Abstract
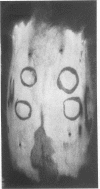
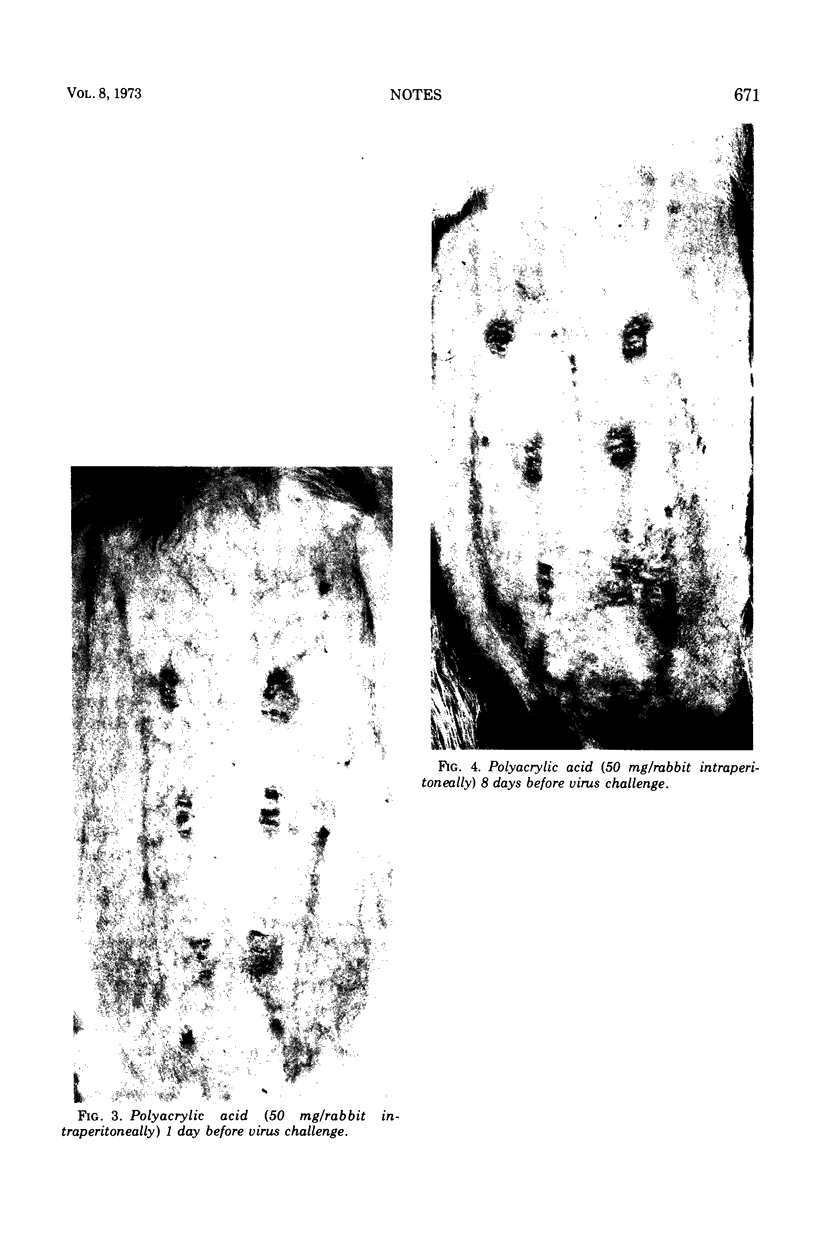
Brucella abortus and polyacrylic acid, two agents whose interferon-inducing capacity in the mouse has been well established, failed to stimulate circulating interferon production in rabbits, yet protected rabbits against local vaccinia virus infection.
Full text
PDF
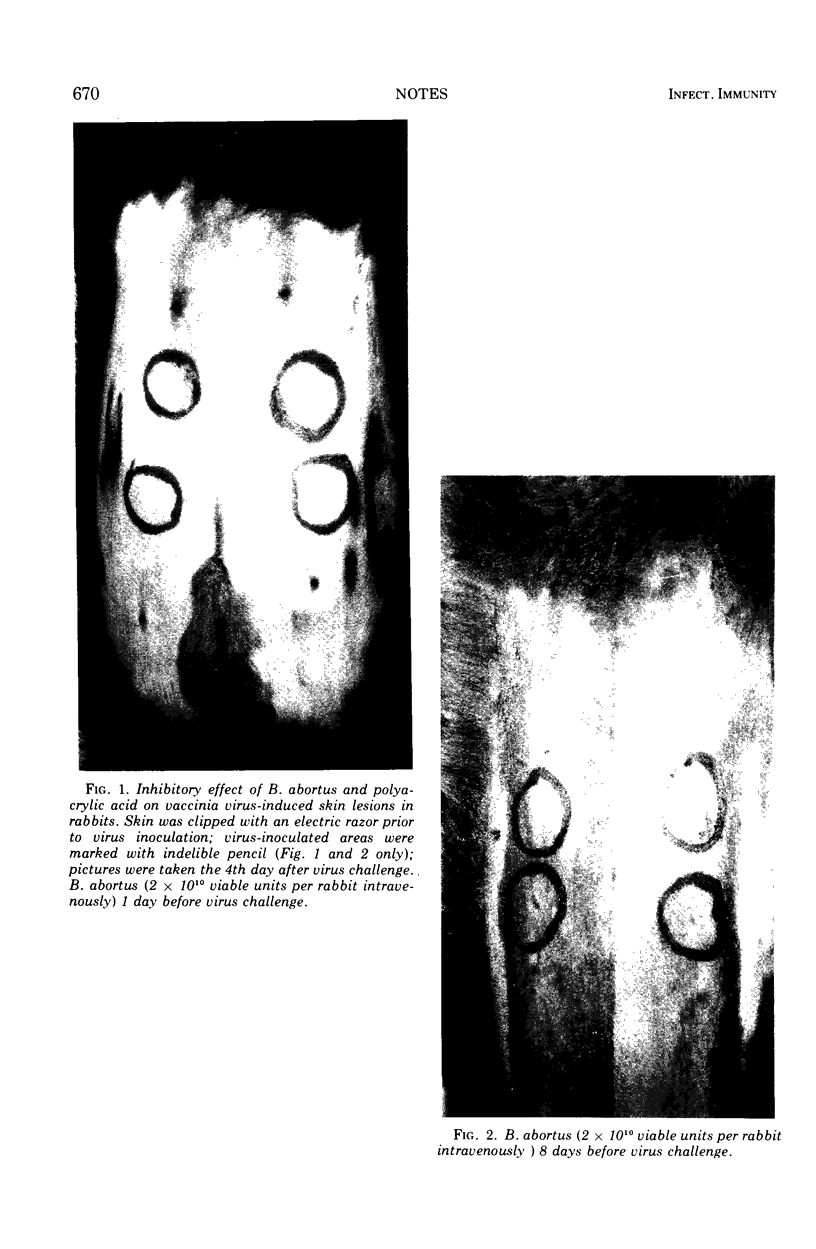

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billiau A., Desmyter J., De Somer P. Antiviral activity of chlorite-oxidized oxyamylose, a polyacetal carboxylic acid. J Virol. 1970 Mar;5(3):321–328. doi: 10.1128/jvi.5.3.321-328.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A., Muyembe J. J., De Somer P. Mechanism of antiviral activity in vivo of polycarboxylases which induce interferon production. Nat New Biol. 1971 Aug 11;232(2):183–186. doi: 10.1038/newbio232183a0. [DOI] [PubMed] [Google Scholar]
- Billiau A., Schonne E., Eyckmans L., De Somer P. Interferon Induction and Resistance to Virus Infection in Mice Infected with Brucella abortus. Infect Immun. 1970 Dec;2(6):698–704. doi: 10.1128/iai.2.6.698-704.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., De Somer P. Effect of interferon, polyacrylin acid, and polymethacrylic acid on tail lesions on mice infected with vaccinia virus. Appl Microbiol. 1968 Sep;16(9):1314–1319. doi: 10.1128/am.16.9.1314-1319.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., De Somer P. Prolonged antiviral protection by interferon inducers. Proc Soc Exp Biol Med. 1969 Nov;132(2):699–703. doi: 10.3181/00379727-132-34291. [DOI] [PubMed] [Google Scholar]
- De Clercq E., De Somer P. Role of interferon in the protective effect of the double-stranded polyribonucleotide against murine tumors induced by Moloney sarcoma virus. J Natl Cancer Inst. 1971 Dec;47(6):1345–1355. [PubMed] [Google Scholar]
- De Clercq E. Hyporeactivity to interferon production by double-stranded RNA associated with hyporeactivity to antiviral protection and hyporeactivity to toxicity. Proc Soc Exp Biol Med. 1972 Oct;141(1):340–345. doi: 10.3181/00379727-141-36772. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Nuwer M. R., Merigan T. C. The role of interferon in the protective effect of a synthetic double-stranded polyribonucleotide against intranasal vesicular stomatitis virus challenge in mice. J Clin Invest. 1970 Aug;49(8):1565–1577. doi: 10.1172/JCI106374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Somer P., De Clercq E., Billiau A., Schonne E., Claesen M. Antiviral activity of polyacrylic and polymethacrylic acids. I. Mode of action in vitro. J Virol. 1968 Sep;2(9):878–885. doi: 10.1128/jvi.2.9.878-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Somer P., De Clercq E., Billiau A., Schonne E., Claesen M. Antiviral activity of polyacrylic and polymethacrylic acids. II. Mode of action in vivo. J Virol. 1968 Sep;2(9):886–893. doi: 10.1128/jvi.2.9.886-893.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober L. L., Friedman-Kien A. E., Havell E. A., Vilcek J. Suppression of the intracellular growth of Shigella flexneri in cell cultures by interferon preparations and polyinosinic-polycytidylic acid. Infect Immun. 1972 Mar;5(3):370–376. doi: 10.1128/iai.5.3.370-376.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C., Finkelstein M. S. Interferon-stimulating and in vivo antiviral effects of various synthetic anionic polymers. Virology. 1968 Jul;35(3):363–374. doi: 10.1016/0042-6822(68)90215-8. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., Regelson W., Munson A. E. Pyran and polyribonucleotides: differences in biological activities. Antimicrob Agents Chemother. 1972 Jul;2(1):16–22. doi: 10.1128/aac.2.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindak F. F. Protection of mice against bacterial infection by interferon inducers. Infect Immun. 1970 Mar;1(3):271–273. doi: 10.1128/iai.1.3.271-273.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Synthetic polyanions protect mice against intracellular bacterial infection. Nature. 1970 Apr 25;226(5243):361–363. doi: 10.1038/226361a0. [DOI] [PubMed] [Google Scholar]
- STINEBRING W. R., YOUNGNER J. S. PATTERNS OF INTERFERON APPEARANCE IN MICE INFECTED WITH BACTERIA OR BACTERIAL ENDOTOXIN. Nature. 1964 Nov 14;204:712–712. doi: 10.1038/204712a0. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Scott W. D., Sulkin S. E. Relative sensitivities of viruses to different species of interferon. J Virol. 1969 Aug;4(2):147–153. doi: 10.1128/jvi.4.2.147-153.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Thacore H. R., Kelly M. E. Sensitivity of ribonucleic acid and deoxyribonucleic acid viruses to different species of interferon in cell cultures. J Virol. 1972 Aug;10(2):171–178. doi: 10.1128/jvi.10.2.171-178.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]