Supplemental Digital Content is available in the text.
Abstract
Background:
The aim of this study is to evaluate and quantify dynamic soft-tissue strain in the human face using real-time 3-dimensional imaging technology.
Methods:
Thirteen subjects (8 women, 5 men) between the ages of 18 and 70 were imaged using a dual-camera system and 3-dimensional optical analysis (ARAMIS, Trilion Quality Systems, Pa.). Each subject was imaged at rest and with the following facial expressions: (1) smile, (2) laughter, (3) surprise, (4) anger, (5) grimace, and (6) pursed lips. The facial strains defining stretch and compression were computed for each subject and compared.
Results:
The areas of greatest strain were localized to the midface and lower face for all expressions. Subjects over the age of 40 had a statistically significant increase in stretch in the perioral region while lip pursing compared with subjects under the age of 40 (58.4% vs 33.8%, P = 0.015). When specific components of lip pursing were analyzed, there was a significantly greater degree of stretch in the nasolabial fold region in subjects over 40 compared with those under 40 (61.6% vs 32.9%, P = 0.007). Furthermore, we observed a greater degree of asymmetry of strain in the nasolabial fold region in the older age group (18.4% vs 5.4%, P = 0.03).
Conclusions:
This pilot study illustrates that the face can be objectively and quantitatively evaluated using dynamic major strain analysis. The technology of 3-dimensional optical imaging can be used to advance our understanding of facial soft-tissue dynamics and the effects of animation on facial strain over time.
Various imaging modalities have been used to characterize facial motion in relation to the soft tissues, skin, and facial skeleton. Conventional radiology, such as computed tomographic scans and magnetic resonance imaging, has been used to evaluate shape and volume of fat, muscle, and bone1,2 and thereby has advanced our understanding of facial anatomy in static subjects. Although 2-dimensional photography remains the most common imaging for patient evaluations, its limitations have fostered technologic advances in 3-dimensional methods such as laser scanning and stereophotogrammetry3 that have begun to better elucidate the multidimensional attributes of the dynamic face.
Facial aging is an intricate and multifactorial process that affects multiple components of the face differentially, resulting in dyspigmentation and loss of skin tone, descent and atrophy of fat and muscle, and remodeling of the underlying facial bone structure.4 The dynamic face is clearly affected by facial aging; however, the relationship between dynamic and static aging remains to be understood, in part, because few objective data defining dynamic facial changes exist. Consequently, an objective method of quantifying age-related dynamic changes of the face is necessary for advancing our understanding of how distinct facial components are specifically affected by animation at baseline and with advancing age. The aim of this pilot study is to evaluate and quantify distinct anatomic changes in the dynamic human face using a novel real-time 3-dimensional imaging technology.
Speckle-tracking photogrammetry, the methodology employed in this study, is an innovative technology that enables the dynamic analysis of both topographic and geometric changes in real time. This 3-dimensional imaging captures position, displacement, geometry, and strain.5,6 Digital image correlation through the ARAMIS system (Trilion Quality Systems, Pa.) is designed to achieve full-field, noncontact, optical measurements for the purpose of characterizing materials in a fluid and dynamic manner. When the surface to be examined is subject to a deformational force, such as stretch or compression, the resulting displacements and strains are measured and then compared to the given surface’s static reference state, which is defined at the onset of the imaging process. This technology has previously successfully characterized the mechanical properties of a variety of biologic materials, including in vivo deformational measurements in frog hearts, local strain behavior in artificial muscle, and bone surface strains secondary to loading in mouse tibias.6–8 We hypothesize that this technology can serve as a valuable tool for the evaluation and understanding of dynamic facial anatomy.
METHODS
Thirteen adult subjects were enrolled in the study. Eight women and 5 men between the ages of 18 and 70 underwent stochastic speckle patterning using black and white colored hairspray applied from the hairline at the scalp superiorly to the neck at the level of the clavicle inferiorly and the tragus laterally (Fig. 1). Patients who had undergone previous surgery or filler injections of the face were excluded, as were patients who had undergone neuromodulator injections less than 12 months before participating in this study. Also excluded were patients who had a history of facial trauma or patients with facial paralysis.
Fig. 1.

A, Speckle patterning applied to a curved surface with white and black color hairspray for detection by the dual-camera charge coupled device system. B, Similar speckle patterning applied to a human face with white and black color hairspray for detection by the dual-camera CCD system.
The subjects were placed at a calibrated working distance from a high-resolution, digital dual-camera charge coupled device system. To obtain an initial reference image, each subject maintained a neutral facial pose for 5 seconds, recorded at a rate of 50 frames per second. An average representative image was selected as each subject’s individual reference.
Each subject was then recorded performing 6 types of facial animation: (1) smile, (2) laughter, (3) surprise, (4) anger, (5) grimace, and (6) lip pursing. Each action was recorded for 10–12 seconds at a rate of 15 frames per second starting from rest to action and back to resting neutral position. As with standard 3-dimensional photogrammetry, 3-dimensional coordinates were calculated, generating each subject’s topographic configuration, displacement, and surface strain of each detected point on the surface of the face relative to its original reference image.8
Positive strain is defined as stretch on the surface of the face occurring with facial animation. Negative strain is defined as compression occurring perpendicular to stretch on the surface of the face with facial animation. Vectors of facial animation were defined using the ARAMIS software in the vertical and horizontal directions, and strain measurements were made using these vectors across regions of the face for each action. The major and minor strains of each tested expression were then calculated using the ARAMIS software system. The program measured both strain and variability in strain, highlighting the areas of the face with maximum and minimum strain.
For each expression, the strain was quantified as a percentage change from baseline and displayed in a “heat map” color scale with red representing the area of greatest strain (stretch) and blue representing the area of least strain, with negative values indicating compression. The strain data for all study subjects were then analyzed and compared. Subgroup analyses were performed, grouping cohorts by age and gender. Pearson’s chi-square or Fisher’s exact tests were used to analyze categorical variables. Wilcoxon rank-sum test was used for continuous variables. All tests were 2-tailed, and statistical significance was defined as P value less than 0.05. All analyses were performed using STATA IC 11.0 (StataCorp, College Station, Tex.).
RESULTS
Eight women and 5 men were included in this pilot study, with a mean age of 40.7 years (± 17.0 years). We compared average stretch and compression strains in different regions of the face between subjects of different ages and different genders. In each subject, gradients of strain were visualized across the whole face as a “heat map” for each action. Interestingly, the greatest stretch and compression were localized to the midface and lower face for all animations (Fig. 2). (See Video 1, Supplemental Digital Content 1, which demonstrates the change in strain over the duration of this patient’s smile, http://links.lww.com/PRSGO/A46; Video 2, Supplemental Digital Content 2, which illustrates the change in strain over the duration of this patient’s smile, http://links.lww.com/PRSGO/A47.) Thus, we further analyzed these regions.
Fig. 2.
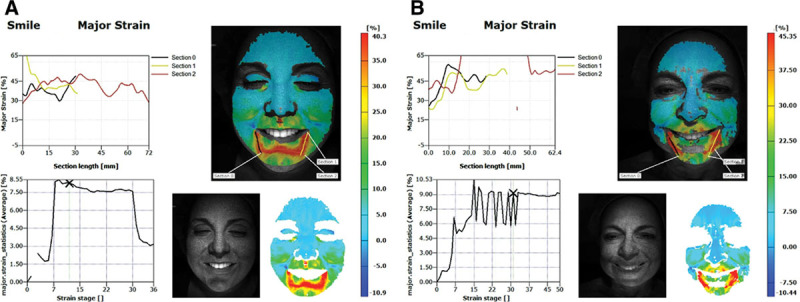
This is an example of major strain analysis in a younger patient who is smiling (A) compared with an older patient who is smiling (B). Strain or stretch, quantified by percentage change, is displayed in a “heat map” color scale with red indicating the area of greatest strain and blue indicating the area of least strain. The sections are representative of the general direction of dynamic facial motion and used for analysis.
Video 1.
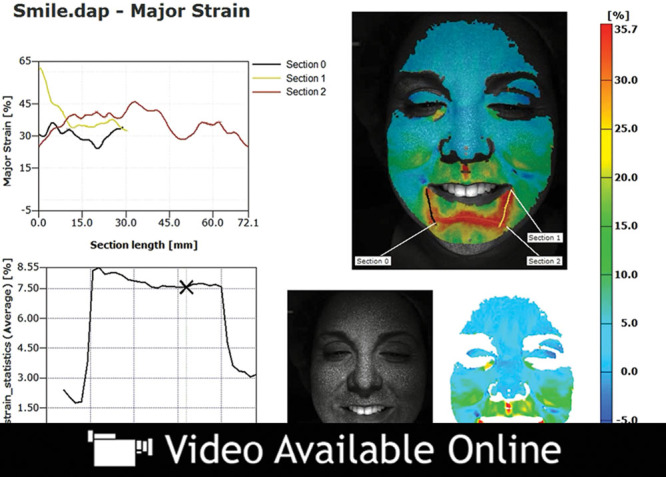
See video, Supplemental Digital Content 1, which demonstrates the change in strain over the duration of this patient’s smile. An example of dynamic major strain analysis in a younger patient who is smiling. Strain or stretch, quantified by percentage change, is displayed in a “heat map” color scale with red indicating the area of greatest strain and blue indicating the area of least strain, http://links.lww.com/PRSGO/A46.
Video 2.
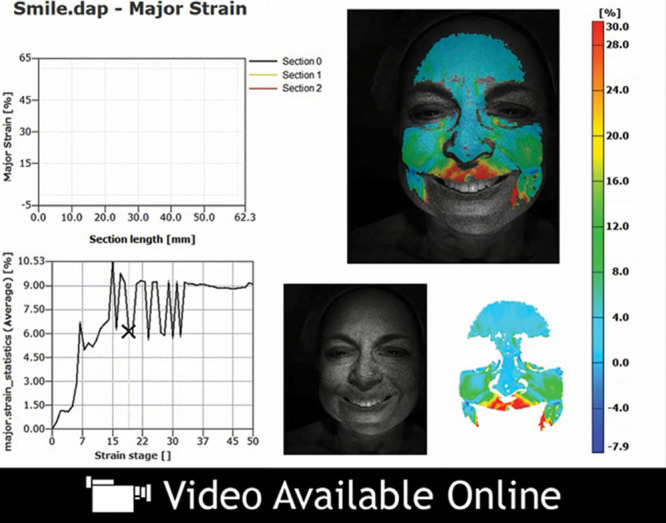
See video, Supplemental Digital Content 2, which illustrates the change in strain over the duration of this patient’s smile. An example of dynamic major strain analysis in an older patient who is smiling, http://links.lww.com/PRSGO/A47.
There were clear and measurable differences in stretch and compression in the midface and lower face between study subjects; however, these differences were not significant among study subjects for the smile, laughter, surprise, anger, or grimace expressions. By contrast, significant differences in stretch and compression were seen during lip pursing (activation of the orbicularis oris) (Fig. 3). (See Video 3, Supplemental Digital Content 3, which demonstrates the change in strain over the duration of this facial animation, http://links.lww.com/PRSGO/A48; Video 4, Supplemental Digital Content 4, which illustrates the change in strain over the duration of this facial animation, http://links.lww.com/PRSGO/A49.) Specific components of lip pursing were subsequently analyzed. In one subgroup analysis, subjects were grouped by age, with those younger than 40 years old (n = 7) compared with those older than 40 years old (n = 6). In subjects over the age of 40, there was a statistically significant greater stretch in the perioral region during lip pursing compared with subjects under the age of 40 (58.4% vs 33.8%, P = 0.015). Moreover, subjects over 40 demonstrated a significantly greater degree of overall strain in the nasolabial fold region compared with those under 40 (61.6% vs 32.9%, P = 0.007) (Fig. 4). The face as a whole was also affected by lip pursing, with a greater compression of the surrounding perioral soft tissues in subjects over the age of 40 compared with subjects under the age of 40 (1.8% vs 0.15%, P = 0.01) (Fig. 5). When separated by laterality, the older cohort also showed a higher degree of stretch at both the left (70.8% vs 32.5%, P = 0.003) and right nasolabial fold regions (52.5% vs 33.4%, P = 0.038) when compared with the younger cohort. We observed a greater degree of unpredictability in the older age group when comparing the symmetry of strain in each subject’s expressions. Interestingly, the asymmetry in strain at the nasolabial fold region was significantly greater in the older age group (18.4% vs 5.4%, P = 0.03) (Fig. 6).
Fig. 3.
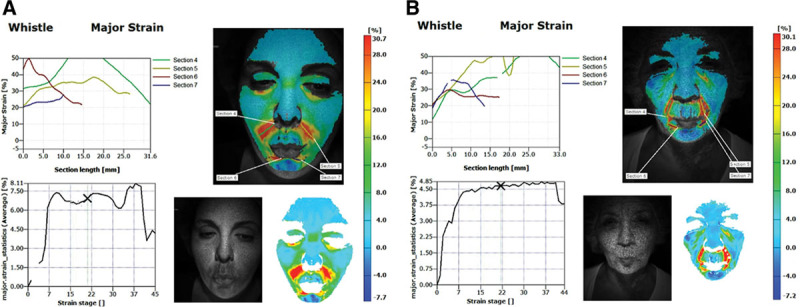
Significant differences were seen in degree of major strain in lip pursing between young (A) and old subjects (B), with a significant degree of contribution at the nasolabial fold region.
Fig. 4.
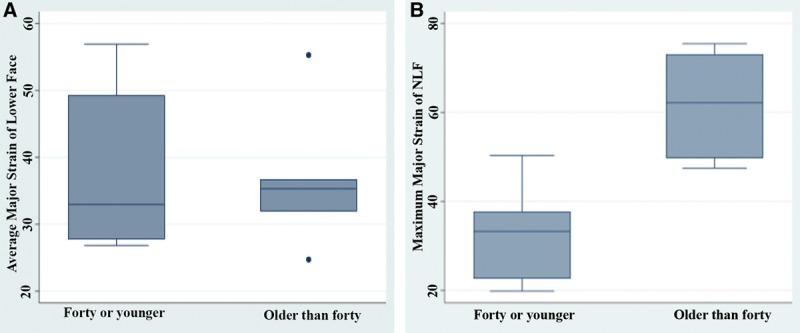
A, In lip pursing, there were significant differences in strain between subjects under 40 years and subjects 40 years and older (58.4% vs 33.8%, P = 0.015). B, Significant differences in strain asymmetry were also demonstrated between subjects older than 40 years and younger than 40 years at the nasolabial fold region (61.6% vs 32.9%, P = 0.007).
Fig. 5.
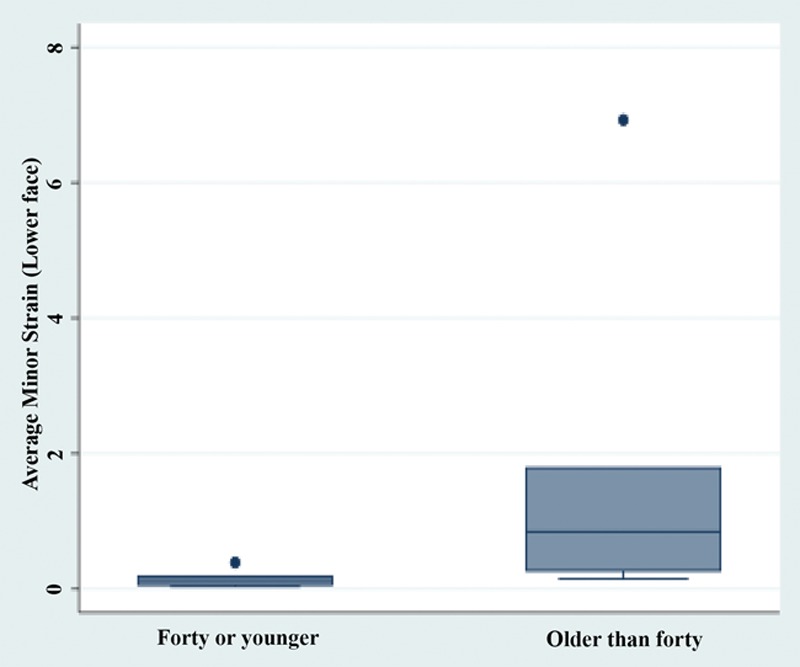
In subjects over the age of 40 compared with subjects under the age of 40, a greater degree of compression of the surrounding soft tissue of the lower face was observed (5.3% vs 3.9%, P = 0.004).
Fig. 6.
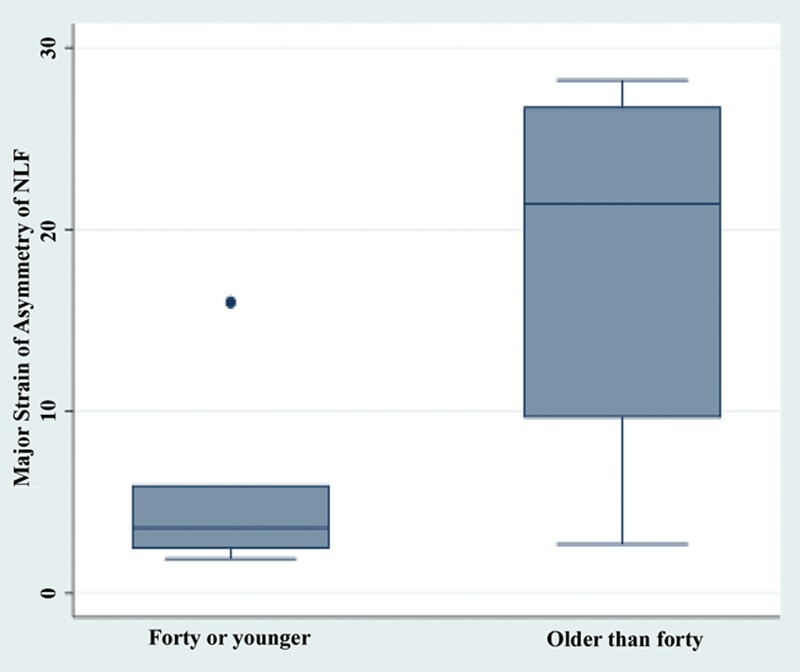
Significant differences in degree of strain asymmetry were demonstrated at the nasolabial fold region between subjects 40 years old or younger and subjects older than 40 years old (18.4% vs 5.4%, P = 0.03).
Video 3.
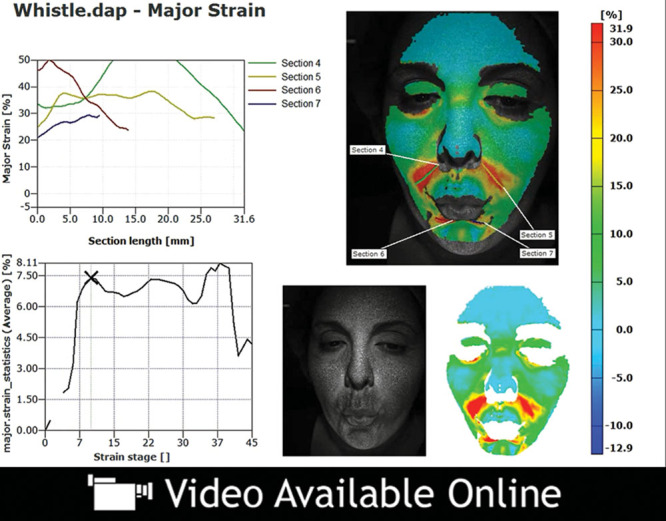
See video, Supplemental Digital Content 3, which demonstrates the change in strain over the duration of this facial animation. An example of dynamic major strain analysis in a younger patient who is lip pursing. Significant differences were seen in the degree of facial strain between younger and older patients while lip pursing, http://links.lww.com/PRSGO/A48.
Video 4.
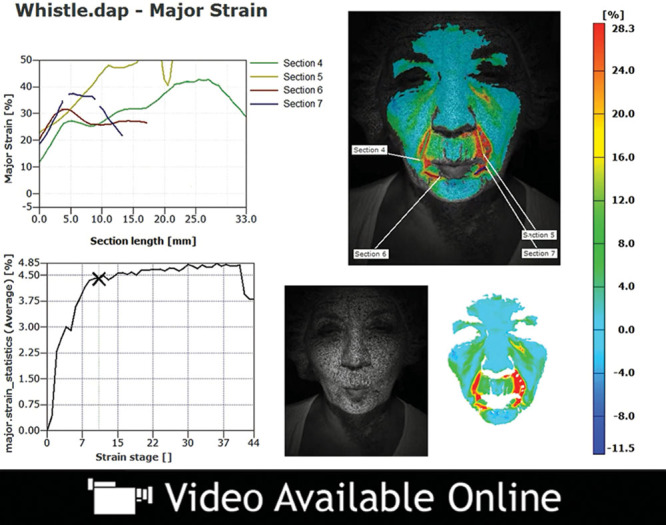
See video, Supplemental Digital Content 4, which illustrates the change in strain over the duration of this facial animation. An example of dynamic major strain analysis in an older patient who is lip pursing, http://links.lww.com/PRSGO/A49.
In another cohort, study subjects were grouped by gender, and strains associated with each facial animation were compared. As seen in the age-defined subgroup analysis, there were no significant differences in strains for any facial expression except lip pursing. The female cohort (n = 8) demonstrated an overall greater degree of stretch in the lower face than the male cohort (n = 5) when pursing their lips (41.1% vs 29.8%, P = 0.04). Notably, the women in this study were also observed as having a significantly greater degree of stretch of the left lower face (41.7% vs 28.9%, P = 0.028) and a nearly significantly greater degree of stretch at the right nasolabial fold region (49.4% vs 30.6%, P = 0.057) while lip pursing.
DISCUSSION
The role of soft-tissue strain in the dynamic face has yet to be characterized and quantified, in part, because current imaging modalities that are static and 2-dimensional are inadequate for analyzing these complex dynamic changes. In this study, we used a dynamic real-time 3-dimensional imaging technology to precisely quantify soft-tissue stretch and compression in the face during animation through the measurement of dynamic, reproducible facial surface strains. We first established that differences in facial strain are not only detectable but also quantifiable using this technology. We subsequently observed that the areas of greatest strain appeared to localize to the midface and lower face and further quantified how stretch and compression differ between patients of different ages and genders in this area of the face.
Prior studies on facial morphology have been conducted using 3-dimensional video analysis and photogrammetry. See et al9 analyzed the changes in topographic facial landmarks that occur between upright and supine positions in mother-daughter pairs and quantified the contribution of gravity using this technology. In a larger study, Iblher et al10 also demonstrated a statistically significant increase in lower face soft-tissue mobility and a decrease in soft-tissue stiffness in older age groups in comparison to younger age groups. Prior investigations in normal facial animation have further shown asymmetry with slightly greater left-sided motion and displacement11,12 and greater displacement in older populations.12 The data we have presented here are consistent with these prior evaluations, thereby validating the application of this novel imaging technology to the dynamic face.
The data from this pilot study confirm the clinical observation that perioral animation is an important contributor to facial aging as demonstrated by significant differences in strain measurements between the younger and older age groups with activation of the perioral musculature. Subjects older than 40 years exhibited significantly greater stretch, compression, variability, and asymmetry in the perioral region compared with subjects younger than 40 years. These data represent the first precise evaluation and quantification of facial dynamics. Because efforts at perioral rejuvenation and reconstruction remain disappointing, we hope these data will serve as a first step for improving our ability to understand and develop more effective treatments for the lower face and beyond.
This pilot study has several limitations. Our sample population is small and potentially underpowered. Although our use of dynamic real-time 3-dimensional imaging technology enables the measurement of dynamic, reproducible facial surface strains, we acknowledge that the specific underlying mechanisms responsible for the observable differences in stretch and compression have yet to be defined and remain beyond the scope of this study. Additionally, the strains associated with each facial expression were subexamined using age and gender as variables, illustrating an association between age and degree of strain but without implication of causality. Moreover, although all patients were white and Fitzpatrick grade 2–3, we did not control for patient variables such as body mass index (BMI), smoking, and photodamage, which undoubtedly have their own contributions to facial dynamics. Further studies using this technology to evaluate facial strain in age-matched patients with low BMI and minimal facial subcutaneous fat versus those with a high BMI and a significant facial fat are warranted. Other variables such as race, Glogau scale, and genetic factors would also be important to consider. The long-term application of this technology for documentation of the aging face in a given individual will permit us to characterize facial aging without inherent variability in facial animation. Of further interest would be a longitudinal investigation of the effects of aesthetic interventions on facial strain within individual patients to quantify dynamic responses to specific rejuvenation therapies, including neuromodulators, facial fillers, and surgical rejuvenation.
The successful use of real-time dynamic 3-dimensional imaging for soft-tissue strain analysis may have broad and functional implications for soft-tissue restoration not only in aesthetic procedures of the face but also in reconstructive surgery. The application of this technology for preoperative assessments of patients for abdominal wall reconstruction, facial reanimation, and delayed facial trauma reconstruction may elucidate the dynamic limitations of previously damaged tissues, resulting in optimized reconstructive surgical plans and patient outcomes.
CONCLUSIONS
We demonstrated that the dynamic face can be objectively and quantitatively evaluated using dynamic principle strain analysis. The midface and lower face exhibit the most amount of dynamic facial strain, a characteristic that increases with advancing age. We propose that dynamic 3-dimensional optical imaging can be used to advance our understanding of soft-tissue dynamics, facial anatomy, and the effects of animation on facial strain over time, thereby leading to improved individualized treatments and new clinical applications for the treatment of facial rejuvenation and reconstruction.
PATIENT CONSENT
Patients provided written consent for the use of their images.
ACKNOWLEDGMENTS
We thank John Tyson, Eric Schwartz, and Raghavendra Saralaya at Trilion Quality Systems for their technical support. This study was reviewed and approved by the Institutional Review Board of the University of Pennsylvania.
Supplementary Material
Footnotes
Presented at the 92nd Annual American Association of Plastic Surgeons Meeting in New Orleans, La., April, 2013, and at the 46th Annual Aesthetic Surgery Meeting in New York, N.Y., April, 2013.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study was funded by the Center for Human Appearance at the University of Pennsylvania. None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382–386. doi: 10.1097/IOP.0b013e3181b2f766. [DOI] [PubMed] [Google Scholar]
- 2.Gosain AK, Amarante MT, Hyde JS, et al. A dynamic analysis of changes in the nasolabial fold using magnetic resonance imaging: implications for facial rejuvenation and facial animation surgery. Plast Reconstr Surg. 1996;98:622–636. doi: 10.1097/00006534-199609001-00005. [DOI] [PubMed] [Google Scholar]
- 3.Honrado CP, Larrabee WF., Jr Update in three-dimensional imaging in facial plastic surgery. Curr Opin Otolaryngol Head Neck Surg. 2004;12:327–331. doi: 10.1097/01.moo.0000130578.12441.99. [DOI] [PubMed] [Google Scholar]
- 4.Coleman SR, Grover R. The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthet Surg J. 2006;26:S4–S9. doi: 10.1016/j.asj.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Techniques, T.Q.S.a.G.O.M. ARAMIS: Optical 3D Deformation Analysis [brochure] 2010. [Google Scholar]
- 6.Tyson J, Psilopoulos J, Schwartz E, et al. Advanced material properties measurements with optical metrology. SAE Int Tech Paper. 2012;1:1–10. [Google Scholar]
- 7.Sztefek P, Vanleene M, Olsson R, et al. Using digital image correlation to determine bone surface strains during loading and after adaptation of the mouse tibia. J Biomech. 2010;43:599–605. doi: 10.1016/j.jbiomech.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Tyson J, Schmidt T, Galanulis DK. Smart biomechanics strain measurements using 3D image correlation photogrammetry. Biophotonics. 2003:8. [Google Scholar]
- 9.See MS, Roberts C, Nduka C. Age- and gravity-related changes in facial morphology: 3-dimensional analysis of facial morphology in mother-daughter pairs. J Oral Maxillofac Surg. 2008;66:1410–1416. doi: 10.1016/j.joms.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 10.Iblher N, Gladilin E, Stark BG. Soft-tissue mobility of the lower face depending on positional changes and age: a three-dimensional morphometric surface analysis. Plast Reconstr Surg. 2013;131:372–381. doi: 10.1097/PRS.0b013e318278d67c. [DOI] [PubMed] [Google Scholar]
- 11.Coulson SE, Croxson GR, Gilleard WL. Three-dimensional quantification of the symmetry of normal facial movement. Otol Neurotol. 2002;23:999–1002. doi: 10.1097/00129492-200211000-00032. [DOI] [PubMed] [Google Scholar]
- 12.Giovanoli P, Tzou CH, Ploner M, et al. Three-dimensional video-analysis of facial movements in healthy volunteers. Br J Plast Surg. 2003;56:644–652. doi: 10.1016/s0007-1226(03)00277-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


