Abstract
Summary:
A recent revival of global interest for reconstruction of long-segment tracheal defects, which represents one of the most interesting and complex problems in head and neck and thoracic reconstructive surgery, has been witnessed. The trachea functions as a conduit for air, and its subunits including the epithelial layer, hyaline cartilage, and segmental blood supply make it particularly challenging to reconstruct. A myriad of attempts at replacing the trachea have been described. These along with the anatomy, indications, and approaches including microsurgical tracheal reconstruction will be reviewed. Novel techniques such as tissue-engineering approaches will also be discussed. Multiple attempts at replacing the trachea with synthetic scaffolds have been met with failure. The main lesson learned from such failures is that the trachea must not be treated as a “simple tube.” Understanding the anatomy, developmental biology, physiology, and diseases affecting the trachea are required for solving this problem.
ANATOMY AND BLOOD SUPPLY
The trachea connects the larynx to the carina, extending from the cricoid cartilage to its bifurcation into the left and right main bronchi. Anteriorly, it is composed of horseshoe-shaped cartilagenous rings making up two thirds of its circumference and posteriorly by a membranous portion connecting the rings.1 In the neck, it is covered by the cervical fascia and infrahyoid muscles, crossed by the isthmus of the thyroid and the jugular venous arch. The carotid sheath and inferior thyroid artery are lateral to the trachea, the esophagus—posterior, and the recurrent laryngeal nerve lies in the groove between the two. In the thorax, it is crossed by the brachiocephalic artery and the left brachiocephalic vein.2
The trachea functions as a conduit for ventilation, clears secretions, warms, humidifies and cleans the air for the respiratory zone, and keeps the airway free of foreign material through coughing and intrinsic defense mechanisms.3,4 The microanatomy of the trachea consists of a pseudostratified ciliated epithelium composed of ciliated cells, goblet cells, basal cells, and neuroendocrine cells4,5 (Fig. 1). The submucosa is rich in elastin, submucosal glands, and smooth muscle. The cartilage is of a hyaline nature.4 The tracheal walls are composed of 15–20 incomplete cartilaginous rings joined together by fibrous tissue and smooth muscle.2 The tracheal lumen is generally ovoid in shape although variations appear even without disease. This lumen flattens anteroposteriorly. Two thirds of the circumference of the trachea is composed of normally C-shaped (or horseshoe-shaped) rings anteriorly while the rest is composed of a flat posterior membranous wall. This posterior wall is made of a thin membrane supported by the trachealis muscle.3 There are about 2 rings per centimeter of trachea (see Figure 2 for photograph of a human trachea).
Fig. 1.
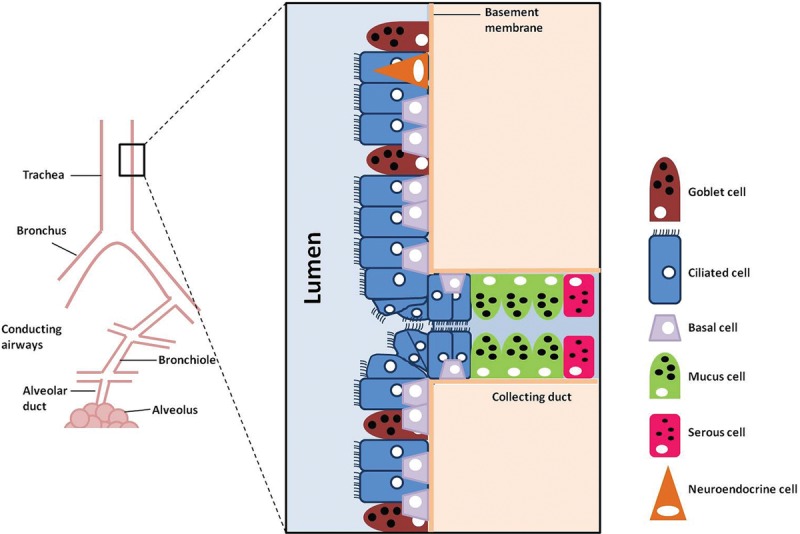
Cellular composition of the human tracheal epithelium.
Fig. 2.
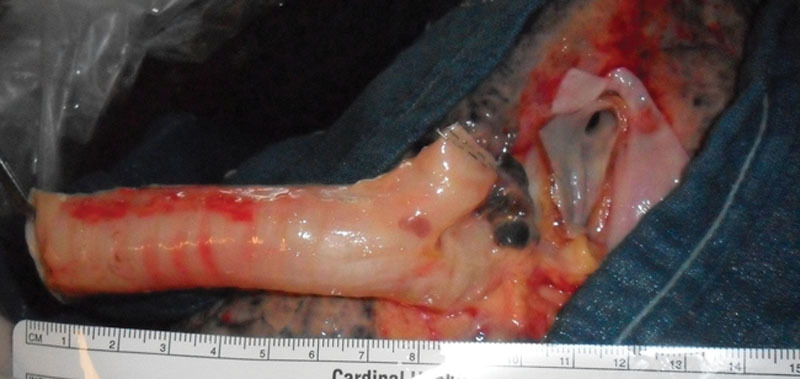
Human trachea harvested intraoperatively from donor lung used for transplantation.
The trachea’s blood supply comes from its lateral pedicles, vessels which originate from the inferior thyroid, subclavian, supreme intercostal, internal thoracic, innominate, and superior and middle bronchial arteries.6 All of these vessels interconnect along the lateral surface and form important longitudinal vascular anastomoses. The lateral and anterior tracheal walls receive their blood supply from transverse segmental vessels which extend from these 2 lateral longitudinal networks and run between the cartilage rings. The transverse vessels feed capillary beds beneath the endotracheal mucosa that nourish the cartilage by diffusion. The esophageal arteries and their subdivisions supply the posterior membranous portion only.6 The trachea’s intricate blood supply makes devascularization easy and reconstruction especially challenging.
TRACHEAL REPLACEMENTS
Indications
The indications for tracheal replacement are lesions that cannot be resected and reconstructed safely with end-to-end anastomosis or long-segment congenital stenosis, which cannot be effectively managed with slide or patch tracheoplasty. Acquired lesions include malignancy, traumatic injury, and subglottic or tracheal stenosis. The general limits for safe resection are about one half of the tracheal length in adults and one third in small children. Very lengthy lesions that cannot be safely removed and reconstructed primarily are managed palliatively with long-term T-tubes or stents. The clinical course of these patients is usually complicated with multiple infections and frequent hospital admissions. Therefore, a safe and dependable tracheal replacement remains an important unmet need.
Requirements
The requirements for tracheal replacements are to be laterally rigid but longitudinally flexible, to have a surface composed of ciliated respiratory epithelium (although some authors have considered this not essential), or at least to have a surface which facilitates epithelial resurfacing. They must also be biocompatible, nontoxic, nonimmunogenic, and noncarcinogenic. They must not dislocate or erode over time, avoid accumulation of secretions, resist bacterial colonization, and must be permanent.
Approaches
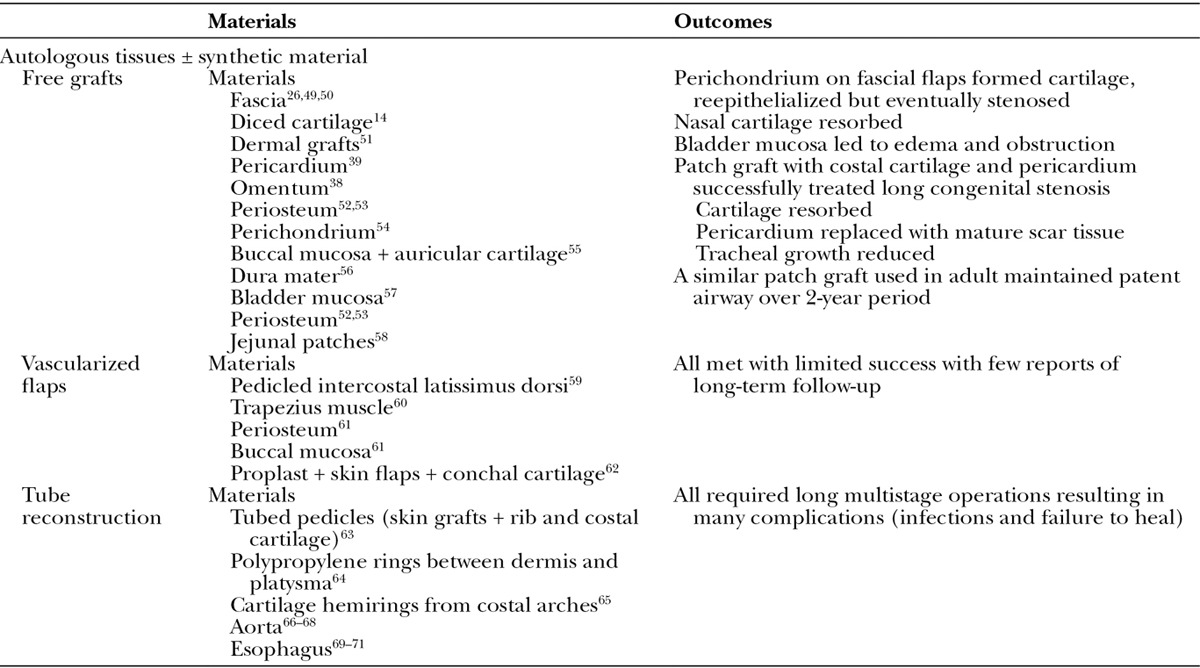
The approaches used for tracheal replacement include stents and synthetic prostheses and scaffolds and are summarized in Table 1. The use of autologous tissues in combination with synthetic material is summarized in Table 2. The most interesting recent advances in the field of tracheal reconstruction pertain to tracheal transplantation and tissue engineering and are described in further detail.
Table 1.
Tracheal Replacements: Stents, Synthetic Prostheses and scaffolds, and Nonviable Tissue
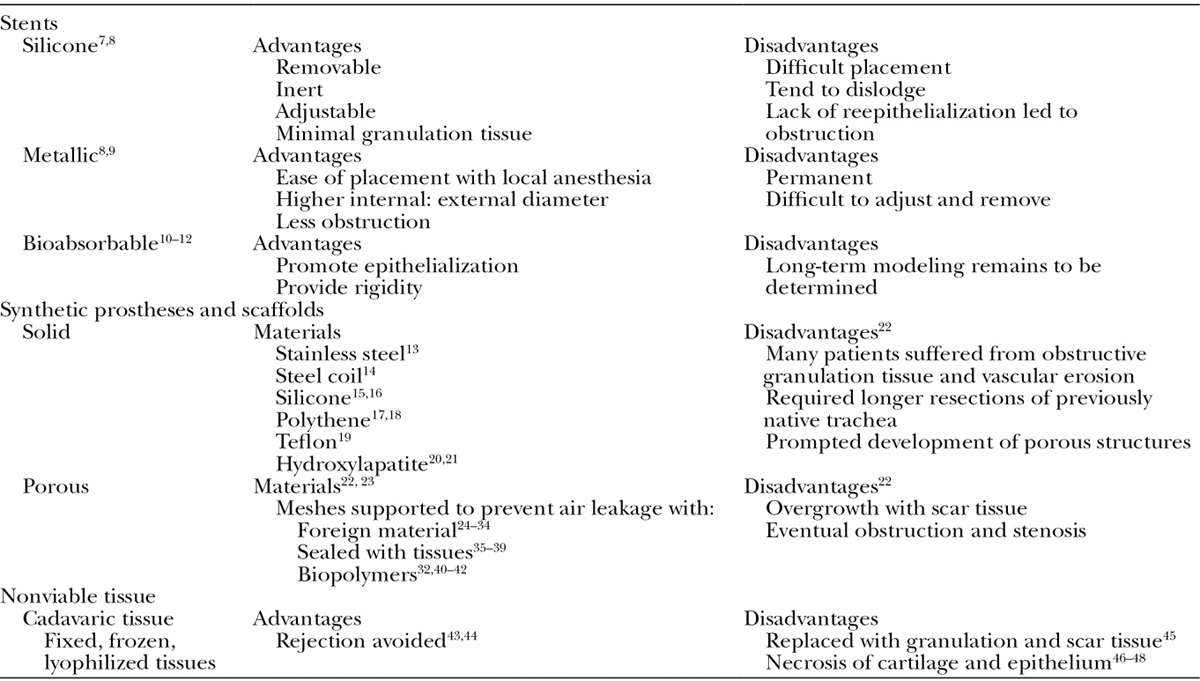
Table 2.
Tracheal Replacements: Autologous Tissues ± Synthetic Material
TRACHEAL TRANSPLANTATION
Nonrevascularized Grafts
Autografts
Tracheal excision and immediate orthotopic reimplantation (fresh autograft) often fail due to a delay in revascularization.46,72–75 However, this depends on the length of the autograft.74,76 Despite possible survival in short segments, the cartilage eventually resorbed and the segment was replaced with fibrous tissue.77 In longer segments, dissolution, stenosis, and obstruction followed due to loss of blood supply.74 A new experimental technique using composite cervical skin and a costal cartilage flap has shown some promise over long segments although long-term follow-up is required.78
Allografts
Fresh tracheal allografts without immunosuppression will lead to rejection.35,76,79,80 Rejection of fresh allografts of any length occurs even with immunosuppression, in the absence of revascularization.26,63,74,77 All these grafts necrose, liquefy, or result in stenosis. Preserved and devascularized allografts also failed due to cartilage resorption, scar replacement, fibrosis, and eventual complete obstruction.46,76,81,82 Cryopreserved allografts for small window defects83 and short segments84 reepithelialized but failed over longer lengths.85 Patients transplanted with chemically fixed allografts for noncircumferential defects required multiple subsequent operations with a decannulation rate of only 60% in children and even lower in the adult population.86 The literature implies that blood supply is critical for successful transplantation.
Vascularized Grafts
Autografts
Revascularization of fresh short-segment tracheal autografts was performed using omentum,87–89 intercostal muscle,90 deltopectoral muscle,91 pectoralis major muscle,92 free costal cartilage grafts,93 chondromuscular flaps,94 musculofascial flaps,95 or other vascular pedicles such as the latissimus dorsi.59,60 Omental flaps longer than 4 cm frequently resulted in ischemic tracheal segments and stenosis.96 Preliminary implantation into a vascularized tissue or flap with delayed transfer into the defect has proven to be more successful.38
Allografts
Nontracheal allografts, such as fresh and cryoperserved allogeneic aorta, required no immunosuppression and had no graft rejection in most cases. However, aortic grafts were deemed unsuitable for tracheal replacement because of failure to regenerate and incorporate recipient tissue, requiring stenting and/or retransplantation.97,98
In tracheal allografts, the epithelium is the major site of antigenicity and removal with a detergent99 or irradiation100 was thought to prevent rejection.101–104 Reepithelialization occurred by migration from the host epithelium105 while the chondrocytes remained of donor origin.106 However, complete epithelial regeneration failed and allografts were eventually rejected.107 Other studies focused on providing immunosuppression, which allows for initial revascularization of a heterotopically transplanted graft to improve success of orthotopic allotransplantation.108,109
The first clinical tracheal allotransplantation was reported in 1979, where a donor trachea was first implanted heterotopically under the sternocleidomastoid muscle and pedicled orthotopically after 3 weeks.110 No immunosuppression was required, and short-term integration with surrounding tissue and reepithelialization was achieved. Another case, later performed with omental revascularization and immunosuppression, eventually led to necrosis and stenosis requiring stent placement.111
Direct Revascularization
The blood supply to the trachea makes it challenging for direct revascularization. A composite graft composed of a thyrotracheal graft with anastomoses of the thyroid artery to the common carotid artery112 has been attempted. Venous anastomosis was also required to prevent soft-tissue necrosis.113,114 Long-term results have not been reported.
There is also an expanding role for free flaps to allow for revascularization of autografts and allografts. Their long-term outcomes for large tracheal defects have been reviewed by Yu et al.115 The free flaps used include radial forearm flap,115–118 anterolateral thigh flap,119,120 sternohyoid muscle,121 and a saphenous corticoperiostal flap.122
Clinically, the transplantation of a fresh laryngeal allograft was performed to replace a stenotic larynx following a motorcycle accident. This allograft also included a 5-ring segment of trachea, thyroid, parathyroids, a portion of the attached pharyngeal wall, both superior laryngeal nerves, and the right recurrent nerve. Arterial, venous, and neural anastomoses were performed, and perfusion was established early in the procedure. Over time, the patient regained vocal cord function and normal deglutition. Despite one episode of rejection, health and function were good at 40 months, with continued immunosuppression.123
In 2010, a donor tracheal allograft was initially heterotopically transplanted under the forearm fascia to allow for indirect revascularization in an immunosuppressed patient. The donor posterior membranous part necrosed and was replaced with the recipient’s buccal mucosa. The graft was subsequently moved to the orthotopic position, by which time the patient no longer required immunosuppression. The graft was fully lined with both donor and recipient epithelium and had viable donor tracheal cartilage surrounded by recipient blood vessels. It was harvested on a radial forearm free flap and inserted into a 4.5-cm defect.124 Recently, they have moved toward the use of autologous cells as reepithelialization was found to be very slow with the use of a buccal mucosa (unpublished results).
TRACHEAL TISSUE ENGINEERING
The long-term risks of chronic immunosuppression and their contraindications in malignant disease have led to interest in tissue-engineering techniques.
The use of the term “tissue engineering” implies the replacement of tissues and organs by isolation and culture of cells outside the body, which are seeded later into a biocompatible scaffold before implantation. The 3 components required for tissue engineering are cells, scaffolds, and bioreactors.
Cells
Epithelial Cells
In the trachea, resident epithelial cells are located along the basal layer. These cells can be isolated, cultured, and differentiated in vitro.125–128 Nontracheal exogenous cells that can be used for epithelial regeneration include embryonic stem cells, induced pluripotent stem cells, and cells from mesenchymal origin such as mesenchymal stem cells, human amniotic fluid stem cells, and umbilical blood cord–derived stem cells.129
Chondrocytes
Regeneration of endogenous cartilage can be stimulated in vivo by implantation of a gelatin sponge slowly releasing basic fibroblast growth factor130,131 or bone morphogenetic protein 2.132,133 The regenerated cartilage is of fibrous rather than hyaline nature. Autologous sources of chondrocytes include the nose, ribs, and ear, and these have been isolated and expanded in vitro in cell flasks and in a 3-dimensional culture system.134–138 Despite the formation of a well-vascularized neotrachea, these scaffold-free constructs showed signs of mechanical failure. Allogeneic chondrocytes have also been used for the repair of joint cartilage and are intriguing due to their low antigenicity.139,140
The exogenous use of autologous stem/progenitor cells has been considered as a safer alternative and a better option for cell amplification. These include autologous adipose-derived stem cells and mesenchymal stromal cells and induced pluripotent stem cells.141
Scaffolds
Synthetic
The advantages of synthetic scaffolds include tailoring of size and shape and the ability to control their properties such as strength, degradation time, porosity, and microstructure. However, they lack the macro- and microanatomic structures of natural scaffolds. There are many potential materials.142–146 The biodegraded molecules from polyglycolic acid led to a low pH environment and excited a vigorous inflammatory response when transplanted.147 Hydrogels also have a slow degradation rate and noncontrolled long-term biologic response.148
Recently, a long-segment circumferential trachea along with the carina and the main bronchi was fabricated from a nanocomposite polymer (POSS) covalently bonded to polyurethane (PCU). The casted form was made into the cartilage “U” shaped rings, and the coagulated form was used for the “connective” tracheal part. It was shown to support recipient progenitor cells and was used clinically.149 Long-term remodeling and outcome remain unknown.
Natural and Decellularized
Natural and decellularized scaffolds are thought to be advantageous because they support adhesion, proliferation, and differentiation of many different cell types.150 They are composed of extracellular matrix material such as collagens,42,134,137,151,152 fibrin/hyaluronic acid,135 and other glycosaminoglycan products. The limitations are their lack of consistency, structure malleability, and biodegradability.
In 2004, a tissue-engineered tracheal patch was used as a bioartificial construct for a tracheal defect in a 58-year-old man.153 It was composed of autologous muscle, fibroblasts, and a collagen matrix from a decellularized porcine proximal jejunum segment. This scaffold was incubated for 3 weeks in a bioreactor before transplantation. After 12 weeks, the bioartificial patch had a ciliated pseudostratified epithelium and was integrated into the adjacent airway.153
In 2008, a 30-year-old woman was the recipient of a decellularized allogeneic trachea for replacement of her left main bronchus. This scaffold required 25 cycles of decellularization based on the absence of major histocompatibility complex markers within the cartilage.154 The scaffold was then recellularized in a bioreactor with primary autologous epithelial cells and mesenchymal stem cell–derived chondrocytes. The patient did not develop antidonor antibodies and did not receive immunosuppressive therapy. The procedure has since been modified to use the recipient’s body as a bioreactor: seeding the scaffold intraoperatively with autologous respiratory epithelial and bone marrow–derived mononuclear cells.155 This in vivo tissue-engineered approach was used in a case series of 9 pediatric and adult patients with benign and malignant diseases on a compassionate basis. No graft-related mortality was reported after follow-up of 12–42 months, with all bioengineered grafts remaining vascularized and lined with healthy respiratory mucosa. However, partial collapse of the scaffolds was noted in 3 patients.156,157 The collapse was thought to be due to degradation of the extracellular matrix architecture and a decrease in the mechanical and angiogenic properties that occurs after long-term storage.158 The group felt that decellularized matrices led to unpredictable results and has since moved on to use an artificial tracheal and bronchial scaffold from a nanocomposite polymeric material.149
Bioreactors
Bioreactors are laboratory tissue-culture devices that provide a controllable, mechanically active environment and can be used to study and improve tissue-engineered structures159 (Figure 3). They enable the cell seeding process, allow for proliferation on a large scale and production of 3D constructs,160,161 and provide an optimal physiological environment for cell adhesion, growth, and differentiation by provision of flow of nutrient media and mechanical stimulation mimicking conditions of growing organ.162 Their operational conditions may be manipulated (such as pH, temperature, oxygen tension, and nutrient supply). Several bioreactors have been described for tracheal tissue engineering,163–167 and a commercial version of this bioreactor launched by Harvard Bioscience currently exists and was used for the first human tissue-engineered tracheal replacement.154 Following the first clinical transplantation, the authors turned to in situ tissue engineering mentioning long-lasting seeding period, high costs, potential risks of cell differentiation instability, and contamination as bottlenecks to integration of bioreactor-seeded tracheas.168
Fig. 3.

Decellularized scaffold and bioreactor setup in incubator. Adapted from Haykal S, Salna M, Zhou Y, et al. Double-chamber rotating bioreactor for dynamic perfusion cell seeding of large segment tracheal allografts: comparison to conventional static methods. Tissue Eng Part C Methods 2014 Mar 5. [Epub ahead of print].169 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
CONCLUSIONS
The anatomical features of the trachea, which include its proximity to major vessels, segmental blood supply, anteroposterior heterogeneity, lateral rigidity, and longitudinal flexibility, make it more complex than a simple conduit. The presence of different tissues, including respiratory epithelium, submucosa, cartilage, and blood vessels, makes reconstruction of the trachea particularly challenging. The attempts that have shown the greatest promise have used tissue-engineered techniques with decellularized allografts. However, there continues to be some significant challenges with biological scaffolds composed of the extracellular matrix particularly related to revascularization. Plastic and reconstructive microsurgeons can significantly contribute to this field by combining free-flap techniques to allow for initial revascularization of these scaffolds followed by a delayed reconstruction, thus providing a novel technique for reconstruction of circumferential long-segment tracheal defects.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Laroia AT, Thompson BH, Laroia ST, et al. Modern imaging of the tracheo-bronchial tree. World J Radiol. 2010;2:237–248. doi: 10.4329/wjr.v2.i7.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burdett E, Mitchell V. Anatomy of the larynx, trachea and bronchi. Anaesth Intensive Care Med. 2008;9:329–333. [Google Scholar]
- 3.Farmer S, Hay D. The Airway Epithelium: Physiology, Pathophysiology, and Pharmacology. New York: M. Dekker; 1991. [Google Scholar]
- 4.Weinberger SE. Principles of Pulmonary Medicine. 4th ed. Philadelphia, PA: W. B. Saunders; 2004. [Google Scholar]
- 5.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salassa JR, Pearson BW, Payne WS. Gross and microscopical blood supply of the trachea. Ann Thorac Surg. 1977;24:100–107. doi: 10.1016/s0003-4975(10)63716-2. [DOI] [PubMed] [Google Scholar]
- 7.Zwischenberger JB, Wittich GR, vanSonnenberg E, et al. Airway simulation to guide stent placement for tracheobronchial obstruction in lung cancer. Ann Thorac Surg. 1997;64:1619–1625. doi: 10.1016/s0003-4975(97)01174-0. [DOI] [PubMed] [Google Scholar]
- 8.Saito Y, Imamura H. Airway stenting. Surg Today. 2005;35:265–270. doi: 10.1007/s00595-004-2942-y. [DOI] [PubMed] [Google Scholar]
- 9.Wood DE. Airway stenting. Chest Surg Clin N Am. 2001;11:841–860. [PubMed] [Google Scholar]
- 10.Liu KS, Liu YH, Peng YJ, et al. Experimental absorbable stent permits airway remodeling. J Thorac Cardiovasc Surg. 2011;141:463–468. doi: 10.1016/j.jtcvs.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Ng AH, Ng NS, Zhu GH, et al. A fully degradable tracheal stent: in vitro and in vivo characterization of material degradation. J Biomed Mater Res B Appl Biomater. 2012;100:693–699. doi: 10.1002/jbm.b.32501. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Araki M, Nakajima N, et al. Biodegradable polymer coating promotes the epithelization of tissue-engineered airway prostheses. J Thorac Cardiovasc Surg. 2010;139:26–31. doi: 10.1016/j.jtcvs.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Cotton BH, Hills B, Penido JR. Resection of the trachea for carcinoma; report of two cases. J Thorac Surg. 1952;24:231–245. [PubMed] [Google Scholar]
- 14.Beattie EJ, Jr, Blades B, Keshishian JM. Tracheal reconstruction. J Thorac Surg. 1956;32:707–725; discussion 725–727. [PubMed] [Google Scholar]
- 15.Neville WE, Bolanowski JP, Kotia GG. Clinical experience with the silicone tracheal prosthesis. J Thorac Cardiovasc Surg. 1990;99:604–612; discussion 612–613. [PubMed] [Google Scholar]
- 16.Toomes H, Mickisch G, Vogt-Moykopf I. Experiences with prosthetic reconstruction of the trachea and bifurcation. Thorax. 1985;40:32–37. doi: 10.1136/thx.40.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clagett OT, Moersch HJ, Grindlay JH. Intrathoracic tracheal tumors: development of surgical technics for their removal. Ann Surg. 1952;136:520–532. doi: 10.1097/00000658-195209000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atamanyuk MY, Melrose DG. The treatment of circumferential defects of the trachea. Br J Surg. 1965;52:59–65. doi: 10.1002/bjs.1800520114. [DOI] [PubMed] [Google Scholar]
- 19.Ekestrom S, Carlens E. Teflon prosthesis in tracheal defects in man. Acta Chir Scand Suppl. 1959;(Suppl 245):71–75. [PubMed] [Google Scholar]
- 20.Hirano M, Yoshida T, Sakaguchi S. Hydroxylapatite for laryngotracheal framework reconstruction. Ann Otol Rhinol Laryngol. 1989;98:713–717. doi: 10.1177/000348948909800910. [DOI] [PubMed] [Google Scholar]
- 21.Triglia JM, Scheiner C, Gouvernet J, et al. Hydroxyapatite in experimental laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg. 1993;119:87–91. doi: 10.1001/archotol.1993.01880130089013. [DOI] [PubMed] [Google Scholar]
- 22.Grillo HC. Surgery of the Trachea and Bronchi. BC Decker: Hamilton, Ontario, Canada; 2004. [Google Scholar]
- 23.Wilhelm DL. Regeneration of tracheal epithelium. J Pathol Bacteriol. 1953;65:543–550. doi: 10.1002/path.1700650226. [DOI] [PubMed] [Google Scholar]
- 24.Bailey BJ, Kosoy J. Observations in the development of tracheal prostheses and tracheal transplantation. Laryngoscope. 1970;80:1553–1565. doi: 10.1288/00005537-197010000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Beall AC, Jr, Harrington OB, Greenberg SD, et al. Tracheal replacement with heavy Marlex mesh. Circumferential replacement of the cervical trachea. Arch Surg. 1962;84:390–396. doi: 10.1001/archsurg.1962.01300220014002. [DOI] [PubMed] [Google Scholar]
- 26.Daniel RA., Jr The regeneration of defects of the trachea and bronchi; an experimental study. J Thorac Surg. 1948;17:335–349. [PubMed] [Google Scholar]
- 27.Greenberg SD, Willms RK. Regeneration of respiratory epithelium. An experimental study in dogs. Arch Pathol. 1962;73:53–58. [PubMed] [Google Scholar]
- 28.Moghissi K. Tracheal reconstruction with a prosthesis of Marlex mesh and pericardium. J Thorac Cardiovasc Surg. 1975;69:499–506. [PubMed] [Google Scholar]
- 29.Morfit HM, Neerken AJ, Prevedel A, et al. Sleeve resections of the trachea; experimental studies on regenerative capacity and principles of reconstruction and repair. AMA Arch Surg. 1955;70:654–661. [PubMed] [Google Scholar]
- 30.Pearson FG, Henderson RD, Gross AE, et al. The reconstruction of circumferential tracheal defects with a porous prosthesis. An experimental and clinical study using heavy Marlex mesh. J Thorac Cardiovasc Surg. 1968;55:605–616. [PubMed] [Google Scholar]
- 31.Poticha SM, Lewis FJ. Experimental replacement of the trachea. J Thorac Cardiovasc Surg. 1966;52:61–67. [PubMed] [Google Scholar]
- 32.Sekine T, Nakamura T, Matsumoto K, et al. Carinal reconstruction with a Y-shaped collagen-conjugated prosthesis. J Thorac Cardiovasc Surg. 2000;119:1162–1168. doi: 10.1067/mtc.2000.106652. [DOI] [PubMed] [Google Scholar]
- 33.Shaw RR, Aslami A, Webb WR. Circumferential replacement of the trachea in experimental animals. Ann Thorac Surg. 1968;5:30–35. doi: 10.1016/s0003-4975(10)66306-0. [DOI] [PubMed] [Google Scholar]
- 34.Wykoff TW. A preliminary report on segmental tracheal prosthetic replacement in dogs. Laryngoscope. 1973;83:1072–1077. doi: 10.1288/00005537-197307000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Beigel A, Steffens-Knutzen R, Müller B, et al. Tracheal transplantation. III. Demonstration of transplantation antigens on the tracheal mucosa of inbred rat strains. Arch Otorhinolaryngol. 1984;241:1–8. doi: 10.1007/BF00457910. [DOI] [PubMed] [Google Scholar]
- 36.Cahan WG. Carcinoma of intrathoracic trachea: excision and repair by tantalum gauze-fascia lata graft; report of a case. J Thorac Surg. 1952;23:513–527. [PubMed] [Google Scholar]
- 37.Li J, Xu P, Chen H. Successful tracheal autotransplantation with two-stage approach using the greater omentum. Ann Thorac Surg. 1997;64:199–202. doi: 10.1016/s0003-4975(97)82828-7. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Xu P, Chen H, et al. Improvement of tracheal autograft survival with transplantation into the greater omentum. Ann Thorac Surg. 1995;60:1592–1596. doi: 10.1016/0003-4975(95)00839-x. [DOI] [PubMed] [Google Scholar]
- 39.Nelson RJ, Goldberg L, White RA, et al. Neovascularity of a tracheal prosthesis/tissue complex. J Thorac Cardiovasc Surg. 1983;86:800–808. [PubMed] [Google Scholar]
- 40.Tatekawa Y, Kawazoe N, Chen G, et al. Tracheal defect repair using a PLGA-collagen hybrid scaffold reinforced by a copolymer stent with bFGF-impregnated gelatin hydrogel. Pediatr Surg Int. 2010;26:575–580. doi: 10.1007/s00383-010-2609-2. [DOI] [PubMed] [Google Scholar]
- 41.Teramachi M, Okumura N, Nakamura T, et al. Intrathoracic tracheal reconstruction with a collagen-conjugated prosthesis: evaluation of the efficacy of omental wrapping. J Thorac Cardiovasc Surg. 1997;113:701–711. doi: 10.1016/S0022-5223(97)70227-7. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita M, Kanemaru S, Hirano S, et al. Tracheal regeneration after partial resection: a tissue engineering approach. Laryngoscope. 2007;117:497–502. doi: 10.1097/MLG.0b013e31802e223d. [DOI] [PubMed] [Google Scholar]
- 43.Scherer MA, Ascherl R, Geissdörfer K, et al. Experimental bioprosthetic reconstruction of the trachea. Arch Otorhinolaryngol. 1986;243:215–223. doi: 10.1007/BF00464433. [DOI] [PubMed] [Google Scholar]
- 44.Bujía J, Wilmes E, Hammer C. [Immunologic behavior of human tracheal transplants]. Laryngorhinootologie. 1992;71:353–358. doi: 10.1055/s-2007-997313. [DOI] [PubMed] [Google Scholar]
- 45.Grillo HC, Mckhann CF. The acceptance and evolution of dermal homografts freed of viable cells. Transplantation. 1964;2:48–59. doi: 10.1097/00007890-196401000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Bjork VO, Rodriguez LE. Reconstruction of the trachea and its bifurcation; an experimental study. J Thorac Surg. 1958;35:596–603. [PubMed] [Google Scholar]
- 47.Jacobs JP, Elliott MJ, Haw MP, et al. Pediatric tracheal homograft reconstruction: a novel approach to complex tracheal stenoses in children. J Thorac Cardiovasc Surg. 1996;112:1549–1558; discussion 1559–1560. doi: 10.1016/S0022-5223(96)70014-4. [DOI] [PubMed] [Google Scholar]
- 48.Elliott MJ, Haw MP, Jacobs JP, et al. Tracheal reconstruction in children using cadaveric homograft trachea. Eur J Cardiothorac Surg. 1996;10:707–712. doi: 10.1016/s1010-7940(96)80328-9. [DOI] [PubMed] [Google Scholar]
- 49.Davis JS. II. The transplantation of free flaps of fascia: an experimental study. Ann Surg. 1911;54:734–748. doi: 10.1097/00000658-191112000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swift EA, Grindlay JH, Clagett OT. The repair of tracheal defects with fascia and tantalum mesh: an experimental study. J Thorac Surg. 1952;24:482–492. [PubMed] [Google Scholar]
- 51.Michelson E, Solomon R, Maun L, et al. Experiments in tracheal reconstruction. J Thorac Cardiovasc Surg. 1961;41:748–759. [PubMed] [Google Scholar]
- 52.Fonkalsrud EW, Plested WG. Tracheobronchial reconstruction with autologous periosteum. J Thorac Cardiovasc Surg. 1966;52:666–674. [PubMed] [Google Scholar]
- 53.Cohen RC, Filler RM, Konuma K, et al. A new model of tracheal stenosis and its repair with free periosteal grafts. J Thorac Cardiovasc Surg. 1986;92:296–304. [PubMed] [Google Scholar]
- 54.Eckersberger F, Moritz E, Wolner E. Circumferential tracheal replacement with costal cartilage. J Thorac Cardiovasc Surg. 1987;94:175–180. [PubMed] [Google Scholar]
- 55.Farkas LG, Farmer AW, McCain WG, et al. Replacement of a tracheal defect in the dog by a preformed composite graft. A later report. Plast Reconstr Surg. 1972;50:238–241. doi: 10.1097/00006534-197209000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Sabás AA, Uez JB, Rojas O, et al. Replacement of the trachea with dura mater. Experimental work. J Thorac Cardiovasc Surg. 1977;74:761–765. [PubMed] [Google Scholar]
- 57.Barker WS, Litton WB. Bladder osteogenesis aids tracheal reconstruction. Arch Otolaryngol. 1973;98:422–425. doi: 10.1001/archotol.1973.00780020436016. [DOI] [PubMed] [Google Scholar]
- 58.Jones RE, Morgan RF, Marcella KL, et al. Tracheal reconstruction with autogenous jejunal microsurgical transfer. Ann Thorac Surg. 1986;41:636–638. doi: 10.1016/s0003-4975(10)63078-0. [DOI] [PubMed] [Google Scholar]
- 59.Ishida I, Oura H, Niikawa H, et al. Non-circumferential tracheal resection with muscle flap reconstruction for adenoid cystic carcinoma. Gen Thorac Cardiovasc Surg. 2012;60:603–606. doi: 10.1007/s11748-012-0062-y. [DOI] [PubMed] [Google Scholar]
- 60.Jana T, Khabbaz E, Bush CM, et al. The body as a living bioreactor: a feasibility study of pedicle flaps for tracheal transplantation. Eur Arch Otorhinolaryngol. 2013;270:181–186. doi: 10.1007/s00405-012-2105-5. [DOI] [PubMed] [Google Scholar]
- 61.Akl BF, Mittelman J, Smith DE, et al. A new method of tracheal reconstruction. Ann Thorac Surg. 1983;36:265–269. doi: 10.1016/s0003-4975(10)60127-0. [DOI] [PubMed] [Google Scholar]
- 62.Kaneko K, Sakaguchi K, Takano A, et al. Tracheal reconstruction using S-shaped skin flaps and a conchal cartilage graft. Ann Thorac Surg. 2011;92:e111–e112. doi: 10.1016/j.athoracsur.2011.05.076. [DOI] [PubMed] [Google Scholar]
- 63.Edgerton MT, Zovickian A. Reconstruction of the trachea and infraglottic larynx. Plast Reconstr Surg (1946) 1954;13:167–192. doi: 10.1097/00006534-195403000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Grillo HC, Dignan EF, Miura T. Experimental reconstruction of cervical trachea after circumferential resection. Surg Gynecol Obstet. 1966;122:733–738. [PubMed] [Google Scholar]
- 65.Serrano A, Ortiz-Monasterio F, Andrade-Pradillo J. Reconstruction of the cervical trachea. Reconstruction of the cervical trachea. A technique to obtain a permanently patent airway. Plast Reconstr Surg Transplant Bull. 1959;24:333–340. doi: 10.1097/00006534-195910000-00003. [DOI] [PubMed] [Google Scholar]
- 66.Anoosh F, Hodjati H, Dehghani S, et al. Tracheal replacement by autogenous aorta. J Cardiothorac Surg. 2009;4:23. doi: 10.1186/1749-8090-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinod E, Seguin A, Pfeuty K, et al. Long-term evaluation of the replacement of the trachea with an autologous aortic graft. Ann Thorac Surg. 2003;75:1572–1578; discussion 1578. doi: 10.1016/s0003-4975(03)00120-6. [DOI] [PubMed] [Google Scholar]
- 68.Azorin JF, Bertin F, Martinod E, et al. Tracheal replacement with an aortic autograft. Eur J Cardiothorac Surg. 2006;29:261–263. doi: 10.1016/j.ejcts.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 69.Ein SH, Friedberg J, Williams WG, et al. Tracheoplasty—a new operation for complete congenital tracheal stenosis. J Pediatr Surg. 1982;17:872–878. doi: 10.1016/s0022-3468(82)80459-4. [DOI] [PubMed] [Google Scholar]
- 70.Fonkalsrud EW, Martelle RR, Maloney JV., Jr Surgical treatment of tracheal agenesis. J Thorac Cardiovasc Surg. 1963;45:520–525. [PubMed] [Google Scholar]
- 71.Fonkalsrud EW, Sumida S. Tracheal replacement with autologous esophagus for tracheal stricture. Arch Surg. 1971;102:139–142. doi: 10.1001/archsurg.1971.01350020049013. [DOI] [PubMed] [Google Scholar]
- 72.Borrie J, Redshaw NR. Prosthetic tracheal replacement. J Thorac Cardiovasc Surg. 1970;60:829–835. [PubMed] [Google Scholar]
- 73.Strandness DE, Jr, Gustafson IJ, Payne JT. Surgical resection of the thoracic trachea: an experimental study in dogs. J Thorac Surg. 1957;34:269–277. [PubMed] [Google Scholar]
- 74.Neville WE, Bolanowski PJ, Soltanzadeh H. Homograft replacement of the trachea using immunosuppression. J Thorac Cardiovasc Surg. 1976;72:596–601. [PubMed] [Google Scholar]
- 75.Nakanishi R, Shirakusa T, Takachi T. Omentopexy for tracheal autografts. Ann Thorac Surg. 1994;57:841–845. doi: 10.1016/0003-4975(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 76.Pacheco CR, Rivero O, Porter JK. Experimental reconstructive surgery of the trachea. J Thorac Surg. 1954;27:554–564. [PubMed] [Google Scholar]
- 77.Aronstam EM, Nims RM, Winn DF., Jr Studies in segmental replacement of the thoracic trachea. J Surg Res. 1961;1:108–110. doi: 10.1016/s0022-4804(61)80005-x. [DOI] [PubMed] [Google Scholar]
- 78.Fabre D, Singhal S, De Montpreville V, et al. Composite cervical skin and cartilage flap provides a novel large airway substitute after long-segment tracheal resection. J Thorac Cardiovasc Surg. 2009;138:32–39. doi: 10.1016/j.jtcvs.2008.11.071. [DOI] [PubMed] [Google Scholar]
- 79.Bujia J, Wilmes E, Hammer C, et al. Tracheal transplantation: demonstration of HLA class II subregion gene products on human trachea. Acta Otolaryngol. 1990;110:149–154. doi: 10.3109/00016489009122530. [DOI] [PubMed] [Google Scholar]
- 80.Kalb TH, Chuang MT, Marom Z, et al. Evidence for accessory cell function by class II MHC antigen-expressing airway epithelial cells. Am J Respir Cell Mol Biol. 1991;4:320–329. doi: 10.1165/ajrcmb/4.4.320. [DOI] [PubMed] [Google Scholar]
- 81.Jackson TL, O’Brien EJ, Tuttle W, et al. The experimental use of homogenous tracheal transplants in the restoration of continuity of the tracheobronchial tree. J Thorac Surg. 1950;20:598–612; passim. [PubMed] [Google Scholar]
- 82.Davies OG, Edmiston JM, McCorkle HJ. The repair of experimental tracheal defects with fresh and preserved homologous tracheal grafts. J Thorac Surg. 1952;23:367–376. [PubMed] [Google Scholar]
- 83.Messineo A, Filler RM, Bahoric A, et al. Repair of long tracheal defects with cryopreserved cartilaginous allografts. J Pediatr Surg. 1992;27:1131–1134; discussion 1134–1135. doi: 10.1016/0022-3468(92)90574-q. [DOI] [PubMed] [Google Scholar]
- 84.Inutsuka K, Kawahara K, Takachi T, et al. Reconstruction of trachea and carina with immediate or cryopreserved allografts in dogs. Ann Thorac Surg. 1996;62:1480–1484. doi: 10.1016/0003-4975(96)00473-0. [DOI] [PubMed] [Google Scholar]
- 85.Lenot B, Macchiarini P, Dulmet E, et al. Tracheal allograft replacement. An unsuccessful method. Eur J Cardiothorac Surg. 1993;7:648–652. doi: 10.1016/1010-7940(93)90261-9. [DOI] [PubMed] [Google Scholar]
- 86.Propst EJ, Prager JD, Meinzen-Derr J, et al. Pediatric tracheal reconstruction using cadaveric homograft. Arch Otolaryngol Head Neck Surg. 2011;137:583–590. doi: 10.1001/archoto.2011.85. [DOI] [PubMed] [Google Scholar]
- 87.Hirata T, Yamazaki F, Fukuse T, et al. Omentopexy for revascularization of free tracheal grafts in rats. Thorac Cardiovasc Surg. 1992;40:178–181. doi: 10.1055/s-2007-1020143. [DOI] [PubMed] [Google Scholar]
- 88.Messineo A, Filler RM, Bahoric B, et al. Successful tracheal autotransplantation with a vascularized omental flap. J Pediatr Surg. 1991;26:1296–1300. doi: 10.1016/0022-3468(91)90603-q. [DOI] [PubMed] [Google Scholar]
- 89.Borro JM, Chirivella M, Vila C, et al. Successful revascularization of large isolated tracheal segments. Eur J Cardiothorac Surg. 1992;6:621–623; discussion 624. doi: 10.1016/1010-7940(92)90137-m. [DOI] [PubMed] [Google Scholar]
- 90.Fell SC, Mollenkopf FP, Montefusco CM, et al. Revascularization of ischemic bronchial anastomoses by an intercostal pedicle flap. J Thorac Cardiovasc Surg. 1985;90:172–178. [PubMed] [Google Scholar]
- 91.Kumaran S, Nambi GI, Kingsly Paul M, et al. Post-electrical-burn tracheal-defect reconstruction with pre-fabricated deltopectoral flap—a case report. J Plast Reconstr Aesthet Surg. 2009;62:e93–e94. doi: 10.1016/j.bjps.2008.08.052. [DOI] [PubMed] [Google Scholar]
- 92.He J, Xu X, Chen M, et al. Novel method to repair tracheal defect by pectoralis major myocutaneous flap. Ann Thorac Surg. 2009;88:288–291. doi: 10.1016/j.athoracsur.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 93.Nakahira M, Nakatani H, Takeuchi S, et al. Safe reconstruction of a large cervico-mediastinal tracheal defect with a pectoralis major myocutaneous flap and free costal cartilage grafts. Auris Nasus Larynx. 2006;33:203–206. doi: 10.1016/j.anl.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 94.Guerrissi JO, Guerrissi JA, Miranda MG. Functional reconstruction of the trachea: prelaminated chondromuscular flap. J Craniofac Surg. 2009;20:868–871. doi: 10.1097/SCS.0b013e3181a86e91. [DOI] [PubMed] [Google Scholar]
- 95.Masuda M, Kamizono K, Ejima M, et al. Tracheal reconstruction with a modified infrahyoid myocutaneous flap. Laryngoscope. 2012;122:992–996. doi: 10.1002/lary.23194. [DOI] [PubMed] [Google Scholar]
- 96.Nakanishi R, Shirakusa T, Mitsudomi T. Maximum length of tracheal autografts in dogs. J Thorac Cardiovasc Surg. 1993;106:1081–1087. [PubMed] [Google Scholar]
- 97.Tsukada H, Ernst A, Gangadharan S, et al. Tracheal replacement with a silicone-stented, fresh aortic allograft in sheep. Ann Thorac Surg. 2010;89:253–258. doi: 10.1016/j.athoracsur.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 98.Wurtz A, Hysi I, Zawadzki C, et al. Construction of a tube-shaped tracheal substitute using fascial flap-wrapped revascularized allogenic aorta. Eur J Cardiothorac Surg. 2012;41:663–668. doi: 10.1093/ejcts/ezr012. [DOI] [PubMed] [Google Scholar]
- 99.Liu Y, Nakamura T, Yamamoto Y, et al. Immunosuppressant-free allotransplantation of the trachea: the antigenicity of tracheal grafts can be reduced by removing the epithelium and mixed glands from the graft by detergent treatment. J Thorac Cardiovasc Surg. 2000;120:108–114. doi: 10.1067/mtc.2000.106655. [DOI] [PubMed] [Google Scholar]
- 100.Yokomise H, Inui K, Wada H, et al. High-dose irradiation prevents rejection of canine tracheal allografts. J Thorac Cardiovasc Surg. 1994;107:1391–1397. [PubMed] [Google Scholar]
- 101.Balderman SC, Weinblatt G. Tracheal autograft revascularization. J Thorac Cardiovasc Surg. 1987;94:434–441. [PubMed] [Google Scholar]
- 102.Tojo T, Niwaya K, Sawabata N, et al. Tracheal replacement with cryopreserved tracheal allograft: experiment in dogs. Ann Thorac Surg. 1998;66:209–213. doi: 10.1016/s0003-4975(98)00270-7. [DOI] [PubMed] [Google Scholar]
- 103.Mukaida T, Shimizu N, Aoe M, et al. Experimental study of tracheal allotransplantation with cryopreserved grafts. J Thorac Cardiovasc Surg. 1998;116:262–266. doi: 10.1016/s0022-5223(98)70125-4. [DOI] [PubMed] [Google Scholar]
- 104.Kawahara K, Inutsuka K, Hiratsuka M, et al. Tracheal transplantation for carinal reconstruction in dogs. J Thorac Cardiovasc Surg. 1998;116:397–401. doi: 10.1016/S0022-5223(98)70004-2. [DOI] [PubMed] [Google Scholar]
- 105.Mukaida T, Shimizu N, Aoe M, et al. Origin of regenerated epithelium in cryopreserved tracheal allotransplantation. Ann Thorac Surg. 1998;66:205–208. doi: 10.1016/s0003-4975(98)00154-4. [DOI] [PubMed] [Google Scholar]
- 106.Tojo T, Kitamura S, Gojo S, et al. Epithelial regeneration and preservation of tracheal cartilage after tracheal replacement with cryopreserved allograft in the rat. J Thorac Cardiovasc Surg. 1998;116:624–627. doi: 10.1016/S0022-5223(98)70169-2. [DOI] [PubMed] [Google Scholar]
- 107.Moriyama H, Sasajima T, Hirata S, et al. Revascularization of canine cryopreserved tracheal allografts. Ann Thorac Surg. 2000;69:1701–1706. doi: 10.1016/s0003-4975(00)01297-2. [DOI] [PubMed] [Google Scholar]
- 108.Delaere PR, Liu ZY, Hermans R, et al. Experimental tracheal allograft revascularization and transplantation. J Thorac Cardiovasc Surg. 1995;110:728–737. doi: 10.1016/S0022-5223(95)70105-2. [DOI] [PubMed] [Google Scholar]
- 109.Delaere PR, Liu Z, Sciot R, et al. The role of immunosuppression in the long-term survival of tracheal allografts. Arch Otolaryngol Head Neck Surg. 1996;122:1201–1208. doi: 10.1001/archotol.1996.01890230047010. [DOI] [PubMed] [Google Scholar]
- 110.Rose KG, Sesterhenn K, Wustrow F. Tracheal allotransplantation in man. Lancet. 1979;1:433. doi: 10.1016/s0140-6736(79)90902-4. [DOI] [PubMed] [Google Scholar]
- 111.Levashov YuN, Yablonsky PK, Cherny SM, et al. One-stage allotransplantation of thoracic segment of the trachea in a patient with idiopathic fibrosing mediastinitis and marked tracheal stenosis. Eur J Cardiothorac Surg. 1993;7:383–386. doi: 10.1016/1010-7940(93)90071-i. [DOI] [PubMed] [Google Scholar]
- 112.Khalil-Marzouk JF. Allograft replacement of the trachea. Experimental synchronous revascularization of composite thyrotracheal transplant. J Thorac Cardiovasc Surg. 1993;105:242–246. [PubMed] [Google Scholar]
- 113.Macchiarini P, Lenot B, de Montpreville V, et al. Heterotopic pig model for direct revascularization and venous drainage of tracheal allografts. Paris-Sud University Lung Transplantation Group. J Thorac Cardiovasc Surg. 1994;108:1066–1075. [PubMed] [Google Scholar]
- 114.Macchiarini P, Mazmanian GM, de Montpréville VT, et al. Maximal preservation time of tracheal allografts. The Paris-Sud University Lung Transplantation Group. Ann Thorac Surg. 1995;60:1597–1604. doi: 10.1016/0003-4975(95)00811-x. [DOI] [PubMed] [Google Scholar]
- 115.Yu P, Clayman GL, Walsh GL. Long-term outcomes of microsurgical reconstruction for large tracheal defects. Cancer. 2011;117:802–808. doi: 10.1002/cncr.25492. [DOI] [PubMed] [Google Scholar]
- 116.Gilbert RW, Neligan PC. Microsurgical laryngotracheal reconstruction. Clin Plast Surg. 2005;32:293–301, v. doi: 10.1016/j.cps.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 117.Al-Khudari S, Sharma S, Young W, et al. Osteocutaneous radial forearm reconstruction of large partial cricotracheal defects. Head Neck. 2013;35:E254–E257. doi: 10.1002/hed.23088. [DOI] [PubMed] [Google Scholar]
- 118.Maciejewski A, Szymczyk C, Półtorak S, et al. Tracheal reconstruction with the use of radial forearm free flap combined with biodegradative mesh suspension. Ann Thorac Surg. 2009;87:608–610. doi: 10.1016/j.athoracsur.2008.06.062. [DOI] [PubMed] [Google Scholar]
- 119.Caliceti U, Piccin O, Cavicchi O, et al. Anterolateral thigh free flap for tracheal reconstruction after parastomal recurrence. Head Neck. 2009;31:1107–1111. doi: 10.1002/hed.20992. [DOI] [PubMed] [Google Scholar]
- 120.Park CW, Miles BA. The expanding role of the anterolateral thigh free flap in head and neck reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2011;19:263–268. doi: 10.1097/MOO.0b013e328347f845. [DOI] [PubMed] [Google Scholar]
- 121.Icibaci A, de Mello-Filho FV. Tracheal transplant with a prefabricated microsurgical flap. Laryngoscope. 2009;119:2309–2314. doi: 10.1002/lary.20538. [DOI] [PubMed] [Google Scholar]
- 122.Kashiwa K, Kobayashi S, Tono H, et al. Reconstruction of the cervical trachea using a prefabricated corticoperiosteal flap from the femur. Ann Plast Surg. 2009;62:633–636. doi: 10.1097/SAP.0b013e31817f023e. [DOI] [PubMed] [Google Scholar]
- 123.Strome M, Stein J, Esclamado R, et al. Laryngeal transplantation and 40-month follow-up. N Engl J Med. 2001;344:1676–1679. doi: 10.1056/NEJM200105313442204. [DOI] [PubMed] [Google Scholar]
- 124.Delaere P, Vranckx J, Verleden G, et al. Leuven Tracheal Transplant Group. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010;362:138–145. doi: 10.1056/NEJMoa0810653. [DOI] [PubMed] [Google Scholar]
- 125.Yamaya M, Finkbeiner WE, Chun SY, et al. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262(6, Part 1):L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 126.Gray TE, Guzman K, Davis CW, et al. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;14:104–112. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 127.Yoon JH, Kim KS, Kim SS, et al. Secretory differentiation of serially passaged normal human nasal epithelial cells by retinoic acid: expression of mucin and lysozyme. Ann Otol Rhinol Laryngol. 2000;109:594–601. doi: 10.1177/000348940010900612. [DOI] [PubMed] [Google Scholar]
- 128.Sachs LA, Finkbeiner WE, Widdicombe JH. Effects of media on differentiation of cultured human tracheal epithelium. In Vitro Cell Dev Biol Anim. 2003;39:56–62. doi: 10.1290/1543-706X(2003)039<0056:EOMODO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 129.Chistiakov DA. Endogenous and exogenous stem cells: a role in lung repair and use in airway tissue engineering and transplantation. J Biomed Sci. 2010;17:92. doi: 10.1186/1423-0127-17-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Igai H, Yamamoto Y, Chang SS, et al. Tracheal cartilage regeneration by slow release of basic fibroblast growth factor from a gelatin sponge. J Thorac Cardiovasc Surg. 2007;134:170–175. doi: 10.1016/j.jtcvs.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 131.Igai H, Chang SS, Gotoh M, et al. Regeneration of canine tracheal cartilage by slow release of basic fibroblast growth factor from gelatin sponge. ASAIO J. 2006;52:86–91. doi: 10.1097/01.mat.0000196513.97411.3d. [DOI] [PubMed] [Google Scholar]
- 132.Igai H, Chang SS, Gotoh M, et al. Tracheal cartilage regeneration and new bone formation by slow release of bone morphogenetic protein (BMP)-2. ASAIO J. 2008;54:104–108. doi: 10.1097/MAT.0b013e31815fd3d3. [DOI] [PubMed] [Google Scholar]
- 133.Okamoto T, Yamamoto Y, Gotoh M, et al. Slow release of bone morphogenetic protein 2 from a gelatin sponge to promote regeneration of tracheal cartilage in a canine model. J Thorac Cardiovasc Surg. 2004;127:329–334. doi: 10.1016/j.jtcvs.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 134.Gong YY, Xue JX, Zhang WJ, et al. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials. 2011;32:2265–2273. doi: 10.1016/j.biomaterials.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 135.Hong HJ, Lee JS, Choi JW, et al. Transplantation of autologous chondrocytes seeded on a fibrin/hyaluronan composite gel into tracheal cartilage defects in rabbits: preliminary results. Artif Organs. 2012;36:998–1006. doi: 10.1111/j.1525-1594.2012.01486.x. [DOI] [PubMed] [Google Scholar]
- 136.Komura M, Komura H, Kanamori Y, et al. An animal model study for tissue-engineered trachea fabricated from a biodegradable scaffold using chondrocytes to augment repair of tracheal stenosis. J Pediatr Surg. 2008;43:2141–2146. doi: 10.1016/j.jpedsurg.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 137.Walles T, Giere B, Macchiarini P, et al. Expansion of chondrocytes in a three-dimensional matrix for tracheal tissue engineering. Ann Thorac Surg. 2004;78:444–448; discussion 448–449. doi: 10.1016/j.athoracsur.2004.02.122. [DOI] [PubMed] [Google Scholar]
- 138.Weidenbecher M, Tucker HM, Awadallah A, et al. Fabrication of a neotrachea using engineered cartilage. Laryngoscope. 2008;118:593–598. doi: 10.1097/MLG.0b013e318161f9f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu Y, Adkisson HD, Bogdanske J, et al. In vivo transplantation of neonatal ovine neocartilage allografts: determining the effectiveness of tissue transglutaminase. J Knee Surg. 2005;18:31–42. doi: 10.1055/s-0030-1248155. [DOI] [PubMed] [Google Scholar]
- 140.Weinand C, Peretti GM, Adams SB, Jr, et al. Healing potential of transplanted allogeneic chondrocytes of three different sources in lesions of the avascular zone of the meniscus: a pilot study. Arch Orthop Trauma Surg. 2006;126:599–605. doi: 10.1007/s00402-005-0100-7. [DOI] [PubMed] [Google Scholar]
- 141.Imaizumi M, Nomoto Y, Sato Y, et al. Evaluation of the use of induced pluripotent stem cells (iPSCs) for the regeneration of tracheal cartilage. Cell Transplant. 2013;22:341–353. doi: 10.3727/096368912X653147. [DOI] [PubMed] [Google Scholar]
- 142.Fishman JM, De Coppi P, Elliott MJ, et al. Airway tissue engineering. Expert Opin Biol Ther. 2011;11:1623–1635. doi: 10.1517/14712598.2011.623696. [DOI] [PubMed] [Google Scholar]
- 143.Kim J, Suh SW, Shin JY, et al. Replacement of a tracheal defect with a tissue-engineered prosthesis: early results from animal experiments. J Thorac Cardiovasc Surg. 2004;128:124–129. doi: 10.1016/j.jtcvs.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 144.Lee CJ, Moon KD, Choi H, et al. Tissue engineered tracheal prosthesis with acceleratedly cultured homologous chondrocytes as an alternative of tracheal reconstruction. J Cardiovasc Surg (Torino) 2002;43:275–279. [PubMed] [Google Scholar]
- 145.Li Z, Zhang M. Chitosan-alginate as scaffolding material for cartilage tissue engineering. J Biomed Mater Res A. 2005;75:485–493. doi: 10.1002/jbm.a.30449. [DOI] [PubMed] [Google Scholar]
- 146.Yang L, Korom S, Welti M, et al. Tissue engineered cartilage generated from human trachea using DegraPol scaffold. Eur J Cardiothorac Surg. 2003;24:201–207. doi: 10.1016/s1010-7940(03)00263-x. [DOI] [PubMed] [Google Scholar]
- 147.Britt JC, Park SS. Autogenous tissue-engineered cartilage: evaluation as an implant material. Arch Otolaryngol Head Neck Surg. 1998;124:671–677. doi: 10.1001/archotol.124.6.671. [DOI] [PubMed] [Google Scholar]
- 148.Temenoff JS, Mikos AG. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2000;21:2405–2412. doi: 10.1016/s0142-9612(00)00108-3. [DOI] [PubMed] [Google Scholar]
- 149.Jungebluth P, Alici E, Baiguera S, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. 2011;378:1997–2004. doi: 10.1016/S0140-6736(11)61715-7. [DOI] [PubMed] [Google Scholar]
- 150.Sutherland RS, Baskin LS, Hayward SW, et al. Regeneration of bladder urothelium, smooth muscle, blood vessels and nerves into an acellular tissue matrix. J Urol. 1996;156(2, Part 2):571–577. doi: 10.1097/00005392-199608001-00002. [DOI] [PubMed] [Google Scholar]
- 151.Galois L, Hutasse S, Cortial D, et al. Bovine chondrocyte behaviour in three-dimensional type I collagen gel in terms of gel contraction, proliferation and gene expression. Biomaterials. 2006;27:79–90. doi: 10.1016/j.biomaterials.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 152.Sato M, Kikuchi M, Ishihara M, et al. Tissue engineering of the intervertebral disc with cultured annulus fibrosus cells using atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS scaffold). Med Biol Eng Comput. 2003;41:365–371. doi: 10.1007/BF02348444. [DOI] [PubMed] [Google Scholar]
- 153.Macchiarini P, Walles T, Biancosino C, et al. First human transplantation of a bioengineered airway tissue. J Thorac Cardiovasc Surg. 2004;128:638–641. doi: 10.1016/j.jtcvs.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 154.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 155.Bader A, Macchiarini P. Moving towards in situ tracheal regeneration: the bionic tissue engineered transplantation approach. J Cell Mol Med. 2010;14:1877–1889. doi: 10.1111/j.1582-4934.2010.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elliott MJ, De Coppi P, Speggiorin S, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380:994–1000. doi: 10.1016/S0140-6736(12)60737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Laurance J. British boy receives trachea transplant built with his own stem cells. BMJ. 2010;340:c1633. doi: 10.1136/bmj.c1633. [DOI] [PubMed] [Google Scholar]
- 158.Baiguera S, Del Gaudio C, Jaus MO, et al. Long-term changes to in vitro preserved bioengineered human trachea and their implications for decellularized tissues. Biomaterials. 2012;33:3662–3672. doi: 10.1016/j.biomaterials.2012.01.064. [DOI] [PubMed] [Google Scholar]
- 159.Freed LE, Guilak F, Guo XE, et al. Advanced tools for tissue engineering: scaffolds, bioreactors, and signaling. Tissue Eng. 2006;12:3285–3305. doi: 10.1089/ten.2006.12.3285. [DOI] [PubMed] [Google Scholar]
- 160.Tan Q, Steiner R, Hoerstrup SP, et al. Tissue-engineered trachea: history, problems and the future. Eur J Cardiothorac Surg. 2006;30:782–786. doi: 10.1016/j.ejcts.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 161.Pörtner R, Nagel-Heyer S, Goepfert C, et al. Bioreactor design for tissue engineering. J Biosci Bioeng. 2005;100:235–245. doi: 10.1263/jbb.100.235. [DOI] [PubMed] [Google Scholar]
- 162.Badylak SF, Weiss DJ, Caplan A, et al. Engineered whole organs and complex tissues. Lancet. 2012;379:943–952. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 163.Asnaghi MA, Jungebluth P, Raimondi MT, et al. A double-chamber rotating bioreactor for the development of tissue-engineered hollow organs: from concept to clinical trial. Biomaterials. 2009;30:5260–5269. doi: 10.1016/j.biomaterials.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 164.Lin CH, Hsu SH, Huang CE, et al. A scaffold-bioreactor system for a tissue-engineered trachea. Biomaterials. 2009;30:4117–4126. doi: 10.1016/j.biomaterials.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 165.Miller C, George S, Niklason L. Developing a tissue-engineered model of the human bronchiole. J Tissue Eng Regen Med. 2010;4:619–627. doi: 10.1002/term.277. [DOI] [PubMed] [Google Scholar]
- 166.Tan Q, Hillinger S, van Blitterswijk CA, et al. Intra-scaffold continuous medium flow combines chondrocyte seeding and culture systems for tissue engineered trachea construction. Interact Cardiovasc Thorac Surg. 2009;8:27–30. doi: 10.1510/icvts.2008.179804. [DOI] [PubMed] [Google Scholar]
- 167.Vunjak-Novakovic G, Martin I, Obradovic B, et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 168.Kalathur M, Baiguera S, Macchiarini P. Translating tissue-engineered tracheal replacement from bench to bedside. Cell Mol Life Sci. 2010;67:4185–4196. doi: 10.1007/s00018-010-0499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Haykal S, Salna M, Zhou Y, et al. Double-chamber rotating bioreactor for dynamic perfusion cell seeding of large segment tracheal allografts: comparison to conventional static methods. Tissue Eng Part C Methods. 2014 doi: 10.1089/ten.tec.2013.0627. Mar 5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


