Abstract
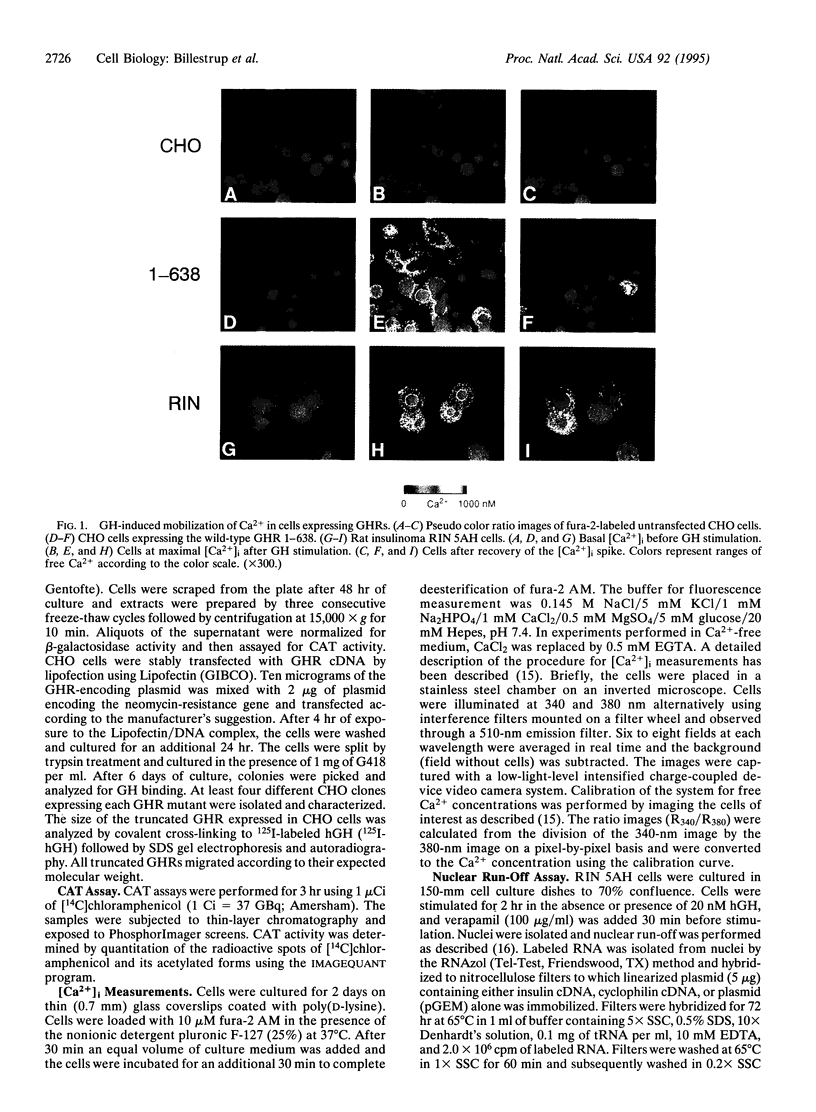
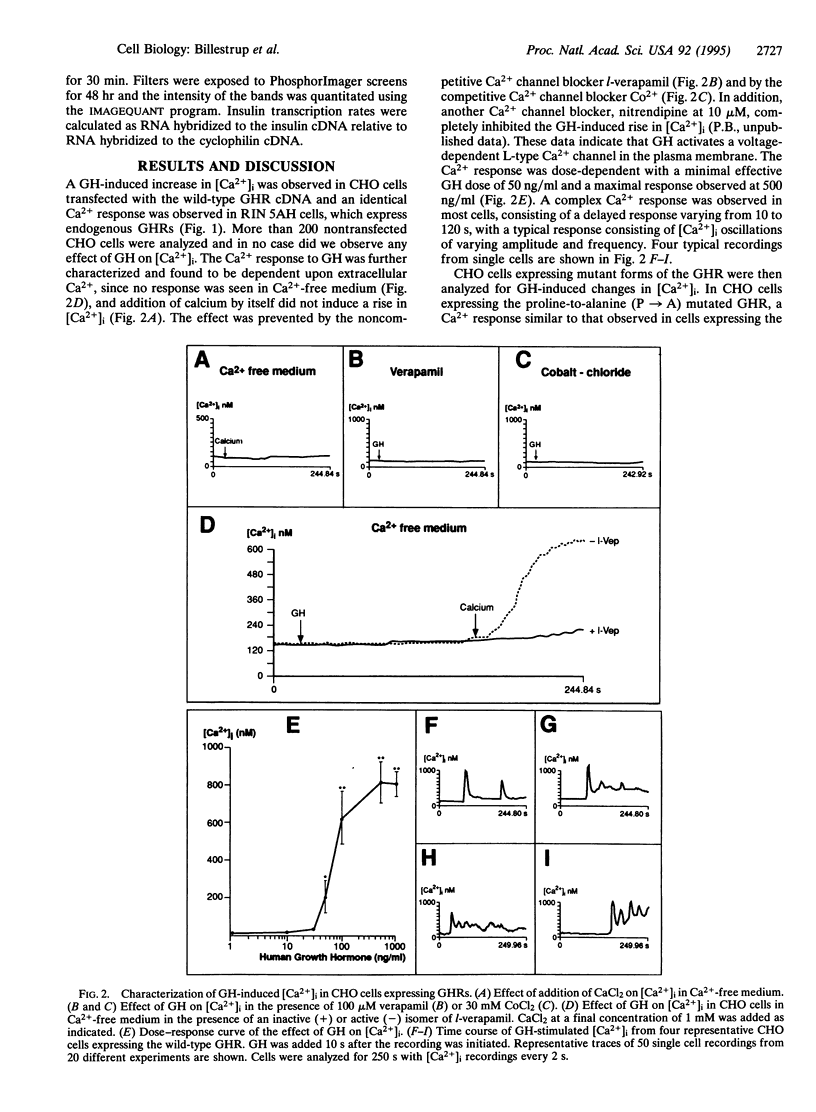
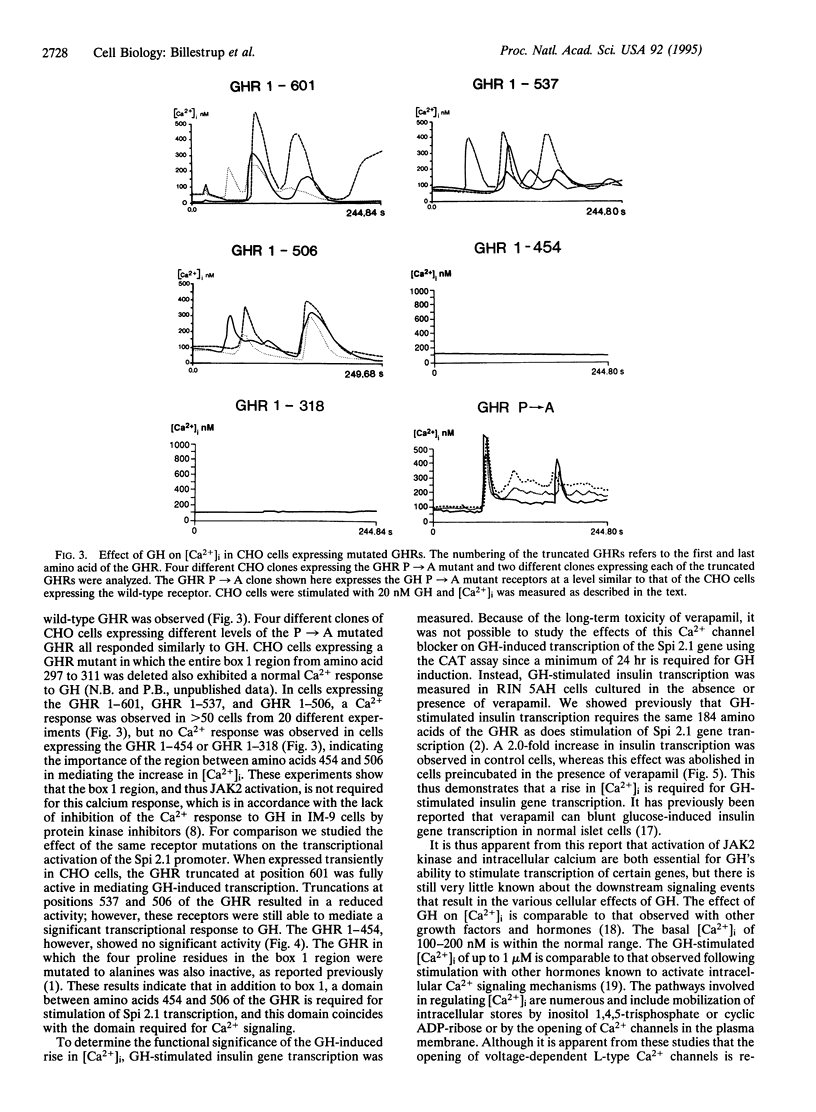
The biological effects of growth hormone (GH) are initiated by its binding to the GH receptor (GHR) followed by association and activation of the tyrosine kinase JAK2. Here we report that GH can stimulate an increase in intracellular free Ca2+ concentration ([Ca2+]i) in cells expressing wild-type GHRs and receptor mutants lacking up to 132 amino acids of the C terminus, whereas GHRs lacking a further 52 amino acids in the C terminus are unable to induce Ca2+ signaling. The GH-induced rise in [Ca2+]i was dependent upon extracellular Ca2+ and the response consisted of GH-induced Ca2+ oscillations of varying frequency and amplitude. GH-induced transcription of the serine protease inhibitor 2.1 gene required the same C-terminal 52-amino acid domain of the receptor as for Ca2+ signaling. Mutation of the four proline residues in the conserved box 1 region of the GHR, which is responsible for binding and activation of JAK2 kinase, completely abolished GH-induced gene transcription but did not affect the GH-induced rise in [Ca2+]i. The Ca2+ channel blocker verapamil prevented GH-induced Ca2+ signaling as well as GH-induced gene transcription in cells expressing endogenous GHRs. These findings indicate that the GHR can initiate two independent signaling pathways, one requiring the box 1 region and the other requiring the region between amino acids 454 and 506, and suggest that both of these pathways are required for GH-induced gene transcription.
Full text
PDF
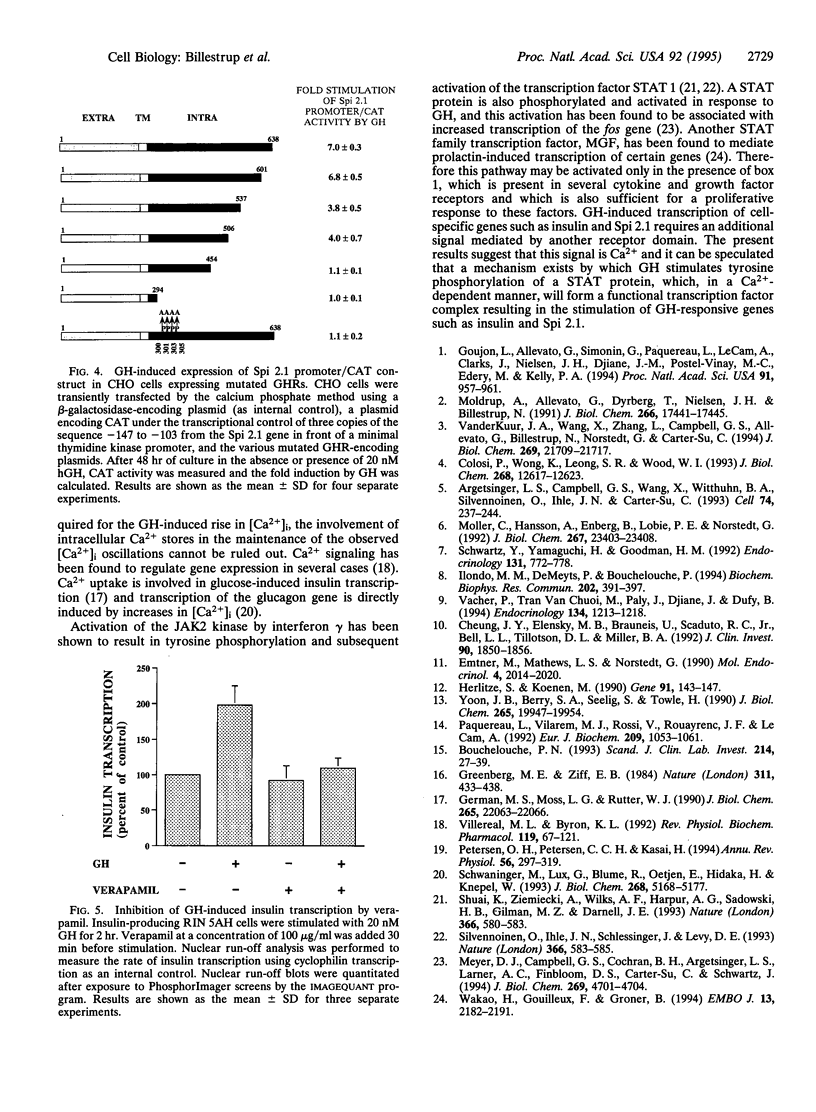
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- Bouchelouche P. N. Dynamic, real time imaging of ion activities in single living cells using fluorescence video microscopy and image analysis. Scand J Clin Lab Invest Suppl. 1993;214:27–39. [PubMed] [Google Scholar]
- Cheung J. Y., Elensky M. B., Brauneis U., Scaduto R. C., Jr, Bell L. L., Tillotson D. L., Miller B. A. Ion channels in human erythroblasts. Modulation by erythropoietin. J Clin Invest. 1992 Nov;90(5):1850–1856. doi: 10.1172/JCI116061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosi P., Wong K., Leong S. R., Wood W. I. Mutational analysis of the intracellular domain of the human growth hormone receptor. J Biol Chem. 1993 Jun 15;268(17):12617–12623. [PubMed] [Google Scholar]
- Emtner M., Mathews L. S., Norstedt G. Growth hormone (GH) stimulates protein synthesis in cells transfected with GH receptor complementary DNA. Mol Endocrinol. 1990 Dec;4(12):2014–2020. doi: 10.1210/mend-4-12-2014. [DOI] [PubMed] [Google Scholar]
- German M. S., Moss L. G., Rutter W. J. Regulation of insulin gene expression by glucose and calcium in transfected primary islet cultures. J Biol Chem. 1990 Dec 25;265(36):22063–22066. [PubMed] [Google Scholar]
- Goujon L., Allevato G., Simonin G., Paquereau L., Le Cam A., Clark J., Nielsen J. H., Djiane J., Postel-Vinay M. C., Edery M. Cytoplasmic sequences of the growth hormone receptor necessary for signal transduction. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):957–961. doi: 10.1073/pnas.91.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Herlitze S., Koenen M. A general and rapid mutagenesis method using polymerase chain reaction. Gene. 1990 Jul 2;91(1):143–147. doi: 10.1016/0378-1119(90)90177-s. [DOI] [PubMed] [Google Scholar]
- Ilondo M. M., De Meyts P., Bouchelouche P. Human growth hormone increases cytosolic free calcium in cultured human IM-9 lymphocytes: a novel mechanism of growth hormone transmembrane signalling. Biochem Biophys Res Commun. 1994 Jul 15;202(1):391–397. doi: 10.1006/bbrc.1994.1940. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Campbell G. S., Cochran B. H., Argetsinger L. S., Larner A. C., Finbloom D. S., Carter-Su C., Schwartz J. Growth hormone induces a DNA binding factor related to the interferon-stimulated 91-kDa transcription factor. J Biol Chem. 1994 Feb 18;269(7):4701–4704. [PubMed] [Google Scholar]
- Möller C., Hansson A., Enberg B., Lobie P. E., Norstedt G. Growth hormone (GH) induction of tyrosine phosphorylation and activation of mitogen-activated protein kinases in cells transfected with rat GH receptor cDNA. J Biol Chem. 1992 Nov 15;267(32):23403–23408. [PubMed] [Google Scholar]
- Møldrup A., Allevato G., Dyrberg T., Nielsen J. H., Billestrup N. Growth hormone action in rat insulinoma cells expressing truncated growth hormone receptors. J Biol Chem. 1991 Sep 15;266(26):17441–17445. [PubMed] [Google Scholar]
- Paquereau L., Vilarem M. J., Rossi V., Rouayrenc J. F., Le Cam A. Regulation of two rat serine-protease inhibitor gene promoters by somatotropin and glucocorticoids. Study with intact hepatocytes and cell-free systems. Eur J Biochem. 1992 Nov 1;209(3):1053–1061. doi: 10.1111/j.1432-1033.1992.tb17381.x. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Petersen C. C., Kasai H. Calcium and hormone action. Annu Rev Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- Schwaninger M., Lux G., Blume R., Oetjen E., Hidaka H., Knepel W. Membrane depolarization and calcium influx induce glucagon gene transcription in pancreatic islet cells through the cyclic AMP-responsive element. J Biol Chem. 1993 Mar 5;268(7):5168–5177. [PubMed] [Google Scholar]
- Schwartz Y., Yamaguchi H., Goodman H. M. Growth hormone increases intracellular free calcium in rat adipocytes: correlation with actions on carbohydrate metabolism. Endocrinology. 1992 Aug;131(2):772–778. doi: 10.1210/endo.131.2.1639023. [DOI] [PubMed] [Google Scholar]
- Shuai K., Ziemiecki A., Wilks A. F., Harpur A. G., Sadowski H. B., Gilman M. Z., Darnell J. E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993 Dec 9;366(6455):580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Ihle J. N., Schlessinger J., Levy D. E. Interferon-induced nuclear signalling by Jak protein tyrosine kinases. Nature. 1993 Dec 9;366(6455):583–585. doi: 10.1038/366583a0. [DOI] [PubMed] [Google Scholar]
- Vacher P., Tran Van Chuoi M., Paly J., Djiane J., Dufy B. Short term effect of prolactin on intracellular calcium in Chinese hamster ovary cells stably transfected with prolactin receptor complementary deoxyribonucleic acid. Endocrinology. 1994 Mar;134(3):1213–1218. doi: 10.1210/endo.134.3.8119161. [DOI] [PubMed] [Google Scholar]
- VanderKuur J. A., Wang X., Zhang L., Campbell G. S., Allevato G., Billestrup N., Norstedt G., Carter-Su C. Domains of the growth hormone receptor required for association and activation of JAK2 tyrosine kinase. J Biol Chem. 1994 Aug 26;269(34):21709–21717. [PubMed] [Google Scholar]
- Villereal M. L., Byron K. L. Calcium signals in growth factor signal transduction. Rev Physiol Biochem Pharmacol. 1992;119:67–121. doi: 10.1007/3540551921_4. [DOI] [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994 May 1;13(9):2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. B., Berry S. A., Seelig S., Towle H. C. An inducible nuclear factor binds to a growth hormone-regulated gene. J Biol Chem. 1990 Nov 15;265(32):19947–19954. [PubMed] [Google Scholar]