Abstract
Background:
Treatment of burned patients is a tricky clinical problem not only because of the extent of the physiologic abnormalities but also because of the limited area of normal skin available.
Methods:
Literature indexed in the National Center (PubMed) has been reviewed using combinations of key words (burns, children, skin graft, tissue engineering, and keratinocyte grafts). Articles investigating the association between burns and graft therapeutic modalities have been considered. Further literature has been obtained by analysis of references listed in reviewed articles.
Results:
Severe burns are conventionally treated with split-thickness skin autografts. However, there are usually not enough skin donor sites. For years, the question of how covering the wound surface became one of the major challenges in clinical research area and several procedures were proposed. The microskin graft is one of the oldest methods to cover extensive burns. This technique of skin expansion is efficient, but results remain inconsistent. An alternative is to graft cultured human epidermal keratinocytes. However, because of several complications and labor-intensive process of preparing grafts, the initial optimism for cultured epithelial autograft has gradually declined. In an effort to solve these drawbacks, isolated epithelial cells from selecting donor site were introduced in skin transplantation.
Conclusions:
Cell suspensions transplanted directly to the wound is an attractive process, removing the need for attachment to a membrane before transfer and avoiding one potential source of inefficiency. Choosing an optimal donor site containing cells with high proliferative capacity is essential for graft success in burns.
Methods for handling extensive burn wounds have changed in recent decades, and an increasingly surgical approach with early excision and wound closure is being applied for patient survival. The procedures, involving removal of necrotic burned tissue and subsequent graft, reduce bacterial colonization of wounds, reduce systemic sepsis, and preserve as much underlying viable tissue as possible.1 However, disadvantages of skin autografts for coverage include limited healthy donor sites in extensive burns and donor-site morbidity. An alternative approach to address these drawbacks is to graft in vitro-expanded epithelial keratinocytes, using a reliable method of culturing human epidermal keratinocytes in stratified and coherent layers.2–6 These autologous epidermal sheets have been successfully used, in addition to split-thickness skin grafts, in treating major burns.7 However, widespread use of these cultured epithelial autografts (CEAs) has been hampered by the long in vitro expansion times, sensitivity to infection, mechanical fragility of the sheets, and labor-intensive process of preparing grafts, associated with the requirement of the relevant laboratory expertise.8–10 In addition, clinical results have not been as satisfactory as expected, with reports of occurrences of hyperkeratosis and scar contracture.11–13 Over the recent past years, great strides have been made in the identification, isolation, and characterization of epidermal stem cells.14–23 Among this potential keratinocyte donor sites, the foreskin seemed to be a promising cell source for graft. Eighty-eight articles investigated for the review of literature and pointed out a correlation between burns and graft therapeutic modalities have been considered. Further literature has been referenced by analysis of references listed in reviewed articles. Twenty articles analyzing keratinocyte grafts have been obtained, and only 8 of them raise the importance of keratinocyte donor sites. Recent studies also suggest that the embryonic cells and induced pluripotent stem cells could be an attractive source for skin graft.
For years, how to cover large surface wounds has been one of the major questions in clinical research. Detailed below, the different approaches were summarized in Table 1.
Table 1.
Therapeutic Means for Epidermal Wound Healings
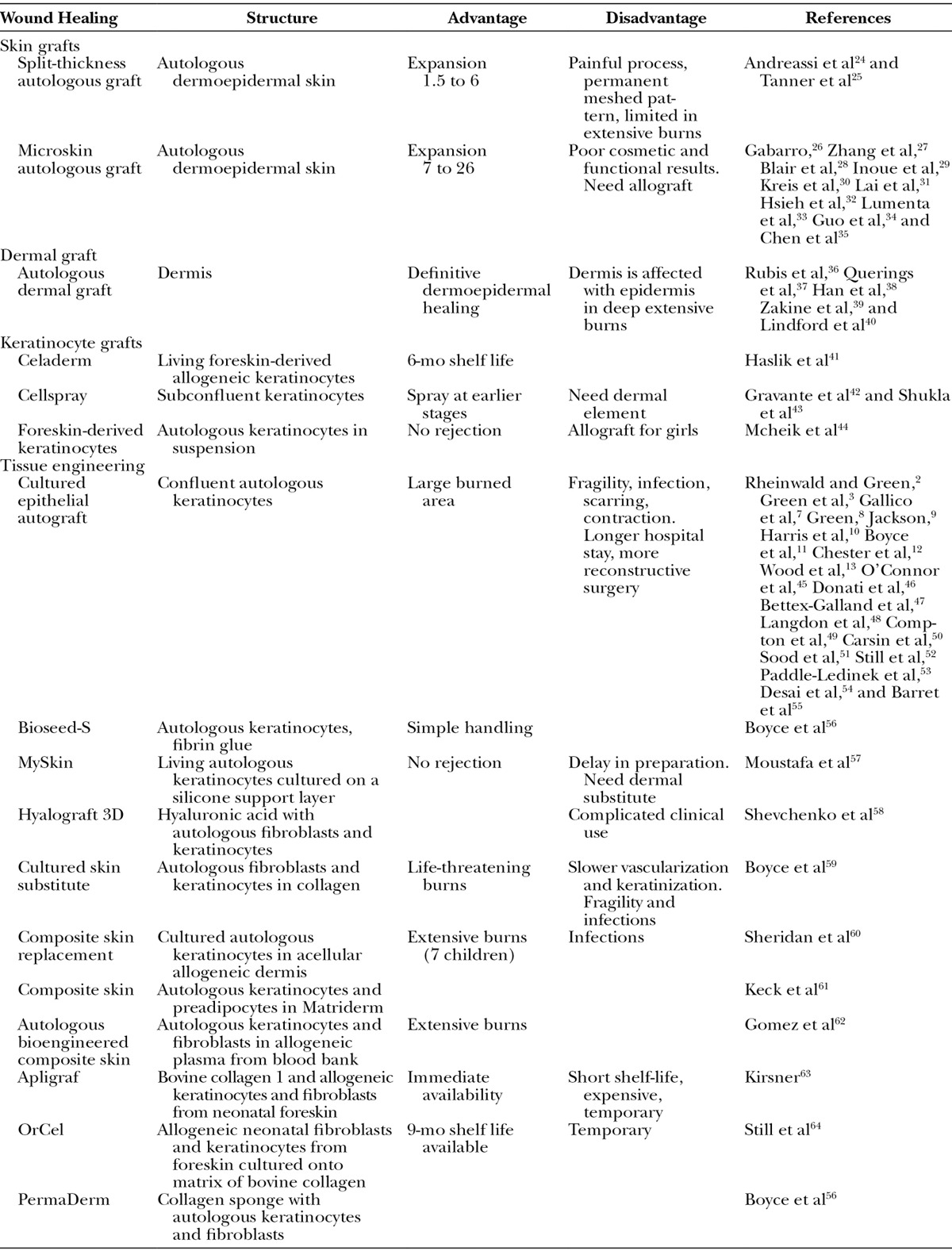
SKIN GRAFT
Split-thickness Skin Grafts
For burn treatments, split-thickness autologous skin grafts (STSGs) are still the gold standard of care.24 The technique of skin harvesting and transplantation was initially described approximately 3000 years ago with HindouTilemaker Caste in which skin grafting was used to reconstruct noses that were amputated as a mean of judicial punishment. Depth graft is usually limited to the upper third of the dermis, leaving sufficient number of epidermal cells in skin appendages. The donor sites heal via reepithelialization from the remaining skin appendage epidermal elements within 7–14 days. However, high rate of morbidity at the donor sites as pain and unsightly scar was noted. Currently, a nearly 95% success rate is the standard of care for skin grafting.
Meshed Split-thickness Grafts
To cover extensive burn areas regarding the limited availability of donor sites, the technique of meshed STSG was employed. The functional result from grafting with 1.5:1 meshed autograft is equivalent to that obtained by sheet graft.25 However, the meshed pattern is permanent, and the scar is not as cosmetically appealing as sheet autograft. Furthermore, hypertrophic scarring and pigmentation changes are not uncommon in donor sites and donor sites are painful. When burns are larger than 30% of total body surface area (TBSA), widely spread meshed autografts (3:1 or 6:1) are commonly used, providing less engraftment and rarely an acceptable cosmetic or functional cover. When the burn area exceeds 40% TBSA, it is not usually possible to cover the entire burns with autologous grafts, and another alternative cover is needed.
Microskin Autologous Graft
Microskin graft technique may be a solution for treating major burns.26 Small pieces of 200 × 200 μm “diced” skin grafts were obtained, and the expansion ratio is more than 10:1. Successful microskin graft was reported in rabbit and pig with expansion ratios from 7:1 to 26:1 and a healing time from 19 to 35 days.27,28 In clinical practice, several authors suggested this procedure for grafting on granulating wounds under poor conditions29–31 and integrated it in a whole treatment concept for management of severely burned patients.32,33 Such procedure enabled early excision and graft, thus reducing morbidity and mortality in 31 of 54 patients with extensive burns (80% TBSA). In this study, the expansion ratio was 15:1, and the wound can be completely resurfaced in 45 days.34 As well, in 63 severely burned patients (85% TBSA), microskin autografting yielded an overall survival rate of 64%, and the wound-healing rate was 75% between 35 and 55 days.35 Overall, we can report that this procedure remains a lifesaving technique.
DERMAL GRAFT
The autologous dermal graft has been used in reconstructive plastic surgery for almost 80 years. Split-thickness dermal graft as an alternative to the STSG was used, comparing their ability to resurface full-thickness skin defects in a pig model. Epithelialization of the split-thickness dermal graft and STSG was complete at 4 weeks.36 Indeed, meshed dermal graft is an appropriate technique for reconstruction of large defects of sole and scalp, and excellent long-term functional results were reported.37 Moreover, dermis graft technique for wound coverage in patients was aesthetically and functionally superior to the regular skin graft technique in both the recipient and donor sites.38–40
CULTURED EPITHELIAL AUTOGRAFT
CEA is an alternative approach to obtain an epidermal graft with a reliable method of culturing human epidermal keratinocytes in stratified and coherent layers.2,3 CEA has become available as an alternative measure to the use of expanded skin autografts, and epidermal sheets have been used in treating major burns.7,11,45–49 From 1991 to 1996, CEA was applied to 30 patients with 78% TBSA. The survival was 90%, and the final CEA engraftment was 69%. Younger age was significantly associated with better CEA success graft.50 In a large study,51 an 18-year experience concerning 88 patients from 6 months to 73 years (including 20 children) and from 28% to 98% TBSA was presented. The mean final CEA engraftment was 73%, and the overall patient survival rate was 91%. Complications were classified as early and late, including blistering and shearing (31%), pruritus (5%), and wound contractures (66%). These results demonstrated that CEA is an adjunct to achieve a high survival rate in child and adult burn patients having a poor prognosis.
However, disappointing results with CEA grafts were reported.52 Thereby, the widespread use of these CEA has been hampered by the long in vitro expansion times and the sensitivity to infection.53 In addition, manufacturing of CEA needs a labor-intensive process of preparing grafts associated with the requirement of a relevant laboratory expertise,9,10 and clinical results reported occurrences of blistering, hyperkeratosis, and scar contracture.54 In 32 burned children (90% TBSA) from 1988 to 1998, CEA graft allowed a survival rate of 60%, but children had longer hospital stay and required more reconstructive procedures during the first 2 years.55 To summarize, a critical review of the literature studying issues associated with CEA pointed out the factors potentially limiting this procedure, the time necessary to expand cells before transplantation, the reliability and vulnerability of grafts on the newly healed surface, the long-term durability, and the high cost implications of such treatment.13 They concluded by the question, “Does CEA have a role in the treatment of major burns?” Taken together, the initial optimism for CEA has gradually declined. Nowadays, we could consider that CEA is valuable as a lifesaving measure for early closure and it is not suitable as permanent coverage.
ISOLATED KERATINOCYTE GRAFTS
Transplantation with isolated keratinocytes marks a turning point in skin grafting.65 Human keratinocytes seem to have a finite culture lifetime, and the plating efficiency of the epidermal cells isolated directly from the skin of newborns is 1–5%.66 As discussed below, the choice of donor sites is a crucial parameter in this procedure. In clinical practice, 80% of patients attending for plastic surgery would accept a tissue-engineered product, and autologous cells were the preferred choice.67
In an effort to solve these drawbacks with CEA,68 epithelial cells in a preconfluent state were introduced in cell transplantation before they form a sheet and thus differentiation and fusion occurred in vivo. This method has the advantages of reducing the culture period before clinical use, minimizing the enzymatic degradation of cell surface proteins or cellular basal membranes, and allowing the transplanted cells to proliferate more actively and to generate a more developed and robust dermal epithelialization.69 Keratinocyte graft in suspension is effective even without a complex delivery system.
Keratinocyte Allografts
Transplanted organs contain resident leucocytes, including Langerhans cells that initiate the rejection of a transplant by expressing foreign class II histocompatibility molecules. In vitro, the Langerhans cells are lost after 7–10 days.70,71 To determine the survival of cultured allogeneic keratinocytes transplanted to a deep dermal bed, cultured allogeneic keratinocytes from donors of the opposite sex were used. If all wounds heal by 3 weeks, no male cells were identified in a biopsy specimen from female patients transplanted with boy cultured keratinocytes.72 By further in vivo studies, they concluded that allogeneic keratinocytes temporarily “take” to the wound, contribute to rapid wound closure, and are replaced by the patient’s epidermal cells after about 1 week.73 After graft, keratinocytes interact with the other cell populations and accelerate wound healing by expressing favorable keratinocyte-derived cytokines and growth factors.41 Recently, 205 adult outpatients from 28 centers in the United States and Canada with persistent ulcer had foreskin keratinocyte grafts. The primary outcome analysis showed a significantly greater mean reduction of wound area associated with active treatment compared to vehicle, and the dose of 0.5 × 106 cells/mL every 14 days showed the largest improvement.23
Keratinocyte Autografts
Few burn units implement the graft of freshly isolated autologous keratinocytes for adult patients and more rarely for children. However, this approach has been initiated 60 years ago in rabbits, and a comparative study was performed in pigs.74 After a period of neglect, this procedure raises and became trendy.17,19,52,75–78 In 2005, the use of the standardized Recell device (Avita medical, Melbourn, United Kingdom) was introduced into human clinical practice, allowing the immediate processing of small split-thickness biopsies to isolate and graft keratinocytes in wounds.42,43 Recently, in a preliminary study in pediatric department of CHU of Poitiers, we grafted burns in boys with a suspension of noncultured autologous foreskin-isolated keratinocytes. Epithelialization and accelerated wound healing were obtained, and the quality of scarring and pigmentation was improved compared to classical skin grafts.44 We emphasized the aesthetic and high quality of the skin: vascularity, pigmentation, and pliability without hypertrophy or pruritus, as reflected by a reliable Vancouver scar scale. In this study, we investigated the reliability of noncultured keratinocyte grafting in 11 boys with a limited average size of TBSA (10%). In a near future, we will propose this procedure for severe deep burns in a multicenter trial study.
DONOR SITES: A CRITICAL CHOICE FOR SUCCESSFUL GRAFTING
The comparison of the characteristics of donor sites and their ability to regenerate human epithelia (3D) in vitro has been poorly studied. In adult patients, human scalp seems a promising suitable keratinocyte donor site for the development of therapeutic cell transplantation,21,79 and hair follicle represents a major source of keratinocyte stem cells. Follicular bulge stem cells are potentially bipotent, giving rise to hair follicle and epidermis.80,81 However, it seems difficult at the present time to extract the epithelial stem cell population deeply located in the basal layer of the outer root sheath, at the level of the hair follicle bulge. Searching for alternative sites, scalp, auricular skin, and chest skin were compared for their capacity to generate keratinocytes with promising graft potentiality. The proportion of K19-positive cells (stem cell marker) harvested from auricular skin was about twice that of the scalp, and surprisingly, the number of K19-positive cells estimated in situ on skin sections was about double in scalp than in auricular skin. Chest skin had the lowest number of K19-positive cells. These results indicate that in addition to the choice of an adult anatomic site featuring a high number of stem cells in situ, the quality of the cultures greatly depends on the ability to extract stem cells from the skin biopsy.82
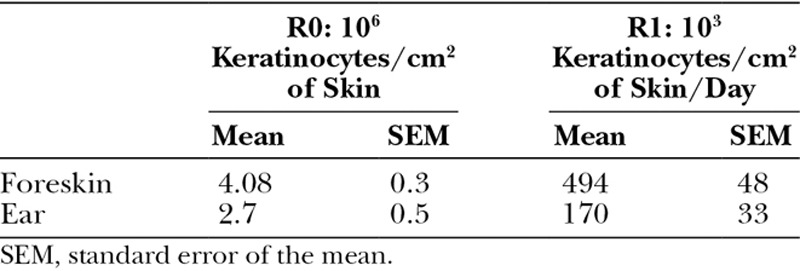
Preputial skin is a thin full-thickness and expandable skin that can be easily harvested. Given the difficulties to harvest deep keratinocyte scalp in children and given our previously in vitro results on foreskin-keratinocyte regenerative capacities, we have proposed to graft noncultured foreskin keratinocytes.22,83 Pediatric foreskin tissues produced more viable keratinocytes than the skin of pinna (Table 2), and the proliferative capacity is higher for foreskin keratinocytes. In addition, we reported that cultured keratinocytes from foreskin express lower amounts of differentiation markers and higher amounts of stem cell markers than those from auricular area, and keratinocytes from foreskin exhibit a high epidermal reconstruction capability.44
Table 2.
Cell Recovery from Tissues (R0: after Skin Enzymatic Digestion) and Cell Production Capacity at Passage 1 (R1: after Culture)
Finally, the possible therapeutic use of somatic cells derived from embryonic stem cells is currently a hot topic in regenerative medicine. Although skin biopsies are a regular source of keratinocytes and epidermal stem cells, recent research suggests that the generation of keratinocytes from human embryonic stem cells could be a useful technique.16 Researchers have also managed to derive induced pluripotent stem cells (iPS) from differentiated cells.84 Advances in this area have been truly breathtaking, and the challenge in the coming years will be to actually transfer all these breakthroughs in molecular and cell biology to the routine clinical practice.
TISSUE ENGINEERING AND CELL GRAFTS
Various engineered tissue formats have been used for skin grafts, including allogeneic fibroblasts and keratinocytes in a bovine collagen sponge (OrCel, Ortec International, Atlanta, Ga.)64 or a bilayered living skin equivalent, Apligraf (Organogenesis, Inc., Canton, Mass.). Derived from the foreskin, cultured neonatal fibroblasts are combined with bovine type I collagen to form a neodermis. Cultured neonatal keratinocytes seeded on this neodermis proliferate and differentiate. Approved in several countries, Apligraf as an allograft has been used in acute wounds such as surgical excision sites and partial thickness donor sites.63 In the same way, cultured autologous fibroblasts and keratinocytes onto collagen-glycosaminoglycan constitute another skin substitute59 grafted in 17 patients; authors noted that skin substitute prepared from autologous cells might be a safe and efficacious alternative to classical autograft for life-threatening burns. However, multiple early deficiencies of this cell-biopolymer graft were observed, including slower vascularization, slower keratinization, greater graft loss from microbial contamination, and greater mechanical fragility. On the other hand, the feasibility of combined cultured autologous keratinocytes and dermal scaffold as a cellular human dermis (Alloderm, Life cell, NJ) was investigated in a porcine model. Both successful histointegration of the in vivo composite grafts and a reduced wound contraction, compared with epithelial grafts,60 were observed. More sophisticated skin substitutes were also described “Human preadipocytes from the subcutaneous tissue and cultured keratinocytes seeded onto a scaffold (Matriderm, MedSkin Solutions Dr Suwelack, Billerbeck, Germany).” Three weeks later, a simultaneous growth of keratinocytes and preadipocytes was observed: keratinocytes adhered to the surface of the matrix and formed a confluent epidermis-like layer and preadipocytes adhered and penetrated into the deeper layers of the matrix. This approach toward a multilayered skin substitute could be a useful asset for future reconstructive surgery.61 To obtain an inexpensive substitute, another evaluation strategy was to graft autologous fibroblasts and keratinocytes with plasma from the blood bank.62 In this study, 25 burned patients (74% TBSA) were treated with a final 49% successful engraftment. Fewer infections concomitant to graft were significantly associated with better engraftment, and cosmetic and functional outcomes were satisfactory 4 years later.
A systematic review of the literature assessed the safety and efficacy of bioengineered skin substitutes in comparison with standard methods in the management of burns. Twenty randomized controlled trials were included in this review, but the numerous subgroup analyses and the diversity of skin substitutes limited the ability to draw conclusions. However, bioengineered skin substitutes, namely, Biobrane (composed of a knitted nylon mesh that is bonded to a thin, silicone membrane and coated with porcine polypeptides; Dow Hickam/Bertex pharmaceuticals, Sugar land, Tex.), TransCyte (composed of a semipermeable silicone membrane and human newborn fibroblast cells cultured on a porcine collagen-coated nylon mesh; Advanced tissue sciences, Inc., La Jolla, Caif.), Dermagraft (polygalactin mesh seeded with allogeneic neonatal fibroblasts), Apligraf (composed of type I bovine collagen and allogeneic keratinocytes and fibroblasts obtained from neonatal foreskin), and CEA, were at least as safe as the classical skin replacements or topical agents/wound dressings. For partial thickness burns (less than 15% TBSA), Biobrane and TransCyte seemed to be more effective than silver sulfadiazine, avoiding the need for painful daily dressing changes and long stay in hospital. TransCyte seemed to be effective for facial burns, providing good adherence to the contours of the face. For burns between 20% and 50% TBSA, CEA, Dermagraft, and Apligraf combined with autograft seemed to be effective. Integra (Integra Life Science Corp., Plainsboro, NJ) may be better suited in patients with limited burns (45% TBSA). Taken together, authors concluded that rigorous randomized controlled trials with long-term follow-up would strengthen the evidence base for the use of bioengineered skin substitutes.85
CONCLUSIONS
At the present time, most of the deep burns are treated with split-thickness skin autografts that could be painful and source of unsightly scars in donor and grafted areas. When the burns are extensive, there are usually not enough skin donor sites. Currently, we can consider that microskin graft and CEA are valuables as lifesaving measures but are not suitable as permanent coverage. In the procedures, functional and aesthetic results remain inconsistent. Numerous “plastic” synthetic materials for dermal substitution have been developed. They seem to work as well as allograft as a temporary wound cover. However, they do not vascularize, their conformability varies, they have little resistance to infection, and they often do not adhere to the wounds. Advances in the development of tissue-engineered skin have led to several new products aimed to improve wound healing. Designed as a skin substitute, dermal equivalents have been investigated for over a decade. Although dermal substitutes may provide wound coverage and reduce pain, they still require an epidermal component. In this case, adjuvant keratinocyte transplantation could have a prominent place. Cell suspensions can be also extemporaneously transplanted directly into the wound. However, questions related to optimal cell type, carrier and transfer modality, as well as the final outcome, the ability to generate an epithelium after transplantation, and the scar quality are still not fully answered. For this reason, choosing an optimal donor-site possessing cells with high proliferative capacity is essential for wound healing success.44 A high number of keratinocytes can be harvested in a few hours, and the graft can be extemporaneously considered as soon as the wound surface is optimally prepared. Large injured areas can be successfully grafted in a few days after burns although a common skin graft is insufficient. Keratinocyte graft in suspension also allows the harvesting and grafting of the entire epithelial cell population.86,87 Successful clinical results and the easy management of the keratinocyte isolation procedure in the operating room allowed us to design the noncultured autologous keratinocyte transplantation as a standard procedure, which can be added to the arsenal of therapies for burned patients in surgical units everywhere.
ACKNOWLEDGMENTS
We thank Sevdy Peci and Elodie Paintoux, laboratory technicians in BIOalternatives Laboratory, for their expert advice on cell culture. We thank Nathalie Coussay for English corrections and reading.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Atiyeh BS, Costagliola M. Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns. 2007;33:405–413. doi: 10.1016/j.burns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 3.Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979;76:5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyce ST. Design principles for composition and performance of cultured skin substitutes. Burns. 2001;27:523–533. doi: 10.1016/s0305-4179(01)00019-5. [DOI] [PubMed] [Google Scholar]
- 5.Jones I, Currie L, Martin R. A guide to biological skin substitutes. Br J Plast Surg. 2002;55:185–193. doi: 10.1054/bjps.2002.3800. [DOI] [PubMed] [Google Scholar]
- 6.Gerlach J, Wolf SE, Johnen C. Principles of Regenerative Medicine No. 76. Gerlach J. Elsevier, ed. Burlington, MA: Elsevier/Academic Press; 2008. Innovative regenerative medicine approaches to skin cell-based therapy for patients with burn injuries. pp. 1298–1321. [Google Scholar]
- 7.Gallico GG, III, O’Connor NE, Compton CC, et al. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 8.Green H. Cultured cells for the treatment of disease. Sci Am. 1991;265:96–102. doi: 10.1038/scientificamerican1191-96. [DOI] [PubMed] [Google Scholar]
- 9.Jackson DM. The evolution of burn treatment in the last 50 years. Burns. 1991;17:329–334. doi: 10.1016/0305-4179(91)90050-q. [DOI] [PubMed] [Google Scholar]
- 10.Harris PA, Leigh IM, Navsaria HA. Pre-confluent keratinocyte grafting: the future for cultured skin replacements? Burns. 1998;24:591–593. doi: 10.1016/s0305-4179(98)00131-4. [DOI] [PubMed] [Google Scholar]
- 11.Boyce ST, Kagan RJ, Meyer NA, et al. The 1999 clinical research award. Cultured skin substitutes combined with Integra artificial skin to replace native skin autograft and allograft for the closure of excised full-thickness burns. J Burn Care Rehabil. 1999;20:453–461. doi: 10.1097/00004630-199920060-00006. [DOI] [PubMed] [Google Scholar]
- 12.Chester DL, Balderson DS, Papini RP. A review of keratinocyte delivery to the wound bed. J Burn Care Rehabil. 2004;25:266–275. doi: 10.1097/01.bcr.0000124749.85552.cd. [DOI] [PubMed] [Google Scholar]
- 13.Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn injuries: a critical review of the literature. Burns. 2006;32:395–401. doi: 10.1016/j.burns.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Papini S, Cecchetti D, Campani D, et al. Isolation and clonal analysis of human epidermal keratinocyte stem cells in long-term culture. Stem Cells. 2003;21:481–494. doi: 10.1634/stemcells.21-4-481. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180:273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guenou H, Nissan X, Larcher F, et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study. Lancet. 2009;374:1745–1753. doi: 10.1016/S0140-6736(09)61496-3. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser HW, Stark GB, Kopp J, et al. Cultured autologous keratinocytes in fibrin glue suspension, exclusively and combined with STS-allograft (preliminary clinical and histological report of a new technique). Burns. 1994;20:23–29. doi: 10.1016/0305-4179(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann B, Ekkernkamp A, Johnen C, et al. Sprayed cultured epithelial autografts for deep dermal burns of the face and neck. Ann Plast Surg. 2007;58:70–73. doi: 10.1097/01.sap.0000250647.39784.bb. [DOI] [PubMed] [Google Scholar]
- 19.Wood FM, Stoner ML, Fowler BV, et al. The use of a non-cultured autologous cell suspension and Integra dermal regeneration template to repair full-thickness skin wounds in a porcine model: a one-step process. Burns. 2007;33:693–700. doi: 10.1016/j.burns.2006.10.388. [DOI] [PubMed] [Google Scholar]
- 20.Larouche D, Tong X, Fradette J, et al. Vibrissa hair bulge houses two populations of skin epithelial stem cells distinct by their keratin profile. FASEB J. 2008;22:1404. doi: 10.1096/fj.07-8109com. [DOI] [PubMed] [Google Scholar]
- 21.Schlabe J, Johnen C, Schwartlander R, et al. Isolation and culture of different epidermal and dermal cell types from human scalp suitable for the development of a therapeutical cell spray. Burns. 2008;34:376–384. doi: 10.1016/j.burns.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 22.McHeik JN, Barrault C, Bernard FX, et al. Quantitative and qualitative study in keratinocytes from foreskin in children: perspective application in paediatric burns. Burns. 2010;36:1277–1282. doi: 10.1016/j.burns.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Kirsner RS, Marston WA, Snyder RJ, et al. Spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: a Phase 2, multicentre, double blind, randomised, placebo-controlled trial. Lancet. 2012;12:60644–60648. doi: 10.1016/S0140-6736(12)60644-8. [DOI] [PubMed] [Google Scholar]
- 24.Andreassi A, Bilenchi R, Biagioli M, et al. Classification and pathophysiology of skin grafts. Clin Dermatol. 2005;23:332–337. doi: 10.1016/j.clindermatol.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Tanner JC, Jr, Vandeput J, Olley JF. The mesh skin graft. Plast Reconstr Surg. 1964;34:287–292. [PubMed] [Google Scholar]
- 26.Gabarro P. A new method of grafting. Br Med J. 1943;1:723–724. doi: 10.1136/bmj.1.4301.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang ML, Chang ZD, Han X, et al. Microskin grafting. I. Animal experiments. Burns Incl Therm Inj. 1986;12:540–543. doi: 10.1016/0305-4179(86)90002-1. [DOI] [PubMed] [Google Scholar]
- 28.Blair SD, Nanchahal J, Backhouse CM, et al. Microscopic split-skin grafts: a new technique for 30-fold expansion. Lancet. 1987;2:483–484. doi: 10.1016/s0140-6736(87)91795-8. [DOI] [PubMed] [Google Scholar]
- 29.Inoue Y, Tanabe H, Tai Y. A method for the preparation of microskin grafts using skin-graft meshers. Plast Reconstr Surg. 1994;94:890. doi: 10.1097/00006534-199411000-00031. [DOI] [PubMed] [Google Scholar]
- 30.Kreis RW, Mackie DP, Hermans RP, et al. Expansion technique for skin grafts: comparison between mesh and Meek island (sandwiched-) grafts. Burns. 1994;20:39–42. doi: 10.1016/0305-4179(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 31.Lai CS, Lin SD, Tsai CC, et al. An easy way to prepare microskin grafts. Burns. 1994;20:151–153. doi: 10.1016/s0305-4179(06)80013-6. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh CS, Schuong JY, Huang WS, et al. Five years’ experience of the modified Meek technique in the management of extensive burns. Burns. 2008;34:350–354. doi: 10.1016/j.burns.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Lumenta DB, Kamolz LP, Frey M. Adult burn patients with more than 60% TBSA involved-Meek and other techniques to overcome restricted skin harvest availability—the Viennese Concept. J Burn Care Res. 2009;30:231–242. doi: 10.1097/BCR.0b013e318198a2d6. [DOI] [PubMed] [Google Scholar]
- 34.Guo F, Chen XL, Wang YJ, et al. Management of burns of over 80% of total body surface area: a comparative study. Burns. 2009;35:210–214. doi: 10.1016/j.burns.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Chen XL, Liang X, Sun L, et al. Microskin autografting in the treatment of burns over 70% of total body surface area: 14 years of clinical experience. Burns. 2011;37:973–980. doi: 10.1016/j.burns.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Rubis BA, Danikas D, Neumeister M, et al. The use of split-thickness dermal grafts to resurface full thickness skin defects. Burns. 2002;28:752–759. doi: 10.1016/s0305-4179(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 37.Querings K, Bachter D, Balda BR. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122–126. doi: 10.1046/j.1524-4725.2002.01076.x. [DOI] [PubMed] [Google Scholar]
- 38.Han SK, Yoon TH, Kim JB, et al. Dermis graft for wound coverage. Plast Reconstr Surg. 2007;120:166–172. doi: 10.1097/01.prs.0000263536.55077.7e. [DOI] [PubMed] [Google Scholar]
- 39.Zakine G, Mimoun M, Pham J, et al. Reepithelialization from stem cells of hair follicles of dermal graft of the scalp in acute treatment of third-degree burns: first clinical and histologic study. Plast Reconstr Surg. 2012;130:42–50. doi: 10.1097/PRS.0b013e318254fa21. [DOI] [PubMed] [Google Scholar]
- 40.Lindford AJ, Kaartinen IS, Virolainen S, et al. The dermis graft: another autologous option for acute burn wound coverage. Burns. 2012;38:274–282. doi: 10.1016/j.burns.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Haslik W, Kamolz LP, Lumenta DB, et al. The treatment of deep dermal hand burns: how do we achieve better results? Should we use allogeneic keratinocytes or skin grafts? Burns. 2010;36:329–334. doi: 10.1016/j.burns.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Gravante G, Di Fede MC, Araco A, et al. A randomized trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns. 2007;33:966–972. doi: 10.1016/j.burns.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Shukla VK, Tiwary SK, Barnwal S, et al. Effect of autologous epidermal cell suspension transplantation in chronic nonhealing wounds: a pilot study. Can J Surg. 2010;53:6–10. [PMC free article] [PubMed] [Google Scholar]
- 44.Mcheik JN, Barrault C, Pedretti N, et al. Foreskin-isolated kératinocytes provide successful extemporaneous autologous paediatric skin grafts. J Tissue Eng Regen Med. 2013 March 14 doi: 10.1002/term.1690. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.O’Connor N, Mulliken J, Banks-Schlegel S, et al. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981;1:75–78. [PubMed] [Google Scholar]
- 46.Donati L, Magliacani G, Bormioli M, et al. Clinical experiences with keratinocyte grafts. Burns. 1992;18:19–26. doi: 10.1016/0305-4179(92)90106-5. [DOI] [PubMed] [Google Scholar]
- 47.Bettex-Galland M, Slongo T, Hunziker T, et al. Use of cultured keratinocytes in the treatment of severe burns. Z Kinderchir. 1988;43:224–228. doi: 10.1055/s-2008-1043461. [DOI] [PubMed] [Google Scholar]
- 48.Langdon RC, Cuono CB, Birchall N, et al. Reconstitution of structure and cell function in human skin grafts derived from cryopreserved allogeneic dermis and autologous cultured keratinocytes. J Invest Dermatol. 1988;91:478–485. doi: 10.1111/1523-1747.ep12476623. [DOI] [PubMed] [Google Scholar]
- 49.Compton CC, Hickerson W, Nadire K, et al. Acceleration of skin regeneration from cultured epithelial autografts by transplantation to homograft dermis. J Burn Care Rehabil. 1993;14:653–662. doi: 10.1097/00004630-199311000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Carsin H, Ainaud P, Le Bever H, et al. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single-center experience with 30 patients. Burns. 2000;26:379–387. doi: 10.1016/s0305-4179(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 51.Sood R, Roggy D, Zieger M, et al. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patients: the Indiana University experience. J Burn Care Res. 2010;31:559–568. doi: 10.1097/BCR.0b013e3181e4ca29. [DOI] [PubMed] [Google Scholar]
- 52.Still JM, Jr, Orlet HK, Law EJ. Use of cultured epidermal autografts in the treatment of large burns. Burns. 1994;20:539–541. doi: 10.1016/0305-4179(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 53.Paddle-Ledinek JE, Cruickshank DG, Masterton JP. Skin replacement by cultured keratinocyte grafts: an Australian experience. Burns. 1997;23:204–211. doi: 10.1016/s0305-4179(96)00123-4. [DOI] [PubMed] [Google Scholar]
- 54.Desai MH, Mlakar JM, McCauley RL, et al. Lack of long-term durability of cultured keratinocyte burn-wound coverage: a case report. J Burn Care Rehabil. 1991;12:540–545. doi: 10.1097/00004630-199111000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Barret JP, Wolf SE, Desai MH, et al. Cost-efficacy of cultured epidermal autografts in massive pediatric burns. Ann Surg. 2000;231:869–876. doi: 10.1097/00000658-200006000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyce ST, Kagan RJ, Greenhalgh DG, et al. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J Trauma. 2006;60:821–829. doi: 10.1097/01.ta.0000196802.91829.cc. [DOI] [PubMed] [Google Scholar]
- 57.Moustafa M, Bullock AJ, Creagh FM, et al. Randomized, controlled, single-blind study on use of autologous keratinocytes on a transfer dressing to treat nonhealing diabetic ulcers. Regen Med. 2007;2:887–902. doi: 10.2217/17460751.2.6.887. [DOI] [PubMed] [Google Scholar]
- 58.Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7:229–258. doi: 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boyce ST, Goretsky MJ, Greenhalgh DG, et al. Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann Surg. 1995;222:743–752. doi: 10.1097/00000658-199512000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheridan RL, Morgan JR, Cusick JL, et al. Initial experience with a composite autologous skin substitute. Burns. 2001;27:421–424. doi: 10.1016/s0305-4179(00)00156-x. [DOI] [PubMed] [Google Scholar]
- 61.Keck M, Haluza D, Lumenta DB, et al. Construction of a multi-layer skin substitute: simultaneous cultivation of keratinocytes and preadipocytes on a dermal template. Burns. 2011;37:626–630. doi: 10.1016/j.burns.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 62.Gómez C, Galán JM, Torrero V, et al. Use of an autologous bioengineered composite skin in extensive burns: clinical and functional outcomes. A multicentric study. Burns. 2011;37:580–589. doi: 10.1016/j.burns.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Kirsner RS. The use of Apligraf in acute wounds. J Dermatol. 1998;25:805–811. [PubMed] [Google Scholar]
- 64.Still J, Glat P, Silverstein P, et al. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns. 2003;29:837–841. doi: 10.1016/s0305-4179(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 65.Gage FH. Cell therapy. Nature. 1998;392(6679 Suppl):18–24. [PubMed] [Google Scholar]
- 66.De Corte P, Verween G, Verbeken G, et al. Feeder layer- and animal product-free culture of neonatal foreskin keratinocytes: improved performance, usability, quality and safety. Cell Tissue Bank. 2012;13:175–189. doi: 10.1007/s10561-011-9247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clover AJ, O’Neill BL, Kumar AH. Analysis of attitudes toward the source of progenitor cells in tissue-engineered products for use in burns compared with other disease states. Wound Repair Regen. 2012;20:311–316. doi: 10.1111/j.1524-475X.2012.00779.x. [DOI] [PubMed] [Google Scholar]
- 68.Stenn KS, Link R, Moellmann G, et al. Dispase, a neutral protease from Bacillus polymyxa, is a powerful fibronectinase and type IV collagenase. J Invest Dermatol. 1989;93:287–290. doi: 10.1111/1523-1747.ep12277593. [DOI] [PubMed] [Google Scholar]
- 69.Adams JC, Watt FM. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990;63:425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- 70.Morhenn VB, Benike CJ, Cox AJ, et al. Cultured human epidermal cells do not synthesize HLA-DR. J Invest Dermatol. 1982;78:32–37. doi: 10.1111/1523-1747.ep12497875. [DOI] [PubMed] [Google Scholar]
- 71.Hefton JM, Amberson JB, Biozes DG, et al. Loss of HLA-DR expression by human epidermal cells after growth in culture. J Invest Dermatol. 1984;83:48–50. doi: 10.1111/1523-1747.ep12261671. [DOI] [PubMed] [Google Scholar]
- 72.Brain A, Purkis P, Coates P, et al. Survival of cultured allogeneic keratinocytes transplanted to deep dermal bed assessed with probe specific for Y chromosome. BMJ. 1989;298:917–919. doi: 10.1136/bmj.298.6678.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pokorná E, Broz L, Veselý P, et al. Y chromosome and vimentin used to trace the fate of allogeneic keratinocytes delivered to the wound by the recombined human/pig skin. Folia Biol (Praha) 2001;47:128–134. doi: 10.14712/fb2001047040128. [DOI] [PubMed] [Google Scholar]
- 74.Billingham RE, Reynolds J. Transplantation studies on sheets of pure epidermal epithelium and on epidermal cell suspensions. Br J Plast Surg. 1952;5:25–36. doi: 10.1016/s0007-1226(52)80004-9. [DOI] [PubMed] [Google Scholar]
- 75.Hunyadi J, Farkas B, Bertényi C, et al. Keratinocyte grafting: a new means of transplantation for full-thickness wounds. J Dermatol Surg Oncol. 1988;14:75–78. doi: 10.1111/j.1524-4725.1988.tb03343.x. [DOI] [PubMed] [Google Scholar]
- 76.Fraulin FO, Bahoric A, Harrop AR, et al. Autotransplantation of epithelial cells in the pig via an aerosol vehicle. J Burn Care Rehabil. 1998;19:337–345. doi: 10.1097/00004630-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 77.Gerlach JC, Johnen C, McCoy E, et al. Autologous skin cell spray-transplantation for a deep dermal burn patient in an ambulant treatment room setting. Burns. 2011;37:19–23. doi: 10.1016/j.burns.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 78.Navarro FA, Stoner ML, Park CS, et al. Sprayed keratinocyte suspensions accelerate epidermal coverage in a porcine microwound model. J Burn Care Rehabil. 2000;21:513–518. doi: 10.1097/00004630-200021060-00007. [DOI] [PubMed] [Google Scholar]
- 79.Limat A, Noser FK. Serial cultivation of single keratinocytes from the outer root sheath of human scalp hair follicles. J Invest Dermatol. 1986;87:485–488. doi: 10.1111/1523-1747.ep12455548. [DOI] [PubMed] [Google Scholar]
- 80.Taylor G, Lehrer MS, Jensen PJ, et al. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 81.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lavoie A, Fugère C, Fradette J, et al. Considerations in the choice of a skin donor site for harvesting keratinocytes containing a high proportion of stem cells for culture in vitro. Burns. 2011;37:440–447. doi: 10.1016/j.burns.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Mcheik JN, Barrault C, Vincent G, et al. Modèles de cultures cellulaires kératinocytaires de prépuce. Possibilité d’application chez les enfants brûlés. Ann Chir Plast Esthet. 2009;54:528–532. doi: 10.1016/j.anplas.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 84.Uitto J. Regenerative medicine for skin diseases: iPS cells to the rescue. J Invest Dermatol. 2011;131:812–814. doi: 10.1038/jid.2011.2. [DOI] [PubMed] [Google Scholar]
- 85.Pham C, Greenwood J, Cleland H, et al. Bioengineered skin substitutes for the management of burns: a systematic review. Burns. 2007;33:946–957. doi: 10.1016/j.burns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 86.Mulekar SV, Ghwish B, Al Issa A, et al. Treatment of vitiligo lesions by ReCell vs. conventional melanocyte-keratinocyte transplantation: a pilot study. Br J Dermatol. 2008;158:45–49. doi: 10.1111/j.1365-2133.2007.08216.x. [DOI] [PubMed] [Google Scholar]
- 87.Cervelli V, De Angelis B, Spallone D, et al. Use of a novel autologous cell-harvesting device to promote epithelialization and enhance appropriate pigmentation in scar reconstruction. Clin Exp Dermatol. 2009;35:776–780. doi: 10.1111/j.1365-2230.2009.03728.x. [DOI] [PubMed] [Google Scholar]


