Abstract
In mammals, the early-gestation fetus has the regenerative ability to heal skin wounds without scar formation. This observation was first reported more than 3 decades ago, and has been confirmed in a number of in vivo animal models. Although an intensive research effort has focused on unraveling the mechanisms underlying scarless fetal wound repair, no suitable model of in vitro fetal skin healing has been developed. In this article, we report a novel model for the study of fetal wound healing. Fetal skin from gestational day 16.5 Balb/c mice (total gestation, 20 days) was grafted onto the chorioallantoic membrane of 12-day-old chicken embryos and cultured for up to 7 days. At 48 hours postengraftment, circular wounds (diameter = 1 mm) were made in the fetal skin using a rotating titanium sapphire laser (N = 45). The tissue was examined daily by visual inspection to look for signs of infection and ischemia. The grafts and the surrounding host tissue were examined histologically. In all fetal skin grafts, the wounds completely reepithelialized by postinjury day 7, with regeneration of the dermis. Fetal mouse skin xenografts transplanted onto the chorioallantoic membrane of fertilized chicken eggs provides a useful model for the study of fetal wound healing. This model can be used as an adjunct to traditional in vivo mammalian models of fetal repair.
Keywords: fetal wound healing, scarless healing, chorioallantoic membrane, CAM, wound healing model, ex vivo model, laser injury
In contrast to postnatal wound healing, the early-gestation fetus heals skin wounds without scar. This observation was first reported more than 3 decades ago.1 Since that time, an intensive research effort has focused on elucidating the mechanisms responsible for scarless fetal wound repair.
Scarless wound healing has been observed in vivo in the fetuses of mice, rats, pigs, monkeys, and humans.1 In fetal mammals, the ability to repair wounds scarlessly is dependent on gestational age.2,3 In other words, fetal skin heals scarlessly prior to a certain gestational age, after which point scar formation occurs. In humans, scarring of wounds begins at approximately 24 weeks of gestation, whereas in mice, scarring of wounds begins on embryonic day 18.5 (average gestation period for mice is 20 days).4–6 This transition point, however, is modulated by wound size. For example, as wound size increases in fetal lambs, the ability to heal scarlessly is lost earlier during gestation.7
Prior to embryonic day 17.5, fetal mice have the ability to regenerate a nondisrupted collagen matrix that is identical to that of the original tissue.8–10 In addition, dermal structures such as sebaceous glands and hair follicles form normally after fetal injury.11 After embryonic day 17.5, fetal wound healing in mouse results in the typical fibroproliferative response, which is characterized by incomplete regeneration of the epidermal appendages and excessive production of an unorganized collagen meshwork (scar tissue).
As the fetus progresses through development, dramatic changes occur in the extracellular matrix, cellular mediators, stem cell function, and gene expression profiles of the developing skin. The fetal skin is constantly evolving on many levels, which makes performing single variable experimental analysis difficult for investigators. Grafting fetal skin onto the chorioallantoic membrane (CAM) of the chick embryo may allow tissue to develop in a more controlled environment. Moreover, in vivo fetal wound experiments have a high mortality rate and the system is not amendable to perturbation. The CAM model allows for molecular perturbation through the manipulation of gene expression at the ex vivo wound site.
The CAM is an extraembryonic membrane that functions as a gas exchange surface, much like the lung. The CAM is formed by the fusion of the splanchnic mesoderm of the allantois and the somatic mesoderm of the chorion, and represents the outermost lining of the noncellular eggshell membrane. The CAM begins forming on the fourth day of incubation and will ultimately cover the entire inner surface of the eggshell by day 12 of incubation. The chick will normally hatch by day 21 of incubation.12–14 As the CAM develops, an extensive network of extraembryonic capillaries forms over its surface. These blood vessels play a role in gas exchange (respiratory function) and also store excretory urinary products (excretory function). Due to its extensive vascularization, the CAM provides an ideal surface for the in vivo study of various biologic processes, such as angiogenesis, tumor biology, tissue engineering, and wound healing.15,16
The chick embryo CAM is a well-known model system dating back to the last century. Due to its rich vasculature, the CAM has the ability to support the growth of a variety of different tissue types.17–21 The CAM model represents an intermediate system that lies somewhere between in vivo and in vitro systems. Moreover, the developing chick embryo is completely immunodeficient during the first 11 days of incubation. In the developing chick, B cells are first detected on day 11 and T cells appear on day 13. Therefore, heterogenic xenografts will not be rejected.22
In 1912, Murphy first reported that heterologous tissue could be grafted onto the CAM.23 Between 2 and 3 days after transplantation, xenografts become nourished by the CAM vasculature. In 1938, Goodpasture et al showed that human skin could be grown on the CAM for up to 10 days.24 Since that time, the CAM has been used extensively as a model system for support of vascularized tissue grafts. In fact, the CAM itself has been advocated as a model to study wound healing.25 However, no study has investigated fetal skin wound healing using a CAM system. In this article, we describe the first full-thickness fetal skin wound healing assay using the CAM as a vascular bed to support the fetal skin graft.
MATERIALS AND METHODS
Animals
Fertilized eggs from Lohman white leghorn chickens (Gallus domesticus) were obtained from commercial sources. The fertilized eggs were incubated at 38°C and 60% relative humidity, and were rotated hourly. The eggs were prepared for tissue grafting on day 5 of incubation.
Impregnated Balb/c mice carrying gestational age E16.5 mice were killed and the embryos were harvested for dissection. All procedures with animals were conducted in accordance with Stanford University-approved protocols according to the National Institutes of Health guidelines.
Chick CAM Preparation
On day 5 of incubation, the CAM was prepared using the modification of a previously described technique.26 First, eggs were fumigated and washed with warm 70% ethanol. An oval portion (1 × 1.5 cm) of each egg’s shell was resected without disturbing the gestating embryo inside. Using a 21-gauge needle, 2 mL of albumin was withdrawn through the large blunt end of the egg. The window was covered with a minimal amount of clear (scotch) tape, which prevented dehydration and allowed for visualization of the developing embryo (Fig. 1). The “windowed” eggs were returned to a nonrocking 38°C incubator. On day 7 of incubation, fetal mouse skin was grafted onto the CAM.
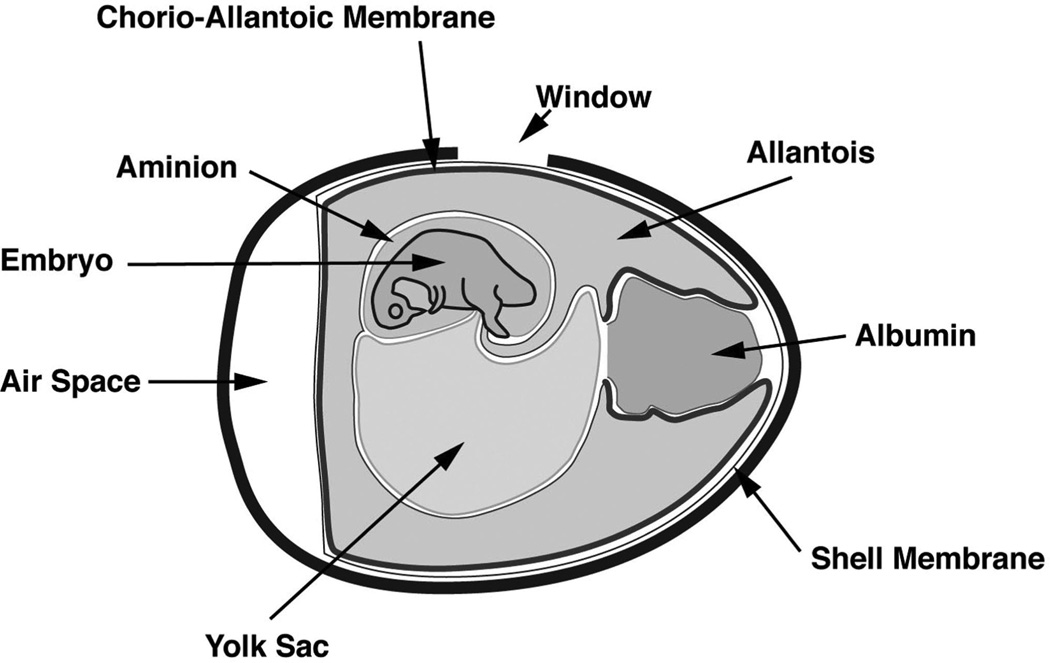
FIGURE 1.
Schematic of chorioallantoic membrane (CAM). Schematic of anatomic structures of a fertilized chicken egg, showing the location of the CAM. A 1.5 × 1.0 cm oval “window” is cut at the top of the egg and is used for visualization of transplanted fetal skin grafts.
Fetal Skin Specimens and Grafting Procedure
All steps were performed under sterile conditions. Pregnant mice were put under general anesthesia, and the E16.5 embryos were harvested from the womb according to our previously described protocol.27 The dorsal skin from the mid scapula down to the most distal aspect of the lumbar region was excised from the embryo. Great care was taken only to harvest the thin epidermis and dermis, and no muscle or subcutaneous fat was incorporated into the graft (Fig. 2). The specimens were transferred to a sterile dish containing cold sterile-filtered phosphate-buffered saline with antibiotics (1% penicillin/streptomycin). The harvested skin was then transferred onto a ring-shaped support structure created from sterilized Whatman filter paper using scissors and a hole punch. The donut-shaped support structure allowed the skin to be stretched flat, like the surface of a drum, without occluding the exposure of the dermis. Once mounted on the Whatman paper, the skin was transplanted through the eggshell window and onto the CAM of the 7-day-old developing chick embryo. Then, a sterile 6-mm donut was fashioned using sterile Whatman paper. Each CAM received only one 1 cm × 1 cm fetal skin graft. The oval window was covered with scotch tape, and the egg was returned to the incubator at 38°C without rocking.
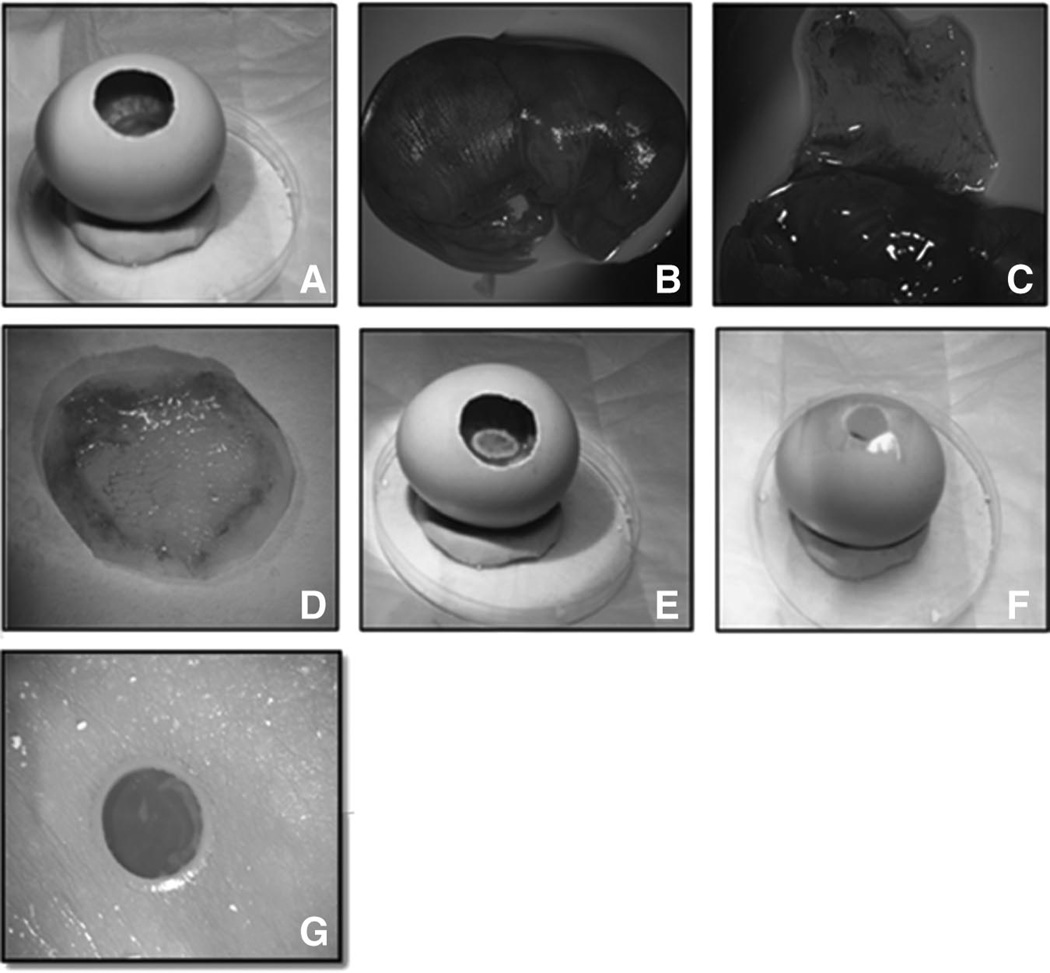
FIGURE 2.
Ex vivo CAM model allowing for fetal wound manipulation. Procuring fetal skin, implantation onto the CAM, and laser wound injury is depicted. A, Fertilized chicken egg with a 1.5 × 1 cm oval window for visualization of grafts and tissue reaction. B, E16.5 mouse fetus. C, Dorsal skin flap is elevated on E16.5 fetus. D, Skin flap is excised prior to grafting. E, Donor fetal skin graft is transplanted onto the CAM. F, Oval window is covered with clear scotch tape. G, Fetal skin xenograft 2 hours after laser injury, and 48 hours post transplantation onto the CAM.
Laser Injury of Fetal Skin Grafts
The developing CAM has a rich vascular structure that is characterized by numerous blood vessels. The fetal skin was grafted onto the CAM, and by 48 hours the xenografts had acquired a substantial vascular supply, which allowed for nutrient delivery and waste removal. Successfully transplanted skin typically appeared healthy and pink, whereas unsuccessful transplants appeared pale and necrotic. Successfully transplanted skin was then wounded in ovo at 48 hours postgrafting. The tape over the eggshell window was carefully removed to expose the fetal skin graft. A circular wound (diameter = 1 mm) was made using a rotating Ti:Sapph plasma laser (Fig. 3). The operating wavelength of the laser was 800 nm, the repetition rate was 240 Hz, and the pulse energy was 750 µJ/pulse. To create the circular wound, the laser made 5 revolutions (1 revolution/s). The wounds were full-thickness through the grafts, but not through the underlying CAM. The laser replicated precise wound area and depth. Once the transplanted skin had been wounded, the host egg was placed back inside incubators at 38°C.
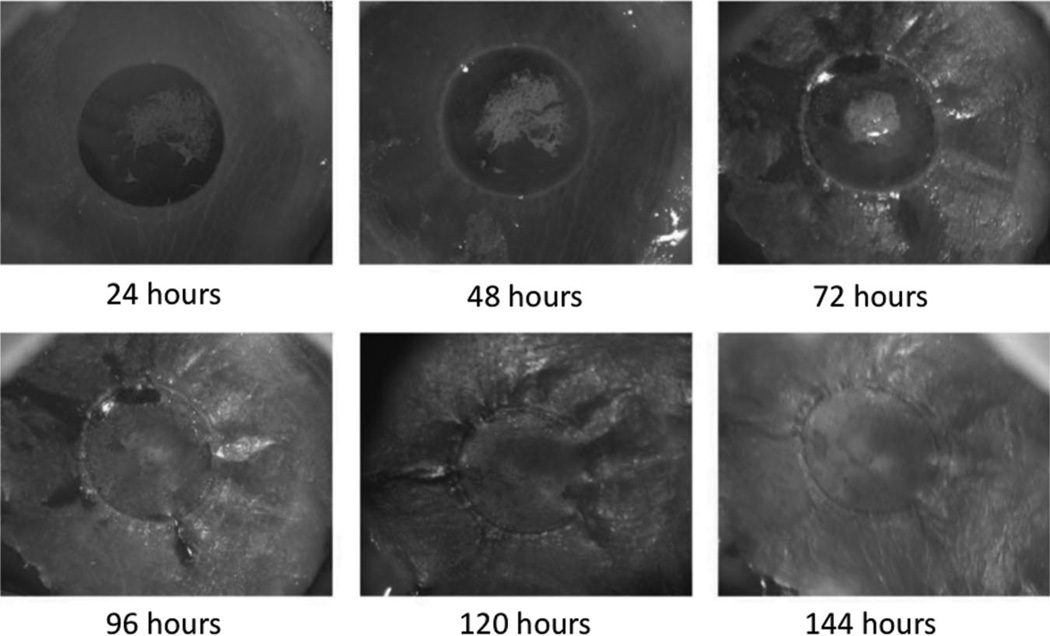
FIGURE 3.
Healing timeline for E16.5 fetal skin xenografts after laser wound injury. Gross photography demonstrates reepithelialization by 144 hours.
Assessment of Scar Formation After Wounding
The status of each wound was recorded daily using an imaging stereoscope. Operated eggs were killed before the host chicks hatched at 7 days postwounding. Xenografts were excised together with the surrounding CAM, embedded in optimal cutting temperature compound, snap-frozen in liquid nitrogen, and stored at −20°C until sectioning. Cryosections of 6 µm were mounted on poly-L-lysine-coated slides and stained with hematoxylin and eosin for histopathology. Tissue integrity, cell viability, neovascularization, ischemic injury, and its associated tissue responses (ie, fibrosis and necrosis) were evaluated.
Picrosirius Red-Polarized Staining
Sections were dewaxed in 100% xylene, followed by washing in 100% ethanol and water. The slides were then incubated in 0.1% Sirius Red F3BA (BDH Laboratory Supplies, Poole, United Kingdom) in saturated picric acid for 1 hour at room temperature. After washing in water, they were placed in 0.1N HCl for 2 minutes followed by another wash in water.28 The sections were then dehydrated through ethanol and xylene before being permanently mounted in Permount (Fisher Scientific, NJ, US).
Microscopy and Image Capture
To visualize the birefringent collagen, a Leica DM 5000B EP600 microscope (Leica microscope, Wetzlar Germany) was used. The polarizing filter was applied through which the background appeared black and the stained collagen fibers were displayed as bright green (predominant collagen III) or red/yellow (predominantly collagen I).
RESULTS
Fetal skin grafts were monitored closely in ovo. Graft take was evident when the fetal skin had a normal texture and a light rose color. The fetal skin grafts were fully adherent to the CAM after 24 hours. Angiogenesis was apparent 48 hours after transplantation and was indicated by new capillaries arranged in a wheel-spoke pattern around the xenograft. Graft loss was evident when the fetal skin did not retain its normal texture, the skin color became gray, and the graft was not fixed on the CAM. In these cases, angiogenesis did not occur and the xenograft became necrotic. Once the technique was mastered, the overall success rate for this model was 80% by postgrafting day 9. The most common reason for graft failure was infection.
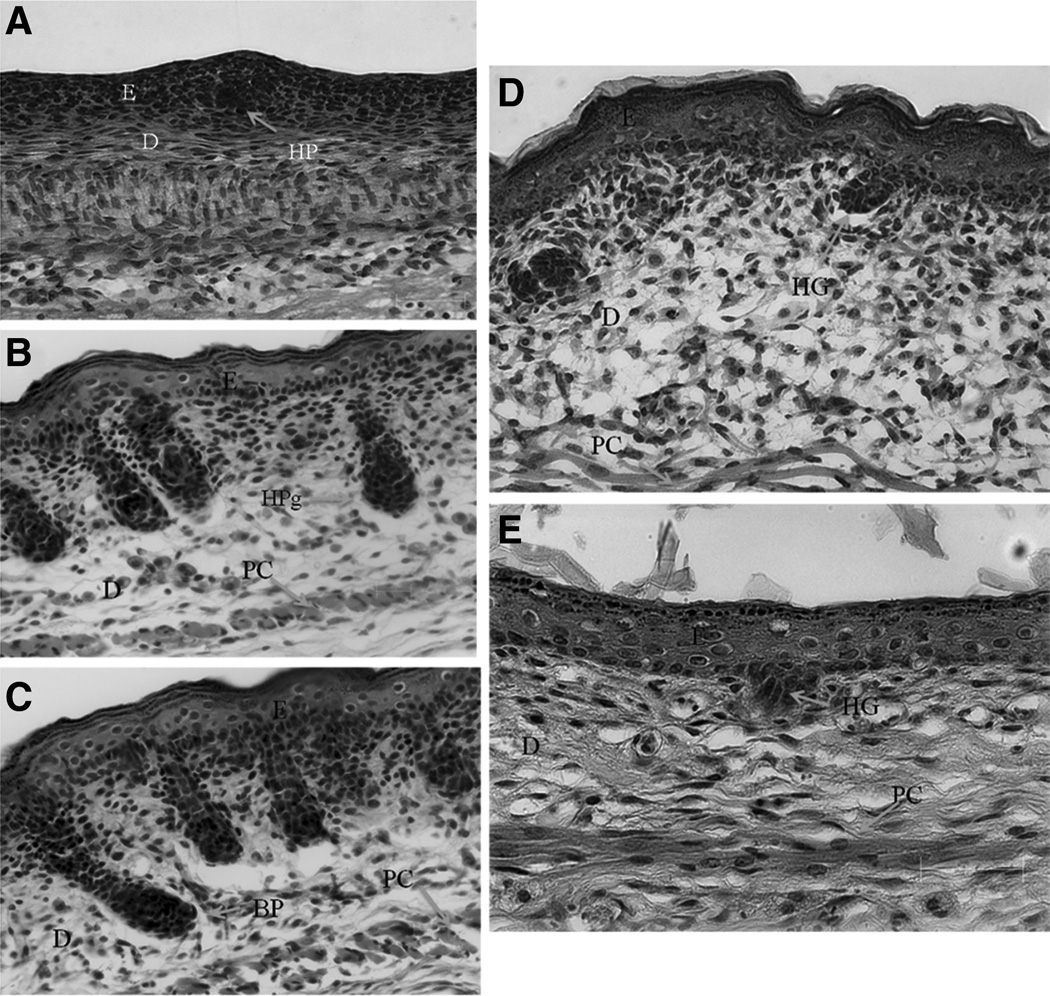
In all viable fetal skin grafts, the wounds completely reepithelialized by postinjury day 6, with regeneration of the epidermis and no dermal collagen scar formation (Fig. 4). Picrosirius-polarized staining revealed that the distribution and orientation of the newly synthesized collagen was similar to that in the adjacent unwounded dermis and without evidence of collagen scar formation (Fig. 5). The collagen fiber pattern in the wound was a reticular mixture of red/yellow (collagen I) and green (collagen III) fibers. The explanted fetal skin grafts did not differentiate at the same rate compared with the normal fetal skin maturation rate (Fig. 6). The graft skin differentiation rate was delayed. Little differentiation occurred as measured by dermal thickening or hair follicle maturation during the experimental period. The E16.5 grafts differentiated histologically to that of E17.5 skin while cultured on the CAM for 9 to 10 days (Fig. 6). In other words, the grafts differentiated an equivalent of 24 hours compared with the in vivo fetal skin differentiation rate. In summary, fetal skin grafted onto the CAM retained its ability to heal without collagen scar while demonstrating a slow rate of maturation compared with in vivo.
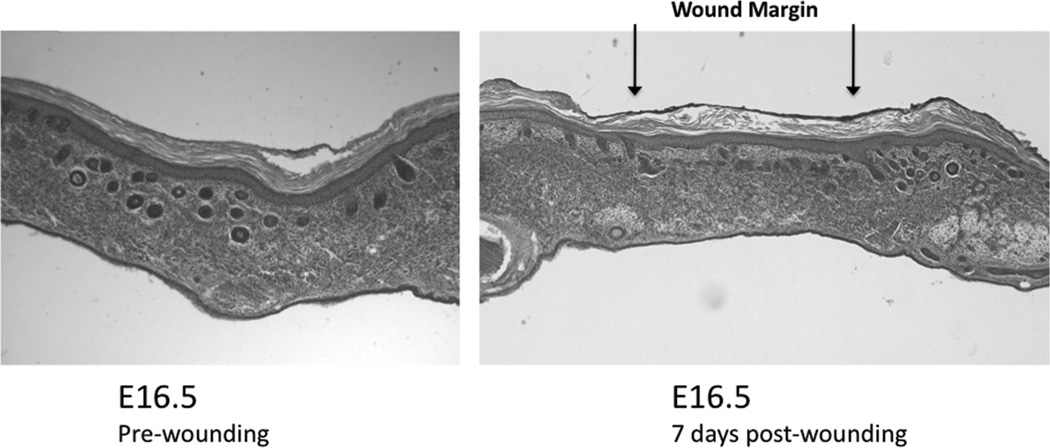
FIGURE 4.
Histology of wounded fetal skin xenografts. Normal E16.5 fetal skin (left) compared with E16.5 fetal skin transplanted onto the CAM and injured with a laser at 7 days post injury (right). The fetal skin graft wound was marked with green dye at the time of harvest. The skin wound healed with complete reepithelialization. The dermis healed with a normal extracellular matrix pattern but with a lack of rete pegs and follicular appendages. The graft skin shows little differentiation since transplantation.
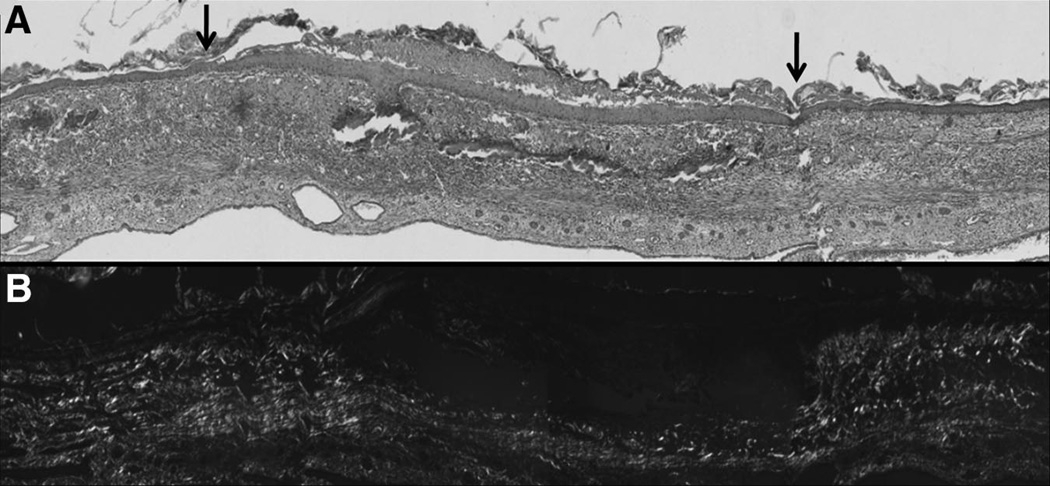
FIGURE 5.
Wound collagen deposition in fetal skin xenografts showing no scars. Picrosirius red demonstrates the orientation of collagens present in the healing xenograft wound at 7 days after injury. A, Hematoxylin and eosin stain histology of E 16.5 fetal skin graft at 7 days after full-thickness laser injury. The wounded area has reepithelialized and the dermis has healed with a lack of rete pegs and follicular appendages. The arrows represent the wound edges. B, Picrosiruis red staining of the adjacent section shown in A. A thin layer of noncollagenous tissue is present between the epidermis and reticular dermis. The collagen pattern in the wound area is indistinguishable from the unwounded surrounding dermis. No collagen scar formed in the fetal skin graft wound.
FIGURE 6.
The unwounded areas of mouse E16.5 grafts at 7 days after laser injury showing morphology similar to in vivo E17.5 unwounded skin. The rate of fetal skin graft differentiation on the CAM compared with normal in vivo skin development is delayed. Unwounded fetal skin was harvested at E16.5, E17.5, E18.5, and E19.5; and compared with E16.5 fetal skin that was grafted, wounded 2 days after grafting, and harvested at 7 days post wounding. A, Unwounded fetal skin at gestational age E16.5. B, Unwounded fetal skin at gestational age E18.5 (63× magnification). C, Unwounded fetal skin at gestational age E19.5 (63× magnification). D, Unwounded fetal skin at gestational age E17.5. E, Unwounded area of E16.5 skin graft at 7 days postlaser injury. The unwounded grafted skin had similar morphology as unwounded E17.5 skin. The hair germ morphology in the graft was similar to natural E17.5 skin. Hair germs have not yet formed in natural E16.5 skin, but have differentiated into hair pegs by E18.5. At 9 days after their initial harvest and grafting, the E16.5 fetal skin grafts have differentiated to E17.5 morphology. They are far behind the level of differentiation of their chronological time-matched counter parts, which is postnatal day 3 (E16.5 + 9 days). Postnatal day 3 skin has fully differentiated hair follicles and appendages. The P3 dermis is thicker and less cellular with greater amounts of collagens and other extracellular matrix proteins present.
D indicates dermis; E, epithelium; HP, hair placode; HG, hair germ; HPg, hair Peg; BP, bullous peg; PC, panniculus carnosus muscle.
DISCUSSION
The aim of the current study was to develop the CAM of the chick embryo as a novel model system for the study of fetal wound healing. Using this model, fetal skin can be kept viable and can heal full-thickness open wounds outside the uterus. We demonstrate the retention of the fetal wound healing phenotype for at least 7 days posttransplantation, which is the limit of the model due to the underlying developing chick. Fetal skin xenografts transplanted onto the CAM completely reepithelialized while healing the dermis without collagen scar formation. Picrosirius-polarized data showed that the collagen in the wound was a mixture of collagen III (green) and collagen I (red). The wound collagen pattern was indistinguishable from that of the adjacent unwounded dermis. Collagen III and collagen I were present in the healed dermal wound similar to unwounded fetal skin collagen organization.29 Furthermore, the orientation of the collagen fibers and the type of collagens in the wound were similar to those in the intact dermis surrounding the wound bed (Fig. 5). However, the regeneration of the papillary dermis was incomplete with little collagen in the area of rete pegs and hair follicles. The paucity of collagen and appendages in the papillary dermis indicates that the wound has not regenerated perfect skin architecture. However, the pattern and the birefringence of the newly synthesized collagen fibers in the wound were identical to the unwounded adjacent dermis. The collagen fibers in the wound were similar in type and alignment with the collagen fibers in the unwounded adjacent dermis. These findings support the notion that the wound in the xenograft healed in fetal-like manner with no collagen scar formation.
The CAM system offers a number of advantages over other model systems for studying fetal wound healing. Due to its low cost, simplicity of use, and the ability to directly visualize graft sites, the CAM might offer a good alternative method for studying the mechanisms underlying scarless fetal wound healing.
The study of fetal wound healing traditionally requires complicated operations, and does not provide continuous visualization of the fetal tissue. Using this model, the effect of exogenous growth factors, cytokines, small molecules, and other drugs can be readily investigated. Factors can be added or blocked and their effect on the regenerative process can be studied. These types of experiments are not feasible with in vivo fetal wound models. The ability to continuously monitor the test site through the eggshell window makes this model suitable for rapid screening of treatments. Also, as the CAM model is such a well-established system, the biology/physiology underlying this model is well-known.
However, the CAM model does have some disadvantages that must be considered. The chick gestation time is 21 days, we placed the xenografts on the CAM at gestational day 12, and the laser injury was performed 48 hours later. Hence, the maximum time of healing in this model is about 7 to 8 days. Therefore, this model is not suitable for studying long-term outcomes in wound healing studies. Also, because of the limited space available on the CAM, each egg can support only a small number of fetal skin grafts. The CAM also has the potential of being contaminated by dust from the eggshell window, which can trigger inflammation and confound experiments.30 Furthermore, the fetal skin xenograft has a delayed differentiation rate on the CAM compared with in vivo fetal skin differentiation. Xenografts were placed onto the CAM at gestational age E16.5 and, after 7 days of incubation, exhibited histologic findings similar to E17.5 fetal skin (Fig. 6). The delayed differentiation rate must be considered when planning experiments in this model. The lack of differentiation is useful in that effects of differentiation signals from repair signals can be separated by this model.
On the basis of our findings, the CAM model is a highly suitable alternative to the in vivo animal models for studying fetal wound healing. However, further characterization of this model is currently under investigation and questions still remain. For example, the contribution to the repair process by chick cells is not yet known. However, in other xenograft systems, the donor host contributes very little if host tissue is not injured. In a model of skin grafted into subcutaneous pockets in adult mice, no mouse response was noted if the host was not injured. However, when the host was wounded, then a large host response occurred.27
In summary, our results demonstrate that fetal mouse skin xenografts transplanted onto the CAM of fertilized chicken eggs provide a useful model for the study of fetal wound healing. Fetal skin grafted onto the CAM remained viable and healed wounds without collagen scar. Chorioallantoic engrafting may represent a valuable tool for investigating the process by which fetal wounds heal and offers a perspective that is different from that given by in vivo analysis. Thus, the CAM model is an adjunct to traditional mammalian models for studying fetal wound healing.
Acknowledgments
Supported by Oak Foundation, Plastic Surgery Education Foundation, and Hagey Laboratory for Pediatric Regenerative Medicine.
REFERENCES
- 1.Colwell AS, Longaker MT, Lorenz HP. Mammalian fetal organ regeneration. Adv Biochem Eng Biotechnol. 2005;93:83–100. doi: 10.1007/b99972. [DOI] [PubMed] [Google Scholar]
- 2.Cass DL, Bullard KM, Sylvester KG. Wound size and gestational age modulate scar formation in fetal wound repair. J Pediatr Surg. 1997;32:411–415. doi: 10.1016/s0022-3468(97)90593-5. [DOI] [PubMed] [Google Scholar]
- 3.Somasundaram K, Prathap K. Intra-uterine healing of skin wounds in rabbit wounds in rabbit fetuses. J Pathol. 1970;100:81–86. doi: 10.1002/path.1711000202. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz HP, Whitby DJ, Longaker MT, et al. Fetal wound healing. The ontogeny of scar formation in the non-human primate. Ann Surg. 1993;217:391–396. doi: 10.1097/00000658-199304000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colwell AS, Krummel TM, Longaker MT, et al. An in vivo mouse excisional wound model of scarless healing. Plast Reconstr Surg. 2006;117:2292–2296. doi: 10.1097/01.prs.0000219340.47232.eb. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz HP, Lin RY, Longaker MT, et al. The fetal fibroblast: the effector cell of scarless fetal skin repair. Plast Reconstr Surg. 1995;96:1251–1259. doi: 10.1097/00006534-199511000-00002. discussion 1260–1251. [DOI] [PubMed] [Google Scholar]
- 7.Cass D, Bullard K, Sylvester K. Wound size and gestational age modulate scar formation in fetal wound repair. J Pediatr Surg. 1997;32:411–415. doi: 10.1016/s0022-3468(97)90593-5. [DOI] [PubMed] [Google Scholar]
- 8.Longaker MT, Whitby DJ, Adzick NS, et al. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25:63–68. doi: 10.1016/s0022-3468(05)80165-4. discussion 68–69. [DOI] [PubMed] [Google Scholar]
- 9.Whitby DJ, Ferguson MW. The extracellular matrix of lip wounds in fetal, neonatal, and adult mice. Development. 1991;112:651–668. doi: 10.1242/dev.112.2.651. [DOI] [PubMed] [Google Scholar]
- 10.Beanes SR, Hu FY, Soo C, et al. Confocal microscopic analysis of scarless repair in the fetal rat: defining the transition. Plast Reconstr Surg. 2002;109:160–170. doi: 10.1097/00006534-200201000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamamichi S, Nishigori H. Establishment of a chick embryo shell-less culture system and its use to observe change in behavior caused by nicotine and substances from cigarette smoke. Toxicol Lett. 2001;119:95–102. doi: 10.1016/s0378-4274(00)00300-3. [DOI] [PubMed] [Google Scholar]
- 13.Kamihira M, Oguchi S, Tachibana A, et al. Improved hatching for in vitro quail embryo culture using surrogate eggshell and artificial vessel. Dev Growth Differ. 1998;40:449–455. doi: 10.1046/j.1440-169x.1998.t01-2-00010.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 15.Ausprunk DH, Knighton DR, Folkman J. Vascularization of normal and neoplastic tissues grafted to the chick chorioallantois. Am J Pathol. 1975;79:597–618. [PMC free article] [PubMed] [Google Scholar]
- 16.Valdes TI, Kreutzer D, Moussy F. The chick chorioallantoic membrane as a novel in vivo model for the testing of biomaterials. J Biomed Mater Res. 2002;62:273–282. doi: 10.1002/jbm.10152. [DOI] [PubMed] [Google Scholar]
- 17.Nakada K, Mashima J, Yao Y, et al. Developmental stage-dependent expression of troponin T isoforms in chicken embryonic breast muscles grafted on chorioallantoic membrane. Zool Sci. 1998;15:729–736. [Google Scholar]
- 18.Murphy JB. The effect of adult chicken organ grafts on the chick embryo. J Exp Med. 1916;24:1–5. doi: 10.1084/jem.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willier BH. The endocrine glands and the development of the chick. Am J Anat. 1924;33:67–103. [Google Scholar]
- 20.Hoadley L. The differentiation of isolated chick primordia in chorio-allantoic grafts. J Exp Zool. 1925;42:143–162. [Google Scholar]
- 21.Sandstrom RH. The differentiation of hepatic and pancreatic tissues of the chick embryo in chorio-allantoic grafts. Physiol Zool. 1934;7:226–246. [Google Scholar]
- 22.Janse ME, Jeurissen SM. Ontogeny and function of two non-lymphoid cell populations in the chick embryo. Immunobiology. 1991;182:472–481. doi: 10.1016/s0171-2985(11)80211-1. [DOI] [PubMed] [Google Scholar]
- 23.Murphy JB. Transplantability of malignant tumours to the embryos of a foreign species. JAMA. 1912;59:874–875. [Google Scholar]
- 24.Goodpasture EW, Douglas B, Anderson K. A study of human skin grafted upon the chorioallantois of chick embryos. J Exp Med. 1938;68:891–904. doi: 10.1084/jem.68.6.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribatti D, Vacca A, Roncali L, et al. The chick embryo chorioallantoic membrane as an in vivo wound healing model. Path Res Pract. 1996;192:1068–1076. doi: 10.1016/S0344-0338(96)80050-1. [DOI] [PubMed] [Google Scholar]
- 26.Auerbach R, Kubai L, Knighton D, et al. A simple procedure for the long-term cultivation of chick embryos. Dev Biol. 1974;41:391–394. doi: 10.1016/0012-1606(74)90316-9. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz H, Longaker M, Perkocha L, et al. Scarless wound repair: a human fetal skin model. Development. 1992;114:253–259. doi: 10.1242/dev.114.1.253. [DOI] [PubMed] [Google Scholar]
- 28.Martes G, Junqueira L. The use of picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz. 1983;86(suppl 3):1–11. doi: 10.1590/s0074-02761991000700002. [DOI] [PubMed] [Google Scholar]
- 29.Cuttle L, Nataatmadja M, Hayes M, et al. Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen. 2005;13:198–204. doi: 10.1111/j.1067-1927.2005.130211.x. [DOI] [PubMed] [Google Scholar]
- 30.Klueh U, Dorsky DI, Moussy F, et al. Ex ova chick chorioallantoic membrane as a novel model for evaluation of tissue responses to biomaterials and implants. J Biomed Mater Res. 2003;67:838–843. doi: 10.1002/jbm.a.10059. [DOI] [PubMed] [Google Scholar]