Abstract
Background
Oral (mobile) tongue squamous cell carcinoma (SCC) is characterized by a highly variable prognosis in early-stage disease (T1/T2 N0M0). The ability to classify early oral tongue SCCs into low-risk and high-risk categories would represent a major advancement in their management.
Methods
Depth of invasion, tumor budding, histologic risk-assessment score (HRS), and cancer-associated fibroblast (CAF) density were studied in 233 cases of T1/T2 N0M0 oral tongue SCC managed in 5 university hospitals in Finland.
Results
Tumor budding (≥5 clusters at the invasive front of the tumor) and depth of invasion (≥4 mm) were associated with poor prognosis in patients with early oral tongue SCC (hazard ratio [HR], 2.04; 95% confidence interval [CI], 1.17–3.55; HR, 2.55; 95% CI, 1.25–5.20, respectively) after multivariate analysis. The HRS and CAF density did not predict survival. However, high-risk worst pattern of invasion (WPOI), a component of HRS, was also an independent prognostic factor (HR, 4.47; 95% CI, 1.59–12.51).
Conclusion
Analyzing the depth of invasion, tumor budding, and/or WPOI in prognostication and treatment planning of T1/T2 N0M0 oral tongue SCC is recommended.
Keywords: oral tongue squamous cell carcinoma, tumor budding, depth of invasion, worst pattern of invasion, histologic risk score, cancer-associated fibroblast, disease-specific mortality, prognosis
Introduction
Detection of oral (mobile) tongue squamous cell carcinoma (SCC) at an early-stage (T1/T2N0M0) does not always portend good prognosis as evidence shows that 20% to 40% already have occult metastasis at presentation.1,2 The purpose of prognostic studies in early-stage mobile tongue cancer is to identify a subset of patients who are at a risk of adverse outcome, and will therefore need a more aggressive treatment, such as multimodality therapy, in contrast with another subset who have increased chances of a favorable outcome. Local surgical treatment alone should be adequate for this latter group.3 Clinical size (T1 or T2) of early oral tongue SCC (N0) by itself has consistently failed in differentiating these 2 groups.4
The tongue has characteristic structural features including a high content of muscle bundles and a rich lymphatic network that may influence the properties of tumor spread in it. Current literature includes a number of studies hypothesizing that histomorphologic parameters may be used to prognosticate oral tongue SCC and SCC of other oral subsites and may be helpful in stratifying patients into low-risk and high-risk categories. We therefore chose those previously suggested histomorphologic parameters (depth of tumor invasion, tumor budding, histologic risk assessment score, and the density of cancer-associated fibroblasts) that are easy and practical to evaluate, and suggested as having important prognostic relevance in oral tongue SCC. All the parameters used in this study are defined in the “Methods” section.
Tumor budding is an expression of 2 properties of malignancy: loss of cellular cohesion and active invasive movement. It has been associated with poor prognosis in tongue carcinoma.5 The depth of invasion (or tumor thickness) is also a measure of tumor invasion.
Brandwein–Gensler et al6 proposed a multiparametric histologic risk assessment score (HRS) model that was reported to predict the survival of patients with T1 to T4 oral SCC and capable of differentiating high-risk and low-risk patients. Other similar models, such as those of Jakobsson et al,7 Anneroth et al,8 Bryne et al,9 and Martínez–Gimeno et al,10 have also been suggested for the same purposes. Although some studies found them useful for prognostication of oral tongue SCC,11–13 most models are either too cumbersome for use in clinical diagnostics or have not shown prognostic significance, particularly for oral tongue SCC.14–17 Consequently, we have not used these models in our study. Similarly, we also excluded tumor margins status because other studies have shown that the status of the margins does not seem to bear a strong relationship to prognosis in T1 and T2 tumors.6,18
Our group19 and others3,20 have described a strong association between the density of cancer-associated fibroblasts (CAFs; increased α-smooth muscle actin [α-SMA] immunostaining) and a higher mortality in oral tongue SCC and oral SCC. Based on our own experience, immunohistochemistry using α-SMA antibody analysis for CAFs is a simple method to include in any diagnostic or prognostic protocol.
Patients and Methods
Patients
The diagnostic histological slides of 340 patients with T1/T2 N0M0 oral tongue SCC managed between 1979 and 2009 from the University Hospitals of Helsinki, Oulu, Turku, Tampere, and Kuopio were collected from the hospitals’ archives. The criteria for inclusion of cases were as previously described15: (1) samples were from the surgical resection specimen and (2) at least 3 different interface tumor slides were available if the whole tumor was not embedded. In addition, patients must not have had any prior treatment for oral tongue SCC. One hundred seven cases did not meet these criteria and were excluded, leaving a total of 233 cases for analysis. The use of patient samples and the data inquiry were approved by the University Hospital Ethics Committees of all 5 hospitals and by the National Supervisory Authority for Welfare and Health (VALVIRA).
Tumor budding, depth of invasion, and histologic risk assessment score
All samples were evaluated in the light microscope independently by 2 investigators (A.A. and I.O.B.), and then jointly for consensus. A critical review of all cases was then carried out together with an experienced head and neck pathologist (I.L.). All 3 investigators were blinded to the clinicopathologic data at the time of the evaluation. Before the evaluation process, a pilot study of 30 cases had been carried out to standardize the evaluation criteria for the 3 investigators (coordinated by I.L.).
Depth of invasion was measured from the surface of the tumor to the deepest point of invasive tumor in paraffin-embedded sections.21 The cutoff point used to stratify the patients with oral tongue SCC into the low-risk and high-risk tumors was 4 mm.22
Tumor budding was defined as a single cancer cell or a cluster of <5 cancer cells in the stroma of the invasive front.23 Tumor budding of <5 was considered low risk, whereas that ≥5 was considered high risk. Slides were scanned using a ×4 microscope objective to select areas with highest tumor budding. Budding was counted using a ×20 objective and the highest count per case was used as the score of budding5 (Figure 1).
Figure 1.
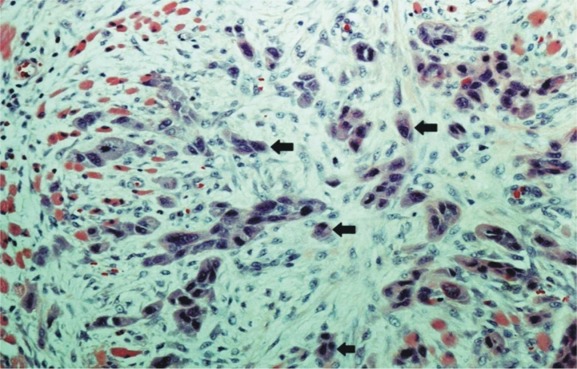
Histological appearance of tumor budding at the invasive front of early oral tongue squamous cell carcinoma (SCC); tumor budding shown by arrows as an isolated single cancer cell or a cluster composed of <5 cancer cells. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The histologic risk assessment score was composed of the worst pattern of invasion (WPOI), lymphocytic host response (LHR), and perineural invasion (PNI), as previously described6 (Figure 2A–C). High-risk WPOI was marked by small tumor islands <15 cells and satellite tumor(s) located at least 1 mm away from the main tumor or the nearest satellite. This was in contrast to low-risk WPOI, which comprised tumors with pushing borders, finger-like growth, or large cohesive invasion. Presence of large PNI and little or no LHR were also considered high risk.
Figure 2.
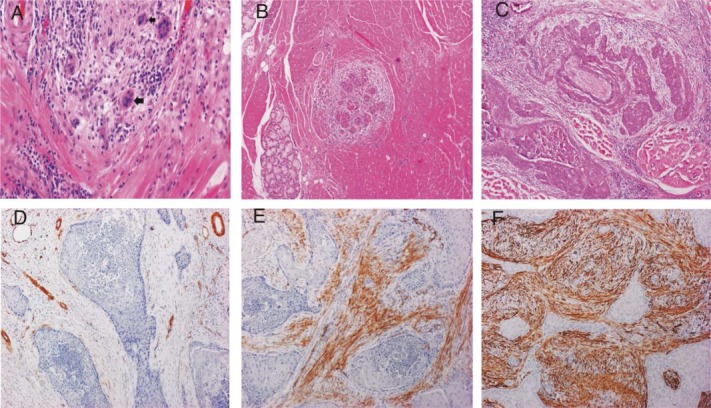
Histologic risk assessment model, (A) worst pattern of invasion type 4 associated with weak lymphocytic host response (type 3); (B) worst pattern of invasion type 5 (tumor satellite); and (C) perineural invasion (small nerve). Cancer associated fibroblasts, (D) poor; (E) medium; and (F) rich. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Immunohistochemical staining for α-SMA was performed and scored as previously described.19 Each tumor received the score according to its most intensive staining (Figure 2D–F). For immunohistochemistry, new slides had to be prepared, and the blocks of only 82 cases were available for staining (T1, n = 31 and T2, n = 51).
Statistical analysis
The prognosis of patients in relation to the parameters of this study was analyzed in relation to disease-specific mortality (death from oral tongue SCC), and mortality resulting from other causes of death. Kaplan–Meier plots were constructed to present cumulative survival outcomes and compared using the log-rank test for both groups. The prognostic strength of each marker was assessed using Cox proportional hazard regression model. The unadjusted (univariate) model was used for single parameters. The adjusted (multivariate) model was used for those parameters considered to have a strong association with prognosis. The adjusted model was fitted with categorical covariates: the age (≤60 years vs >60 years), the sex, tumor grade, and center in which the patient was managed, to assess the independent prognostic strength of the marker. Statistical significance was set at p < .05. All statistics were done using IBM SPSS version 20.
Results
The distribution of patients by demographic and clinicopathologic factors in the 5 centers is shown in Table 1. The mean follow-up period was 67 months (range, 1–267 months). Fifty-five patients died of oral tongue SCC and 63 died of other causes, whereas 115 were alive at the end of the follow-up period.
Table 1.
Demographic and clinicopathological features of 233 patients with early oral tongue squamous cell carcinoma (T1/T2N0M0).
| Clinicopathological variable | No. of patients (%) | Oulu (n = 58) | Helsinki (n = 58) | Turku (n = 52) | Tampere (n = 37) | Kuopio (n = 28) |
|---|---|---|---|---|---|---|
| Years under review | 1985–2007 | 1993–2003 | 1998–2009 | 1997–2007 | 1979–1997 | |
| Age, y | ||||||
| ≤60 | 86 (36.9) | 18 (31.0) | 30 (51.7) | 14 (26.9) | 14 (37.8) | 10 (35.7) |
| >60 | 147 (63.1) | 40 (69.0) | 28 (48.3) | 38 (73.1) | 23 (62.2) | 18 (64.3) |
| Range | 10–95 | 26–89 | 23–91 | 25–95 | 37–89 | 10–82 |
| Median | 65 | 70.0 | 59.5 | 68.5 | 65.0 | 64 |
| Sex | ||||||
| Male | 109 (46.8) | 24 (41.4) | 29 (50.0) | 28 (53.8) | 13 (35.1) | 15 (53.6) |
| Female | 124 (53.2) | 34 (58.6) | 29 (50.0) | 24 (46.2) | 24 (64.9) | 13 (46.4) |
| Grade | ||||||
| I | 83 (35.6) | 27 (46.6) | 19 (32.8) | 9 (17.3) | 17 (45.9) | 11 (39.3) |
| II | 109 (46.8) | 29 (50.0) | 26 (44.8) | 25 (48.1) | 15 (40.5) | 14 (50.0) |
| III | 41 (17.6) | 2 (3.4) | 13 (22.4) | 18 (34.6) | 5 (13.5) | 3 (10.7) |
| Clinical stage | ||||||
| I | 113 (48.5) | 24 (41.4) | 27 (46.6) | 33 (63.5) | 16 (43.2) | 13 (46.4) |
| II | 120 (51.5) | 34 (58.6) | 31 (53.4) | 19 (36.5) | 21 (56.8) | 15 (53.6) |
| Recurrence | ||||||
| Absent | 160 (68.6) | 36 (62.1) | 41 (70.7) | 38 (73.1) | 28 (76.0) | 17 (60.7) |
| Present | 73 (31.4) | 22 (37.9) | 17 (29.3) | 14 (26.9) | 9 (24.0) | 11 (39.3) |
| Status | ||||||
| Alive | 115 (49.4) | 32 (55.2) | 25 (43.1) | 31 (59.6) | 17 (45.9) | 10 (35.7) |
| Dead of oral tongue SCC | 55 (23.6) | 9 (15.5) | 16 (27.6) | 13 (25.0) | 7 (18.9) | 10 (35.7) |
| Dead of other causes | 63 (27.0) | 17 (29.3) | 17 (29.3) | 8 (15.4) | 13 (35.2) | 8 (28.6) |
| Tumor budding | ||||||
| Low (<5 buds) | 152 (65.2) | 38 (65.5) | 36 (62.1) | 41 (78.8) | 25 (67.6) | 12 (42.9) |
| High (≥5 buds) | 81 (34.8) | 20 (34.5) | 22 (37.9) | 11 (21.2) | 12 (32.4) | 16 (57.1) |
| Tumor depth | ||||||
| Low (<4 mm) | 80 (34.3) | 20 (34.5) | 16 (27.6) | 32 (61.5) | 7 (18.9) | 5 (17.9) |
| High (≥4 mm) | 153 (65.7) | 38 (65.5) | 42 (72.4) | 20 (38.5) | 30 (81.1) | 23 (82.1) |
| Histologic risk assessment score | ||||||
| Low risk (<3) | 111 (47.6) | 35 (60.3) | 34 (58.6) | 14 (26.9) | 19 (51.4) | 9 (32.1) |
| High risk (≥3) | 122 (52.4) | 23 (39.7) | 24 (41.4) | 38 (73.1) | 18 (48.6) | 19 (67.9) |
| CAF score* | ||||||
| Low (0–1) | 27 (32.9) | 9 (24.3) | 6 (33.3) | 0 | 0 | 12 (44.4) |
| Medium (2–3) | 40 (48.8) | 23 (62.2) | 8 (44.5) | 9 (33.3) | ||
| High (4) | 15 (18.3) | 5 (13.5) | 4 (22.2) | 6 (22.2) |
Abbreviations: SCC, squamous cell carcinoma; CAF, cancer-associated fibroblast.
Only 82 cases (37 from Oulu, 18 from Helsinki, and 27 from Kuopio) were available for this analysis as some blocks no longer have tumor tissue.
Survival outcomes
Increased tumor budding and depth of invasion were strongly associated with increased oral tongue SCC-related mortality (log-rank p = .009 and .023, respectively), whereas a strong association was not found for HRS and CAF density (Figure 3). The data for the 3 constituent parameters of HRS (ie, WPOI, LHR, and PNI) were then further analyzed separately. When WPOI was divided in a 2-tiered system (score 0 as low, and scores 1 and 3 as high), there was a statistically significant association with mortality from oral tongue SCC (p = .005; Figure 4). LHR and PNI did not show a strong association with mortality from oral tongue SCC when similarly grouped in any 2-tiered system (p > .05, not shown).
Figure 3.
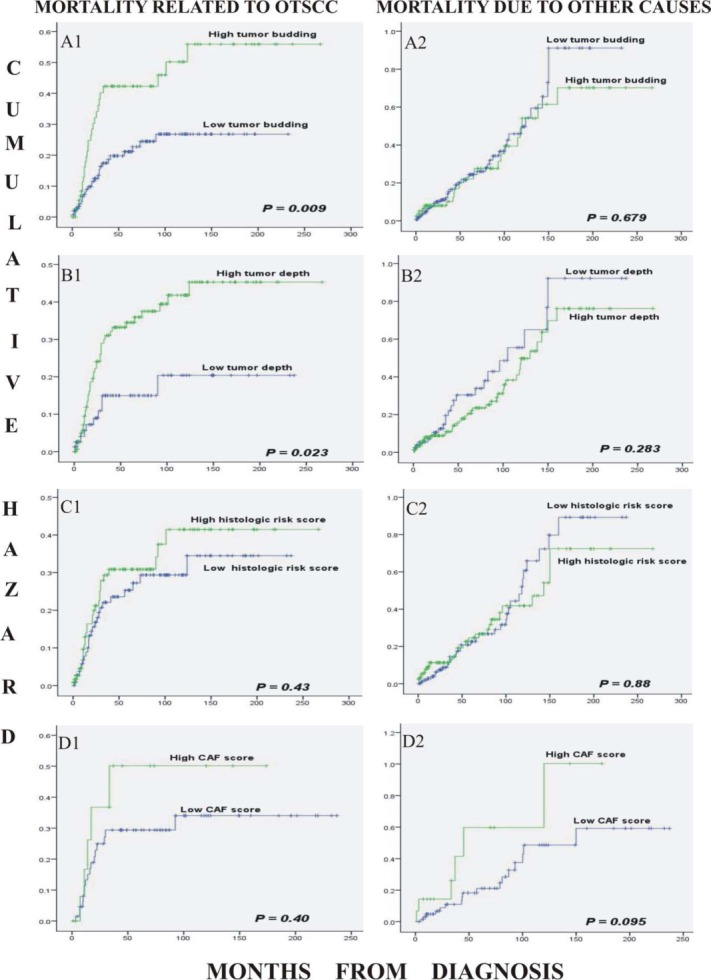
Kaplan–Meier curves describing the cumulative mortality of patients during the follow-up period from oral tongue squamous cell carcinoma (A1–D1) and from other causes of death (A2–D2). The markers were tumor budding (high = ≥5 buds; low = <5 buds; A1, A2); tumor depth (high = ≥4 mm; low = <4 mm; B1, B2); histologic risk score (low = <3; high = ≥3; C1, C2); and cancer-associated fibroblast (CAF) score (high = high CAF density; low = medium and low CAF density; D1, D2). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 4.
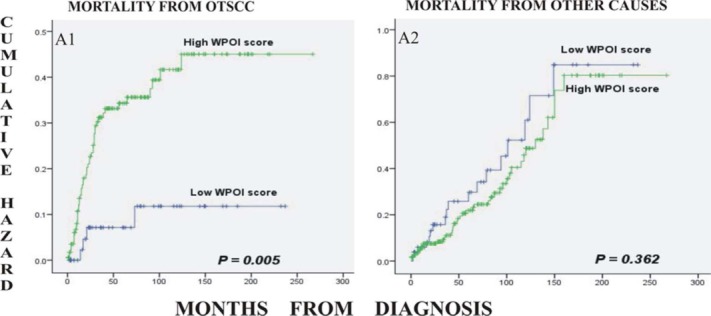
Kaplan–Meier curves for cumulative mortality of patients from oral tongue squamous cell carcinoma (SCC; A1), and from other causes (A2) in relation to the worst pattern of invasion (WPOI). The patients with high WPOI (<15 cells in an invasive island, single cells, or satellite tumor cells) were associated with a higher mortality compared to those with a low WPOI score (pushing borders, finger-like, and cohesive invasion). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In our previous study, we classified all stages of oral tongue SCC into CAF-poor, CAF-medium, and CAF-rich patterns for CAF density analysis (Table 1).19 However, because this study was aimed at dividing the patients into 2 risk groups, the analysis for CAF score was modified into a 2-tiered system combining low and intermediate scores versus high score. For comparison with our previous work,19 we reanalyzed them with the 3-tiered system (not shown). In both instances, we found no significant association (p > .05). None of the analyzed histological parameters were associated with deaths from other causes (Figure 3).
Regression analysis
In the unadjusted model, the age of the patient, tumor budding, and depth of invasion were the parameters that were positively and strongly associated with mortality from early-stage oral tongue SCC (Table 2). Analyzed separately, WPOI was also strongly associated with mortality from oral tongue SCC (Table 3). Age was the only variable that was associated with mortality from other causes and consequently further exploration of its effect was considered unnecessary. However, it was still included in the adjusted model.
Table 2.
Unadjusted (univariate) Cox proportional hazard models for all variables analyzed fitted for mortality because of oral tongue squamous cell carcinoma and mortality because of other causes.
| Mortality related to oral tongue SCC | Mortality related to other causes | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Events (55) | HR | (95% CI) | p value | Events (63) | HR | (95% CI) | p value |
| Age | ||||||||
| ≤60 | 13 | 1 | 17 | 1 | ||||
| >60 | 42 | 2.44 | 1.31–4.56 | .005 | 46 | 2.77 | 1.57–4.87 | .001 |
| Sex | ||||||||
| Male | 20 | 1 | 34 | 1 | ||||
| Female | 35 | 0.67 | 0.39–1.16 | .16 | 29 | 1.59 | 0.96–2.61 | .07 |
| Grade | ||||||||
| I | 15 | 1 | 23 | 1 | ||||
| II | 29 | 1.62 | 0.87–3.03 | .13 | 30 | 1.17 | 0.68–2.01 | .57 |
| III | 11 | 1.92 | 0.88–4.18 | .10 | 10 | 1.25 | 0.60–2.64 | .55 |
| Clinical stage | ||||||||
| I | 22 | 1 | 27 | 1 | ||||
| II | 33 | 1.50 | 0.87–2.57 | .14 | 36 | 1.42 | 0.86–2.34 | .17 |
| Centers | ||||||||
| Oulu | 9 | 1 | 17 | 1 | ||||
| Helsinki | 16 | 0.93 | 0.34–2.48 | .87 | 17 | 1.12 | 0.54–2.31 | .77 |
| Kuopio | 10 | 1.40 | 0.58–3.41 | .46 | 8 | 0.78 | 0.38–1.60 | .49 |
| Turku | 13 | 2.26 | 0.86–5.94 | .10 | 8 | 1.05 | 0.44–2.54 | .91 |
| Tampere | 7 | 2.72 | 1.07–6.92 | .035 | 13 | 1.86 | 0.73–4.73 | .19 |
| Tumor budding | ||||||||
| Low (<5) | 27 | 1 | 41 | 1 | ||||
| High (≥5) | 28 | 2.00 | 1.17–3.40 | .01 | 22 | 0.90 | 0.53–1.51 | .68 |
| Tumor depth | ||||||||
| Low (<4) | 10 | 1 | 23 | 1 | ||||
| High (≥4) | 45 | 2.17 | 1.09–4.31 | .027 | 40 | 0.76 | 0.45–1.26 | .29 |
| Histologic risk score | ||||||||
| Low risk (<3) | 25 | 1 | 33 | 1 | ||||
| High risk (≥3) | 30 | 1.24 | 0.73–2.11 | .43 | 30 | 0.96 | 0.59–1.58 | .88 |
| CAF score* | ||||||||
| Low + medium (<4) | 17 | 1 | 17 | 1 | ||||
| High (4) | 5 | 1.53 | 0.56–4.14 | .41 | 6 | 2.17 | 0.85–5.54 | .10 |
Abbreviations: SCC, squamous cell carcinoma; HR, hazard ratio; CI, confidence interval.
The figures in boldface represent significant association with mortality.
Only 82 cases were available for cancer-associated fibroblast (CAF) score analyses.
Table 3.
Analysis (unadjusted Cox proportional hazard models) of the association of the components of the histologic risk assessment score and patients’ mortality.
| Mortality related to oral tongue SCC | Mortality related to other causes | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Events (55) | HR | (95% CI) | p value | Events (63) | HR | (95% CI) | p value |
| WPOI | ||||||||
| Low | 4 | 1 | 18 | 1 | ||||
| High | 51 | 3.86 | 1.39–10.68 | 0.009 | 45 | 0.77 | 0.45–1.34 | 0.36 |
| LHR | ||||||||
| Low | 34 | 1 | 40 | 1 | ||||
| High | 21 | 1.56 | 0.87–2.79 | 0.13 | 23 | 1.86 | 1.03–3.36 | 0.04 |
| PNI | ||||||||
| None | 45 | 1 | 52 | 1 | ||||
| Present | 10 | 1.31 | 066–2.61 | 0.44 | 11 | 1.06 | 0.56–2.05 | 0.85 |
Abbreviations: SCC, squamous cell carcinoma; HR, hazard ratio; CI, confidence interval; WPOI, worst pattern of invasion; LHR, lymphocytic host response; PNI, perineural invasion.
The figures in boldface represent significant association with mortality.
In the adjusted model, increased tumor budding, depth of invasion, and WPOI retained their strong prognostic association with mortality because of oral tongue SCC (Table 4).
Table 4.
Adjusted (multivariate) Cox proportional hazard model for mortality because of oral tongue squamous cell carcinoma. Each variable was entered into a model comprising age (≤60 and >60), sex, grade, and centers where patients were managed.
| HR | 95% CI | p value | |
|---|---|---|---|
| Tumor budding | |||
| <5 | 1 | ||
| ≥5 | 2.04 | 1.17–3.55 | 0.01 |
| Tumor depth | |||
| <4 mm | 1 | ||
| ≥4 mm | 2.55 | 1.25–5.20 | 0.01 |
| WPOI | |||
| 0 (low) | 1 | ||
| 1 or 3 (high) | 4.47 | 1.59–12.51 | 0.004 |
Abbreviations: SCC, squamous cell carcinoma; HR, hazard ratio; CI, confidence interval; WPOI, worst pattern of invasion; LHR, lymphocytic host response; PNI, perineural invasion.
The figures in boldface represent significant association with mortality.
Discussion
Management of patients with oral tongue SCC is still based mainly on the clinical (TNM) staging of the patient, despite large numbers of reported histological, immunohistochemical, and molecular biomarkers in the literature.24,25 Based on our previous findings, tumor staging may be more powerful in prognostication and treatment planning for the later stages of oral tongue SCC, and much less so for the early stages (T1/T2N0M0).12 Although a recent study11 has suggested that a more relaxed approach to management (surgery without extensive ablation of margins or use of multimodality treatment) of these lesions may be as good as aggressive treatment, there is no question that a small percentage of patients with early oral tongue SCC constitute a high-risk group that will benefit from multimodality treatment.
The present study assessed the effectiveness of histomorphologic parameters in predicting the mortality of patients with early-stage oral tongue SCC. The purpose was to group the patients into a high-risk category that would benefit from multimodality treatments, and a low-risk category in which local surgical treatment would be sufficient. We found that the depth of invasion, tumor budding, and WPOI were the only parameters that independently predicted the prognosis of patients with T1/T2N0M0 oral tongue SCC, whereas we could not find such results with the HRS and density of CAFs.
The depth of invasion was an independent prognostic factor in early-stage oral tongue SCC. This observation confirms several previous findings.26,27 Although various cutoff points have been suggested in different studies, depth of invasion of 4 mm seems to be common, and the optimal point by a meta-analytic review of the subject.22 One study that reported no association between tumor thickness and prognosis was carried out on 26 patients only, and the measurements were taken from the base of the overlying epithelium.28 The negative findings from that study may be because of the difference in the method of measurement or the small sample size. To test the erratic effects of a small sample size, we analyzed 28 patients from one of our centers (Kuopio) and could not confirm a significant relationship between the depth of invasion and patient mortality (not shown).
Tumor budding has been related to the prognosis of patients in various types of epithelial cancers, such as esophageal,29 lung,30 colorectal,23 and endometrial31 carcinomas. In tongue carcinoma, a recent study involving patients with tumors of all clinical stages, and of both oral and posterior locations, found tumor budding to be an adverse prognostic parameter.5 Here, we could demonstrate that tumor budding and depth of invasion independently predicted the mortality of patients with oral tongue SCC with a T1/T2N0M0 tumor.
The HRS model studies by Brandwein–Gensler et al6,15 have been performed on a tumor material containing all clinical stages of oral SCC, similarly to those other studies that have replicated the findings.32,33 Our results on T1 to T2N0M0 oral tongue SCC indicate that the HRS in our hands offered little or no insight into the prognosis in this subset of patients. HRS did not stratify these patients into risk groups. The inclusion of T1 to T4 tumors in studies validating the model may have contributed to the positive predictive value of the model. Moreover, the inclusion of SCCs from all oral subsites together with the oropharynx, which appear biologically different from oral tongue SCC, may also have contributed to those results. However, further exploration of the components of the HRS showed that unfavorable WPOI is strongly associated with mortality. Several studies have previously confirmed the relationship of unfavorable WPOI with poor prognosis in oral SCC.34–36 A recent study on oral tongue SCC did not find any correlation of WPOI with prognosis or response to treatment, but a dense LHR was associated with complete response to therapy. This latter study comprised all stages of oral tongue SCC, in addition to using only biopsy specimens.37 These 2 factors may have influenced their findings.
In our previous study,19 we showed that high density of CAFs, as demonstrated by α-SMA immunohistochemistry, is a strong poor prognosticator in oral tongue SCC. That finding was based on a study material comprising all stages of oral tongue SCC. A review of studies on oral SCC has suggested that CAFs play a significant role in the adverse prognosis of epithelial oral cancers.38 We were unable to replicate this finding in the present cohort of patients with T1/T2N0M0 SCC. Analyzing our present finding (CAFs vs oral tongue SCC outcome) based on the 3-tier system (low, medium, and high), we noted that our cumulative hazard (Kaplan–Meier) curve followed a trend similar to the previous study but did not achieve statistical significance in the present study. Our finding showed that in T1/T2N0M0 oral tongue SCC, it is unusual to have a high density of CAFs in these tumors. It is conceivable that CAF density is a time-dependent phenomenon in oral tongue SCC as CAFs are believed to be recruited into the tumor from a variety of sources, and not only from local resting fibroblasts.39 Our previous finding might have been influenced in part by the inclusion of patients with stages III and IV oral tongue SCC.
One of the arguments against the usefulness of the conventional histological and morphological parameters is that they can be best evaluated only after the patient has undergone surgical treatment of the tumor. Therefore, the treatment already planned may in some cases have to be modified based on the results obtained from the surgical resection specimen. However, a preoperative determination of tumor depth through MRI and ultrasonography has been suggested by several groups.26,40 Measurement of tumor depth may therefore be advocated as part of a routine clinical workup of the patient. It may be helpful toward stratification of patients in early-stage oral tongue SCC and in treatment planning with further reassurance by examining the tumor budding and WPOI from the surgical specimen. In addition, based on this study, we suggest that the predictive value of measuring tumor depth, budding, or WPOI from frozen sections of T1/T2 N0 oral tongue SCC during the surgery should be studied. The results obtained should then be compared with those of postoperative surgical paraffin-embedded tumors. In case this unrefined estimation of the above parameters from a frozen section assigns an outcome similar to that in the evaluation of the paraffin sections, it could be used in treatment planning. Specifically, in aggressive T1/T2 N0 cases (ie, depth of ≥4 mm, budding of ≥5 clusters at the invasive front or high-risk WPOI), evacuation of the neck lymph nodes during the primary surgery should be considered as a routine treatment plan. Furthermore, the use of multimodality treatment, including radiation and/or chemotherapy, should also be considered in these latter patients.
In conclusion, there are patients with T1/T2N0M0 SCC with aggressive high-risk disease for whom the use of multimodality treatment would be beneficial regardless of treatment approaches. Those patients could be selected by evaluating the depth of invasion, tumor budding, or WPOI, all parameters that can be easily and rapidly analyzed from the routine hematoxylin-eosin–stained tumor. Moreover, this study was based on a large patient material from the central university hospitals in Finland, and our findings may be a step forward in individualized patient management in early tongue cancer.
Acknowledgments
The first author acknowledges the support of Dr. Kowan Jee of the Department of Pathology, University of Helsinki.
References
- Ganly I, Patel S, Shah J. Early stage squamous cell cancer of the oral tongue—clinicopathologic features affecting outcome. Cancer. 2012;118:101–111. doi: 10.1002/cncr.26229. [DOI] [PubMed] [Google Scholar]
- Ho CM, Lam KH, Wei WI, Lau SK, Lam LK. Occult lymph node metastasis in small oral tongue cancers. Head Neck. 1992;14:359–363. doi: 10.1002/hed.2880140504. [DOI] [PubMed] [Google Scholar]
- Kellermann MG, Sobral LM, da Silva SD, et al. Myofibroblasts in the stroma of oral squamous cell carcinoma are associated with poor prognosis. Histopathology. 2007;51:849–853. doi: 10.1111/j.1365-2559.2007.02873.x. [DOI] [PubMed] [Google Scholar]
- Keski–Säntti H, Atula T, Tikka J, Hollmén J, Mäkitie AA, Leivo I. Predictive value of histopathologic parameters in early squamous cell carcinoma of oral tongue. Oral Oncol. 2007;43:1007–1013. doi: 10.1016/j.oraloncology.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Wang C, Huang H, Huang Z, et al. Tumor budding correlates with poor prognosis and epithelial-mesenchymal transition in tongue squamous cell carcinoma. J Oral Pathol Med. 2011;40:545–551. doi: 10.1111/j.1600-0714.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandwein–Gensler A, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- Jakobsson PA, Eneroth CM, Killander D, Moberger G, Mårtensson B. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys Biol. 1973;12:1–8. doi: 10.3109/02841867309131085. [DOI] [PubMed] [Google Scholar]
- Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res. 1987;95:229–249. doi: 10.1111/j.1600-0722.1987.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989;18:432–437. doi: 10.1111/j.1600-0714.1989.tb01339.x. [DOI] [PubMed] [Google Scholar]
- Martínez–Gimeno C, Rodríguez EM, Vila CN, Varela CL. Squamous cell carcinoma of the oral cavity: a clinicopathologic scoring system for evaluating risk of cervical lymph node metastasis. Laryngoscope. 1995;105(7 Pt 1):728–733. doi: 10.1288/00005537-199507000-00011. [DOI] [PubMed] [Google Scholar]
- Odell EW, Jani P, Sherriff M, et al. The prognostic value of individual histologic grading parameters in small lingual squamous cell carcinomas. The importance of the pattern of invasion. Cancer. 1994;74:789–794. doi: 10.1002/1097-0142(19940801)74:3<789::aid-cncr2820740302>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Kantola S, Parikka M, Jokinen K, et al. Prognostic factors in tongue cancer—relative importance of demographic, clinical and histopathological factors. Br J Cancer. 2000;83:614–619. doi: 10.1054/bjoc.2000.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa H, Zhang M, Matsumoto S, et al. The high prognostic value of the histologic grade at the deep invasive front of tongue squamous cell carcinoma. J Oral Pathol Med. 2005;34:329–333. doi: 10.1111/j.1600-0714.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- Po Wing Yuen A, Lam KY, Lam LK, et al. Prognostic factors of clinically stage I and II oral tongue carcinoma—a comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martínez–Gimeno score, and pathologic features. Head Neck. 2002;24:513–520. doi: 10.1002/hed.10094. [DOI] [PubMed] [Google Scholar]
- Brandwein–Gensler M, Smith RV, Wang B, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34:676–688. doi: 10.1097/PAS.0b013e3181d95c37. [DOI] [PubMed] [Google Scholar]
- Weijers M, Snow GB, Bezemer PD, van der Waal I. Malignancy grading is no better than conventional histopathological grading in small squamous cell carcinoma of tongue and floor of mouth: retrospective study in 128 patients. J Oral Pathol Med. 2009;38:343–347. doi: 10.1111/j.1600-0714.2009.00751.x. [DOI] [PubMed] [Google Scholar]
- Silveira EJ, Godoy GP, Lins RD, et al. Correlation of clinical, histological, and cytokeratin profiles of squamous cell carcinoma of the oral tongue with prognosis. Int J Surg Pathol. 2007;15:376–383. doi: 10.1177/1066896907304992. [DOI] [PubMed] [Google Scholar]
- Barry CP, Katre C, Papa E, et al. De-escalation of surgery for early oral cancer—is it oncologically safe? Br J Oral Maxillofac Surg. 2013;51:30–36. doi: 10.1016/j.bjoms.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Bello IO, Vered M, Dayan D, et al. Cancer-associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011;47:33–38. doi: 10.1016/j.oraloncology.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Marsh D, Suchak K, Moutasim KA, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223:470–481. doi: 10.1002/path.2830. [DOI] [PubMed] [Google Scholar]
- Jerjes W, Upile T, Petrie A, et al. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1-T2 oral squamous cell carcinoma patients. Head Neck Oncol. 2010;2:9. doi: 10.1186/1758-3284-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SH, Hwang D, Lockwood G, Goldstein DP, O’Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009;115:1489–1497. doi: 10.1002/cncr.24161. [DOI] [PubMed] [Google Scholar]
- Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC. A new prognostic staging system for rectal cancer. Ann Surg. 2004;240:832–839. doi: 10.1097/01.sla.0000143243.81014.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (II) Oral Oncol. 2010;46:636–643. doi: 10.1016/j.oraloncology.2010.06.008. [DOI] [PubMed] [Google Scholar]
- da Silva SD, Ferlito A, Takes RP, et al. Advances and applications of oral cancer basic research. Oral Oncol. 2011;47:783–791. doi: 10.1016/j.oraloncology.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Jung J, Cho NH, Kim J, et al. Significant invasion depth of early oral tongue cancer originated from the lateral border to predict regional metastases and prognosis. Int J Oral Maxillofac Surg. 2009;38:653–660. doi: 10.1016/j.ijom.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Fakih AR, Rao RS, Borges AM, Patel AR. Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. Am J Surg. 1989;158:309–313. doi: 10.1016/0002-9610(89)90122-0. [DOI] [PubMed] [Google Scholar]
- Morton RP, Ferguson CM, Lambie NK, Whitlock RM. Tumor thickness in early tongue cancer. Arch Otolaryngol Head Neck Surg. 1994;120:717–720. doi: 10.1001/archotol.1994.01880310023005. [DOI] [PubMed] [Google Scholar]
- Koike M, Kodera Y, Itoh Y, et al. Multivariate analysis of the pathologic features of esophageal squamous cell cancer: tumor budding is a significant independent prognostic factor. Ann Surg Oncol. 2008;15:1977–1982. doi: 10.1245/s10434-008-9901-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Ishii G, Kojima M, et al. Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J Thorac Oncol. 2010;5:1361–1368. doi: 10.1097/JTO.0b013e3181eaf2f3. [DOI] [PubMed] [Google Scholar]
- Koyuncuoglu M, Okyay E, Saatli B, Olgan S, Akin M, Saygili U. Tumor budding and E-Cadherin expression in endometrial carcinoma: are they prognostic factors in endometrial cancer? Gynecol Oncol. 2012;125:208–213. doi: 10.1016/j.ygyno.2011.12.433. [DOI] [PubMed] [Google Scholar]
- Vered M, Dobriyan A, Dayan D, et al. Tumor-host histopathologic variables, stromal myofibroblasts and risk score, are significantly associated with recurrent disease in tongue cancer. Cancer Sci. 2010;101:274–280. doi: 10.1111/j.1349-7006.2009.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenblatt Rde C, Martinez GL, Silva LE, Faria PS, Camisasca DR, Lourenço Sde Q. Oral squamous cell carcinoma grading systems–analysis of the best survival predictor. J Oral Pathol Med. 2012;41:34–39. doi: 10.1111/j.1600-0714.2011.01068.x. [DOI] [PubMed] [Google Scholar]
- Søland TM, Brusevold IJ, Koppang HS, Schenck K, Bryne M. Nerve growth factor receptor (p75 NTR) and pattern of invasion predict poor prognosis in oral squamous cell carcinoma. Histopathology. 2008;53:62–72. doi: 10.1111/j.1365-2559.2008.03063.x. [DOI] [PubMed] [Google Scholar]
- Chang YC, Nieh S, Chen SF, Jao SW, Lin YL, Fu E. Invasive pattern grading score designed as an independent prognostic indicator in oral squamous cell carcinoma. Histopathology. 2010;57:295–303. doi: 10.1111/j.1365-2559.2010.03616.x. [DOI] [PubMed] [Google Scholar]
- Dissanayaka WL, Pitiyage G, Kumarasiri PV, Liyanage RL, Dias KD, Tilakaratne WM. Clinical and histopathologic parameters in survival of oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:518–525. doi: 10.1016/j.oooo.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Lundqvist L, Stenlund H, Laurell G, Nylander K. The importance of stromal inflammation in squamous cell carcinoma of the tongue. J Oral Pathol Med. 2012;41:379–383. doi: 10.1111/j.1600-0714.2011.01107.x. [DOI] [PubMed] [Google Scholar]
- Thode C, Jørgensen TG, Dabelsteen E, Mackenzie I, Dabelsteen S. Significance of myofibroblasts in oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:201–207. doi: 10.1111/j.1600-0714.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- Liu M, Xu J, Deng H. Tangled fibroblasts in tumor-stroma interactions. Int J Cancer. 2011;129:1795–1805. doi: 10.1002/ijc.26116. [DOI] [PubMed] [Google Scholar]
- Mark Taylor S, Drover C, Maceachern R, et al. Is preoperative ultrasonography accurate in measuring tumor thickness and predicting the incidence of cervical metastasis in oral cancer? Oral Oncol. 2010;46:38–41. doi: 10.1016/j.oraloncology.2009.10.005. [DOI] [PubMed] [Google Scholar]


