Abstract
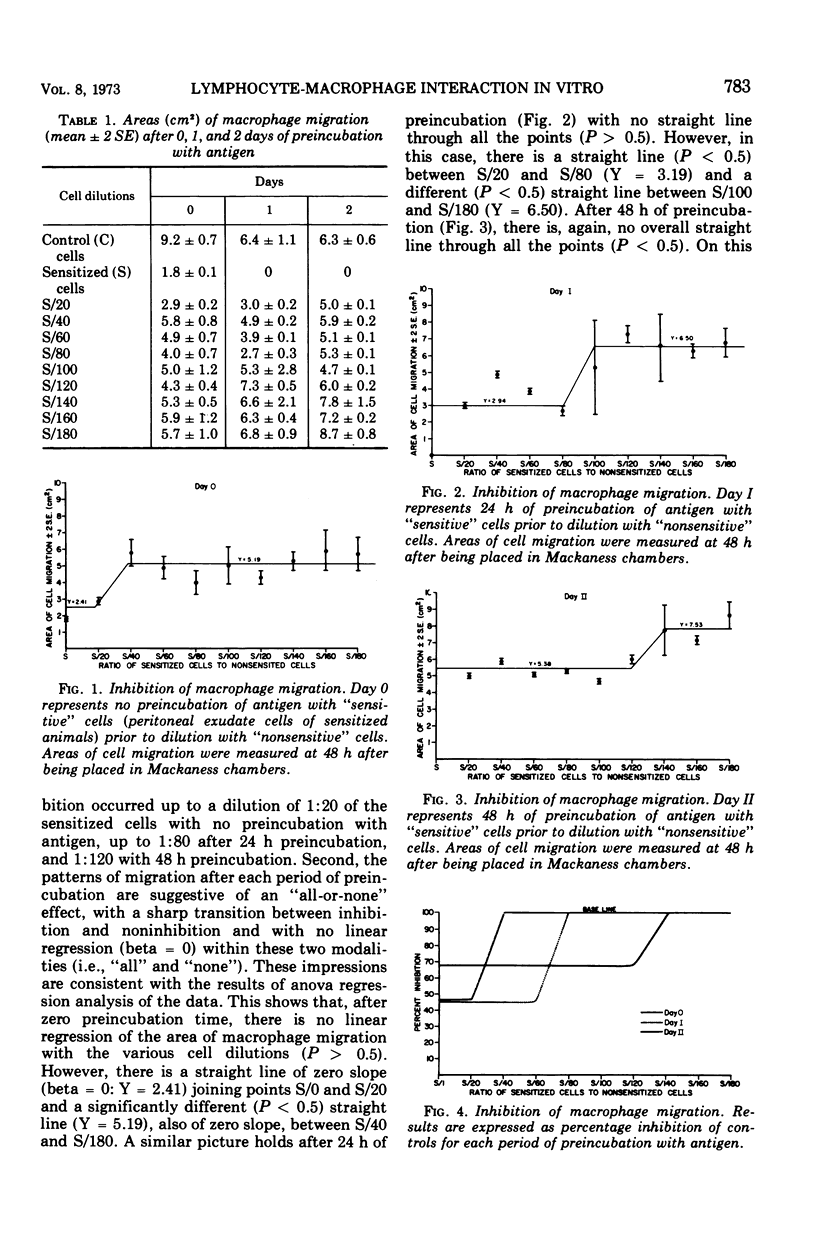
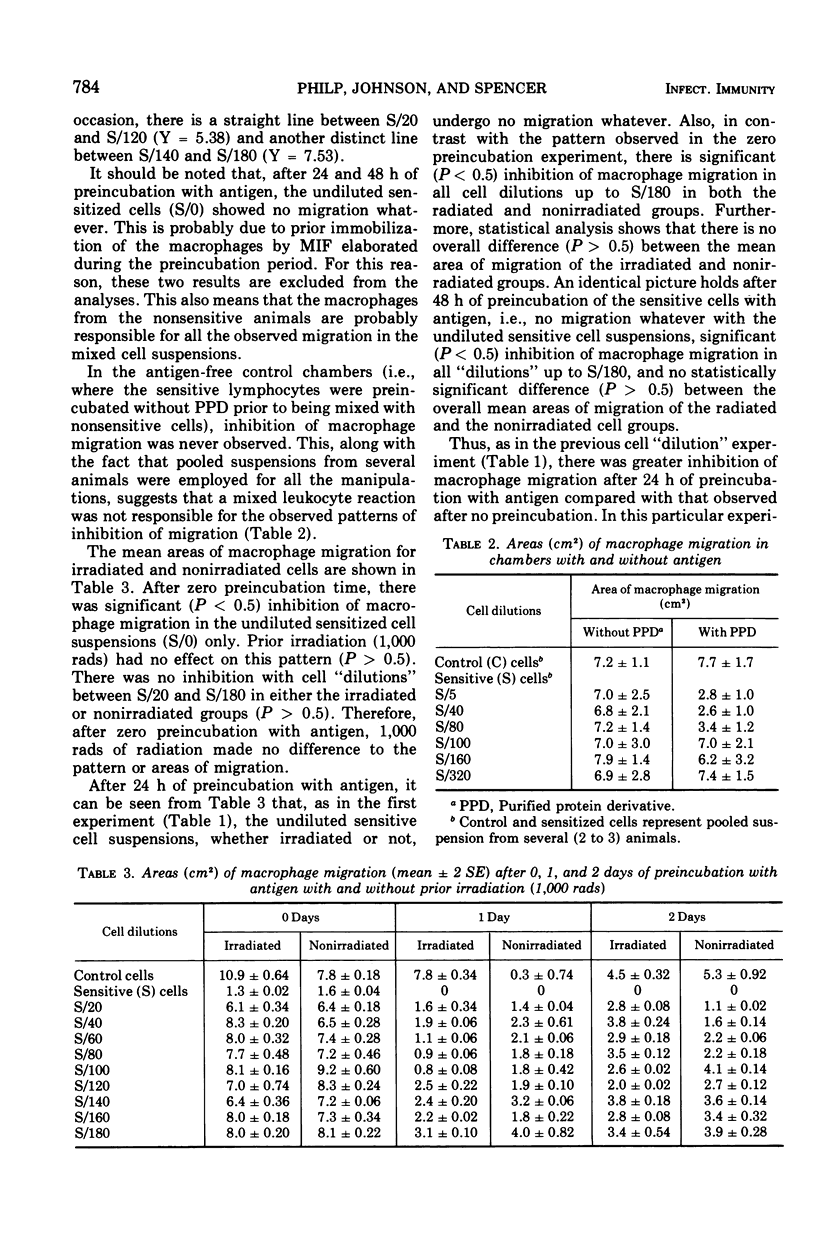
When lymphocyte-macrophage suspensions from sensitized animals are preincubated with specific antigen for 24 or more h, the following results are observed. (i) In a standard capillary macrophage migration test, there is complete inhibition of migration. (ii) When the preincubated cell suspension is mixed in varying proportions with a similar suspension from nonsensitized animals and a macrophage migration test is performed, there is no linear relationship between the degree of inhibition of migration and the proportion of sensitized lymphocytes initially present. Inhibition thus appears to be an “all-or-none” effect. (iii) In spite of the second observation, increasing periods of preincubation with antigen result in increasing inhibition. (iv) These results suggest the existence of a complex amplifying mechanism operating within the early period of exposure to antigen. (v) To test the possibility that cell proliferation contributes to this amplification, cells from sensitized guinea pigs were irradiated with a dose of 1,000 rads prior to preincubation with antigen. Despite this dose, which virtually abolishes cell division in other systems, no diminution whatever in the amplification of inhibition was observed. These results suggest the existence of an early phase of increased production of migratory inhibition factor that is not dependent on cell division but that may be related to “recruitment” of nonsensitized lymphocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brent L., Medawar P. Quantitative studies on tissue transplantation immunity. 8. The effects of irradiation. Proc R Soc Lond B Biol Sci. 1966 Oct 11;165(1001):413–423. doi: 10.1098/rspb.1966.0074. [DOI] [PubMed] [Google Scholar]
- David J. R. Macrophage migration. Fed Proc. 1968 Jan-Feb;27(1):6–12. [PubMed] [Google Scholar]
- David J. R. Suppression of delayed hypersensitivity in vitro by inhibition of protein synthesis. J Exp Med. 1965 Dec 1;122(6):1125–1134. doi: 10.1084/jem.122.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence H. S. In vitro correlates of delayed hypersensitivity. Introductory remarks. Fed Proc. 1968 Jan-Feb;27(1):3–5. [PubMed] [Google Scholar]
- Lolekha S., Dray S., Gotoff S. P. Macrophage aggregation in vitro: a correlate of delayed hypersensitivity. J Immunol. 1970 Feb;104(2):296–304. [PubMed] [Google Scholar]
- Marshall W. H., Valentine F. T., Lawrence H. S. Cellular immunity in vitro. Clonal proliferation of antigen-stimulated lymphocytes. J Exp Med. 1969 Aug 1;130(2):327–343. doi: 10.1084/jem.130.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp J. R., Crooks J., Macgregor A. G., McIntosh J. A. The short term effects of x-radiation on goitrogen induced growth of the rat thyroid. Br J Cancer. 1969 Sep;23(3):524–535. doi: 10.1038/bjc.1969.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp J. R., McIntosh J. A. The long-term effects of x-radiation on the goitrogenic response of and iodide trapping by the rat thyroid. Am J Roentgenol Radium Ther Nucl Med. 1970 Feb;108(2):386–391. doi: 10.2214/ajr.108.2.386. [DOI] [PubMed] [Google Scholar]



