandiflorum | L | 20 | 0.14 | |
| Ipomoea longituba | L | 20 | 0.12 | |
| Craterospermum sp. | L | 20 | 0.00 | |
| Diascorea nako | L | 20 | 0.00 | |
| Unidentified species 2 | L | 20 | 0.00 | |
| Unidentified species 3 | L | 20 | 0.00 | |
| Xerosicyos sp. | L | 10 | 0.90 | |
| Polygala humbertii | L | 10 | 0.08 | |
| Cissampelos pareira | L | 10 | 0.06 | |
| Adenia elegans | L | 10 | 0.00 | |
| Ipomea sp. | L | 10 | 0.00 | |
| Unidentified species 4 | L | <10 | 0.18 | |
| Clerodendrum sp. | L | <10 | 0.01 |
Note:T, tree; L, liana. Food tree and liana abundance were evaluated using different methods and are here considered separately. The top ten contributors to the diet of L. leucopus are highlighted in bold.
We included most (25 of 32) of the food tree species via the point-quarter method and calculated food availability scores for these 25 species. Food availability varied seasonally (Fig. 1). While leaves contributed most to available food, flowers and fruits played only a minor role. During the early wet season, young leaves dominated, while mature leaves were most abundant during the late wet and early dry season and reached a low during the late dry season. The animals did not switch to a different diet during the lean season. Instead, during each of the four seasons the animals fed predominantly on the leaves of A. procera, followed by M. parvifolia during the late wet and early dry season or Alantsilodendron alluaudianum during the late dry and early wet season, respectively (Table2). Metaporana parvifolia contributed importantly during all four seasons, whereas A. alluaudianum was not among the top 5 contributors during the late wet season.
Figure 1.
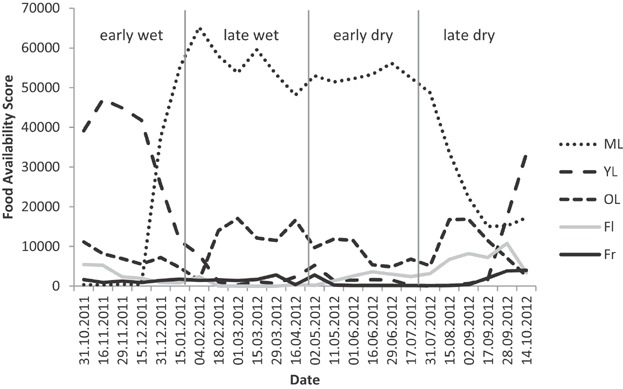
Seasonal food availability based on 25 identified tree species that were used as a food sources by L. leucopus (ML, mature leaves; YL, young leaves; OL, old leaves; Fl, flowers; Fr, fruits).
TABLE II.
Top Five Contributors to L. leucopus Diet According to Usage Intensity (% Feeding Time) Considered Separately for Each of the Four Seasons (L, Leaves; Fl, Flowers)
| Scientific name | Item | Early wet | Late wet | Early dry | Late dry |
|---|---|---|---|---|---|
| Alluaudia procera | L | 27.9 | 23.8 | 34.7 | 36.3 |
| Metaporana parvifolia | L | 7.5 | 14.4 | 18.5 | 6.6 |
| Alantsilodendron alluaudianum | L | 12.0 | 4.2 | 10.2 | |
| Alluaudia procera | Fl | 6.9 | |||
| Paederia sp. | L | 8.5 | 15.7 | 5.9 | |
| Grewia grevei | L | 5.0 | 3.1 | ||
| Commiphora humbertii | L | 4.7 | |||
| Salvadora angustifolia | L | 7.3 | |||
| Commiphora orbicularis | L | 4.3 |
Competitive Regime
We observed displacement from food patches of an adult individual by another adult individual belonging to the same social unit only three times during 524.2 hr of observation where the focal animal was in sight, resulting in an average rate of 0.006 displacements/hr. In all three cases, it was a female who displaced a male from the food patch. Furthermore, displacements from food trees were never observed between individuals belonging to different social units. Across all seasons, a pair used on average 37 ± 9 food patches (N = 52 simultaneous observation nights). Of these food patches, an average of only 3 ± 2 (or 8 ± 5%) were used by both members of a pair during the same night. On only four occasions did we see adult individuals foraging simultaneously in the same food patch. We never saw individuals belonging to different social units feeding simultaneously in the same food patch. We identified on average 189 ± 21 (N = 7) food patches within a single territory across all four seasons. Only 32 ± 8 (or 17 ± 5%) of these were used by both adults of a social unit. Of the total of 1,320 food patches only five were used by individuals from different social units, in each case neighboring females.
Seasonal Influences
Season did not have a significant effect on the amount of food patches that were used by both pair-partners (MLM: χ2(3) = 1.36, P = 0.71), on their average cohesion (MLM: χ2(3) = 0.49, P = 0.92), on space-use sharing (i.e., UDOI) of core areas (MLM: χ2(3) = 0.67, P = 0.88), or on their average rates of agonism (Friedman's ANOVA: χ2(3) = 5.49, P = 0.14; Table3). However, season did have a significant effect on the amount of time animals spent resting (MLM: χ2(3) = 16.24, P = 0.001), eating (χ2(3) = 12.72, P = 0.01) and traveling (χ2(3) = 20.25, P < 0.01; Fig. 2). Time spent resting was significantly higher during the early dry, compared to the late wet season (Tukey's post hoc test: Z = 2.967, P = 0.02). In addition, the animals spent significantly less time resting during the late dry compared to the early dry season (Z = −4.283, P < 0.01). The animals spent significantly more time eating during the late dry, compared to the early wet (Z = 2.767, P = 0.03) and early dry season (Z = 3.387, P < 0.01). The animals traveled significantly less during the early dry compared to the early wet (Z = −5.066, P < 0.01) and late wet season (Z = −2.906, P = 0.02) as well as significantly less during the late dry compared to the early wet season (Z = −2.746, P = 0.03).
TABLE III.
Seasonal Values (Average ± SD) for the Percentage of Food Patches Used by Both Pair-Partners, for Cohesion Measured as Average Distance Between Pair-Partners, for Space-Use Sharing of Core Areas by Pair-Partners Based on UDOI, and for the Rate of Agonistic Interactions Between Pair Partners (N = 7)
| Season | Food patch sharing (%) | Cohesion (m) | UDOI of core area | Agonistic interactions/h |
|---|---|---|---|---|
| Early wet | 8.98 ± 2.28 | 33.92 ± 10.00 | 0.12 ± 0.08 | 0.08 ± 0.08 |
| Late wet | 7.32 ± 4.42 | 33.39 ± 5.81 | 0.10 ± 0.05 | 0.05 ± 0.05 |
| Early dry | 8.29 ± 3.97 | 33.77 ± 4.54 | 0.12 ± 0.07 | 0.01 ± 0.02 |
| Late dry | 7.00 ± 4.17 | 34.99 ± 8.31 | 0.10 ± 0.06 | 0.01 ± 0.02 |
Figure 2.
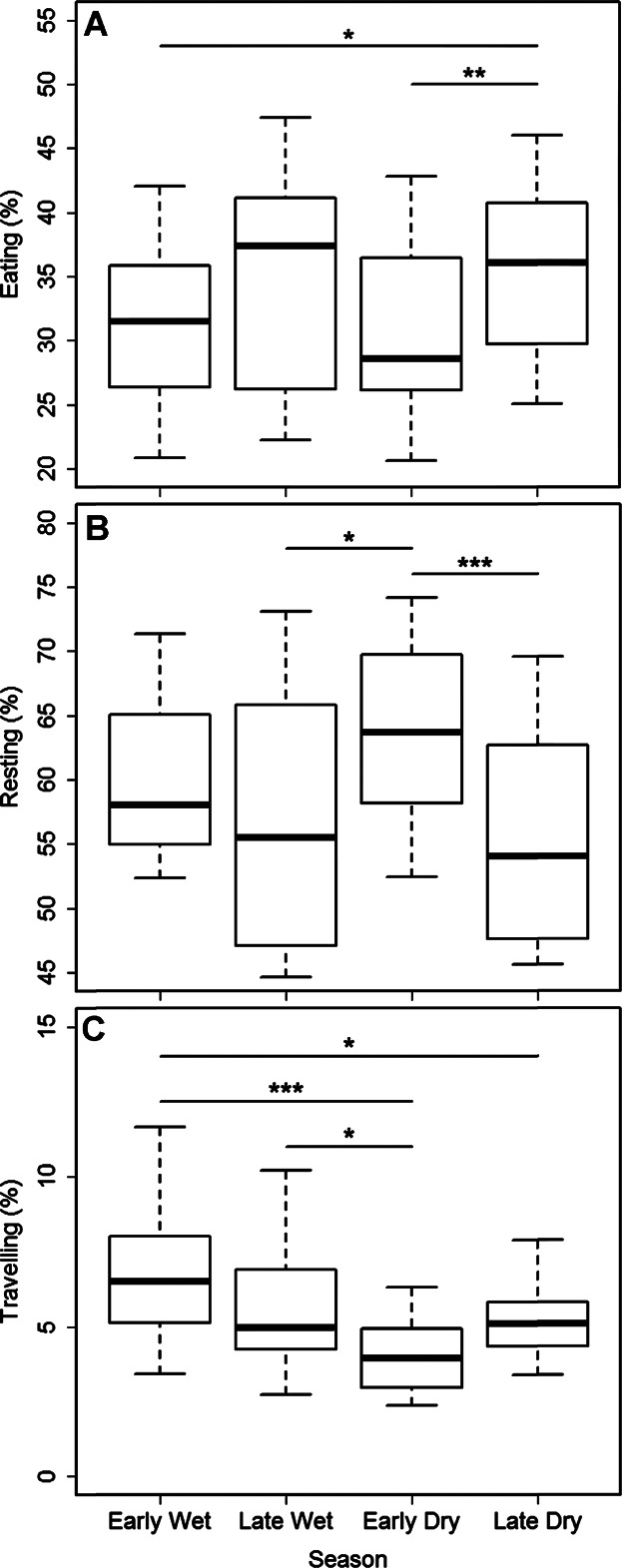
Boxplots showing medians and quartiles of the proportions of total observation time Lepilemur leucopus spent eating (A), resting (B) and traveling (C) across four seasons (N = 14; multilevel modeling (MLM) for repeated measures, *P < 0.05, **P < 0.01, ***P < 0.001).
Predation Pressure
Between October 2011 and August 2013, we recorded the death or disappearance of 9 of 21 individually known animals. In two cases we found the dead body without signs of external injury. In four cases we could assign the death of the individual to predation. On several occasions we observed introduced African wild cats (Felis silvestris) to approach or attack L. leucopus. We found the remains (guts, head and tail) of one victim, and characteristic tooth marks on the radio-collars of three disappeared individuals. In three additional cases animals disappeared and were never re-sighted. Therefore, during a period of about 2 years the disappearance of at least 19% and perhaps up to 33% of the study animals could be attributed to predation.
DISCUSSION
Competitive Regime
Contest feeding competition between neighboring social units of L. leucopus is presumably very weak, as we did not observe any displacements from food patches. Similarly, within social units, displacements from food patches occurred at a negligible rate. When they occurred, it was the female that displaced the male, which may indicate female dominance, a widespread characteristic of other lemurs [Kappeler, 1993]. Scramble competition between social units of L. leucopus was also not pronounced, as a negligible number of food patches was used by individuals of neighboring social units, perhaps because adjacent home ranges overlap little [Dröscher & Kappeler, 2013]. Finally, the number of shared food patches within pairs was also low, despite extensive mutual home range overlap.
Solitary foraging per se cannot explain the absence of feeding competition in L. leucopus as other species of solitary foragers were found to experience feeding competition. The competitive regime of M. berthae, for example, is characterized by within-group scramble competition, whereas the competitive regime of M. murinus is additionally characterized by between-group contest [Dammhahn & Kappeler, 2009]. Microcebus berthae mainly feeds on the secretion of homopteran larvae, which occur in small dispersed patches that can be depleted by a single individual, whereas M. murinus spends a substantial amount of time foraging on gum and fruit trees, which are large, high-quality resources that can be monopolized. In M. berthae, females that overlap with many other females have larger home ranges and range farther than females that overlap with fewer other females. In M. murinus displacement from high-quality resource patches occurs, but aggression is mainly targeted at individuals that are not part of female sleeping associations.
The competitive regime of P. pallescens includes strong within-group scramble and contest competition [Schülke, 2003]. The most important food species is a relatively rare gum-producing tree, and the majority of the trees are used by both pair-partners. Direct contest for food is reflected by a high rate of agonistic inter-sexual interactions. Females displace males from food trees, and avoidance of direct competition is achieved by differential timing of resource use. Physical condition of females is negatively correlated with family size, indicating strong within-group scramble competition. In contrast, L. leucopus relied on common tree and liana species, similar to L. edwardsi [Thalmann, 2001].
Theory suggests that high selectivity for uncommon food items distributed in clumped patches creates the potential for food competition [Grueter et al., 2009], while competition can be expected to be low when the diet is based on abundant and evenly distributed food resources [Terborgh & Janson, 1986]. Based on these principles, the absence of competition in L. leucopus might be explained by their low selectivity in dietary choice, as they primarily used the most abundant plant species.
Seasonality
Despite seasonal variation in food availability, with a minimum of food availability during the late dry season, season affected neither the intensity of competition nor the nature of social interactions in L. leucopus. This pattern contrasts with that seen in spectral tarsiers (Tarsius spectrum), in which a seasonal decrease in food abundance leads to decreased cohesion between family members and increased territorial disputes [Gursky, 2000]. This finding, together with the overall low rates of competition regardless of season, suggests that feeding competition cannot be regarded as a dominant ecological pressure in L. leucopus, at least at this site in years with average, or better, rainfall.
A steep decline in food availability can lead to physiological and behavioral costs in folivorous primates [Harris et al., 2010], and one may expect adaptive strategies such as dietary specialization, ranging and/or physiological adaptations to overcome periods of food scarcity [Hemingway & Bynum, 2005]. Lepilemur leucopus did not switch to a different diet during the late dry season; instead, it fed predominantly on leaves of A. procera regardless of season. Similarly, food choice and dietary diversity were similar during the wet to the dry season in Lepilemur petteri [Nash, 1998]. Lepilemur leucopus minimized its energy expenditure during the early dry season by resting more and traveling less. At this time of year, temperatures reached a minimum, but leaves were still abundant. During the late dry season, when temperatures increased again but food availability reached a low, activity returned to pre-dry season levels, indicating that L. leucopus might be seasonally more affected by cold stress than by food limitation. Likewise, L. petteri conserved energy during the cool dry season by resting more and travelling less [Nash, 1998], and L. ruficaudatus did not experience energetic constraints due to restricted food supply during the dry season as indicated by their body condition [Ganzhorn, 2002]. Thus, L. leucopus may be more constrained by food quality than abundance. In addition, sportive lemurs are characterized by low resting metabolic rates [Schmid & Ganzhorn, 1996]. Further studies investigating C-peptide levels, an indicator of energy balance, across different seasons might provide more insights into possible physiological costs of reduced food availability [Harris et al., 2010].
Resource Defense as a Male Strategy
While resource-defense is a common mating strategy among birds [Emlen & Oring, 1977], males defend resources to attract females in only a few mammalian species [Greenwood, 1980]. However, males play an important role in resource defense in several primate species [spider monkeys: Aureli et al., 2006; capuchins: Crofoot, 2007; guerezas: Fashing, 2001; bamboo lemurs: Nievergelt et al., 1998; tamarins: Peres, 1989; sakis: Thompson et al., 2012; chimpanzees: Williams et al., 2004]. For example, in frugivorous chimpanzees (Pan troglodytes), males defend a feeding territory for themselves and the resident females (Williams et al., 2004). In folivorous guerezas (Colobus guereza), intensity of intergroup aggression between adult males is related to the frequency of food patch use at intergroup encounter sites [Fashing, 2001]. In contrast, males of L. leucopus did not engage in intergroup aggression related to food resources.
Males are expected to adopt the resource defense strategy when food is limiting and distributed in defensible patches, when females are reproductively monopolizable, and when females choose to mate with males that defend resources [Fashing, 2001]. Although Lepilemur females are reproductively monopolizable, as mate-guarding is intense during the short mating season [Hilgartner et al., 2012], food is not distributed in defensible patches, as indicated by the preferential use of the most abundant plant species with random spatial distribution. Moreover, females of L. ruficaudatus were never observed to terminate a pair-bond or to try to repel a new immigrant male [Hilgartner et al., 2012]. Likewise, none of our study females was observed to transfer to another social unit. Although long-term data are required to obtain better information on the role of female choice in this species, it seems that Lepilemur males cannot achieve greater reproductive success by defending resources for females.
The resource defense hypothesis also predicts that an expansion in territory size should lead to an increase in female reproductive rates due to an increase in food availability [Williams et al., 2004]. In contrast, the mate defense hypothesis predicts that an expansion in territory size should lead instead to an increase in the number of adult females [Wrangham, 1979]. The two males in our population that defended the largest territories were associated with two females each [Dröscher & Kappeler, 2013]. These females had the smallest and the third-smallest home range, respectively, indicating that females do not necessarily benefit from male range expansion in terms of increased food availability. However, we assume that differences in territory quality are related to territory size in this species and further studies incorporating more precise measures of territory quality are required to advance our understanding of the importance of male resource defense as a mating strategy in L. leucopus.
Intersexual Spatial Avoidance
Sportive lemurs are characterized by low spatial cohesiveness, including active avoidance of pair partners [Hilgartner et al., 2012; Dröscher & Kappeler, 2013]. The observed intersexual avoidance cannot be explained by avoidance of competition over food resources, as conflicts over food resources were rarely observed, and inter-individual avoidance did not increase when food availability was low.
Diurnal primates benefit from living in cohesive groups as it provides enhanced protection against predation [Dehn, 1990], and groups are larger and more cohesive where individuals are exposed to a high predation risk [Clutton-Brock & Janson, 2012]. While diurnal social primates rely on early detection and warning of approaching predators, solitariness and crypsis is a viable alternative anti-predator strategy for solitary foragers [Terborgh & Janson, 1986]. The fossa is a specialist predator of lemurs [Dollar et al., 2007; Karpanty & Wright, 2007], and half its prey items are lemurs with a high prevalence of medium-sized nocturnal species [Hawkins & Racey, 2008]. While fossas are no longer present at the study site, we could confirm the presence of African wild cats, which have been observed to prey upon much larger Verreaux's sifaka [Propithecus verreauxi; Brockman et al., 2008].
Predation poses an important ecological pressure for L. leucopus as mortality rates due to predation were high. Similarly, predation rate on L. ruficaudatus was 36% during a 4-year study [Hilgartner et al., 2008]. Sportive lemurs produce alarm calls only when predators directly attack them [Fichtel, 2007]. The lack of an early warning system against predators seems to be a reason why sportive lemurs do not spend more time together [Fichtel et al., 2011]. Thus, anti-predator benefits of crypsis may explain intersexual spatial avoidance of pair partners as this strategy decreases conspicuousness. However, L. leucopus produces loud calls to communicate with pair-partners and/or neighbors. As L. leucopus are highly territorial, a trade-off between the need to signal their presence in their territory [Fichtel & Hilgartner, 2013; Rasoloharijaona et al., 2006] and the need to avoid detection by predators seems to exist. Further studies should investigate if and how individuals adjust their loud-calling behavior during times of potentially heightened predation risk such as during the dry season when crown coverage is reduced, or during full moon when ambient light levels are increased.
Intrasexual Spatial Avoidance
The spatio-temporal distribution of females is one of the main aspects underlying variation among mating and social systems [Arnold & Duvall, 1994], and spatial dispersion of females appears to be the best predictor of pair-living in mammals [Komers & Brotherton, 1997; Lukas & Clutton-Brock, 2013]. High levels of female intrasexual avoidance are indicated by a virtual absence of home range overlap in L. leucopus [Dröscher & Kappeler, 2013]. According to Koenig et al. [2013], possible determinants of female spatial dispersion are anti-predator benefits of crypsis [Clutton-Brock & Janson, 2012], the dependence on non-divisible resources [Schülke & Kappeler, 2003] or a low abundance of large resources [Delgado & van Schaik, 2000].
While an anti-predator strategy based on crypsis may explain at least partly female spatial avoidance in L. leucopus, other factors may be important as well. Female reproductive success is generally limited by access to resources [Emlen & Oring, 1977]. When food is spatially clumped, females are expected to be more aggregated and less territorial, as food resources are not economically defendable. In contrast, when food is spatially dispersed, it can be expected that interactions among females are decreased and the costs of home range defense are reduced, and that females are more territorial [Maher and Lott, 2000; Schubert et al., 2009]. As we included only food patches that were actually visited in our nearest-neighbor analyses, the results presented above are biased against finding random patterns. Nevertheless, in most cases the main food resources showed a random or even distribution. To examine in more detail whether scramble competition for food reduces the potential for female association, future studies on the effects of territory size and quality on female reproductive success of females would be required [Koenig, 2002].
CONCLUSIONS
Competitive costs of feeding competition were negligible within and between social units of L. leucopus, presumably due to low dietary selectivity and reliance on the most common food species. As seasonal food scarcity was not reflected by feeding competition, L. leucopus is ecologically more constrained by food quality than quantity. Pair-living in this species is probably not the result of male resource defense. Intersexual avoidance between pair-partners is best explained by anti-predator benefits related to crypsis. The factors favoring female-female avoidances may include crypsis-related anti-predator benefits and feeding competition, but they could not be conclusively identified by the present study.
Acknowledgments
We wish to thank the family de Heaulme for the permission to study the sportive lemurs and for accommodation at Berenty Nature Reserve. We thank Dr. Daniel Rakotondravony, Dr. Rodin Rasoloarison and the other members of the Département Biologie Animale de l'Université d'Antananarivo for their cooperation and administrative support. We are grateful to blow-darting expert Mamitiana Razafindrasamba as well as Mahamaro and Jo from Berenty for help during animal capture. We wish to thank Rina Razafindraibe for help during vegetation mapping. We are thankful to field assistant Mahefa Mbeloamanitra Rahajarivony for continued assistance during data collection, Hajarimanitra Rambeloarivony for logistic support in the field as well as Sonomena for daily care. We wish to thank two anonymous reviewers and Marina Cords for helpful comments on an earlier version of the manuscript. The research permit was provided by Commission Tripartite CAFF (Madagascar).
Appendix A
Summary of continuous focal animal observations conducted simultaneously on adult male and female range-mates of L. leucopus throughout the year (Season: 1 = early wet, 2 = late wet, 3 = early dry, 4 = late dry). The calendar date is given as day-month-year.
| Social Unit | Date | Season | Female | Hours | Male | Hours |
|---|---|---|---|---|---|---|
| 1 | 05.11.2011 | 1 | f1B | 10:20:39 | m1B | 10:11:48 |
| 1 | 08.01.2012 | 1 | f1B | 9:45:12 | m2 | 8:26:21 |
| 1 | 21.02.2012 | 2 | f1B | 10:21:18 | m2 | 10:31:47 |
| 1 | 05.06.2012 | 3 | f1B | 12:02:42 | m10 | 11:52:59 |
| 1 | 11.07.2012 | 3 | f1B | 11:14:51 | m10 | 11:42:50 |
| 1 | 26.08.2012 | 4 | f1B | 11:19:59 | m10 | 11:12:35 |
| 1 | 04.10.2012 | 4 | f1B | 10:23:12 | m10 | 10:26:52 |
| 2 | 23.10.2011 | 1 | f2 | 10:29:55 | m2 | 10:35:38 |
| 2 | 05.12.2011 | 1 | f2 | 9:55:23 | m9 | 10:27:46 |
| 2 | 06.02.2012 | 2 | f2 | 10:07:07 | m9 | 9:59:52 |
| 2 | 23.03.2012 | 2 | f2 | 11:06:12 | m9 | 11:17:18 |
| 2 | 09.05.2012 | 3 | f2 | 11:36:48 | m9 | 12:10:32 |
| 2 | 27.06.2012 | 3 | f2 | 12:08:50 | m9 | 12:27:34 |
| 2 | 17.08.2012 | 4 | f2 | 11:18:55 | m9 | 11:56:01 |
| 2 | 26.09.2012 | 4 | f2 | 10:47:48 | m9 | 10:56:07 |
| 3 | 23.12.2011 | 1 | f3 | 9:33:38 | m3 | 10:22:11 |
| 3 | 31.03.2012 | 2 | f3 | 11:00:27 | m3 | 11:27:25 |
| 3 | 04.05.2012 | 3 | f3 | 11:40:27 | m3 | 11:53:50 |
| 3 | 23.06.2012 | 3 | f3 | 11:47:57 | m3 | 12:01:37 |
| 3 | 01.08.2012 | 4 | f3 | 11:20:53 | m3 | 11:25:55 |
| 3 | 12.09.2012 | 4 | f3 | 10:31:20 | m3 | 11:25:36 |
| 4 | 13.12.2011 | 1 | f4 | 8:59:38 | m4 | 9:19:01 |
| 4 | 25.01.2012 | 1 | f4 | 9:57:12 | m4 | 10:23:16 |
| 4 | 19.03.2012 | 2 | f4 | 11:07:14 | m4 | 11:53:16 |
| 4 | 30.04.2012 | 2 | f4 | 11:43:01 | m4 | 11:57:32 |
| 4 | 18.06.2012 | 3 | f4 | 12:07:22 | m4 | 12:35:29 |
| 4 | 25.07.2012 | 3 | f4 | 11:00:38 | m4 | 11:47:10 |
| 4 | 08.09.2012 | 4 | f4 | 11:03:23 | m4 | 11:19:02 |
| 4 | 18.10.2012 | 4 | f4 | 10:24:15 | m4 | 10:35:59 |
| 5 | 23.11.2011 | 1 | f5 | 9:57:44 | m5 | 9:56:24 |
| 5 | 03.01.2012 | 1 | f5 | 9:35:30 | m5 | 9:38:24 |
| 5 | 25.02.2012 | 2 | f5 | 10:29:40 | m5 | 10:37:44 |
| 5 | 09.04.2012 | 2 | f5 | 11:26:57 | m5 | 11:12:07 |
| 5 | 10.06.2012 | 3 | f5 | 12:15:05 | m5 | 12:35:24 |
| 5 | 15.07.2012 | 3 | f5 | 12:08:52 | m5 | 12:00:20 |
| 5 | 30.08.2012 | 4 | f5 | 11:27:43 | m5 | 10:52:24 |
| 5 | 08.10.2012 | 4 | f5 | 10:41:58 | m5 | 10:44:01 |
| 6 | 09.12.2011 | 1 | f6 | 9:44:33 | m6 | 9:38:39 |
| 6 | 10.02.2012 | 2 | f6 | 10:22:28 | m6 | 9:34:37 |
| 6 | 27.03.2012 | 2 | f6 | 11:21:19 | m6 | 11:22:33 |
| 6 | 30.05.2012 | 3 | f6 | 12:26:08 | m6 | 12:17:21 |
| 6 | 02.07.2012 | 3 | f6 | 12:21:02 | m6 | 12:07:54 |
| 6 | 21.08.2012 | 4 | f6 | 11:32:52 | m6 | 11:19:12 |
| 6 | 30.09.2012 | 4 | f6 | 12:00:46 | m6 | 10:53:25 |
| 7 | 18.11.2011 | 1 | f7 | 9:29:35 | m7 | 9:57:00 |
| 7 | 21.01.2012 | 1 | f7 | 9:36:58 | m7 | 9:50:44 |
| 7 | 04.03.2012 | 2 | f7 | 10:20:57 | m7 | 10:40:44 |
| 7 | 25.04.2012 | 2 | f7 | 11:46:32 | m7 | 11:47:35 |
| 7 | 14.06.2012 | 3 | f7 | 12:27:43 | m7 | 12:41:50 |
| 7 | 20.07.2012 | 3 | f7 | 11:53:03 | m7 | 12:24:17 |
| 7 | 03.09.2012 | 4 | f7 | 10:43:39 | m7 | 11:21:36 |
| 7 | 12.10.2012 | 4 | f7 | 10:42:20 | m7 | 10:39:13 |
REFERENCES
- Arnold SJ, Duvall D. Animal mating systems—a synthesis based on selection theory. American Naturalist. 1994;143:317–348. [Google Scholar]
- Aureli F, Schaffner CM, Verpooten J, Slater K, Ramos-Fernandez G. Raiding parties of male spider monkeys: insights into human warfare. American Journal of Physical Anthropology. 2006;497:486–497. doi: 10.1002/ajpa.20451. [DOI] [PubMed] [Google Scholar]
- Balmford A, Rosser AM, Albon SD. Correlates of female choice in resource-defending antelope. Behavioral Ecology and Sociobiology. 1992;31:107–114. [Google Scholar]
- Borries C, Larney E, Lu A, Ossi K, Koenig A. Costs of group size: lower developmental and reproductive rates in larger groups of leaf monkeys. Behavioral Ecology. 2008;19:1186–1191. [Google Scholar]
- Brockman DK, Godfrey LR, Dollar LJ, Ratsirarson J. Evidence of invasive Felis silvestris predation on Propithecus verreauxi at Beza Mahafaly Special Reserve, Madagascar. International Journal of Primatology. 2008;29:135–152. [Google Scholar]
- Calenge C. The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling. 2006;197:516–519. [Google Scholar]
- Clutton-Brock T, Janson C. Primate socioecology at the crossroads: past, present, and future. Evolutionary Anthropology. 2012;21:136–150. doi: 10.1002/evan.21316. [DOI] [PubMed] [Google Scholar]
- Crofoot M. Mating and feeding competition in white-faced capuchins (Cebus capucinus): the importance of short- and long-term strategies. Behaviour. 2007;144:1473–1495. [Google Scholar]
- Dammhahn M, Kappeler PM. Females go where the food is: does the socio-ecological model explain variation in social organisation of solitary foragers. Behavioral Ecology and Sociobiology. 2009;63:939–952. [Google Scholar]
- Dehn MM. Vigilance for predators: detection and dilution effects. Behavioral Ecology and Sociobiology. 1990;26:337–342. [Google Scholar]
- Delgado RA, van Schaik CP. The behavioral ecology and conservation of the orangutan (Pongo pygmaeus): a tale of two islands. Evolutionary Anthropology. 2000;9:201–218. [Google Scholar]
- De Solla SR, Bonduriansky R, Brooks RJ. Eliminating autocorrelation reduces biological relevance of home range estimates. Journal of Animal Ecology. 1999;68:221–234. [Google Scholar]
- Dollar L, Ganzhorn JU, Goodman SM. Primates and other prey in the seasonally variable diet of Cryptoprocta ferox in the dry deciduous forest of western Madagascar. In: Gursky-Doyen S, Nekaris KAI, editors. Primate anti-predator strategies development. New York, NY: Springer; 2007. pp. 63–76. [Google Scholar]
- Dröscher I, Kappeler PM. Defining the low end of primate social complexity: the social organization of the nocturnal white-footed sportive lemur (Lepilemur leucopus. International Journal of Primatology. 2013;34:1224–1243. doi: 10.1007/s10764-013-9735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Fashing P. Male and female strategies during intergroup encounters in guerezas (Colobus guereza): evidence for resource defense mediated through males and a comparison with other primates. Behavioral Ecology and Sociobiology. 2001;50:219–230. [Google Scholar]
- Fichtel C. Avoiding predators at night: antipredator strategies in red-tailed sportive lemurs (Lepilemur ruficaudatus. American Journal of Primatology. 2007;69:611–624. doi: 10.1002/ajp.20363. [DOI] [PubMed] [Google Scholar]
- Fichtel C, Hilgartner R. Noises in the dark: vocal communication in Lepilemur ruficaudatus and other nocturnal pair-living primates. In: Masters J, Gamba M, Genin F, editors. Leaping ahead. Advances in prosimian biology. New York, NY: Springer; 2013. pp. 297–304. [Google Scholar]
- Fichtel C, Zucchini W, Hilgartner R. Out of sight but not out of mind? Behavioral coordination in red-tailed sportive lemurs (Lepilemur ruficaudatus. International Journal of Primatology. 2011;32:1383–1396. doi: 10.1007/s10764-011-9551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieberg J, Kochanny CO. Quantifying home-range overlap: the importance of the utilization distribution. Journal of Wildlife Management. 2005;69:1346–1359. [Google Scholar]
- Field A, Miles J, Field Z. Discovering statistics using R. Los Angeles, CA: Sage; 2012. [Google Scholar]
- Ganzhorn JU. Distribution of a folivorous lemur in relation to seasonally varying food resources: integrating quantitative and qualitative aspects of food characteristics. Oecologia. 2002;131:427–435. doi: 10.1007/s00442-002-0891-y. [DOI] [PubMed] [Google Scholar]
- Ganzhorn JU. Habitat description and phenology. In: Setchell JM, Curtis DJ, editors. Field and laboratory methods in primatology. Cambridge, UK: Cambridge University Press; 2003. pp. 40–56. [Google Scholar]
- Goodman SM, O'Connor S, Langrand O. A review of predation on lemurs: implications for the evolution of social behavior in small, nocturnal primates. In: Kappeler PM, Ganzhorn JU, editors. Lemur social systems and their ecological basis. New York, NY: Plenum Press; 1993. pp. 51–66. [Google Scholar]
- Greenwood PJ. Mating systems, philopatry and dispersal in birds and mammals. Animal Behaviour. 1980;28:1140–1162. [Google Scholar]
- Grueter CC, Li D, Ren B, Wei F, van Schaik CP. Dietary profile of Rhinopithecus bieti and its socioecological implications. International Journal of Primatology. 2009;30:601–624. doi: 10.1007/s10764-009-9363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursky S. Effect of seasonality on the behavior of an insectivorous primate, Tarsius spectrum. International Journal of Primatology. 2000;21:477–495. [Google Scholar]
- Harris TR, Chapman CA, Monfort SL. Small folivorous primate groups exhibit behavioral and physiological effects of food scarcity. Behavioral Ecology. 2010;21:46–56. [Google Scholar]
- Hawkins CE, Racey PA. Food habits of an endangered carnivore, Cryptoprocta ferox, in the dry deciduous forests of western Madagascar. Journal of Mammalogy. 2008;89:64–74. [Google Scholar]
- Hemingway C, Bynum N. The influence of seasonality on primate diet and ranging. In: Brockman D, van Schaik C, editors. Seasonality in primates: studies of living and extinct human and non-human primates. Cambridge, UK: Cambridge University Press; 2005. pp. 57–104. [Google Scholar]
- Hilgartner R, Fichtel C, Kappeler PM, Zinner D. Determinants of pair-living in red-tailed sportive lemurs (Lepilemur ruficaudatus. Ethology. 2012;118:466–479. doi: 10.1111/j.1439-0310.2012.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgartner R, Zinner D, Kappeler PM. Life history traits and parental care in Lepilemur ruficaudatus. American Journal of Primatology. 2008;70:2–11. doi: 10.1002/ajp.20410. [DOI] [PubMed] [Google Scholar]
- Hladik CM, Charles-Dominique P. The behavior and ecology of the sportive lemur (Lepilemur mustelinus) in relation to its dietary peculiarities. In: Martin RD, Doyle GA, Walker AC, editors. Prosimian biology. London, UK: Duckworth; 1974. pp. 23–37. [Google Scholar]
- Hooge PN, Eichenlaub B. Animal movement extension to arcview Version 1.1. Anchorage, AK: Alaska Science Center—Biological Science Office, U.S. Geological Survey; 1997. [Google Scholar]
- Isbell LA. Contest and scramble competition: patterns of female aggression and ranging behavior among primates. Behavioral Ecology. 1991;2:143–155. [Google Scholar]
- Jolly A, Dobson A, Rasamimanana HM, et al. Demography of Lemur catta at Berenty Reserve, Madagascar: effects of troop size, habitat and rainfall. International Journal of Primatology. 2002;23:327–353. [Google Scholar]
- Jolly A, Koyama N, Rasamimanana H, Crowley H, Williams G. Berenty Reserve: a research site in southern Madagascar. In: Jolly A, Sussman R, Koyama N, Rasamimanana H, editors. Ringtailed lemur biology: Lemur catta. New York, NY: Springer Press; 2006. pp. 32–42. in Madagascar. [Google Scholar]
- Kappeler PM. Female dominance in primates and other mammals. In: Bateson PPG, Klopfer PH, Thompson NS, editors. Perspectives in ethology. Vol. 10: behavior and evolution. New York, NY: Plenum Press; 1993. pp. 143–158. [Google Scholar]
- Karpanty SM, Wright PC. Predation on lemurs in the rainforest of Madagascar by multiple predator species: observations and experiments. In: Gursky-Doyen S, Nekaris KAI, editors. Primate anti-predator strategies development. New York, NY: Springer; 2007. pp. 77–99. [Google Scholar]
- Koenig A. Competition for resources and its behavioral consequences among female primates. International Journal of Primatology. 2002;23:759–783. [Google Scholar]
- Koenig A, Beise J, Chalise MK, Ganzhorn JU. When females should contest for food—testing hypotheses about resource density, distribution, size, and quality with Hanuman Langurs (Presbytis entellus. Behavioral Ecology and Sociobiology. 1998;42:225–237. [Google Scholar]
- Koenig A, Scarry CJ, Wheeler BC, Borries C. Variation in grouping patterns, mating systems and social structure: what socio-ecological models attempt to explain. Philosophical Transactions of the Royal Society of London Series B. 2013;368:20120348. doi: 10.1098/rstb.2012.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komers PE, Brotherton PN. Female space use is the best predictor of monogamy in mammals. Philosophical Transactions of the Royal Society of London Series B. 1997;264:1261–1270. doi: 10.1098/rspb.1997.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341:526–530. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- Maher CR, Lott DF. A review of ecological determinants of territoriality within vertebrate species. American Midland Naturalist. 2000;143:1–29. [Google Scholar]
- Méndez-Cárdenas MG, Zimmermann E. Duetting—a mechanism to strengthen pair bonds in a dispersed pair-living primate (Lepilemur edwardsi. American Journal of Physical Anthropology. 2009;139:523–532. doi: 10.1002/ajpa.21017. [DOI] [PubMed] [Google Scholar]
- Nash LT. Vertical clingers and sleepers: seasonal influences on the activities and substrate use of Lepilemur leucopus at Beza Mahafaly Special Reserve, Madagascar. Folia Primatologica. 1998;69:204–217. [Google Scholar]
- Nievergelt CM, Mutschler T, Feistner AT. Group encounters and territoriality in wild Alaotran gentle lemurs (Hapalemur griseus alaotrensis. American Journal of Primatology. 1998;46:251–258. doi: 10.1002/(SICI)1098-2345(1998)46:3<251::AID-AJP5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Norscia I, Palagi E. Berenty 2006: census of Propithecus verreauxi and possible evidence of population stress. International Journal of Primatology. 2008;29:1099–1115. [Google Scholar]
- Parker GA. Assessment strategy and the evolution of fighting behaviour. Journal of Theoretical Biology. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. [DOI] [PubMed] [Google Scholar]
- Pereira ME, Kappeler PM. Divergent systems of agonistic behaviour in lemurid primates. Behaviour. 1997;134:225–274. [Google Scholar]
- Peres CA. Costs and benefits of territorial defense in wild golden lion tamarins, Leontopithecus rosalia. Behavioral Ecology. 1989;25:227–233. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D Development Core Team. 2013. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3. 1–113.
- Rasoloarison RM, Rasolonandrasana BPN, Ganzhorn JU, Goodman SM. Predation on vertebrates in the Kirindy Forest, western Madagascar. Ecotropica. 1995;1:59–65. [Google Scholar]
- Rasoloharijaona S, Randrianambinina B, Braune P, Zimmermann E. Loud calling, spacing, and cohesiveness in a nocturnal primate, the Milne Edwards' sportive lemur (Lepilemur edwardsi. American Journal of Physical Anthropology. 2006;129:591–600. doi: 10.1002/ajpa.20342. [DOI] [PubMed] [Google Scholar]
- R Core Development Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Rooney SM, Wolfe A, Hayden TJ. Autocorrelated data in telemetry studies: time to independence and the problem of behavioural effects. Mammal Review. 1998;28:89–98. [Google Scholar]
- Schmid J, Ganzhorn JU. Resting metabolic rates of Lepilemur ruficaudatus. American Journal of Primatology. 1996;38:169–174. doi: 10.1002/(SICI)1098-2345(1996)38:2<169::AID-AJP5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Schubert M, Pillay N, Ribble DO, Schradin C. The round-eared sengi and the evolution of social monogamy: factors that constrain males to live with a single female. Ethology. 2009;115:972–985. [Google Scholar]
- Schülke O. To breed or not to breed? Food competition and other factors involved in female breeding decisions in the pair-living nocturnal fork-marked lemur (Phaner furcifer. Behavioral Ecology and Sociobiology. 2003;55:11–21. [Google Scholar]
- Schülke O, Kappeler PM. So near and yet so far: territorial pairs but low cohesion between pair partners in a nocturnal lemur, Phaner furcifer. Animal Behaviour. 2003;65:331–343. [Google Scholar]
- Schülke O, Ostner J. Predation on a Lepilemur by a Harrier hawk and implications of sleeping site quality. Lemur News. 2001;6:5–6. [Google Scholar]
- Silverman BW. Density estimation for statistics and data analyses. London, UK: Chapman and Hall; 1986. [Google Scholar]
- Snaith TV, Chapman CA. Towards an ecological solution to the folivore paradox: patch depletion as an indicator of within-group scramble competition in red colobus monkeys (Piliocolobus tephrosceles. Behavioral Ecology and Sociobiology. 2005;59:185–190. [Google Scholar]
- Sussman RW. Primate ecology and social structure. Vol 1: lorises, lemurs and tarsiers. New York, NY: Pearson Custom Publishing; 1999. [Google Scholar]
- Terborgh J, Janson CH. The socioecology of primate groups. Annual Review of Ecology and Systematics. 1986;17:111–135. [Google Scholar]
- Thalmann U. Food resource characteristics in two nocturnal lemurs with different social behavior: Avahi occidentalis and Lepilemur edwardsi. International Journal of Primatology. 2001;22:287–324. [Google Scholar]
- Thompson CL, Whitten PL, Norconk MA. Why fight? Selective forces favoring between-group aggression in a variably pair-living primate, the white-faced saki (Pithecia pithecia. Behaviour. 2012;149:795–820. [Google Scholar]
- Vander Wal E, Rodgers AR. An individual-based quantitative approach for delineating core areas of animal space use. Ecological Modelling. 2012;224:48–53. [Google Scholar]
- Van Schaik CP, Dunbar RIM. The evolution of monogamy in large primates: a new hypothesis and some crucial tests. Behaviour. 1990;115:30–61. [Google Scholar]
- Williams JM, Oehlert GW, Carlis JV, Pusey AE. Why do male chimpanzees defend a group range. Animal Behaviour. 2004;68:523–532. [Google Scholar]
- Worton BJ. Kernel methods for estimating the utilization distribution in home-range studies. Ecology. 1989;70:164–168. [Google Scholar]
- Wrangham RW. On the evolution of ape social systems. Social Science Information. 1979;18:335–368. [Google Scholar]
- Zinner D, Hilgartner RD, Kappeler PM, Pietsch T, Ganzhorn JU. Social organization of Lepilemur ruficaudatus. International Journal of Primatology. 2003;24:869–888. [Google Scholar]


