Abstract
Resistance nodulation division (RND) efflux pumps, such as the SmeIJK pump of Stenotrophomonas maltophilia, are known to contribute to the multidrug resistance in Gram-negative bacteria. However, some RND pumps are constitutively expressed even though no antimicrobial stresses occur, implying that there should be some physical implications for these RND pumps. In this study, the role of SmeIJK in antimicrobials resistance, envelope integrity, and σE-mediated envelope stress response (ESR) of S. maltophilia was assessed. SmeIJK was involved in the intrinsic resistance of S. maltophilia KJ to aminoglycosides and leucomycin. Compared with the wild-type KJ, the smeIJK deletion mutant exhibited growth retardation in the MH medium, an increased sensitivity to membrane-damaging agents (MDAs), as well as activation of an σE-mediated ESR. Moreover, the expression of smeIJK was further induced by sub-lethal concentrations of MDAs or surfactants in an σE-dependent manner. These data collectively suggested an alternative physiological role of smeIJK in cell envelope integrity maintenance and σE-mediated ESR beyond the efflux of antibiotics. Because of the necessity of the physiological role of SmeIJK in protecting S. maltophilia from the envelope stress, smeIJK is constitutively expressed, which, in turn, contributes the intrinsic resistance to aminoglycoside and leucomycin. This is the first demonstration of the linkage among RND-type efflux pump, cell envelope integrity, and σE-mediated ESR in S. maltophilia.
Introduction
Efflux pump systems are present in all organisms and recognized to transport a range of structurally unrelated compounds and confer clinically relevant resistances to antimicrobial agents. There are five families of efflux pumps associated with multidrug resistance (MDR): the ATP binding cassette (ABC) family, the multidrug and toxic-compound extrusion (MATE) family, the major facilitator (MFS) family, the resistance nodulation division (RND) family, and the small multidrug resistance (SMR) family [1]. The RND efflux pumps consist of a RND-type transporter protein, which is located in the inner membrane; a membrane-fusion protein (MFP), which is located in the periplasmic space; and an outer membrane protein, which is located in the outer membrane of the bacterium. Many studies have evidenced that the RND-type efflux pumps not only confer resistance to drugs but also have a physiological role in stress adaption [2]. It is possible that the efflux pump-mediated antibiotics resistance is a by-product of the physiological role(s) of these pumps, especially for the constitutively expressed efflux pumps. Several proposed examples can evidence this viewpoint; for example, the AcrAB-TolC system of Escherichia coli and the CmeABC system of Campylobacter jejuni can export noxious metabolites, such as bile acid, produced by the host organism [3], [4]. In addition, several RND-type efflux pumps display inducibly expression upon the treatment of stresses, further linking the stress adaption and the efflux pumps.
Bacteria encounter an array of potentially growth compromising conditions in nature. Cell envelope is the major component of the defense against environmental threats for bacteria. Bacterial cells possess a variety of mechanisms to monitor and keep the cell envelope integrity. A variety of stresses, which affect components of the cell envelope, will intrigue the envelope stress responses (ESRs) [5]. In response to the various extracellular changes, extracytoplasmic function (ECF) σ factor provides a mean to sense external changes and regulate gene expression to prevent or repair cellular damages caused by stresses [6]. One of the best-studied ECF σ factors is the σE, which is a key regulator of ESRs in E. coli and other Gram-negative bacteria [7]. In unstressed cells, σE generally interacts with an antisigma factor RseA, a single-pass inner membrane protein, that prevents σE from interacting with RNA polymerase and keeps σE inactive. When envelope stresses occur, inhibition of σE is relieved by the complete degradation of RseA via regaulted intramembrane proteolysis (RIP) [8]. The activated σE acts as a transcription factor to affect the expression of σE regulon genes. In most cases, the rpoE and rseA genes are organized in an operon.
Stenotrophomonas maltophilia, ubiquitous in environment, is an opportunistic pathogen involved in many nosocomial infections [9]. The genome information of S. maltophilia K279a predicted that there are eight putative RND efflux pumps, SmeABC, SmeDEF, SmeGH, SmeIJK, SmeMN, SmeOP, SmeVWX, and SmeYZ [10]. Of them, the SmeIJK pump is special because it consists of two different RND-type transporters, SmeJ and SmeK. It has been reported that the smeIJK operon is intrinsically expressed and can be further overexpressed in some mutants. The expression of smeIJK confers the resistance to aminoglycosides, tetracycline, minocycline, ciprofloxacine, and levofloxacin [11]. However, the constitutive expression of the smeIJK operon in strains maintained in the absence of antibiotic selective pressure raises the possibility that drug extrusion is not the only or main function of the SmeIJK pump. Accordingly, the physiological function of smeIJK was further assessed in this study.
Materials and Methods
Bacterial strains and culture conditions
A complete list of strains, plasmids, and primers used in this study is shown in Table S1.
Construction of deletion mutants
Four PCR amplicons (labeled as I-IV in Fig. S1) were amplified using primer sets of SmeI5-F/SmeI5-R, SmeJ5-F/SmeJ5-R, SmeK5-F/SmeK5-R, and SmeK3-F/SmeK3-R (Table S1), respectively. Amplicons II and III were subsequently cloned into pEX18Tc to yield the recombinant plasmid pΔSmeJ, in which the cloned smeJ gene was partially deleted. Similar constructs for pΔSmeK and pΔSmeIJK were done by assembling the amplicons of III and IV as well as I and IV respectively, yielding pΔSmeK and pΔSmeIJK. Three PCR amplicons (labeled as I-III in Fig. S2) were amplified using primer sets of RpoE5-F/RpoE5-R, RpoE3-F/RpoE3-R, and RseA3-F/RseA3-R (Table S1), respectively. Recombinant plasmids pΔRpoE and pΔRseA were obtained by subsequently cloning the amplicons I and II as well as II and III into pEX18Tc. The plasmids mobilization, transconjugants selection, and mutant confirmation were performed as described previously [12]. The smeJK and rpoErseA double mutants were constructed from a single mutant.
Construction of the rpoE expression plasmid, pRpoE
The intact rpoE gene was PCR amplified from genomic DNA template of S. maltophilia KJ by using primer sets of RpoE5-F and RpoE3-R and then cloned into plasmid pRK415, generating plasmid pRpoE.
Susceptibility testing
The antibacterial activities of agents were determined on Mueller-Hinton agar plates. Agar plates were prepared by the twofold agar dilution technique recommended by Clinical and Laboratory Standards Institute (CLSI) [13]. The MIC was defined as the lowest concentrations that inhibited bacterial cell growth. All antimicrobial agents used were purchased from Sigma Aldrich.
In vitro growth curves
Overnight cultures of S. maltophilia were inoculated into the fresh medium to the A 450 of 0.15. Cells were grown aerobically and the A 450 was measured every 3 h.
Osmotic challenge assay
The 24-h cultured bacterial cells of strains KJ, KJΔIJK, KJΔJ, and KJΔK were inoculated into fresh LB or MH broths containing different concentrations of NaCl with an A450 nm of 0.15. The bacteria were further cultured for 5 h and the A450 nm were recorded. The relative survival percentage of individual mutant to the wild-type KJ, at each cultured condition, was calculated. These experiments were performed at least three times.
Sodium dodecyl sulfate (SDS) survival analysis
Overnight cultures of the tested strains were diluted to A 450 nm of 0.15 with LB or MH broth. Cells grown to stationary phase were adjusted to A 450 nm of 1.0 with the same broth. The cells were treated with or without 0.02% SDS. A final A 450 nm measurement was taken after 10 min of incubation without shaking. 100% survival was defined as the absorbance of each strain without SDS. The percentage of survival was defined as the A 450 nm ratio of the SDS-additive group to the SDS-free counterpart.
Polymyxin E susceptibility test
LB or MH plates were streaked with a cotton swab soaked in S. maltophilia cell suspension of 107 cells/ml. The commercial discs of 10 mg polymyxin E was placed at the centre onto the agar surface. The culture was then incubated at 37°C for 24 hours. The diameter of a zone of inhibition was measured (in millimeters). Each experiment was repeated at least three times.
Construction of PsmeI-xylE and PrpoE-xylE transcription fusion, pSmeIxylE and pRpoExylE
The 465-bp DNA fragment upstream of smeIJK operon and the 414-bp DNA fragment upstream of the rpoE-rseA-mucD operon were obtained by PCR using primer sets SmeI5-F/SmeI5-R (Fig. S1 & Table S1) and RpoE5-F/RpoE5-R (Fig. S2 & Table S1), respectively. The plasmid-borne transcription fusions, pSmeIxylE and pRpoExylE, were constructed by ligating the 465-and 414-bp DNA fragment into the xylE-reporter plasmid pRKXylE, respectively. The plasmids, pSmeIxylE and pRpoExylE, were mobilized into S. maltophilia strains indicated for promoter activity assay.
Stress challenge assays
Overnight cultures of KJ(pSmeIxylE) were subcultured into fresh LB medium with anA450 nm of 0.15. After 5 h incubation (37°C, 170 rpm), the KJ(pSmeIxylE) cells were divided into two parts. One part served as a non-treated control and the second part received different stress challenges for 3 h. The stress included Triton X-100 (100 µg/ml), benzalkonium chloride (BC) (10 µg/ml), cetyltributylammonium bromide (CTAB) (10 µg/ml), gentamicin (5 µg/ml), amikacin (5 µg/ml), and leucomycin (1 µg/ml). All antimicrobial agents used were purchased from Sigma Aldrich. The C23O activity of KJ(pSmeIxylE) was determined. The C23O activity determined from the non-treated control was regarded as 100%.
Catechol 2,3-dioxygenase (C23O) activity assay
Catechol-2,3-dioxygenase is encoded by the xylE gene and its activity was measured as the rate of increase in A375 nm following the addition of 100 mM catechol, as described elsewhere [14]. The rate of hydrolysis was calculated by using 44,000 M−1cm−1 as the extinction coefficient. One unit of enzyme activity (U) was defined as the amount of enzyme that converts 1 nmole substrate per minute. The specific activity was expressed as U/OD450 nm.
Results
Either SmeJ or SmeK protein can support the SmeIJK pump function
Eight RND-type efflux systems were predicted to be present in the S. maltophilia K279a genome [10]. Of them, the smeIJK is unique for the presence of two tandem RND-type inner membrane transporters (Fig. S1). The SmeI protein, encoded by the annotated Smlt4279 gene, was predicted to be an MFP, as well as SmeJ and SmeK, encoded by the annotated Smlt4280 and Smlt4281 genes respectively, to be RND-type inner membrane transporters. SmeJ and SmeK showed a significant similarity (42% identity and 59% similarity). To assess whether both RND transporters are required for pump function or either RND transporter can independent associate with SmeI and cognate outer membrane protein for the assembly of functional tripartite pumps, we constructed strains with various combinations of deletion in RND transporters and assessed efflux pump function by the susceptibility test. KJΔJ, KJΔK, KJΔJK, and KJΔIJK were the smeJ, smeK, smeJK, and smeIJK isogenic mutants of wild-type KJ, respectively (Fig. S1). The consideration of polar effect in KJΔJ was assessed by qRT-PCR. The smeK transcript of KJΔJ was comparable to that of the wild-type KJ, indicating that inactivation of smeJ had no polar effect on the expression of downstream smeK gene. Eight- to 16-fold increase in the susceptibility to aminoglycoside and 4-fold decrease in the resistance to leucomycin were observed for KJΔJK, as compared to the MICs of the parent strain KJ. Deletion of smeJ or smeK alone slightly altered the susceptibility of strain KJ to the aminoglycosides tested (two-fold MIC difference) (Table 1). Although the acceptable inaccuracy for the susceptibility test is in the range of 2-fold MIC value, it cannot be immediately ruled out that SmeJ and SmeK may be partially functionally redundant and simultaneous inactivation of smeJK may has an additive effect on aminoglycosides extrusion. Furthermore, Table 1 also demonstrates that mutants KJΔJK and KJΔIJK displayed the same susceptibility to all the antimicrobials tested, supporting that smeI deletion makes little contribution to the additive phenotype of smeJK mutant.
Table 1. Antimicrobial susceptibilities of S. maltophilia KJ and its derived deletion mutants.
| Antimicrobial | MIC (µg/ml) | ||||
| KJ | KJΔJ | KJΔK | KJΔJK | KJΔIJK | |
| Chloramphenicol | 8 | 8 | 8 | 8 | 8 |
| Quinolone | |||||
| Nalidixic acid | 8 | 8 | 8 | 8 | 8 |
| Norfloxacin | 16 | 16 | 16 | 16 | 16 |
| Tetracycline | |||||
| Deoxycycline | 1 | 1 | 1 | 0.5 | 0.5 |
| Tetracycline | 16 | 16 | 16 | 8 | 8 |
| Aminoglycoside | |||||
| Amikacin | 1024 | 512 | 512 | 64 | 64 |
| Gentamicin | 1024 | 512 | 512 | 64 | 64 |
| Kanamycin | 256 | 128 | 128 | 32 | 32 |
| Tobramycin | 512 | 256 | 256 | 64 | 64 |
| Macrolide | |||||
| Erythromycin | 64 | 64 | 64 | 64 | 64 |
| Leucomycin | 256 | 256 | 256 | 64 | 64 |
| Rokitamycin | 512 | 512 | 512 | 512 | 512 |
The smeIJK mutant displays a compromised growth in Mueller-Hinton (MH) medium, but not in Luria-Bertani (LB) medium
The phenotype of KJΔIJK grown in the MH agar attracted our attention during the susceptibility test. In comparison, bacterial lawn of KJΔIJK on the MH agar was homogeneously smaller and thinner than those of KJ, KJΔJ, and KJΔK. Nevertheless, the bacterial lawns of KJ, KJΔJ, KJΔK, and KJΔIJK were almost the same size in the LB agar.
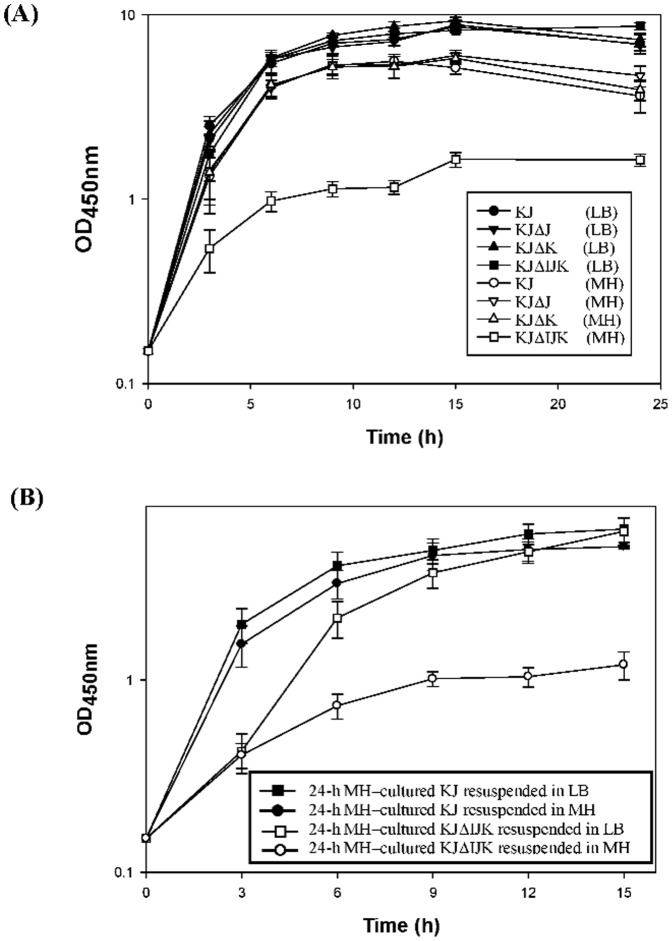
To further clarify this observation, the growth curves of KJ, KJΔJ, KJΔK, and KJΔIJK in MH and LB broth were monitored. As shown in Fig. 1A, KJ, KJΔJ, and KJΔK grown in the MH broth exhibited slightly reduced growth rates compared to those grown in the LB broth. However, the growth patterns of KJΔIJK were distinctly different when it grew in MH and LB broth. In the MH broth, the doubling time of KJΔIJK was approximately 1.71-fold longer than that of KJ, KJΔJ, and KJΔK. In the meantime, the maximum OD450 achieved for KJΔIJK in the MH broth was approximately 1.63, which was significantly lower than those for KJ, KJΔJ, and KJΔK in the MH broth. Nevertheless, the growth curve for KJΔIJK grown in the LB broth was indistinguishable from those for KJ, KJΔJ, and KJΔK (Fig. 1A).
Figure 1. Growth of KJ, KJΔJ, KJΔK, and KJΔIJK in Luria-Bertani (LB) and Mueller-Hinton (MH) media.
(A) Growth curves of KJ, KJΔJ, KJΔK, and KJΔIJK in LB and MH broth. (B) The growth curve of 24-h MH-cultured KJ and KJΔIJK cells subcultured into the MH or LB media.
The growth retardation of KJΔIJK in MH medium can be reverted to normal when the bacteria were shifted from MH medium into LB medium
Since RND-type efflux pump has been known to be responsible for extrusion of noxious compounds, we speculated that the growth retardation of smeIJK mutant in the MH broth may result from the accumulation of some noxious metabolites, which should be extruded by the SmeIJK pump. The putative noxious metabolites should be present in the MH broth, but absent in the LB broth. To test this assumption, the bacterial cells of KJ and KJΔIJK grown in the MH broth for 24 h were collected and resuspended into a fresh LB broth and MH broth, respectively, to an A450 nm of 0.15, and then the growth curves of KJΔIJK in the LB broth and MH broth were monitored. If there are noxious metabolites present in the 24-h MH-cultured KJΔIJK cells, the initial growth rate must be significantly retarded when the cells were shifted to the fresh MH broth. However, when they were shifted to the fresh MH medium, the 24-h MH-cultured-KJΔIJK cells still can logarithmically grow for 9 h (Fig. 1B). Furthermore, the growth of 24-h MH-cultured-KJΔIJK cells was gradually reverted to the wild-type pattern after LB broth shift (Fig. 1B). Therefore, the ΔsmeIJK-mediated growth retardation in the MH broth is reversible when the cultured environment is changed from the MH broth to the LB broth, suggesting that a component present in MH medium but absent from LB medium determined the growth compromise in MH medium.
SmeIJK mutant displayed decreased tolerance to hypo-osmolarity and casein hydrolysate
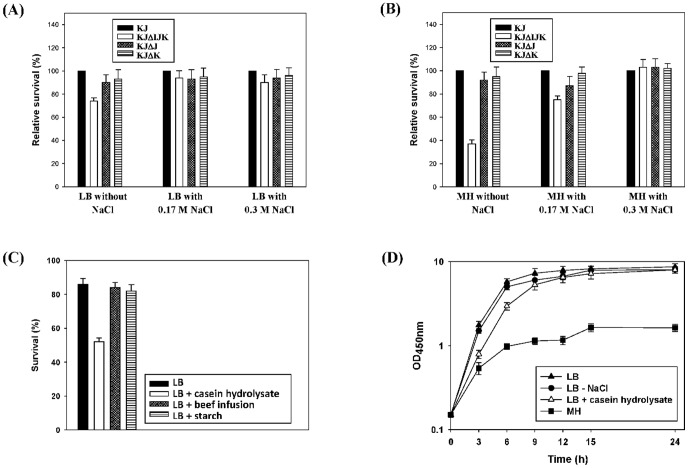
An MH medium typically contains 0.3% beef infusion, 1.75% casein hydrolysate, and 0.15% starch. The ingredients for LB media were 1% tryptone, 0.5% yeast extract, and 1% (0.17 M) NaCl. Given the compositions of the MH and LB media, we speculated that the observed growth retardation in the MH medium was due to the absence of NaCl in the medium. To test this assumption, three LB-based media with a final NaCl concentration of 0, 0.17, and 0.3 M were prepared. The osmotic challenge assay to KJ, KJΔJ, KJΔK, and KJΔIJK cells was determined. KJΔIJK displayed a reduced survival in the 0 M NaCl LB medium when compared to the wild-type KJ. Nevertheless, the survival of KJΔIJK cells restored to nearly wild-type levels in the LB supplemented with 0.17 M and 0.3 M NaCl (Fig. 2A). The phenotype correlated smeIJK to the hypo-osmolarity tolerance. Furthermore, the survivals of KJΔJ and KJΔK were similar to that of the wild-type KJ in spite of the concentration of NaCl in the cultured medium (Fig. 2A). The osmotic challenge assay of KJ, KJΔJ, KJΔK, and KJΔIJK was also performed in three MH-based media containing 0, 0.17, and 0.3 M NaCl. The results concluded from the MH counterpart (Fig. 2B) is consistent with those from the LB counterpart. It is worthily noted that the ΔsmeIJK-mediated compromise in hypo-osmolarity tolerance was more apparent in the MH counterpart than that in the LB counterpart, implying that some components, in addition to NaCl, in the MH medium may cause an envelope stress to S. maltophilia. To test this assumption, we supplemented LB with individual compounds present in MH medium to determine which component affected susceptibility to SDS. Only the addition of casein hydrolysate increased the sensitivity of KJ cells to SDS (Fig. 2C)
Figure 2. The role of SmeIJK pump in the tolerance to hypo-osomolarity and casein hydrolysate.
The data are the average of the measurements made in triplicate. (A) The relative survival of KJΔIJK, KJΔJ, and KJΔK to the wild-type KJ in the LB medium containing different concentrations of NaCl. The relative survival percentage of individual mutant to the wild-type KJ, at each cultured condition, was calculated using the OD450 nm of the wild-type KJ as 100%. (B) The relative survival of KJΔIJK, KJΔJ, and KJΔK to the wild-type KJ in the MH medium containing different concentrations of NaCl. The relative survival percentage of individual mutant to the wild-type KJ, at each cultured condition, was calculated using the OD450 nm of the wild-type KJ as 100%. (C) The sensitivities of KJ to SDS in LB or LB containing casein hydrolysate, beef infusion, or starch were determined by the OD450 nm measurement. The percentage of survival was defined as the OD450 nm ratio of the SDS-additive group to the SDS-free counterpart. (D) Growth curves of KJΔIJK grown in the media of the LB, the LB without NaCl, the LB with casein hydrolysate, and the MH, respectively.
To further elucidate whether the presence of casein hydrolysate and the absence of NaCl result in the ΔsmeIJK-mediated growth compromise in the MH medium. The growth curves of KJΔIJK cells grown in the LB, the LB without NaCl, the LB with casein hydrolysate, and the MH were monitored. As shown in Fig. 2D, the logarithmic-phase growth of KJΔIJK cells was compromised when casein hydrolysate was added into the LB medium; however, the stationary-phase growth of KJΔIJK cells in the LB with casein hydrolysate was comparable to that in the LB.
Loss of SmeIJK compromised the cell envelope integrity
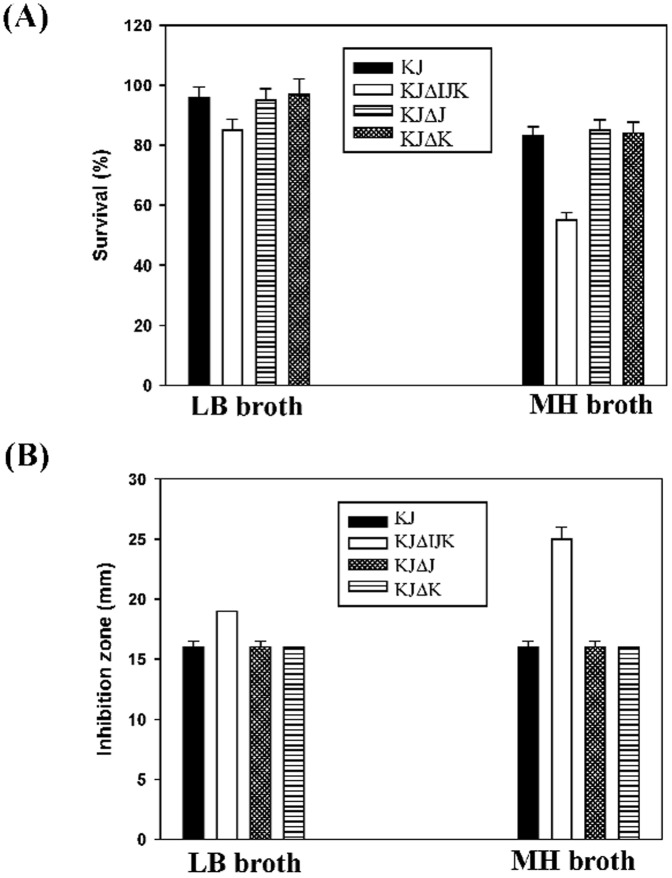
Compared with the wild-type KJ, KJΔIJK was more sensitive to hypo-osmolarity environments (Fig. 2). KJΔIJK can thus exhibit a perturbation in the cell envelope integrity. We performed the survival experiments with a 0.02% SDS challenge for 10 min on cells. KJΔIJK was found to be more susceptible to SDS stress than the parental strain KJ. Moreover, the LB-grown KJΔIJK cells were more tolerant to SDS challenge than the MH-grown ones (Figure 3A). KJΔJ and KJΔK cells kept the similar SDS sensitivity as the wild-type KJ cells did.
Figure 3. Assessment of the cell envelope integrity of S. maltophilia KJ and its derived mutants in different cultured conditions.
(A) Sodium dodecyl sulfate (SDS) survival analysis. The survival of KJ, KJΔIJK, KJΔJ, and KJΔK in LB or MH broth without or with 0.02% SDS was determined by the A450 nm measurement. The percentage of survival was defined as the A450 nm ratio of the SDS-additive group to the SDS-free counterpart. (B) Polymyxin E susceptibility. The polymyxin E susceptibility of KJ, KJΔIJK, KJΔJ, and KJΔK in LB or MH broth was determined by the disc diffusion assay.
Polymyxins can bind to lipopolysaccharide (LPS) in the outer membrane of Gram-negative bacteria, disrupting both the outer and inner membranes. Therefore, the susceptibility to polymyxins should be increased if the cell envelope integrity of bacteria is compromised. The polymyxin E susceptibility of KJ, KJΔIJK, KJΔJ, and KJΔK was thus assessed. KJΔIJK was more sensitivity to polymyxin E than KJ, KJΔJ, and KJΔK, especially in the MH medium (Fig. 3B).
Loss of SmeIJK activated the σE regulon
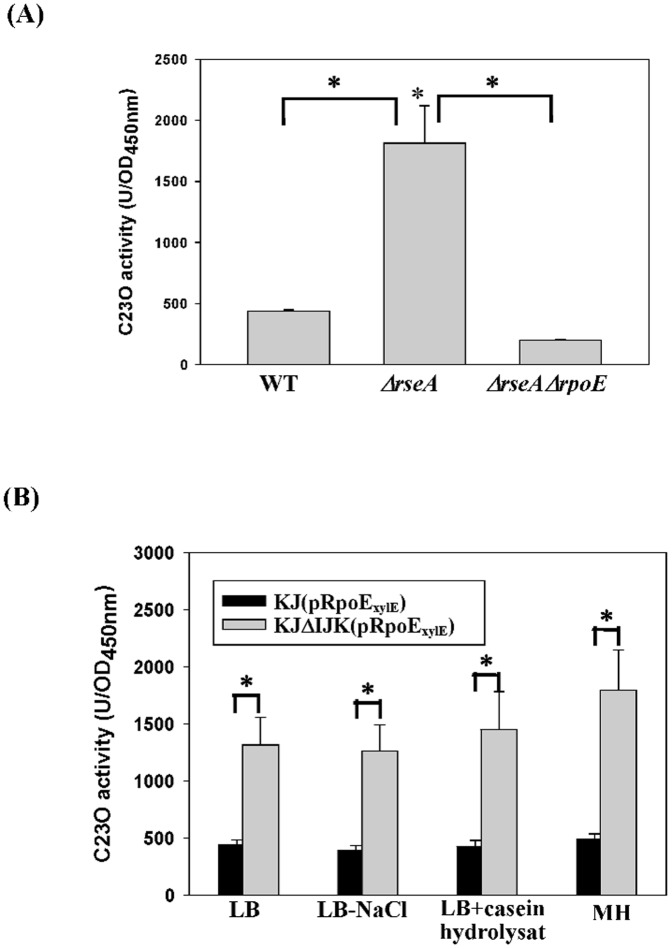
Envelope stress responses (ESRs) are well documented in bacteria, with the σE being a key regulator of ESRs in several bacteria such as E. coli [15], Pseudomonas aeruginosa [16], and Xanthomonas campestris [17]. Because ΔsmeIJK may cause a compromise in the envelope integrity (Fig. 3), whether ΔsmeIJK-mediated ESR triggers rpoE regulon activation in S. maltophilia is of great importance. Based on the conserved genes organization of rpoE and anti-rpoE from various bacterial species, the putative rpoE/anti-rpoE operon was genome-widely searched in S. maltophilia K279a genome. Three flanking genes (Smlt3555-Smlt3554-Smlt3553), which encode putative σE, RseA, and MucD proteins, attracted our attention (Fig. S2). The genomic organization of rpoE-rseA-mucD in S. maltophilia is identical to that in X. campestris pv. campestris [17], and their encoded proteins share the identities of 89%, 48%, and 66% for σE, RseA, and MucD, respectively (Fig. S2). The consensus DNA sequences recognized by σE of X. campestris are -35 (5′-GAACTT-3′) and -10 (5′-TCTCA-3′) [18], which are identified upstream of S. maltophilia rpoE gene (Fig. S2), indicating that the rpoE-rseA-mucD operon can be a member of σE regulon in S. maltophilia, like that in X. campestris [17]. To test it, a PrpoE-xylE transcription-fusion reporter plasmid pRpoExylE was constructed. The plasmid was introduced into the wild-type KJ, KJΔRseA (an rseA isogenic mutant) and KJΔRpoEΔRseA (an rpoE and rseA double mutant), respectively. The PrpoE activity obviously increased when rseA was inactivated, and significantly decreased to the level below the wild-type when rseA and rpoE were simultaneously inactivated (Fig. 4A), indicating that the σE is a member of σE regulon in S. maltophilia, which has been commonly seen in many other bacteria [17]. Therefore, the extent of rpoE expression can be used as the indicator of σE regulon activation.
Figure 4. Determination of PrpoE activities in different strains and culture conditions.
Plasmid containing a transcriptional fusion of the upstream region of rpoE to the xylE gene (pRpoExylE) was transferred into the wild-type KJ and its derived mutants. The C23O activities of the logarithmic-phase cultures of these strains were determined. Each bar represents the mean of three independent experiments. Error bars, where visible, indicate the average deviation. *, p≤0.01 significance calculated by a Student's t-test. (A) The impacts of resA mutant and rseA/rpoE double mutant on the promoter activity of rpoE gene. (B) The impacts of smeIJK mutant and culture media on the promoter activity of rpoE gene.
In view of the involvement of SmeIJK pump in the maintenance of envelope integrity, we further tested whether smeIJK inactivation activates the σE regulon. The rpoE expression in KJ and KJΔSmeIJK cells grown in different culture media was determined. The culture media tested included the LB, the LB without NaCl, the LB with casein hydrolysate, and the MH. Compared with wild-type, smeIJK mutant caused a 3.0-, 3.1-, 3.4-, and 3.7-fold increment in the PrpoE-driven C23O activity in the LB, the LB without NaCl, the LB with casein hydrolysate, and the MH, respectively. (Fig. 4B).
MDA-mediated, σE-dependent SmeIJK up-regulation
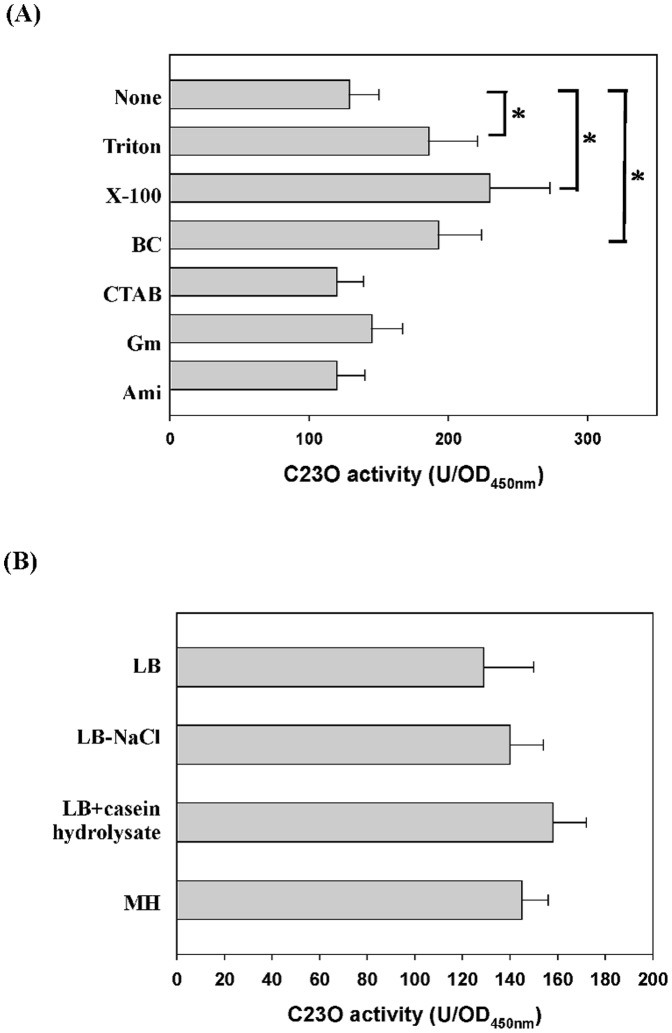
Studies of stresses that alter smeIJK operon expression can give important insights into its physiological function beyond antibiotics extrusion. For this purpose, a PsmeIJK-xylE transcription fusion construct, pSmeIxylE, was prepared. The impact of stresses on the smeIJK expression was evaluated by monitoring the C23O activities of KJ(pSmeIxylE). The addition of membrane-damaging agents (MDAs) (Triton X-100) and surfactants (benzalkonium chloride (BC) and cetyltributylammonium bromide (CTAB)) moderately increased the expression of smeIJK operon (Fig. 5A). However, no significant increase in the C23O activity was seen when KJ(SmeIxylE) cells were treated with gentamicin, amikacin, and leucomycin, which are the known substrates of SmeIJK pump (Fig. 5A). Furthermore, the smeIJK expression of KJ cells was less relevant to the types of culture media tested (Fig. 5B)
Figure 5. The expression of smeIJK operon in different stresses and culture media.
Plasmid containing a transcriptional fusion of the upstream region of smeI to the xylE gene (pSmeIxylE) was transferred into the wild-type KJ. The C23O activities of the logarithmic-phase cultures of KJ(pSmeIxylE) were determined. Each bar represents the mean of three independent experiments. Each bar represents the mean of three independent experiments. *, p≤0.01 significance calculated by a Student's t-test. (A) The expression of smeIJK operon in different stresses. The concentrations of the stressors added were: Triton X-100, 100 µg/ml; benzalkonium chloride (BC), 10 µg/ml; cetyltributylammonium bromide (CTAB), 10 µg/ml; gentamicin (Gm), 1 µg/ml; amikacin (Ami), 1 µg/ml; and leucomycin (Leu), 0.5 µg/ml. (B) The expression of smeIJK operon in different culture media, including the LB, the LB without NaCl, the LB with casein hydrolysate, and the MH.
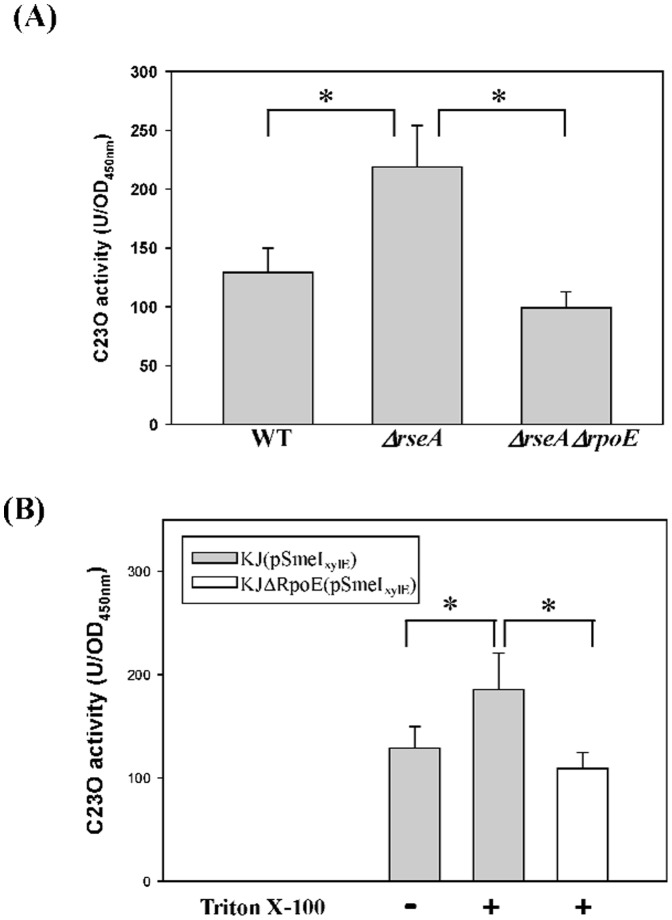
It is well perceived that the treatment of MDAs generally causes an σE-mediated envelope stress response (ESR) in Gram-negative bacteria. The involvement of σE in the MDAs-mediated smeIJK up-regulation is of interest. Firstly, we checked whether the smeIJK is subjected to the regulation of rpoE pathway. Compared with that in the wild-type KJ, the PsmeIJK activity in the rseA mutant increased approximately 1.7 fold and restored to the wild-type level in the ΔrseAΔrpoE double mutant (Fig. 6A), supporting that rpoE activation leads to the up-regulation of smeIJK operon. In addition, the smeIJK expression in the σE-overexpression KJ cells (KJ(pRpoE)) was assessed by qRT-PCR. The smeK transcript in KJ(pRpoE) cells had a 2.1-fold increment compared to that in KJ(pRK415) cells, further verifying that smeIJK is a member of σE regulon.
Figure 6. Determination of PsmeI activities in different strains.
Plasmid containing a transcriptional fusion of the upstream region of smeI to the xylE gene (pSmeIxylE) was transferred into the wild-type KJ and its derived mutants. The C23O activities of the logarithmic-phase cultures of these strains were determined. Each bar represents the mean of three independent experiments. Error bars, where visible, indicate the average deviation. *, p≤0.01 significance calculated by a Student's t-test. (A) The impacts of resA mutant and rseA/rpoE double mutant on the promoter activity of smeIJK operon. (B) The impacts of MDAs treatment on the promoter activity of smeIJK operon in the wild-type and rpoE mutant.
Next, the involvement of rpoE in MDAs-mediated smeIJK expression was further assessed by determining the smeIJK expression in the wild-type KJ and in an rpoE knockout mutant, KJΔRpoE. As shown in Fig. 6B, the PsmeIJK activity increased upon the challenge of Triton X-100 (129±21 vs. 186±35), but returned to the level as low as the non-treated counterpart once the rpoE was inactivated (109±16), indicating that the Triton X-100-mediated smeIJK up-regulation is rpoE dependent.
If the smeIJK operon is directly subjected to the regulation of rpoE system, the consensus DNA sequences recognized by σE should be found upstream of smeIJK. Since the component of rpoE system in S. maltophilia and X. campestris is highly similar (Fig. S2), the consensus σE-binding DNA sequence of X. campestris [18] was tentatively employed as a search query for the σE regulon identification in S. maltophilia. We found a putative σE recognized −35 (5′-GAACCT-3′) and −10 (5′-TCTTA-3′) sequences upstream of the smeIJK operon (Fig. S3). This raises a possibility of direct regulation of rpoE system on the expression of smeIJK.
Discussion
The organization of smeIJK locus is similar to those of mdtABC of E. coli [19], [20], sdeCDE of Serratia macrescens [21], [22], and muxABC of P. aeruginosa [23] in the possession of two RND-type transporters. The identities among these homologues are summarized in Table S2. For the MuxABC pump of P. aeruginosa, both the two RND components are essential for its function [23]. However, the MdtABC system of E. coli is an example to describe that the two RND transporters encoded by the same operon may have its own role [20]. The heteromultimer RND pump, MdtABC-TolC, effluxes bile salts, SDS, and novobiocin. Homomultimer RND pump MdtAC-TolC also displays the efflux function but with a narrow substrate profile limited to bile salts; in contrast, homomultimer RND pump MdtAB-TolC totally loses the efflux function [20]. In this article, with respect to the functions in antimicrobial extrusion, growth ability in MH medium, hypo-osmolarity tolerance, and cell envelope sensitivity, both RND components are not absolutely essential for pump functions, since mutants KJΔJ and KJΔK still keep considerable functions as the wild-type KJ does (Table 1, Fig. 1, 2 & 3). However, a recent study has pointed out that the SmeJ and SmeK RND transporters of S. maltophilia K279a are essential to produce a functional multidrug transporter [11]. The discrepancies in the necessity of two RND transporters between isolates K279a and KJ may be due to the genetic background of the bacterial host.
In this study, we observed that smeIJK mutant displays growth retardation in the MH medium, but not in the LB medium. Although the real mechanism responsible for this phenotype is still unclear after several tries, at least two conclusions can be made from our results. (i) The medium-dependent accumulation of noxious compounds is not the key reason for the phenotype of smeIJK mutant (Fig. 1B) (ii) The casein hydrolysate in the MH medium partially contributes the logarithmic-phase growth compromise of smeIJK mutant in the MH medium (Fig. 2D). A recent study has shown that there is a considerable culture medium-specific variability in the Cpx-mediated ESR in E. coli [24]. Therefore, the impact of culture medium ingredients on the ESR activation and bacterial physiology should be more intricate than we can image.
For survival, bacteria harbor a variety of ESRs to deal with the envelope stresses. Six ESR systems, RpoE, CpxRA, BaeSR, Rcs-phosphorelays, phage shock protein, and vesicle release response, have been well documented in E. coli. Each system has unique sets of inducing stressors and downstream targets. Therefore, the ESRs are highly regulated and there are often multiple response pathways in a given microorganism. The MdtABC, a SmeIJK homologue in E. coli, has been proved to be a member of the BaeSR regulon; however, there is no direct evidence to support the role of MdtABC in dealing with the envelope stress [25]. In this study, we demonstrated that SmeIJK is regulated by RpoE-mediated ESR and SmeIJK has a contribution to the envelope integrity in S. maltophilia. In addition, EmhABC of P. fluorescens cLP6a [26] and MexCD-OprJ of Pseudomonas aeruginosa [27] are known to be involved in the ESR. The expression of emhABC increases when P. florescens is grown at 35°C (7°C up the optimum growth temperature), signifying the role of EmhABC in the management of membrane stress caused by an unfavorable incubation temperature [26]. The MexCD-OprJ efflux system appears to be a component of an ESR in P. aeruginosa because it is induced by a variety of MDAs in an algU-dependent manner [27]. It is worth mentioning that the EmhABC and mexCD-oprJ are constitutively weakly expressed. However, the SmeIJK pump proposed in this study is intrinsically expressed even in the absence of any antibiotics stress. Loss of the constitutively expressed SmeIJK pump may disturb the envelope stability and cause the σE-mediated envelope stress (Fig. 7A). Furthermore, smeIJK can be further upexpressed upon the challenge of MDAs or surfactants. As illustrated in Fig. 7B, treatment with MDAs triggers the σE regulon and, thus, activates an array of genes to alleviate the envelope stresses. The rpoE-resA-mucD and smeIJK operons are the members of σE regulon. The up-regulation of the SmeIJK efflux pump may be a means by which the σE mediates adaptation to the envelope stress. The disinfectants, such as BC and CTAB, are extensively used in hospital. The physiological role of SmeIJK pump may benefit the survival of S. maltophilia against the disinfectants and envelope stress challenges. But, this outcome unfortunately confers the increased resistance of S. maltophilia to aminoglycosides and leucomycin.
Figure 7. The involvement of smeIJK and membrane damaging agents (MDAs) in the σE-mediated envelope stress response (ESR) of S. maltophilia.
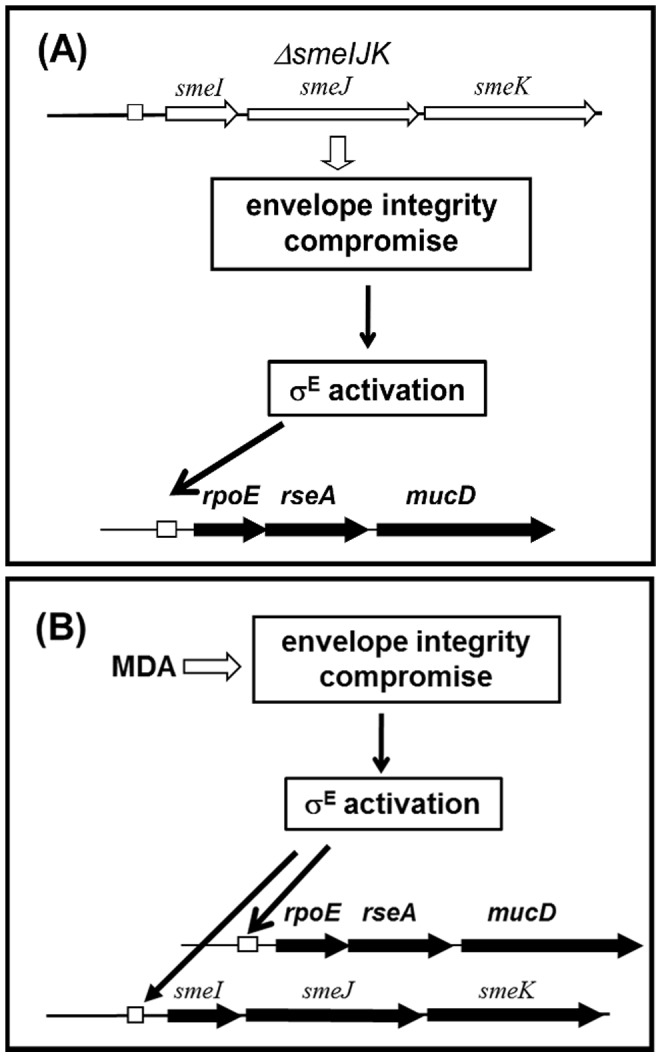
(A) Deletion of smeIJK compromises the cell envelope integrity and activates the rpoE to alleviate the envelope stress. (B) The treatment of MDAs on the wild-type cells activates rpoE system and upregualtes smeIJK expression. SmeIJK operon is a member of rpoE regulon.
Supporting Information
Schematic organization of the smeIJK operon and its derived mutants of S. maltophilia . The smeIJK operon contains genes for a membrane fusion protein (smeI) and two RND transporters (smeJ and smeK). The orientation of gene is indicated by the arrow. The solid lines, labeled as I to IV, represent the PCR amplicons for the construction of recombinant plasmids. The numbers in the brackets represent the PCR amplicon size (bps). The white box indicates the deleted region for each mutant construct.
(DOCX)
Schematic organization of the rpoE-rseA-mucD cluster , its derived mutants and the predicted σE binding site upstream of the rpoE in S. maltophilia . The orientation of gene is indicated by the arrow. The white box indicates the deleted region. The each protein identity of the rpoE region between X. campestris pv. campestris and S. maltophilia is indicated. The gray lines, labeled as I to III, represent the PCR amlpicons for the construction of recombinant plasmids. The numbers in the brackets represent the PCR amplicon size (bps). The sequence of the putative rpoE promoter region is shown below the map. The putative −35/−10 regions are underlined, based on the reported consensus sequence for the σE-regulated promoter elements of X. campestris pv. campestris.
(DOCX)
The DNA sequences upstream of the smeIJK operon. The orientation of gene is indicated by the arrow. The putative −35/−10 regions of the rpoE promoter are boxed, based on the reported consensus sequence for the σE-regulated promoter elements of X. campestris pv.campestris.
(DOCX)
Bacterial strains, plasmids and primers used in this study.
(DOCX)
The homologues of SmeIJK efflux pump.
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
TCY: Ministry of Science and Technology of Taiwan, NSC 101-2320-B-010-053-MY3, http://www.most.gov.tw. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Paulsen IT, Chen J, Nelson KE, Saier MH Jr (2001) Comparative genomics of microbial drug efflux systems. J Mol Microbiol Biotechnol 3: 145–150. [PubMed] [Google Scholar]
- 2. Krulwich TA, Lewinson O, Padan E, Bibi E (2005) Do physiological roles foster persistence of drug/multidrug-efflux transporters? A case study. Nat Rev Microbiol 3: 566–572. [DOI] [PubMed] [Google Scholar]
- 3. Thanassi DG, Cheng LW, Nikaido H (1997) Active efflux of bile salts by Escherichia coli . J Bacteriol 179: 2512–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin J, Sahin O, Michel LO, Zhang Q (2003) Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni . Infect Immun 71: 4250–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raivio TL (2005) Envelope sterss responses and Gram-negative bacterial pathogenesis. Mol Microbiol 56: 1119–1128. [DOI] [PubMed] [Google Scholar]
- 6. Staroń A, Sofia HJ, Dietrich S, Ulrich LE, Liesegang H, et al. (2009) The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Mol Microbiol 74: 557–581. [DOI] [PubMed] [Google Scholar]
- 7. Rowley G, Spector M, Kormanec J, Roberts M (2006) Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat Rev Microbiol 4: 383–394. [DOI] [PubMed] [Google Scholar]
- 8. Missiakas D, Mayer MP, Lemaire M, Georgopoulos C, Raina S (1997) Modulation of the Escherichia coli σE (RpoE) heat-shock transcription-factor activity by the RseA, RseB, and RseC proteins. Mol Microbiol 24: 355–371. [DOI] [PubMed] [Google Scholar]
- 9. Brooke JS (2012) Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25: 2–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crossman LC, Gould VC, Dow JM, Vernikos GS, Okazaki A, et al. (2008) The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Gen Biol 9: R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gould VC, Okazaki A, Avison MB (2013) Coordinate hyper-production of SmeZ and SmeJK efflux pumps extends drug resistance in Stenotrophomonas maltophilia . Antimicrob Agents Chemother 57: 655–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang TC, Huang YW, Hu RM, Huang SC, Lin YT (2009) AmpDI is involved in expression of the chromosomal L1 and L2 β-lactamases of Stenotrophomonas maltophilia . Antimicrob Agents Chemother 53: 2902–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical Laboratory Standards Institute (CLSI). Performance standards for Antimicrobial Susceptibility Testing, 20th Informational Supplement. M7-S20. CLSI. Wayne, PA, USA, 2010.
- 14. Lin CW, Huang YW, Hu RM, Chiang KH, Yang TC (2009) The role of AmpR in regulation of L1 and L2 β-lactamases in Stenotrophomonas maltophilia . Res Microbiol 160: 152–158. [DOI] [PubMed] [Google Scholar]
- 15. Hayden JD, Ades SE (2008) The extracytoplasmic stress factor, σE, is required to maintain cell envelope integrity in Escherichia coli . PLoS ONE 3: e1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wood LF, Ohman DE (2012) Identification of genes in the σ22 regulon of Pseudomonas aeruginosa required for cell envelope homeostasis in either the planktonic or the sessile mode of growth. mBio 3: e00094–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bordes P, Lavatine L, Phok K, Barriot R, Boulanger A, et al. (2011) Insights into the extracytoplasmic stress response of Xanthomonas campestris pv. campestris: Role and regulaton of σE-denpendent activity. J Bacteriol 193: 246–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng CY, Shieh SY, Hsu CC, Yang MT (2008) Characterization and transcriptional analysis of an ECF sigma factor from Xanthomonas campestris pv. campestris. FEMS Microbiol Lett 289: 250–257. [DOI] [PubMed] [Google Scholar]
- 19. Baranova N, Nikaido H (2002) The BaeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases it resistance to novobiocin and deoxycholate. J Bacteriol 184: 4168–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagakubo S, Nishino K, Hirata T, Yamaguchi A (2002) The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J Bacteriol 184: 4161–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar A, Worobec EA (2005) Cloning, sequencing, and characterization of the SdeAB multidrug efflux pump of Serratia marcescens . Antimicrob Agents Chemother 49: 1459–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Begic S, Worobec EA (2008) Characterization of the Serratia marcescens SdeCDE multidrug efflux pump studied via gene knockout mutagenesis. Can J Microbiol 54: 411–416. [DOI] [PubMed] [Google Scholar]
- 23. Mima T, Kohira N, Li Y, Sekiya H, Ogawa W, et al. (2009) Gene cloning and characteristics of the RND-type multidrug efflux pump MuxABC-OpmB possessing two RND components in Pseudomonas aeruginosa . Microbiology 155: 3509–3517. [DOI] [PubMed] [Google Scholar]
- 24. Raivio TL, Leblanc SKD, Price NL (2013) The Escherichia coli Cpx envelope sterss response regulated genes of diverse function that impact antibiotic resistance and membrane integrity. J Bacteriol 195: 2755–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leblanc SK, Oates CW, Raivio TL (2011) Characterization of the induction and cellular role of the BaeSR two-component envelope stress response of Escherichia coli . J Bacteriol 193: 3367–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adebusuyi AA, Foght JM (2011) An alternative physiological role for the EmhABC efflux pump in Pseudomonas fluorecens cLP6a. BMC Microbiol 11: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fraud S, Campigotto AJ, Chen Z, Poole K (2008) MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa: involvement in chlorhexidine resistance and induction by membrane-damaging agents dependent upon the AlgU stress response sigma factor. Antimicrob Agents Chemother 52: 4478–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic organization of the smeIJK operon and its derived mutants of S. maltophilia . The smeIJK operon contains genes for a membrane fusion protein (smeI) and two RND transporters (smeJ and smeK). The orientation of gene is indicated by the arrow. The solid lines, labeled as I to IV, represent the PCR amplicons for the construction of recombinant plasmids. The numbers in the brackets represent the PCR amplicon size (bps). The white box indicates the deleted region for each mutant construct.
(DOCX)
Schematic organization of the rpoE-rseA-mucD cluster , its derived mutants and the predicted σE binding site upstream of the rpoE in S. maltophilia . The orientation of gene is indicated by the arrow. The white box indicates the deleted region. The each protein identity of the rpoE region between X. campestris pv. campestris and S. maltophilia is indicated. The gray lines, labeled as I to III, represent the PCR amlpicons for the construction of recombinant plasmids. The numbers in the brackets represent the PCR amplicon size (bps). The sequence of the putative rpoE promoter region is shown below the map. The putative −35/−10 regions are underlined, based on the reported consensus sequence for the σE-regulated promoter elements of X. campestris pv. campestris.
(DOCX)
The DNA sequences upstream of the smeIJK operon. The orientation of gene is indicated by the arrow. The putative −35/−10 regions of the rpoE promoter are boxed, based on the reported consensus sequence for the σE-regulated promoter elements of X. campestris pv.campestris.
(DOCX)
Bacterial strains, plasmids and primers used in this study.
(DOCX)
The homologues of SmeIJK efflux pump.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.