Abstract
Marine sediments can contain B vitamins, presumably incorporated from settled, decaying phytoplankton and microorganisms associated with decomposition. Because B vitamins may be advantageous for the energetically intensive processes of metamorphosis, post-metamorphic growth, and reproduction, we tested several B vitamins to determine if they would stimulate larvae of the deposit-feeding polychaete Capitella teleta to settle and metamorphose. Nicotinamide and riboflavin individually stimulated larvae of C. teleta to settle and metamorphose, generally within 1–2 hours at nicotinamide concentrations as low as 3 µM and riboflavin concentrations as low as 50 µM. More than 80% of the larvae metamorphosed within 30 minutes at a nicotinamide concentration of 7 µM. The pyridine channel agonist pyrazinecarboxamide also stimulated metamorphosis at very low concentrations. In contrast, neither lumichrome, thiamine HCl, pyridoxine HCl, nor vitamin B12 stimulated larvae of C. teleta to metamorphose at concentrations as high as 500 µM. Larvae also did not metamorphose in response to either nicotinamide or pyrazinecarboxamide in calcium-free seawater or with the addition of 4-acetylpyridine, a competitive inhibitor of the pyridine receptor. Together, these results suggest that larvae of C. teleta are responding to nicotinamide and riboflavin via a chemosensory pyridine receptor similar to that previously reported to be present on crayfish chela and involved with food recognition. Our data are the first to implicate B vitamins as possible natural chemical settlement cues for marine invertebrate larvae.
Introduction
The larvae of many benthic marine invertebrates are planktonic and are thus ‘forced to wander’ the sea until they become competent to metamorphose and locate a site suitable for settlement [1]. Particular chemical cues then promote larval settlement and subsequent metamorphosis by competent larvae of many species [2]–[4]. These chemical cues may indicate the presence of appropriate food, conspecific adults to mate with, or other environmental factors that signal the suitability of a site to live in for juveniles and adults. For example, larvae of the sea urchin Holopneustes purpurascens settle and metamorphose in response to histamine, which leaches away from Delisea pulchra, their algal food source [5]–[7]. Similarly, larvae of the tube-building polychaete worm Phragmatopoma californica settle and metamorphose in response to several different fatty acids that are produced by conspecific tube-dwelling adults, signaling the presence of potential mates [8]. Negative recruitment cues that prevent larvae from metamorphosing in specific areas have also been identified and can also be important in determining species distributions [9]. However, for the vast majority of marine invertebrates, the specific chemical settlement cues remain undefined.
Capitella teleta, previously known as Capitella sp. I, is a small (∼20 mm long×1 mm wide), opportunistic, deposit-feeding marine polychaete found in salt marsh sediments and in disturbed and polluted areas such as busy harbors, sewage outflows, and some regions affected by oil spills [10], [11]. Its planktonic, non-feeding, metatrochophore larvae will typically metamorphose in the presence of salt-marsh sediments, making it a convenient model for studies of substrate selection [12]–[14]. Larvae of C. teleta metamorphose rapidly when in the presence of salt-marsh sediment; in one study 90% of the tested larvae metamorphosed within 30 minutes of treatment [13]. The active component of the natural settlement cue contained within the salt-marsh sediment is currently unknown; however, larvae of C. teleta have been shown to settle and metamorphose preferentially in response to sediments with high organic content, and those with a low carbohydrate to protein ratio [15], [16]. Sediments forced through a 0.45 µm filter can still stimulate larvae to metamorphose; however, filtering the sediment to 0.22 µm removed the cue, suggesting that the cue is bound to small particulates [15]. Also, combusting the sediment at 500°C for 6 h removed the cue from the sediment: the resulting ash did not stimulate metamorphosis, supporting the notion that the cue is organic [15].
Juvenile hormones and juvenile hormone-active chemicals are known to induce settlement and metamorphosis of C. teleta larvae [17], [18]. Extracts prepared from marine sediments displayed juvenile hormone-activity in insect bioassays, suggesting that the chemicals in the sediments that induced settlement and metamorphosis may be similar in structure to juvenile hormones (JHs) or have juvenile hormone-activity. In addition, the induction of settlement and metamorphosis by JH was found to involve activation of protein kinase C and further activation of calcium channels [18]. Exposing C. teleta larvae to 400 nM calcium ionophore A23187, a membrane soluble chemical that shuttles calcium ions into cells, induced all of the larvae to metamorphose in less than one hour. Whether or not the larvae could metamorphose in calcium-free seawater however was not explored.
As has been found for some other invertebrate larvae, larvae of C. teleta settle and metamorphose in response to serotonin and the serotonin-reuptake inhibitor fluoxetine [19], [20], indicating that stimulation of the larval nervous system is involved in mediating the chemosensory response to chemical cues. Furthermore, different inhibitors of nitric oxide synthase such as S-methylisothiourea and aminoguanidine hemisulfate were also found to induce larval settlement and metamorphosis [20], adding C. teleta to a growing list of marine invertebrates, including some gastropods [21]–[23], echinoderms [24], and ascidians [25], [26] whose metamorphosis is inhibited by the presence of endogenous nitric oxide. While we currently know much about the intermediate steps of the signal transduction cascade leading to metamorphosis of C. teleta, we do not know which chemicals are acting as natural settlement cues for larvae of C. teleta or how these chemicals actually initiate this signal transduction cascade.
Larval metamorphosis, juvenile growth, and adult reproduction are all energy-requiring processes; not surprisingly the growth and reproduction of Capitella are affected by diet [27]. Plant nutrients have also been shown to be quickly assimilated into the growing oocytes of Capitella [28] and to supply adequate nutrition for larval development and subsequent metamorphosis. Dietary B vitamins are essential co-factors for biochemical energy production, and are known to be essential for the growth and development of some annelids [29]. In this respect it should be beneficial for larvae of C. teleta to settle and metamorphose in locations with adequate levels of these vitamins to support post-metamorphic growth. Because B vitamins are likely to be found within the marine sediments that the deposit-feeding adults of C. teleta consume and are required for growth and survival, we suspected that the larvae would respond to at least some B vitamins as a natural settlement cue. To test our hypothesis, we added several B vitamins individually to seawater and monitored the metamorphosis of newly-released larvae of C. teleta. In addition we conducted a series of experiments using various pharmacological agents to determine how these vitamins were stimulating metamorphosis.
Results
Effects of different B vitamins
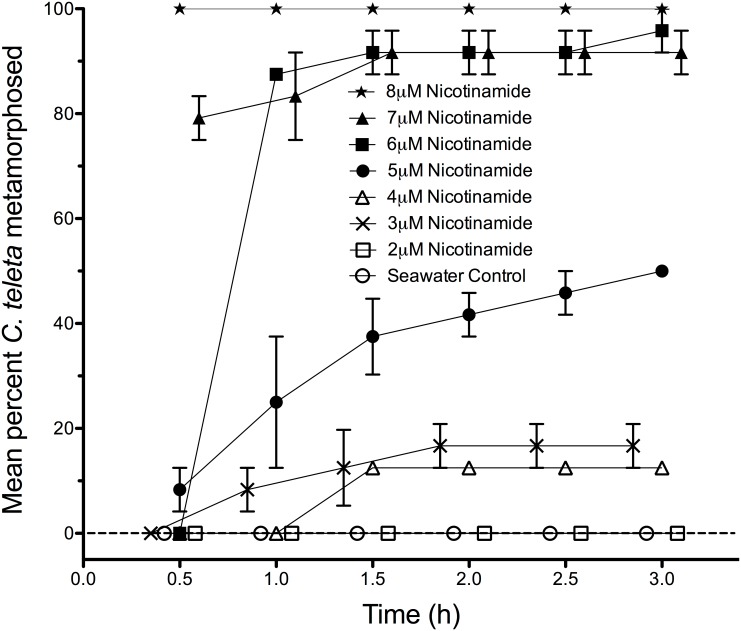
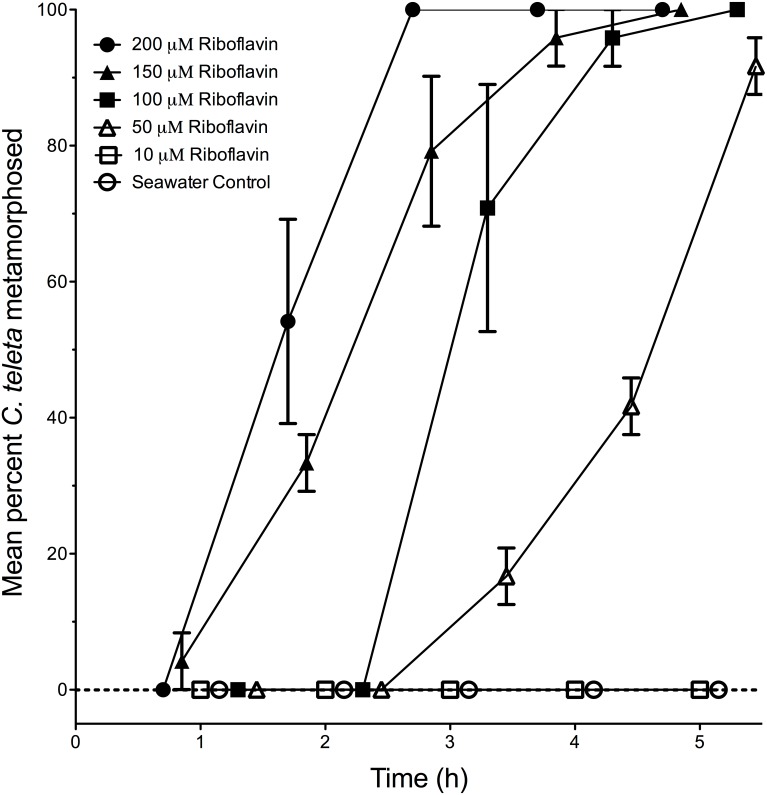
Of the 7 B vitamins tested, only riboflavin (B2), nicotinamide (B3), and nicotinic acid (another form of B3) stimulated metamorphosis in C. teleta. Thiamine HCl (B1), pyridoxine HCl (B6), biotin (B7), and cobalamin (B12) did not stimulate any metamorphosis within 24 h at the tested concentration of 500 µM (data not shown). In contrast, nicotinamide stimulated metamorphosis in a nearly dose-dependent manner at concentrations as low as 3–8 µM, with larvae initiating metamorphosis within 30 min (Figure 1). Nicotinic acid stimulated 70.8% +/−8.3 (s.e.m.) of the treated larvae to metamorphose within 24 h. Also, riboflavin concentrations of 50–200 µM stimulated nearly all larvae of C. teleta to metamorphose within 5 hours (Figure 2). However, the larvae did not respond to the riboflavin breakdown product lumichrome even when tested for 24 h at the maximum concentration of 1 mM (data not shown).
Figure 1. Promotion of metamorphosis by nicotinamide (vitamin B3) in larvae of Capitella teleta.
Each treatment consisted of 3 replicates with 8 larvae per replicate. Larvae were placed in 30 ppt artificial seawater (Instant Ocean) containing the indicated final concentration of nicotinamide. Artificial seawater acted as a negative control. Error bars represent +/−1 s.e.m.
Figure 2. Promotion of metamorphosis by riboflavin (vitamin B2) in larvae of Capitella teleta.
Each treatment consisted of 3 replicates with 8 larvae per replicate. Larvae were placed in 30 ppt artificial seawater (Instant Ocean) containing the indicated final concentration of riboflavin. Artificial seawater acted as a negative control. Error bars represent +/−1 s.e.m.
Effects of pyridine channel activators and inhibitors
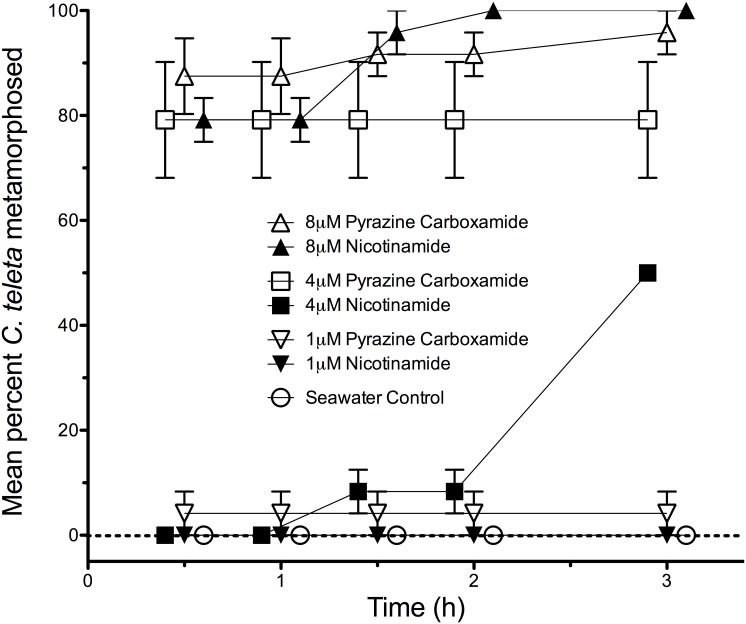
Because concentrations of nicotinamide as low as 3–6 µM stimulated larvae of C. teleta to metamorphose quickly in our experiments, we hypothesized that larvae of C. teleta were sensing nicotinamide via a pyridine activated ion channel, something first characterized in the walking legs of the crayfish Austrapotamobius torrentium [30]–[32]. We therefore compared dose responses between nicotinamide and pyrazinecarboxamide, an agonist of the pyridine-activated ion channel, at 1 µM, 4 µM, and 8 µM. Another commonly occurring nutritive chemical, beta nicotinamide adenine dinucleotide (β-NAD), also stimulated the nicotinamide-activated ion-channel to open, with half-maximal rate of opening (KM) at 1 mM [31]. We therefore treated larvae of C. teleta with β-NAD at the concentrations of 0.5, 1, and 5 mM to determine if β-NAD also stimulated metamorphosis. As shown in Figure 3, the pyridine-activated ion channel agonist pyrazinecarboxamide was found to also stimulate larvae of C. teleta to metamorphose, and did so at similar concentrations as nicotinamide. Although both chemicals stimulated larvae of C. teleta to metamorphose in a dose-dependent fashion, pyrazinecarboxamide stimulated more individuals (about 70%) to metamorphose at 4 µM than did nicotinamide at the same concentration (Figure 3). β-NAD, however, did not stimulate any metamorphosis at 0.5, 1, or 5 mM.
Figure 3. The effects of pyrazinecarboxamide and nicotinamide on metamorphosis of C. teleta when tested equal concentrations.
Each treatment consisted of 3 replicates with 8 larvae per replicate. Artificial seawater (Instant Ocean) acted as a negative control. Error bars represent +/−1 s.e.m.
Effects of calcium free seawater
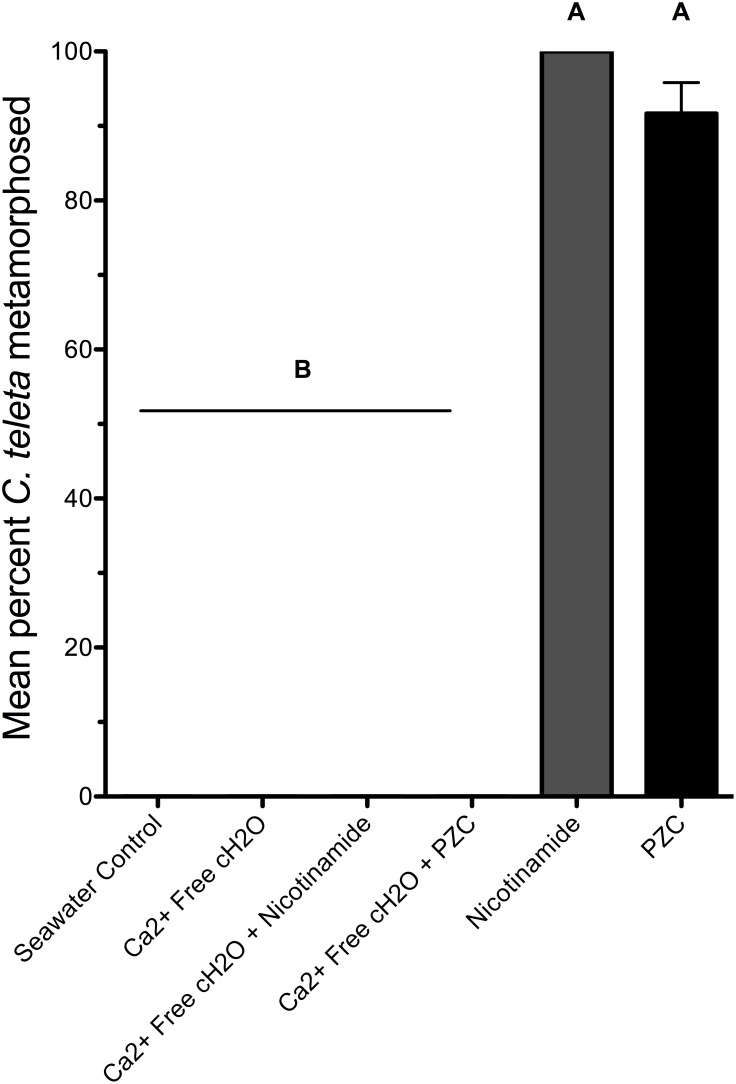
To determine if an influx of extracellular calcium is required for metamorphosis to proceed when pyridine-activated ion channels open, larvae of C. teleta were treated with nicotinamide or pyrazinecarboxamide while bathed in either normal ASW(control) or calcium-free ASW. Pre-treating the larvae in calcium-free ASW inhibited the metamorphic response of C. teleta larvae to both nicotinamide and pyrazinecarboxamide even at the high concentration of 40 µM (one-way ANOVA with Bonferroni post-hoc comparisons, F(5,12) = 234.4, p<0.001) (Figure 4). Larvae in calcium-free ASW settled to the bottom of the dish within a few minutes after the addition of nicotinamide or pyrazinecarboxamide; they then remained stationary, but did not metamorphose over the 24 h observation period. Control larvae in calcium-free ASW continued to swim normally and neither settled nor metamorphosed. At least 90% of larvae exposed to nicotinamide or pyrazinecarboxamide in ASW metamorphosed, indicating that most larvae were competent to metamorphose at the start of this experiment.
Figure 4. The effect of calcium-free seawater on metamorphosis of C. teleta treated with nicotinamide or pyrazinecarboxamide.
Larvae were acclimated to calcium-free seawater for one hour before being transferred to a solution of calcium-free seawater and either nicotinamide or pyrazinecarboxamide (PZC) at 40 µM for 24 h. Each treatment consisted of 3 replicates with 8 larvae per replicate. Artificial seawater (Instant Ocean) acted as a negative control. Letters indicate significant (P<0.05) differences as determined by a Bonferroni post-hoc test. Error bars represent +1 s.e.m.
Effects of 4-acetylpyridine
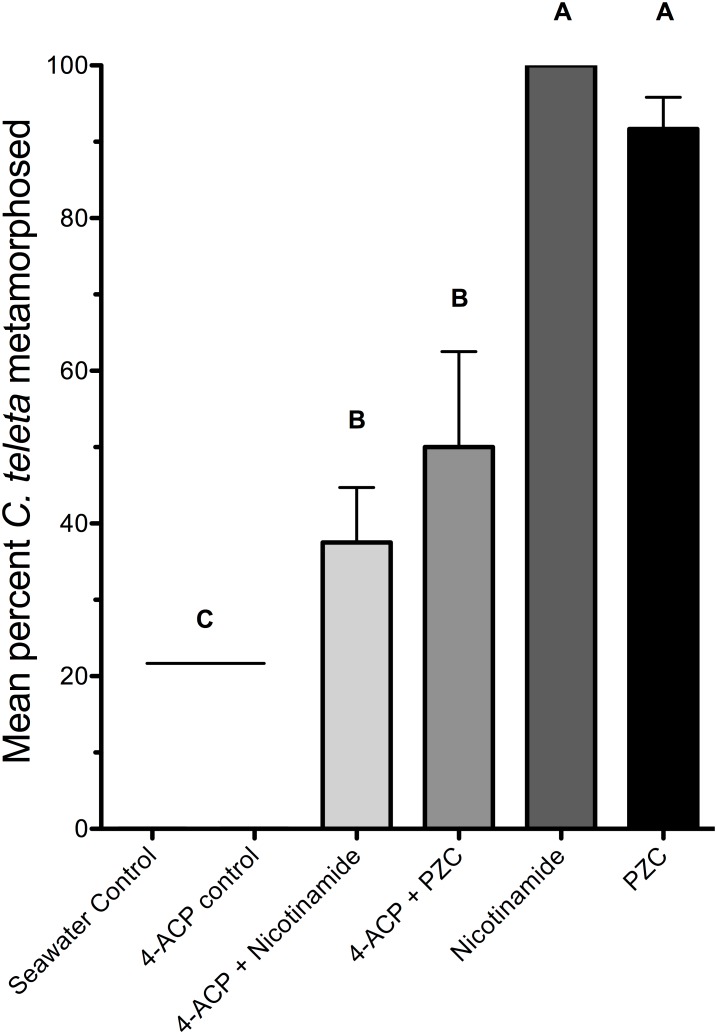
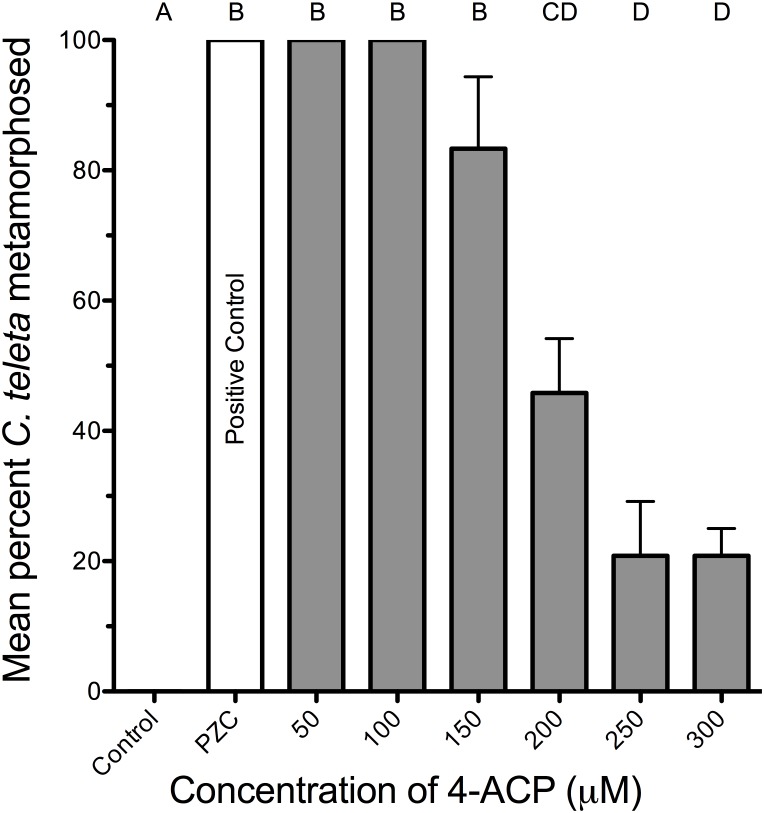
4-acetylpyridine, a specific competitive antagonist of the pyridine-activated ion channel, was also tested on larvae of C. teleta in the presence of either nicotinamide or pyrazinecarboxamide [32]. Treating larvae with 200 µM 4-acetylpyridine inhibited them from metamorphosing in the presence of either 40 µM nicotinamide or 40 µM pyrazinecarboxamide (one-way ANOVA with Bonferroni post-hoc comparisons, F(5,12) = 69.52, p<0.001) (Figure 5). Only about half as many larvae metamorphosed within 24 h when exposed to both 4-acetylpyridine and either nicotinamide or pyrazinecarboxamide, indicating that the inhibitory concentration allowing for 50% settlement and metamorphosis (IC50) is about 200 µM. No larvae exposed to both 4-acetylpyridine and either nicotinamide or pyrazinecarboxamide metamorphosed within the first 5 h of the experiment (data not shown) and no control larvae exposed to 200 µM 4-acetylpyridine alone settled or metamorphosed within 24 h. Again, the high response of larvae in the positive controls shows that most of the larvae were metamorphically competent when tested. Also, larvae were inhibited from metamorphosing in response to pyrazinecarboxamide in a dose-dependent fashion when tested with increasing doses of 4-acetylpyridine (one-way ANOVA with Bonferroni post-hoc comparisons, F(7,16) = 53.92, p<0.001) (Figure 6).
Figure 5. The effect of 4-acetylpyridine on metamorphosis of C. teleta in the presence of nicotinamide or pyrazinecarboxamide.
Larvae were acclimated to 200 µM 4-acetylpyridine (4-ACP) for one hour before being transferred to a solution of 200 µM 4-acetylpyridine and either nicotinamide or pyrazinecarboxamide (PZC) at 40 µM for 24 h. Each treatment consisted of 3 replicates with 8 larvae per replicate. Artificial seawater (Instant Ocean) acted as a negative control. Letters indicate significant (P<0.05) differences as determined by a Bonferroni post-hoc test. Error bars represent + s.e.m.
Figure 6. The inhibitory dose response of 4-acetylpyridine on metamorphosis of C. teleta after 24 h.
Larvae were acclimated to the listed concentrations of 4-acetylpyridine (4-ACP) for one hour before being transferred to a solution of the same concentration of 4-acetylpyridine and pyrazinecarboxamide at 40 µM. Each treatment consisted of 3 replicates with 8 larvae per replicate. Artificial seawater (Instant Ocean) acted as a negative control. The PZC treatment acted as a positive control containing only 40 µM pyrazinecarboxamide and artificial seawater. Letters indicate significant (P<0.05) differences as determined by a Bonferroni post-hoc test. Error bars represent +1 s.e.m.
Effects of ketanserin
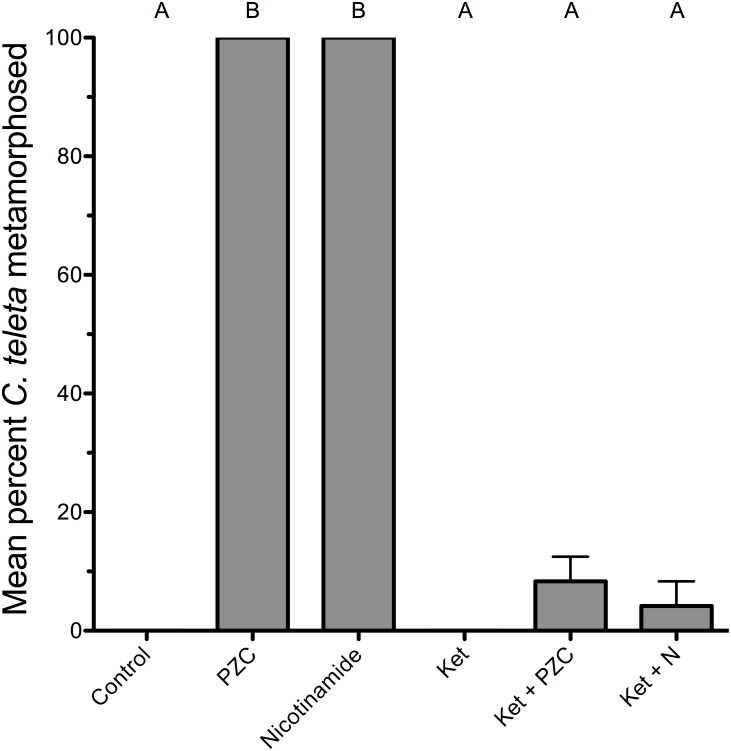
Sensory information relayed by sensory chemoreceptors such as olfactory receptor neurons usually involves sensory transduction through synapses within the central nervous system. In order to assess the involvement of the serotonergic nervous system in mediating the response of the larvae to nicotinamide and pyrazinecarboxamide, the larvae were first pre-exposed to ketanserin, an inhibitor of serotonin 5-HT2B receptors, before induction by nicotinamide and pyrazinecarboxamide. Previous results have demonstrated that ketanserin blocks the response of Capitella larvae to natural mud sediment chemical cues, and also to the nitric oxide synthase inhibitors N-methyl-L-arginine and S-methylisothiourea [19], [20], indicating the involvement of the serotonergic nervous system in mediating the settlement and metamorphosis of C. teleta. Pre-exposing C. teleta larvae to ketanserin inhibited most of the larvae of C. teleta from metamorphosing in response to both nicotinamide and pyrazinecarboxamide (one-way ANOVA with Bonferroni post-hoc comparisons, F(5,12) = 122.5, p<0.001) (Figure 7). No control larvae exposed to 2 µM ketanserin alone settled or metamorphosed within 24 h; however, all of the larvae exposed to the positive controls of nicotinamide and pyrazinecarboxamide alone metamorphosed in less than an hour, indicating that most larvae were competent when tested.
Figure 7. The effect of ketanserin on metamorphosis of C. teleta in the presence of nicotinamide or pyrazinecarboxamide.
Larvae were acclimated to 2 µM ketanserin for three hours before being transferred to a solution of 2 µM ketanserin and either nicotinamide or pyrazinecarboxamide at 40 µM for 24 h. Each treatment consisted of 3 replicates with 8 larvae per replicate. Artificial seawater (Instant Ocean) acted as a negative control. Letters indicate significant (P<0.05) differences as determined by a Bonferroni post-hoc test. Error bars represent + s.e.m.
Discussion
Overall, these data support our hypothesis that at least some B vitamins may act as chemical cues for habitat selection by the larvae of C. teleta and may stimulate the larvae of this species to metamorphose in the field. In particular, nicotinamide (B3), nicotinic acid (B3), and riboflavin (B2) stimulated larvae of C. teleta to metamorphose, whereas thiamine HCl, pyridoxine HCl, biotin, vitamin B12, and the riboflavin breakdown product lumichrome did not. It may be advantageous for marine larvae to settle and metamorphose in response to environmental riboflavin and nicotinamide, as riboflavin is required for the synthesis of FAD, and nicotinamide serves as a building block for the synthesis of NAD; both products are important electron carriers for the electron transport chain, ATP production, and other aspects of cellular metabolism. Substantial concentrations of these vitamins may serve as an adaptive signal that the local environment is suitable for the energetically intensive processes of juvenile development and adult reproduction. In this regard, B vitamins present in ocean waters have been demonstrated to be important for the growth of phytoplankton, zooplankton, and larger marine invertebrates [33]–[35]. However, even if B vitamins are contained within marine sediments, they may not always be bioavailable to deposit feeders.
Although the stimulation of settlement and metamorphosis by vitamins seems not to have been investigated in other marine invertebrate species, the larvae of several other marine invertebrates have previously been demonstrated to settle and metamorphose in response to certain vitamin derivatives [2]. For example, larvae of the ascidian Halocynthia roretzi will settle and metamorphose in response to lumichrome, a photocatalytic breakdown product of riboflavin, although they did not respond to riboflavin itself [36]. This inducer was found to be naturally biosynthesized by the larvae and adults of Halocynthia. Lumichrome, however, did not induce settlement and metamorphosis in other ascidian species, and also did not stimulate metamorphosis in our study, although the larvae of C. teleta did respond to riboflavin. In other studies, larvae of the hydroid Coryne uchidae were found to settle and metamorphose in response to delta-tocopherol epoxides, compounds related in structure to the alpha-tocopherol (vitamin E) produced by the brown algae Sargassum tortile [37].
The vitamin requirements for the growth, reproduction, and development of C. teleta have so far been unexplored, to our knowledge; however, as previously mentioned, we know that growth, reproduction, and development of C. teleta depend strongly on diet and organic content of sediments [27], [38], and that nutrients such as algal pigments, including carotenoids, are rapidly assimilated into developing oocytes [28]. As previously mentioned, larval settlement of C. teleta depends on the organic content of sediments, with larvae preferring sediments with a low carbohydrate to protein ratio [15], [16]. Very little is known about marine annelid nutrition and vitamin requirements; however, an absolute requirement for riboflavin and nicotinamide, along with 6 other vitamins, was demonstrated using artificial culture media for the oligochaete annelid Enchytraeus fragmentosus [29].
We do not currently know the concentrations of riboflavin and nicotinamide present naturally in the environments that larvae of C. teleta settle and metamorphose in. It is likely, however, that recently deposited sediments, particularly those near sewage outflows, contain high levels of these compounds, sufficient to induce metamorphosis. Many phytoplankton species are known to contain large concentrations of B vitamins within their cells [39]. Several marine microorganisms, such as the bacteria Shewanella spp. [40] and Micrococcus luteus [41], and the yeast Candida [42], are also known to produce and release large amounts of riboflavin particularly when grown as biofilms. Remnants of settled, decomposing phytoplankton and the other microbes taking part in decomposition processes are likely to be a large component of marine sediments, which should therefore contain high concentrations of B vitamins. Indeed, biotin (B7), thiamine (B1), and vitamin B12 have been all identified in Pacific marine sediments [43]. Additional studies will be required to determine if riboflavin and/or nicotinamide are present in salt marsh sediments and sediments near sewage outfalls at the low concentrations that we have found to be inductive.
Although the biochemical and sensory mechanisms through which the larvae of C. teleta respond to nicotinamide and riboflavin still need more investigation, our results suggest that larvae may be sensing nicotinamide with chemosensory pyridine receptors similar to those previously found in chemosensory sensilla on the walking legs of crayfish, and which are involved in the foraging behavior of those animals [30]–[32]. The inhibitory effects of 4-acetylpyridine on chemosensation of nicotinamide support this suggestion, as do the stimulatory effects of pyrazinecarboxamide on the larvae of C. teleta, a powerful agonist of these pyridine-activated ion channels [32]. In crayfish, pyrazinecarboxamide was found to be the most effective inducer, followed by nicotinamide; we have found that this is also the case for larvae of C. teleta (Table 1) [32]. Also, the IC50 for 4-acetylpyridine was found to be approximately 200 µM for C. teleta compared with a KI of 70 µM for the crayfish A. torrentium [32]. This difference in effective concentrations for 4-ACP may at least be partly due to the fact that Hatt et al. [32] explored the effects of 4-ACP using isolated chemosensory neurons and electrophysiological techniques, whereas the effects on C. teleta were explored in in vivo studies in which the chemical had to also penetrate the larvae. Interestingly, larvae did not metamorphose when exposed to β-NAD at concentrations as high as 5 mM. While nicotinamide, pyrazinecarboxamide, and nicotinic acid stimulated larvae to metamorphose at concentrations similar to those that stimulated action potentials in the crayfish, it may be possible that other pyridines such as β-NAD do not bind efficiently to this receptor at similar concentrations for both species. It would be interesting in this regard to also try a patch-clamp technique on the C. teleta larvae to demonstrate the presence of pyridine receptors.
Table 1. The lowest stimulatory concentrations of pyridines that induced larvae of C. teleta to metamorphose compared with the KM of these same chemicals on the pyridine-activated ion channel of the crayfish A. torrentium.
| Species | ||
| Chemical | C. teleta | A. torrentium |
| Pyrazinecarboxamide | 1 µM | 1.5 µM |
| Nicotinamide | 3 µM | 10 µM |
| Nicotinic acid | 1 mM | >1 mM |
| 4-acetylpyridine | 200 µM | 70 µM |
The concentration of 4-acetylpyridine that inhibited 50% of the larvae of C. teleta is also compared with the IC50 of 4-acetylpyridine for the crayfish (adapted from Hatt and Schmiedel-Jacob, 1984).
Each year, more ligand-gated ion channels have been characterized as having roles in animal’s taste reception [44], although the presence of pyridine receptors used for chemodetection in other animals besides crustaceans to our knowledge has not been reported. Signal transduction carried out by these ion channels may be calcium dependent [45]. Our results indicating that calcium-free seawater inhibited metamorphosis in response to nicotinamide also therefore agree with these data. Although larvae of C. teleta did not metamorphose in the presence of nicotinamide or pyrazinecarboxamide in calcium-free seawater in our study, they did settle to the bottom in those solutions within several minutes. Most likely, the nicotinamide or pyrazinecarboxamide ligand is binding to the channel and opening it; however, without calcium ions entering through the channel, the signal transduction pathway leading to metamorphosis is probably stalled shortly after initiation. These results parallel the results of Biggers and Laufer [18], showing that settlement and metamorphosis of C. teleta can be induced by very low concentrations of the calcium ionophore A23187.
Our results using the 5-HT2B receptor inhibitor ketanserin suggest that the response to nicotinamide and riboflavin requires participation of the serotonergic nervous system as well, since ketanserin inhibited this response. These data suggest that the larvae detect nicotinamide and riboflavin in the environment via chemosensory neurons likely present in the chemosensory cilia noted by Eckelbarger and Grassle [46]. Sensory depolarization of these receptors may lead to the release of serotonin either directly by the chemosensory neurons or indirectly through synaptic activation of other serotonergic neurons. Nitric oxide may play a role as a secondary messenger between the influx of ions caused by the opening of nicotinamide activated ion channels and serotonergic signaling. Larvae of C. teleta did not metamorphose in the presence NOS inhibitors when treated with ketanserin, a serotonin 5-HT2B receptor antagonist [20]. Serotonin binding to receptors then most likely leads to settlement and metamorphosis. In this regard, the serotonin 5-HT2B receptor is known to be a metabotropic receptor involving G-protein activation, secondary messengers, calcium channel activation, and downstream kinase activation such as through mitogen activated protein kinases (MAPK) involved in mediating gene expression [47]. Other chemosensory structures as reviewed by Lindsay [48] may be also be involved in the detection of nicotinamide and riboflavin by these larvae.
Larvae of C. teleta may be foraging for an environment containing nutrient-rich sediment prior to metamorphosing and thereby may be exhibiting foraging behavior in choosing habitats for settlement and metamorphosis. By responding to the B vitamins nicotinamide and riboflavin as chemical settlement cues, they could be ensuring that a selected environment contains the proper vitamins to successfully grow and reproduce. As deposit feeders, C. teleta can utilize these vitamins only if they are contained within marine sediments. Here, larvae of C. teleta seem to be mirroring how adult crustaceans such as crayfish and lobsters sense nutrients within sediments via chemosensory hairs attached to their legs or antennules. The crayfish A. torrentium can sense nicotinamide and other pyridines while the spiny lobster Panulirus argus can sense ATP using their antennules [49]. Interestingly, the crayfish Astacus astacus has been demonstrated to have specific internal oesophageal receptors for nicotinamide [50], and the external receptors on the walking legs and antennules of crustaceans may represent primitive fore-runners of internal chemoreceptors such as the oesophageal receptors [49]. To our knowledge, the present paper is the first demonstration of the presence of nicotinamide or riboflavin receptors in an annelid or in invertebrate larvae.
Materials and Methods
Ethics statement
No permissions were required to collect sediment from the Little Sippewissett salt marsh (West Falmouth, MA) (41°34′34.4″N 70°38′08.6″W). This activity did not involve endangered or protected species.
Care and maintenance of larvae
Adults of Capitella teleta were provided by Dr. Judith Grassle (Rutgers University) and maintained in the lab since 2012 in 9 cm glass dishes containing 30 psu Instant Ocean artificial seawater (hereafter called ASW) at 18°C. Sediment collected from the Little Sippewissett salt marsh (West Falmouth, MA) was sieved through a 1 mm wire mesh screen, frozen for at least 24 h, aerated, and provided as food ad libitum [13], [14]. Cultures were searched for brooding females every 2–3 days; brooding individuals were then transferred individually to 6 cm glass dishes containing ASW. Dishes containing brooding females were checked daily for swimming larvae; larvae were then pipetted into a separate, clean 6 cm glass dish containing ASW. All experiments used larvae released only within the previous 24 hours. C. teleta larvae are usually competent to settle and metamorphose within minutes after their escape from the brood tube [11], [14], so that all larvae should have been competent at the start of our experiments.
General bioassay protocol
All experiments were conducted using either 12-well tissue-culture plates or 3 cm glass dishes, with each well or bowl containing 4 mL of a vitamin solution in ASW, or a negative control (ASW). Each experiment included 3 replicates per treatment, with 8 larvae per replicate. To avoid diluting the final treatment solution, larvae were first pipetted into an intermediate bath of treatment solution (∼20 mL) before being pipetted into the final treatment vessel. Dishes were examined every 30 or 60 minutes under 20–50x magnification to count the number of newly metamorphosed juveniles. Larvae of C. teleta were considered to have metamorphosed when they elongated, lost their prototroch and telotroch cilia, and began crawling on the bottom of the vessel. All experiments were conducted at room temperature (20–23°C) under laboratory fluorescent lighting.
Testing the effects of B vitamins and other chemicals on larval metamorphosis
Thiamine HCl (B1), riboflavin (B2), nicotinamide (B3), nicotinic acid (another form of B3), pyridoxine HCl (B6), biotin (B7) and cobalamin (B12) were obtained from Sigma Chemical Co. Thiamine HCl, nicotinic acid, pyridoxine HCl, biotin, and cobalamin were all tested on larvae of C. teleta at 500 µM. Nicotinic acid was also tested at 1 mM. Riboflavin was tested on larvae of C. teleta at 10, 50, 100, 150, and 200 µM. Nicotinamide was tested on larvae of C. teleta at 2, 3, 4, 5, 6, 7, and 8 µM. The concentrations tested for nicotinamide and riboflavin were based on preliminary pilot studies. The photo-degradation product of riboflavin, lumichrome, was obtained from Sigma Chemical and tested at concentrations of 100, 500, and 1000 µM. The pyridine receptor antagonists and agonists 4-acetylpyridine, pyrazinecarboxamide, and β-NAD were all purchased from Sigma Chemical Co. and tested in final concentrations as stated in results. The serotonin receptor antagonist ketanserin was purchased from Sigma Chemical Co. and prepared as a 10 mM stock solution in ethanol.
Testing the effects of 4-acetylpyridine, calcium-free ASW, and ketanserin on metamorphosis
Larvae were acclimated to ASW containing 200 µM 4-acetylpyridine for one hour and then transferred to a treatment containing either 200 µM 4-acetylpyridine + 40 µM nicotinamide, 200 µM 4-acetylpyridine + 40 µM pyrazinecarboxamide, or 200 µM 4-acetylpyridine as a negative control. Larvae that were not acclimated to 4-acetylpyradine were transferred to positive controls containing either 40 µM nicotinamide or 40 µM pyrazinecarboxamide.
Calcium-free ASW was prepared by omitting calcium chloride from the recipe given in [51]. Larvae that were in calcium-free ASW treatments were acclimated to calcium-free ASW for one hour and then were transferred to treatments containing either calcium-free ASW+40 µM nicotinamide, calcium-free ASW + 40 µM pyrazinecarboxamide, or calcium-free ASW only (negative control). Larvae that were not acclimated to calcium-free ASW were transferred to positive controls containing either 40 µM nicotinamide or 40 µM pyrazinecarboxamide to assess metamorphic competence.
Larvae were acclimated to ASW containing 2 µM ketanserin for three hours and then transferred to a treatment containing either 2 µM ketanserin + 40 µM nicotinamide, 2 µM ketanserin + 40 µM pyrazinecarboxamide, or 2 µM ketanserin as a negative control. Larvae that were not acclimated to ketanserin were transferred to positive controls containing either 40 µM nicotinamide or 40 µM pyrazinecarboxamide.
Data analysis
In order to determine if calcium-free seawater, 4-acetylpyridine, or ketanserin influenced the number of C. teleta larvae that metamorphosed in the presence of nicotinamide or pyrazinecarboxamide, a one-way analysis of variance was conducted on the numbers of C. teleta that had metamorphosed by 24 h after treatment. If the results of an ANOVA were significant, Bonferroni’s post-hoc test was conducted to determine which treatments were significantly different from each other. For each ANOVA, each replicate was the arcsine transformed ratio of individuals that had metamorphosed at that timepoint. It is important to note that, although the figures represented in this paper reflect the percent of C. teleta larvae that had metamorphosed, all statistical analyses were conducted with arcsine transformed ratio of the larvae that had metamorphosed. All statistical analyses were carried out with GraphPad Prism version 5.0.
Acknowledgments
We thank Dr. Judith Grassle (Rutgers University) for sending us a population of C. teleta to begin our cultures here at Tufts University.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported in part by funding from the Howard Hughes Medical Institute to the Biology Department of Wilkes University and Wilkes University Mentoring Task Force grant to WB, GS, and CL. Wilkes University and Tufts FRAC funded the publication of this manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pechenik JA (1990) Delayed metamorphosis by larvae of benthic marine invertebrates: Does it occur? Is there a price to pay? Ophelia 32: 63–94. [Google Scholar]
- 2.Hadfield MG, Paul VJ (2001) Natural chemical cues for settlement and metamorphosis of marine invertebrate larvae. In: McClintock JB, Baker BJ, editors. Marine chemical ecology. Boca Raton: CRC Press. 431–461.
- 3. Pawlik JR (1992) Chemical ecology of the settlement of benthic marine invertebrates. Oceanogr Mar Biol Annu Rev 30: 273–335. [Google Scholar]
- 4. Rittschof D, Forward RB Jr, Cannon G, Welch JM, McClary M Jr, et al. (1998) Cues and context: Larval responses to physical and chemical cues. Biofouling 12: 31–44. [Google Scholar]
- 5. Swanson RL, Byrne M, Prowse TAA, Mos B, Dworjanyn SA, et al. (2012) Dissolved histamine: a potential habitat marker promoting settlement and metamorphosis in sea urchin larvae. Mar Biol 159: 915–925. [Google Scholar]
- 6. Swanson RL, De Nys R, Huggett MJ, Green JK, Steinberg PD (2006) In situ quantification of a natural settlement cue and recruitment of the Australian sea urchin Holopneustes purpurascens . Mar Ecol Prog Ser 314: 1–14. [Google Scholar]
- 7. Swanson RL, Williamson JE, de Nys R, Kumar N, Bucknall MP, et al. (2004) Induction of settlement of larvae of the sea urchin Holopneustes purpurascens by histamine from a host alga. Biol Bull 206: 161–172. [DOI] [PubMed] [Google Scholar]
- 8. Pawlik JR, Faulkner DJ (1986) Specific free fatty acids induce larval settlement and metamorphosis of the reef-building tube worm Phragmatopoma califomica(Fewkes). J Exp Mar Biol Ecol 102: 301–310. [Google Scholar]
- 9. Woodin SA (1991) Recruitment of infauna: positive or negative cues? Am Zool 31: 797–807. [Google Scholar]
- 10. Blake JA, Grassle JP, Eckelbarger KJ (2009) Capitella teleta, a new species designation for the opportunistic and experimental Capitella sp. I, with a review of the literature for confirmed records. Zoosymposia 2: 25–53. [Google Scholar]
- 11. Grassle J, Grassle JF (1976) Sibling species in the marine pollution indicator Capitella (polychaeta). Science 192: 567–569. [DOI] [PubMed] [Google Scholar]
- 12. Butman CA, Grassle JP, Webb CM (1988) Substrate choices made by marine larvae settling in still water and in a flume flow. Nature 333: 771–773. [Google Scholar]
- 13. Dubilier N (1988) H2S-A Settlement Cue or a Toxic Substance For Capitella sp. I Larvae? Biol Bull 174: 30–38. [DOI] [PubMed] [Google Scholar]
- 14. Pechenik JA, Cerulli TR (1991) Influence of delayed metamorphosis on survival, growth, and reproduction of the marine polychaete Capitella sp. I. J Exp Mar Biol Ecol 151: 17–27. [Google Scholar]
- 15. Cohen RA, Pechenik JA (1999) Relationship between sediment organic content, metamorphosis, and postlarval performance in the deposit-feeding polychaete Capitella sp. I. J Exp Mar Biol Ecol 240: 1–18. [Google Scholar]
- 16. Thiyagarajan V, Soo L, Qian P-Y (2005) The role of sediment organic matter composition in larval habitat selection by the polychaete Capitella sp. I. J Exp Mar Biol Ecol 323: 70–83. [Google Scholar]
- 17. Biggers WJ, Laufer H (1992) Chemical induction of settlement and metamorphosis of Capitella capitata sp-I (polychaeta) larvae by juvenile hormone-active compounds. Invert Reprod Devel 22: 39–46. [Google Scholar]
- 18. Biggers WJ, Laufer H (1999) Settlement and metamorphosis of Capitella larvae induced by juvenile hormone-active compounds is mediated by protein kinase C and ion channels. Biol Bull 196: 187–198. [DOI] [PubMed] [Google Scholar]
- 19. Ricker T, Poesnecker R, Deats S, Biggers WJ (2011) Biochemical regulation of settlement and metamorphosis of larvae of Capitella teleta by serotonin. Integr Comp Biol 51: e243. [Google Scholar]
- 20. Biggers WJ, Pires A, Pechenik JA, Johns E, Patel P, et al. (2012) Inhibitors of nitric oxide synthase induce larval settlement and metamorphosis of the polychaete annelid Capitella teleta . Invert Reprod Devel 56: 1–13. [Google Scholar]
- 21. Bishop CD, Pires A, Norby S-W, Boudko D, Moroz LL, et al. (2008) Analysis of nitric oxide-cyclic guanosine monophosphate signaling during metamorphosis of the nudibranch Phestilla sibogae Bergh (Gastropoda: Opisthobranchia). Evol Dev 10: 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leise EM, Thavaradhara K, Durham NR, Turner BE (2001) Serotonin and nitric oxide regulate metamorphosis in the marine snail Ilyanassa obsoleta . Am Zool 41: 258–267. [Google Scholar]
- 23. Pechenik JA, Cochrane DE, Li W, West ET, Pires A, et al. (2007) Nitric oxide inhibits metamorphosis in larvae of Crepidula fornicata, the slippershell snail. Biol Bull 213: 160–171. [DOI] [PubMed] [Google Scholar]
- 24. Bishop CD, Brandhorst BP (2001) NO/cGMP signaling and HSP90 activity represses metamorphosis in the sea urchin Lytechinus pictus . Biol Bull 201: 394–404. [DOI] [PubMed] [Google Scholar]
- 25. Bishop CD, Bates WR, Brandhorst BP (2001) Regulation of metamorphosis in ascidians involves NO/cGMP signaling and HSP90. J Exp Zool 289: 374–384. [DOI] [PubMed] [Google Scholar]
- 26. Comes S, Locascio A, Silvestre F, d'Ischia M, Russo GL, et al. (2007) Regulatory roles of nitric oxide during larval development and metamorphosis in Ciona intestinalis . Dev Biol 306: 772–784. [DOI] [PubMed] [Google Scholar]
- 27. Gremare A, Marsh AG, Tenore KR (1988) Short-term reproductive responses of Capitella sp. I (Annelida: Polychaeta) fed on different diets. J Exp Mar Biol Ecol 123: 147–162. [Google Scholar]
- 28. Marsh AG, Gremare A, Dawson R, Tenore KR (1990) Translocation of algal pigments to oocytes in Capitella sp. I.(Annelida: Polychaeta). Mar Ecol Prog Ser 67: 301–304. [Google Scholar]
- 29. Gotthold ML, Koch J (1974) Further development of an artificial medium for the axenically cultured annelid Enchytraeus fragmentosus . Comp Biochem Physiol 48: 307–314. [DOI] [PubMed] [Google Scholar]
- 30. Hatt H, Franke C (1987) Taste receptors in crayfish: recording of single nicotinamide-activated channels. Neurosci Lett 73: 137–142. [DOI] [PubMed] [Google Scholar]
- 31. Hatt H, Schmiedel-Jakob I (1984) Electrophysiological studies of pyridine-sensitive units on the crayfish walking leg. J Comp Physiol A 154: 855–863. [Google Scholar]
- 32. Hatt H, Schmiedel-Jakob I (1985) Specific antagonists at the pyridine receptor: evidence from electrophysiological studies with acetylpyridines. Chem Senses 10: 317–323. [Google Scholar]
- 33. Panzeca C, Beck AJ, Tovar-Sanchez A, Segovia-Zavala J, Taylor GT, et al. (2009) Distributions of dissolved vitamin B12 and Co in coastal and open-ocean environments. Estuar Coast Shelf S 85: 223–230. [Google Scholar]
- 34. Giovannoni SJ (2012) Vitamins in the sea. Proc Natl Acad Sci USA 109: 13888–13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sañudo-Wilhelmy SA, Cutter LS, Durazo R, Smail EA, Gómez-Consarnau L, et al. (2012) Multiple B-vitamin depletion in large areas of the coastal ocean. Proc Natl Acad Sci USA 109: 14041–14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsukamoto S, Kato H, Hirota H, Fusetani N (1999) Lumichrome. Eur J Biochem 264: 785–789. [DOI] [PubMed] [Google Scholar]
- 37. Kato T, Kumanireng AS, Ichinose I, Kitahara Y, Kakinuma Y (1975) Active components of Sargassum tortile effecting the settlement of swimming larvae of Coryne uchidai . Experientia 31: 433–434. [DOI] [PubMed] [Google Scholar]
- 38. Tsutsumi H, Fukunaga S, Fujita N, Sumida M (1990) Relationship between growth of Capitella sp. and organic enrichment of the sediment. Mar Ecol Prog Ser 63: 157–162. [Google Scholar]
- 39. Brown MR, Mular M, Miller I, Farmer C, Trenerry C (1999) The vitamin content of microalgae used in aquaculture. J Appl Phycol 11: 247–255. [Google Scholar]
- 40. Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, et al. (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci USA 105: 3968–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sims GK, O'Loughlin EJ (1992) Riboflavin production during growth of Micrococcus luteus on pyridine. Appl Environ Microbiol 58: 3423–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mitra S, Thawrani D, Banerjee P, Gachhui R, Mukherjee J (2012) Induced biofilm cultivation enhances riboflavin production by an intertidally derived Candida famata . Appl Biochem Biotechnol 166: 1991–2006. [DOI] [PubMed] [Google Scholar]
- 43. Ohwada K, Taga N (1972) Distribution and seasonal variation of vitamin B 12, thiamine and biotin in the sea. Mar Chem 1: 61–73. [Google Scholar]
- 44. Pellegrino M, Nakagawa T (2009) Smelling the difference: controversial ideas in insect olfaction. J Exp Biol 212: 1973–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meunier N, Marion-Poll F, Lucas P (2009) Water taste transduction pathway is calcium dependent in Drosophila . Chem Senses 34: 441–449. [DOI] [PubMed] [Google Scholar]
- 46. Eckelbarger KJ, Grassle JP (1987) Interspecific variation in genital spine, sperm, and larval morphology in six sibling species of Capitella . Bull Biol SocWash 7: 62–76. [Google Scholar]
- 47. Launay J-M, Birraux G, Bondoux D, Callebert J, Choi D-S, et al. (1996) Ras involvement in signal transduction by the serotonin 5-HT2B receptor. J Biol Chem 271: 3141–3147. [DOI] [PubMed] [Google Scholar]
- 48. Lindsay SM (2009) Ecology and biology of chemoreception in polychaetes. Zoosymposia 2: 339–367. [Google Scholar]
- 49. Carr WE, Ache BW, Gleeson RA (1987) Chemoreceptors of crustaceans: similarities to receptors for neuroactive substances in internal tissues. Environ Health Perspect 71: 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Altner H, Hatt H, Altner I (1986) Structural and functional properties of the mechanoreceptors and chemoreceptors in the anterior oesophageal sensilla of the crayfish, Astacus astacus . Cell Tissue Res 244: 537–547. [Google Scholar]
- 51. Baloun AJ, Morse DE (1984) Ionic control of settlement and metamorphosis in larval Haliotis rufescens (Gastropoda). Biol Bull 167: 124–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.