Abstract
Aim
An abundance of evidence has firmly established the familial aggregation of schizophrenia. The aim of this study was to examine how age at onset, parental characteristics and season of birth modify the familiality in schizophrenia.
Methods
A population-based cohort was created by linking the Swedish Multi-Generation and Hospital Discharge Registers. Among 5 075 998 full siblings born between 1932 through 1990, 16 346 cases of schizophrenia were identified. Familial aggregation was measured by the sibling recurrence-risk ratio, defined as the risk of schizophrenia among full siblings of schizophrenia patients compared with the risk among siblings of unaffected subjects.
Results
We found a statistically significantly lower recurrence-risk ratio in siblings of later onset cases (7.2; 95%CI 6.7-7.9) than of early onset cases (10.8; 95%CI 9.4-12.2). A lower recurrence-risk ratio was observed among offspring to fathers above 40 years (6.3; 95%CI 5.3-7.3) as compared with offspring of younger fathers (8.6; 95%CI 8.0-9.3). Further, among offspring to parents born outside Sweden the recurrence-risk ratio was statistically significantly lower (maternal immigrants 4.8; 95%CI 4.0-5.7, paternal immigrants 5.7; 95%CI 4.6-6.9) than among offspring to parents born in Sweden.
Conclusions
The familial aggregation of schizophrenia was reduced by higher age at onset, advancing paternal age and immigrant status of parents.
Keywords: schizophrenia, familial aggregation, recurrence-risk ratio, age at onset, paternal age, migration
Background
Schizophrenia is a multi-faceted disorder with a highly familial nature. The life-time risk is approximately 10 times higher in first-degree relatives of patients with schizophrenia than in the general population [1] and twin studies indicate that 81% of the variation in liability can be explained by genes [2]. However, a number of risk factors additional to family history have been identified [3] and it has been proposed that other factors than genetic relatedness to a schizophrenia patient can affect familial risk [4]. Similar to many complex diseases with a variable age at onset, the familiality of schizophrenia seems to be stronger in the early-onset than in the late onset forms of the illness [5-7]. van Os et al. [8] suggest that urban birth and family history interact synergistically, whereas season of birth does not seem to affect the sibling's risk of developing of schizophrenia [9]. Other potential interacting factors related to schizophrenia include paternal age and migration [10, 11].
Aims
The aim of this study was to investigate possible moderating effect of individual characteristics (such as age at onset of disease and season of birth) and parental characteristics (such as paternal age and immigrant status) on familial aggregation of schizophrenia. For this purpose, we investigated the sibling recurrence-risk ratio for schizophrenia by utilising data from several Swedish population-based registers.
Methods
Data sources
The Swedish Multi-Generation Register includes children born since 1932 that are linked to their biological parents. The register comprises 9 million children (index persons) and 11 million unique individuals [12].
The Swedish Hospital Discharge Register contains details on virtually all psychiatric hospitalisations in Sweden from 1973. Dates of each hospital admission, discharge and the main discharge diagnosis assigned by the treating physician (and up to eight secondary diagnoses if occurring) are recorded according to the International Classification of Diseases, Eighth revision (ICD-8) through 1986, Ninth revision (ICD-9) between 1987 and 1996 and Tenth revision (ICD-10) from 1997 and onwards. The register has a nationwide coverage of inpatient treatment facilities and includes care in both psychiatric and somatic hospitals [13]. The discharge diagnosis of schizophrenia has been validated and few false positive cases reported [14, 15].
Patients with schizophrenia were defined as individuals identified in the Swedish Hospital Discharge Register having at least two inpatient hospitalisations with a discharge diagnosis of schizophrenia (ICD-8 and ICD-9 code 295 and ICD-10 codes F20, F23.1, F23.2 and F25). Latent schizophrenia (295.5, 295F and F21) was excluded. The criterion of at least two inpatient hospitalisations was chosen to increase diagnostic precision, providing nearly identical estimates of familial risks as those from the literature [1]. Information about country of birth was derived from the Total Population Register.
Using the unique national registration number, a population-based Swedish database was created by linking the Swedish Hospital Discharge and Multi-Generation Registers. The linkage identified 5 075 998 unique individuals born between 1932 and 1990, where at least one full sibling and both the parents were identified. These individuals were followed up until 2004, resulting in 16 346 individuals who met our criteria for schizophrenia.
Statistical analyses
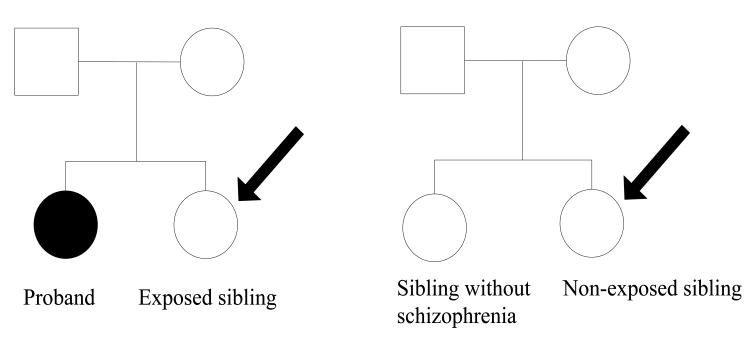
A matched cohort study design was used to estimate the risk of schizophrenia among siblings of patients with schizophrenia compared with the risk among siblings of unaffected individuals [16]. All full siblings of cases with schizophrenia were selected and pairs consisting of these exposed individuals and the schizophrenia patient (the proband) were specified. Thus, several sibling pairs descended from one proband. Each pair with a proband and an exposed sibling was then matched to 10 sibling pairs consisting of an individual who did not meet our criteria for schizophrenia and his or her sibling (Figure 1). Because age, birth cohort and gender can affect the probability a diagnosis of schizophrenia, the members of the pair were matched on these variables. The probands were matched in an attempt to reduce misclassification of exposure and the study individuals (the siblings of the proband) were matched to avoid confounding. To ensure equal follow-up time for schizophrenia, we additionally required that the proband was matched to individuals who were alive and had not been admitted to psychiatric care for schizophrenia in Sweden at the date the proband was first hospitalised. Because the magnitude of the sibling recurrence-risk ratio was not affected by gender of the patient in this population [1], brothers and sisters were analysed together.
Figure 1.
 General population (male/female)
General population (male/female)
 Patients with schizophrenia
Patients with schizophrenia
Sibling recurrence-risk ratio of schizophrenia was calculated comparing exposed/non-exposed siblings (indicated by black arrows). Further, we estimated risk ratios for different levels of individual and parental covariates of the exposed/non-exposed sibling and for different age at onset of the proband.
To estimate the sibling recurrence-risk ratio of schizophrenia, the data were analyzed in a conditional logistic regression model using the proc tphreg procedure in SAS version 9.1. To take into account the dependence of individuals from the same family, confidence intervals were computed by bootstrapping [17]. We created 1000 bootstrap samples, each equal in size to the original sample, by randomly re-sampling with replacement from the original data. For each bootstrap sample, a matched stratum (1 exposed and 10 non-exposed study individuals) was selected at random to be included in the sample and then made available to be selected again for that same sample. Exact, asymmetric confidence intervals were calculated with proc univariate using the 2.5 and 97.5 percentiles to constitute the limits of the 95% confidence interval. Statistically significant differences were assumed when p<0.05 (two-sided).
Two forms of information (individual and parental) were included as interaction terms with proband schizophrenia to examine whether the risk ratio was influenced by covariates. Separate models were fitted for each covariate. Covariates were dichotomised according to predefined categories: early (< 25 years) and late age at onset (≥ 25 years), Swedish and non-Swedish place of birth, younger (< 40 years) and older paternal age (≥ 40 years) and birth in January-April and May-December. The cut-off for advancing paternal age was based on studies showing that major changes in schizophrenia risk have occurred at this age [18, 19]. Summer and winter births were defined according to Hultman et al. [20], following an extensive review by Torrey et al. [21], which concluded maximum schizophrenia birth excess in this period.
Age at onset was defined as age at first hospitalisation (as recorded in the Hospital Discharge Register) of schizophrenia of the proband. First, age at onset was dichotomised into early (< 25 years) and late age at onset (≥ 25 years). In an attempt to further investigate the effect of age at onset we grouped this variable into finer categories: < 20, 21-30, 31-40 and > 40 years, following the categorisation of age at onset in another Swedish cohort [7]. Because the Swedish Hospital Discharge Register started in1973, data on age at first hospitalisation, especially on early onset cases, can only be captured accurately in younger cohorts. Therefore, a subset of schizophrenia cases (n= 5 243) born from 1960 onwards was analysed, ensuring that cases admitted at age 13 years or older were correctly classified.
Results
In total, 35 953 pairs comprising a schizophrenia proband and his or her exposed sibling were matched to 359 102 non-exposed pairs (Table I).
Table I.
Number of schizophrenia cases among 395 055 Swedish siblings. Data stratified by exposure to sibling schizophrenia and different levels of covariates.
| Study subjects exposed to schizophrenia sibling | Study subjects non-exposed to schizophrenia sibling | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Number | Number of schizophrenia cases | % | Number | Number of schizophrenia cases | % | Total number of study subjects | |||
|
|
|
|
|||||||
| Total | 35 953 | 1 386 | 3.86 | 359 102 | 1 752 | 0.49 | 395 055 | ||
| Age at onset of proband | |||||||||
| < 25 years | 10 816 | 484 | 4.47 | 108 006 | 467 | 0.43 | 118 822 | ||
| ≥ 25 years | 25 020 | 898 | 3.59 | 249 927 | 1 283 | 0.51 | 274 947 | ||
| Missing | 117 | 4 | 1 169 | 2 | 1 286 | ||||
| Paternal age | |||||||||
| Father < 40 years | 29 453 | 1 144 | 3.88 | 306 803 | 1 435 | 0.47 | 336 256 | ||
| Father ≥ 40 years | 6 500 | 242 | 3.72 | 52 299 | 317 | 0.61 | 58 799 | ||
| Missing | 0 | 0 | 0 | 0 | 0 | ||||
| Immigrant status | |||||||||
| Mother | |||||||||
| Sweden | 30 027 | 1 153 | 3.84 | 322 504 | 1 451 | 0.45 | 352 531 | ||
| Outside Sweden | 5 392 | 204 | 3.78 | 33 161 | 268 | 0.81 | 38 553 | ||
| Missing | 534 | 29 | 3 437 | 33 | 3 971 | ||||
| Father | |||||||||
| Sweden | 30 556 | 1 168 | 3.82 | 323 614 | 1 490 | 0.46 | 354 170 | ||
| Outside Sweden | 4 443 | 182 | 4.10 | 27 993 | 207 | 0.74 | 32 436 | ||
| Missing | 954 | 36 | 7 495 | 55 | 8 449 | ||||
| Sibling | |||||||||
| Sweden | 33 859 | 1 292 | 3.81 | 346 281 | 1 654 | 0.48 | 380 140 | ||
| Outside Sweden | 2 056 | 94 | 4.57 | 12 500 | 98 | 0.78 | 14 556 | ||
| Missing | 38 | 0 | 324 | 0 | 362 | ||||
| Season of birth | |||||||||
| January-April | 12 467 | 489 | 3.92 | 124 048 | 596 | 0.48 | 136 515 | ||
| May-December | 23 486 | 897 | 3.82 | 235 054 | 1 156 | 0.49 | 258 540 | ||
| Missing | 0 | 0 | 0 | 0 | 0 | ||||
Table I presents the number and percent of schizophrenia cases among the study sample, stratified by exposure to affected sibling and by age at onset of proband, immigrant status of parents and sibling and season of birth in sibling. The percent of cases among study individuals with a sibling with schizophrenia was 3.86%. In Table I the effect of covariates on schizophrenia prevalence is attained by comparing the number of cases among individuals exposed to the covariate to the number of cases among individuals non-exposed to the covariate (row-wise comparisons). The sibling recurrence-risk ratio for schizophrenia can be calculated by comparing the number of schizophrenia cases among individuals exposed to sibling schizophrenia to the number of cases among individuals non-exposed to sibling schizophrenia (column-wise comparisons).
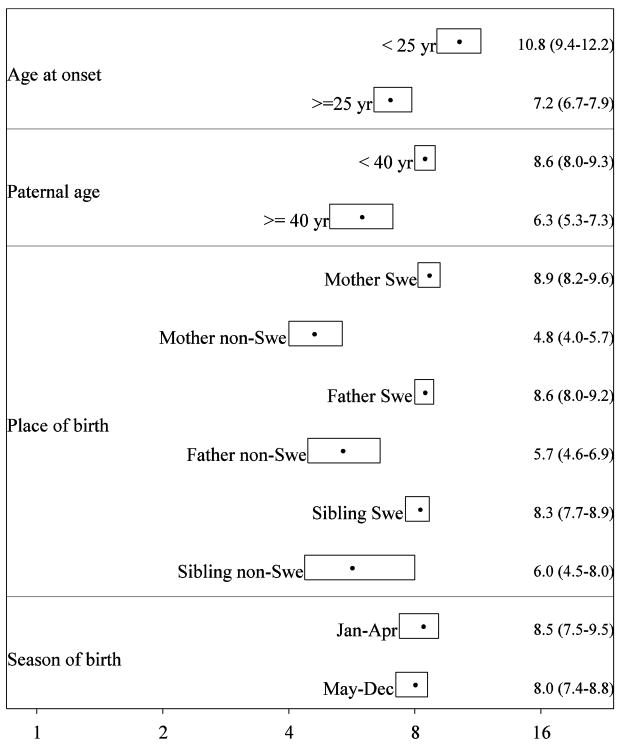
Figure 2 illustrates the results from the statistical analyses, conditioned on the matching factors. We estimated the crude recurrence-risk ratio for schizophrenia in siblings to be 8.2 (95% CI 7.6-8.8).
Figure 2.
Sibling recurrence-risk ratio for schizophrenia from statistical modelling, conditioned on the matching factors of age, gender and year of birth. Point estimates as filled circles surrounded by two-sided 95% confidence intervals as boxes. Exact numbers are given to the right.
Age at onset
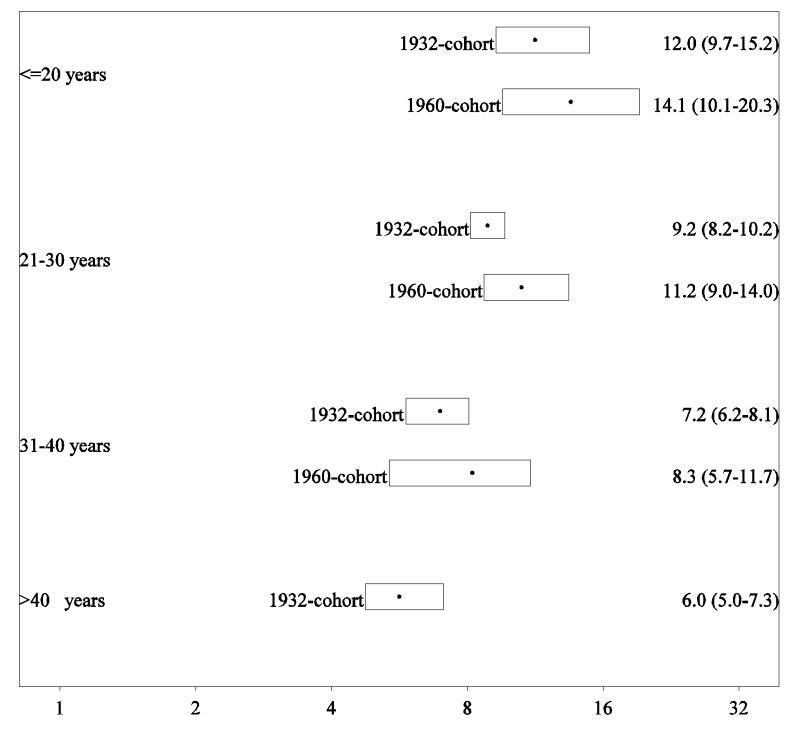
The mean age at onset was 31.6 years (range 14-71 years and interquartile range 24-37 years). We found a statistically significantly higher risk ratio in siblings of earlier onset cases [10.8 (95% CI 9.4-12.2)] than of later onset cases [7.2 (95% CI 6.7-7.9)]. In an additional analysis the risk associated with sibling schizophrenia was calculated in four categories of age at onset. We found a monotonic decrease in the sibling risk ratio with higher age at onset of the proband (Figure 3).
Figure 3.
Sibling recurrence-risk ratio for schizophrenia according to proband age at onset in cohorts from 1932 and 1960 onwards.
Note: The recurrence-risk ratio for the age category > 40 years in the cohort from 1960 was not estimable because of the short follow-up.
In the later 1960 cohort there were 9 311 exposed and 92 951 non-exposed siblings. The mean age at onset was 25.6 years (range 14-42 years and interquartile range 21-29 years). A similar effect of age at onset was found as in the full cohort though the estimates were overall higher, and because of the too short follow-up, we were not able to measure the effect in the oldest age (> 40 years) category (Figure 3).
Paternal age
The mean paternal age was 32.0 years (range 14-80 years and interquartile range was 27-36 years). Advancing paternal age reduced the recurrence-risk ratio in siblings: the recurrence-risk ratio in siblings was statistically significantly lower in the presence of older paternal age [6.3 (95% CI 5.3-7.3)] than in younger paternal age [8.6 (95% CI 8.0-9.3)].
Immigrant status
The sibling recurrence-risk ratio was statistically significantly lower among offspring to parents born outside Sweden. The recurrence-risk ratio among offspring to mothers born outside Sweden was 4.8 (95% CI 4.0-5.7) versus 8.9 (95% CI 8.2-9.6) among offspring to mothers born in Sweden. The corresponding risks based on paternal immigrant status were 5.7 (95% CI 4.6-6.9) and 8.6 (95% CI 8.0-9.2), respectively. We found no statistically significant effect of siblings' place of birth, although the point estimate for births in Sweden was lower than among births outside Sweden.
Season of birth
There was no statistically significant effect of seasonality of birth on the recurrence-risk ratio in siblings. The recurrence-risk ratio among siblings born in January-April [8.5 (95% CI 7.5‐9.5)] was similar to the risk among siblings born in May-December [8.0 (95% CI 7.4-8.8)].
Discussion
In this large, comprehensive population-based study we investigated how the familiality of schizophrenia was influenced by known risk factors of the disease. While the crude sibling risk is in accordance with earlier estimates of familial risk, the variation caused by environmental factors and other covariates can be informative and crucial in unravelling the aetiology of schizophrenia. The familial aggregation, as measured by the sibling recurrence‐risk ratios, was reduced by higher age at onset, advancing paternal age and immigrant status of parents. There was a monotonic decrease in the sibling recurrence-risk ratio with higher age at onset of the proband. However, the recurrence-risk ratio remained high across the influence of the covariates, indicating a high genetic contribution.
We found that patients with early age at onset convey a higher familial risk for schizophrenia than patients with a later age at onset. The familiality became progressively stronger as age at onset decreased. This trend remained and was even more pronounced when analysing a later birth cohort in an endeavour to capture more accurately the true date of first hospitalisation. Our results are consistent with most of those previously reported [5, 6, 22], where the only negative study [23] involved a small sample of participants (n=22). Collectively, these studies provide strong evidence that early age at onset is associated with a higher familiality of schizophrenia.
It has previously been shown that the association between advancing paternal age and schizophrenia increased in people without a family history of schizophrenia [10]. Inversely, our results indicate that familial effects are significantly reduced by advancing paternal age. Germ cells divide continuously in males and because of these numerous divisions, older men have an increased risk of errors in DNA transcription. Malaspina et al. [24] suggest that the association between paternal age and schizophrenia might be due to de novo mutations in paternal germ cells. The authors support their hypothesis by showing that sporadic cases have significantly older fathers [25]. The idea of de novo mutations constitutes an appealing theory in that it has been estimated that the major part of this highly heritable disorder comprise sporadic cases [1].
A personal and family history of migration are both important risk factors for schizophrenia [11]. We found that the familiality was less pronounced in families in which at least one of the parents was born outside Sweden. This effect was reliable for maternal and paternal place of birth and the results from analyses of the siblings' place of birth followed the same pattern.
Replicating the findings from a large population-based Finnish study [9], we detected no interaction between seasonality of birth and familiality of schizophrenia. This observation is further supported by Hettema et al [26], although some older studies on this issue have yielded contradictory results [27, 28]. Overall, the largest studies to date have found no association between season of birth and familiality of schizophrenia [9, 26, 29] and the present nationwide study confirms the lack of association.
The strength of our data is the national coverage of all inpatient treatment facilities including care in psychiatric and somatic clinics. An inherent problem with population-based studies of this kind, however, is the use of diagnostic evaluations made by different clinicians, as well as variation that occurs over time and place. Nevertheless, the reporting of diagnostic information from every hospital discharge to The Swedish National Board of Health and Welfare is standardized to ICD-codes and follows national guidelines. Furthermore, validation studies confirm a very low number of false positive diagnoses [14, 15]. Based on semi-structured interviews and medical records, 94% agreement has been reported between register-based diagnoses of schizophrenia and research diagnoses [15]. Moreover, by restricting our analyses to at least two admissions for schizophrenia, we enhanced specificity of register diagnoses.
There are several limitations that deserve consideration. First, although the siblings were matched on birth cohort, there might be an inherited problem of left-truncation. However, when a subset of schizophrenia cases born from 1960 onwards was analysed for all covariates, the same effect of risk factors on sibling recurrence-risk ratio could be demonstrated as in the full sample (data not shown). Second, even if siblings were matched by age, gender and birth cohort, other unknown confounders might be operating. Third, our study covers a fraction of possible covariates that could potentially influence familial risk. For example, it has been demonstrated that familiality can be affected by urban birth [8] and obstetric complications [30]. Finally, we have examined gene-environment interplay in schizophrenia by use of register-derived variables as proxies for environmental exposure. A similar study design could be applied on data with more detailed information on exposures. We did not find a statistically significant effect of seasonality of birth on the familiality on schizophrenia. However, data on specific factors associated with winter-birth, such as prenatal infection, vitamin D-status and sunlight exposure, would perhaps be more powerful in elucidating interactions.
Conclusions
In conclusion, higher age at onset of proband, advancing paternal age and parents' place of birth outside Sweden reduced the familial aggregation of schizophrenia. Nevertheless, the sibling recurrence-risk ratio was significantly increased across all levels of risk factors of the disease. The current findings demonstrating a large variation in the estimates depending on different levels of other risk factors underscore the importance of both familial and non-familial risk factors in schizophrenia, challenging the assumption of a uniform population-wide sibling risk. It would be tempting to interpret our results as evidence of a gene-environment interaction. However, familiality of schizophrenia, as measured by the sibling risk ratio, consists of both genetic and environmental components. Likewise, some risk factors for schizophrenia have an environmental origin, whereas others might themselves influence or be influenced by genetic factors. Advances in molecular genetics now allow direct assessment of hundreds of thousands of genetic variants spaced across the genome and enable more refined and powerful analyses of gene-environment interactions. At least, our study suggests that genetic influences cannot alone provide an answer to the disease panorama.
Acknowledgments
Funding for this study was provided by the Swedish Council for Working Life and Social Research Grant 2002-2013
References
- 1.Lichtenstein P, Björk B, Hultman CM, Scolnick E, Sklar P, Sullivan PF. Recurrence risks for schizophrenia in a Swedish national cohort. Psychological Medicine. 2006;36(10):1417–25. doi: 10.1017/S0033291706008385. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60(12):1187–92. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 3.Murray RM, Jones PB, Susser E, van Os J, Cannon M. The Epidemiology of Schizophrenia. Cambridge, UK: Cambridge University Press; pp. 2003pp. 5–99. [Google Scholar]
- 4.Kendler KS, Eaves LJ. Models for the joint effect of genotype and environment on liability to psychiatric illness. Am J Psychiatry. 1986;143(3):279–89. doi: 10.1176/ajp.143.3.279. [DOI] [PubMed] [Google Scholar]
- 5.Husted JA, Greenwood CM, Bassett AS. Heritability of schizophrenia and major affective disorder as a function of age, in the presence of strong cohort effects. Eur Arch Psychiatry Clin Neurosci. 2006;256(4):222–9. doi: 10.1007/s00406-005-0629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne M, Agerbo E, Mortensen PB. Family history of psychiatric disorders and age at first contact in schizophrenia: an epidemiological study. Br J Psychiatry Suppl. 2002;43:19–25. doi: 10.1192/bjp.181.43.s19. [DOI] [PubMed] [Google Scholar]
- 7.Sham PC, MacLean CJ, Kendler KS. A typological model of schizophrenia based on age at onset, sex and familial morbidity. Acta Psychiatr Scand. 1994;89(2):135–41. doi: 10.1111/j.1600-0447.1994.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 8.van Os J, Pedersen CB, Mortensen PB. Confirmation of synergy between urbanicity and familial liability in the causation of psychosis. Am J Psychiatry. 2004;161(12):2312–4. doi: 10.1176/appi.ajp.161.12.2312. [DOI] [PubMed] [Google Scholar]
- 9.Suvisaari JM, Haukka JK, Lonnqvist JK. No association between season of birth of patients with schizophrenia and risk of schizophrenia among their siblings. Schizophr Res. 2004;66(1):1–6. doi: 10.1016/s0920-9964(02)00506-6. [DOI] [PubMed] [Google Scholar]
- 10.Sipos A, Rasmussen F, Harrison G, Tynelius P, Lewis G, Leon DA, et al. Paternal age and schizophrenia: a population based cohort study. BMJ. 2004;329(7474):1070. doi: 10.1136/bmj.38243.672396.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162(1):12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- 12.Multi-Generation Register 2004 - A description of contents and quality. Örebro: Statistics Sweden; 2005. [Google Scholar]
- 13.In-patient diseases in Sweden 1987-2006. Stockholm: The National Board of Health and Welfare; 2007. [Google Scholar]
- 14.Dalman C, Broms J, Cullberg J, Allebeck P. Young cases of schizophrenia identified in a national inpatient register--are the diagnoses valid? Soc Psychiatry Psychiatr Epidemiol. 2002;37(11):527–31. doi: 10.1007/s00127-002-0582-3. [DOI] [PubMed] [Google Scholar]
- 15.Ekholm B, Ekholm A, Adolfsson R, Vares M, Osby U, Sedvall GC, et al. Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry. 2005;59(6):457–64. doi: 10.1080/08039480500360906. [DOI] [PubMed] [Google Scholar]
- 16.Susser E, Susser M. Familial aggregation studies. A note on their epidemiologic properties. Am J Epidemiol. 1989;129(1):23–30. doi: 10.1093/oxfordjournals.aje.a115119. [DOI] [PubMed] [Google Scholar]
- 17.Efron B, Tibshirani R. An introduction to the bootstrap. London, UK: Chapman and Hall; 1993. [Google Scholar]
- 18.Byrne M, Agerbo E, Ewald H, Eaton WW, Mortensen PB. Parental age and risk of schizophrenia: a case-control study. Arch Gen Psychiatry. 2003;60(7):673–8. doi: 10.1001/archpsyc.60.7.673. [DOI] [PubMed] [Google Scholar]
- 19.Malaspina D. Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophr Bull. 2001;27(3):379–93. doi: 10.1093/oxfordjournals.schbul.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hultman CM, Sparen P, Takei N, Murray RM, Cnattingius S. Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: case-control study. BMJ. 1999;318(7181):421–6. doi: 10.1136/bmj.318.7181.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torrey EF, Miller J, Rawlings R, Yolken RH. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28(1):1–38. doi: 10.1016/s0920-9964(97)00092-3. [DOI] [PubMed] [Google Scholar]
- 22.Haukka JK, Suvisaari J, Lonnqvist J. Family structure and risk factors for schizophrenia: case-sibling study. BMC Psychiatry. 2004;4:41. doi: 10.1186/1471-244X-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendler KS, Karkowski-Shuman L, Walsh D. Age at onset in schizophrenia and risk of illness in relatives. Results from the Roscommon Family Study. Br J Psychiatry. 1996;169(2):213–8. doi: 10.1192/bjp.169.2.213. [DOI] [PubMed] [Google Scholar]
- 24.Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, et al. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58(4):361–7. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 25.Malaspina D, Corcoran C, Fahim C, Berman A, Harkavy-Friedman J, Yale S, et al. Paternal age and sporadic schizophrenia: evidence for de novo mutations. Am J Med Genet. 2002;114(3):299–303. doi: 10.1002/ajmg.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hettema JM, Walsh D, Kendler KS. Testing the effect of season of birth on familial risk for schizophrenia and related disorders. Br J Psychiatry. 1996;168(2):205–9. doi: 10.1192/bjp.168.2.205. [DOI] [PubMed] [Google Scholar]
- 27.Pulver AE, Liang KY, Brown CH, Wolyniec P, McGrath J, Adler L, et al. Risk factors in schizophrenia. Season of birth, gender, and familial risk. Br J Psychiatry. 1992;160:65–71. doi: 10.1192/bjp.160.1.65. [DOI] [PubMed] [Google Scholar]
- 28.Baron M, Gruen R. Risk factors in schizophrenia. Season of birth and family history. Br J Psychiatry. 1988;152:460–5. doi: 10.1192/bjp.152.4.460. [DOI] [PubMed] [Google Scholar]
- 29.Mortensen PB, Pedersen CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340(8):603–8. doi: 10.1056/NEJM199902253400803. [DOI] [PubMed] [Google Scholar]
- 30.Parnas J, Schulsinger F, Teasdale TW, Schulsinger H, Feldman PM, Mednick SA. Perinatal complications and clinical outcome within the schizophrenia spectrum. Br J Psychiatry. 1982;140:416–20. doi: 10.1192/bjp.140.4.416. [DOI] [PubMed] [Google Scholar]