Abstract
Summary:
Outcome studies help provide the evidence-based science rationalizing treatment end results that factor the experience of patients and the impact on society. They improve the recognition of the shortcoming in clinical practice and provide the foundation for the development of gold standard care. With such evidence, health care practitioners can develop evidence-based justification for treatments and offer patients with superior informed consent for their treatment options. Furthermore, health care and insurance agencies can recognize improved cost-benefit options in the purpose of disease prevention and alleviation of its impact on the patient and society. Health care outcomes are ultimately measured by the treatment of disease, the reduction of symptoms, the normalization of laboratory results and physical measures, saving a life, and patient satisfaction. In this review, we outline the tools available to measure outcomes in plastic surgery and subsequently allow the objective measurements of plastic surgical conditions. Six major outcome categories are discussed: (1) functional measures; (2) preference-based measures and utility outcome scores; (3) patient satisfaction; (4) health outcomes and time; (5) other tools: patient-reported outcome measurement information system, BREAST-Q, and Tracking Operations and Outcomes for Plastic Surgeons; and (6) cost-effectiveness analysis. We use breast hypertrophy requiring breast reduction as an example throughout this review as a representative plastic surgical condition with multiple treatments available.
Plastic surgeons have a substantial variation in surgical practice for similar diagnoses and conditions. For example, women with breast hypertrophy are offered breast reduction surgery with over half a dozen different pedicles and incision types depending on the consultant surgeon and patient preference. This difference in practice is not limited to the health state/diagnosis, borders, race, ethics, or health care policy. Indeed, even within the same institution, patients with the same condition are treated differently with respect to medical and surgical practice. Whose technique is “superior”? Which patient is receiving “better care”? Does the lack of uniformity in surgical practice indicate that all techniques are equal for the care of one condition in different patients? Or does it mean that there is not enough evidence to prove which procedure is “superior”? Outcomes research helps answer such questions by providing the relation between clinical care and their end results.1–3 As Clancy and Eisenberg4 outlined, outcomes research help measure the end results of health care.
Plastic surgical conditions and health states’ severity are often subjectively graded. For example, the amount of breast tissue that requires excision in a breast reduction surgery varies not only according to the patient’s and surgeon’s subjective desires but also according to the breast weight cutoffs that insurance agencies and health care policy makers have subjectively set. Furthermore, are we able to compare the objective value of living with severe breast hypertrophy with other health states within different specialties? For example, can we objectively compare living with severe breast hypertrophy with living with severe renal failure?
Because of the subjective endpoints that categorize most conditions treated by plastic surgeons, there can be a false sense of “less urgency” or “less importance” to their treatment. Coupled with the fact that only a few plastic surgery conditions are life threatening, our specialty may be falsely perceived with “less priority” by insurance agencies and policy makers, thus restricting funding, operative times, and insurance coverage in the future.
Outcomes research provides the scientific evidence relating the treatment end results that factor the experience of patients and the impact on society.5,6 It helps recognize the shortcoming in clinical practice and provide rationale for the development of gold standard care.5,6 With such evidence, health care practitioners can develop evidence-based rationale for treatments and provide patients with informed consent for their treatment options. Furthermore, health care and insurance agencies can identify improved cost-benefit options in the purpose of disease prevention and the alleviation of its impact on the patient and society.5–7 Our aim in this review is to outline the tools available to measure outcomes in plastic surgery and subsequently allow the objective measurements of plastic surgical conditions. We use breast hypertrophy requiring breast reduction as an example throughout this review as a representative plastic surgical condition with multiple treatments available.
TOOLS TO MEASURE OUTCOME STUDIES
Health care outcomes are ultimately measured by the treatment of disease, the reduction of symptoms, the normalization of laboratory results and physical measures, saving a life, and patient satisfaction. Many validated tools exist today to objectively measure such endpoints and can be subdivided into 4 categorical endpoints4: (1) Health perception: a person’s score of overall health; (2) Functional measures: an assessment of the gross impact of health care on health; (3) Preference-based measures: have been developed from business and economy literature as standardized tools to evaluate the functional status. They are designed to place value on particular health states; and (4) Patient satisfaction: factors in interpersonal aspects, psychological behaviors, and technical aspects of care. Many validated outcomes measure instruments available in the literature do provide more than one type of endpoint objective measures. Consequently, it is important to note that many of the available tools can fall into more than 1 of the 4 categories listed. This can be said, for example, for patient-reported outcome measurement information system (PROMIS) and European quality of life (EuroQol). We have categorized the widely used measures into the most fitting category. Tools such as PROMIS however, are validated measures of more than one outcomes endpoint. Consequently, we have created a fifth categorical outcome tools subdivision to include such tools. Moreover, a fifth group can include a combination of the above 4 endpoints or large outcome-specific databases. Figure 1 categorically illustrates the available outcome tools. The choice of which outcome measure to use can depend on the medical or surgical specialty and the specific endpoints in question.
Fig. 1.
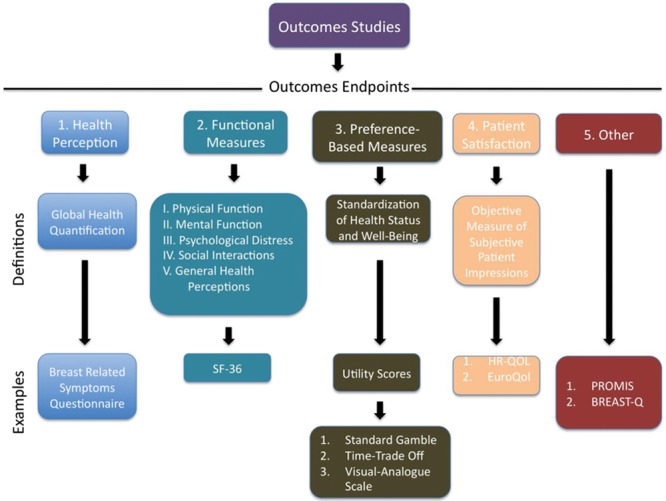
Tools used in outcome studies. Five general subdivisions can be used to categorize outcome studies. A definition and examples are provided below each subdivision.
Plastic surgery is a diverse specialty that encompasses a variety of subspecialties including craniofacial surgery, hand surgery, microsurgery, breast reconstruction, burn care, pediatric plastic surgery, general reconstruction, and aesthetic surgery. Even within each subspecialty, there is a diversity of procedures that are performed for the treatment of the same condition. Alternate interventions can be compared and validated using outcome evidence-based and preference-based measures. These measures will help replace the traditional evaluation of surgical success in terms of subjective aesthetic outcomes to more objective measures such as sustainability of aesthetic results, preservation of function, or pain relief.8
HEALTH PERCEPTION
Health perception is measured by the patient’s symptoms. It helps quantify the global health of an individual and has been shown to be a good predictor of death and seeking health care.9,10 These tests are performed by practitioners inquiring their patients about validated symptoms using published and established standardized questions. For example, in the case of breast hypertrophy, The Breast-Related Symptoms Questionnaire has been used as a validated 13-item analysis for the measure of health perception in patients with severe breast hypertrophy.11 The average scores are linearly transformed to a scale from 0 (greater number, more severe symptoms) to 100 (fewer and less severe symptoms). These scores can be divided by 100 for comparison to other standardized outcome scores.
FUNCTIONAL MEASURES (SF-36)
Functional measures are used to determine the overall influence of health care provided to an individual on his/her global health and the impact on his/her specific disease. They objectively measure the ability of patients to perform relevant activities of daily living. These activities can range from self-care to specific functions of a given anatomical region. These functional measures can be compared to those attained before and after an intervention to assess the effect the intervention has had on the patient’s functional status.
Generally, functional measures should include inquiries on (1) physical function; (2) mental function; (3) psychological distress; (4) social interactions; and (5) general health perceptions.12 The SF-36 is an example of an instrument to attain functional measures that determines health status in outcome studies.13 On a multi-item scale, it measures 8 health concepts that are related to the health problem(s): (1) limitations in physical activities; (2) limitations in social activities; (3) limitations in usual function activities (secondary to physical problems); (4) limitations in usual function activities (secondary to emotional problems); (5) general mental health (psychological distress and well-being); (6) bodily pain; (7) vitality (energy and fatigue); and (8) general health perception. It is easily self-administered and simple to use and thus has become widely used in clinical practice and trials. Other functional measure tools include Veterans RAND-36, Veterans RAND-12, and PROMIS global.
PREFERENCE-BASED MEASURES AND UTILITY OUTCOME SCORES
Preference-based measures allow the objective standardization of health status and well-being. Utility scores are validated preference-based measures that can be used in plastic surgery outcome studies. Utility assessments are an established and published recognized method of determining health state preferences in health economics and medicine.14–18 Utility scores were first introduced to the plastic surgery literature by Kerrigan et al.19,20 These measures range from 0 (death) to 1 (perfect health). As such, different health states can be compared to one another by a numeric value. For example, the utility outcome score of living with severe breast hypertrophy was found to be 0.86.19,20 This utility score can now be compared to other health states such as living with kidney transplantation after severe renal failure (0.84).19,21
To objectify the burden of a particular health state, different tests have been designed to attain utility outcome scores.16,17,19,20 Some of the more popular tools include the standard gamble,22 time trade-off,17 and visual analogue scale.23 The utilization of all 3 tools for obtaining utility scores is optimal to minimize the inherent weaknesses of any single test. In the standard gamble, subjects are asked to choose between 2 choices: to either remain in a given health state or take a chance (gamble) with some probability of success (perfect health) and some probability of failure (death). Percentages of success and failure are systematically alternated until the subject is indifferent between taking the gamble and remaining in the described health state. The utility score is derived from this point of indifference by the following formula: utility health state = (1.00 − risk of death at the point of indifference) ÷ 100. A bisecting search routine algorithm is used to determine the subject’s point of indifference. Typically, 6 iterations are used in the algorithm.20,24 If the subject has declined to accept a 1% risk of death, the test asks whether he or she would accept any chance of death. To avoid the biasing effect of phrasing every question in terms of the risk of death,25 every statement should be rephrased in terms of the probability of living in perfect health. “Smiley faces” and Xs should be used as visual aid tools to facilitate the subject’s comprehension of percentage of perfect health for the time trade-off and life years remaining for the standard gamble.20 Figure 2 demonstrates an example of utility assessment through a standard gamble survey. In the time trade-off, the subject is asked to choose between living a specified number of years in the health state (eg, 36 years) or “trading-off” some of those years to live in perfect health. The number of years traded off in the time trade-off task is systematically alternated using a similar bisecting search routine algorithm until the indifference point of the subject is found (Fig. 3). The utility value is derived from this indifference point: Utility = (number of years specified in the described health state − number of years traded off at the indifference point) ÷ number of years specified in the described health state. In the visual analogue scale (Fig. 4), the subject is asked to score a value of the given health state on a scale from 0 (death) to 100 (perfect health). The utility score is calculated from each subject’s score using the following formula: utility health state = score ÷100.
Fig. 2.
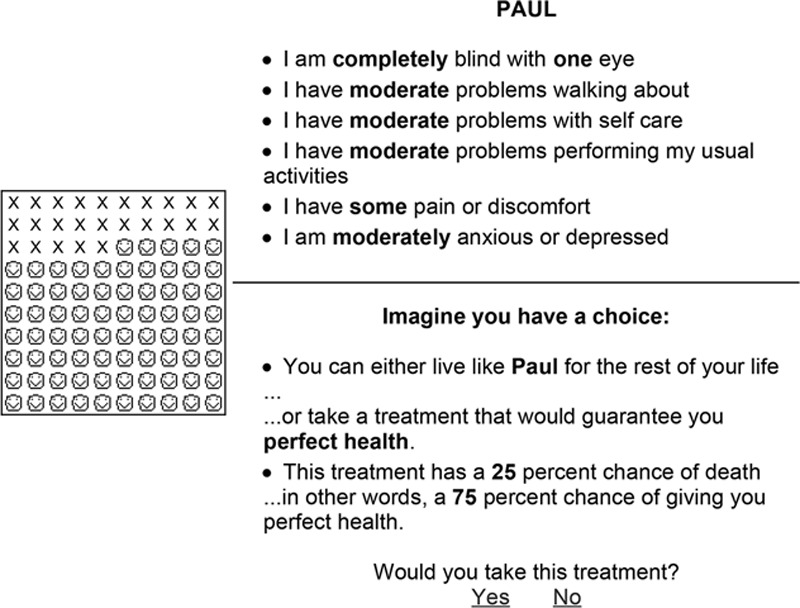
Example of the standard gamble survey for monocular blindness. To the left are smiley faces and Xs that are visual representatives for percentage chance of life and death, respectively. The case scenarios are provided again for each frame.
Fig. 3.

Example of the time trade-off survey for monocular blindness. To the left are smiley faces and Xs that are visual representatives for years of life and death, respectively. The case scenarios are provided again for each frame.
Fig. 4.

Example of the visual analogue scale for blindness. A horizontal bar with a cursor is provided for the volunteers to slide from 0 to 100. The case scenarios are provided again for each frame.
Should utility scores be extracted from patients living with the specific health states, family members of patients, or a sample of the general population? There has been a great debate to answer this question.20 Attaining utility scores from patients directly has the advantage of objectifying and further understanding the physical and psychological impact the disease or condition has on the individual. However, studies have shown that patients living with health states can become accustomed to and habituated to the condition and thus with time become less burdened with the condition. For example, Barker et al26 questioned the objectivity of attaining utility scores from patients with facial disfigurement. They concluded that some patients living with facial disfigurement adjust to their condition and integrate their appearance into their lives. These individuals become accustomed to their appearance and health state to the point that they would be willing to undergo significantly less risk to change their facial appearance.
How about health states where infants are affected, such as cleft lip and palate? Who should the outcome studies be conducted on? In these situations, it would be impossible to survey the patients because of their age and incomprehension of the tests and potential impact of the disease. Some have suggested that family members or caregivers may be adequate alternatives as they may have a better understanding of the condition or disease. However, family members can be less willing to take risk or gamble for a procedure for their children, for example. This finding introduces a risk for bias in attaining objective utility scores. Consequently, the assessment of utility scores should be from a neutral sample of the general population. Moreover, it is the recommendation of the Panel on Cost-Effectiveness in Health and Medicine that utility assessments be performed on a sample from the general population.18 According to these guidelines, we have shown the utility scores of living with severe facial disfigurement requiring facial transplantation to be 0.6627 and those of cleft lip and palate to be 0.84.28
PATIENT SATISFACTION
Patient satisfaction outcome studies attempt to objectify the patient’s subjective impression of their health state or treatment. They can also measure patient satisfaction with their (1) health care experience; (2) outcomes compared to expectations; and (3) health care providers. These studies transcend the definition of a disease or the technical aspect of a procedure. Examples of such measures can be extrapolated from tools such as the health-related quality of life (HR-QOL) and EuroQol.
HR-QOL refers to physical condition and well-being that affect the daily lives of individual patients. It specifically relates to the health domain of the patients’ existence by evaluating measures of patients’ preferences and values, health perceptions, symptoms, and function.29 These factors are converted to numeric values typically ranging from 0 (mortality) to 1 (perfect health/patient satisfaction).
The EuroQol assessment tool was the result of the joint development of the European Quality of life group (Fig. 5).30 It was developed as a standardized non–disease-specific instrument for describing and giving value to HR-QOL.30 This tool was intended to complement other forms of quality of life measures. It was designed as a self-completion survey with 4 instrument components: (1) description of the respondent’s own health; (2) rating of own health by means of visual analogue scale; (3) valuation of standard set of health states; and (4) background information about respondents. Figure 3 is an example of the EuroQol that we have used in studies evaluating outcomes in plastic surgery.27,28,31–34
Fig. 5.
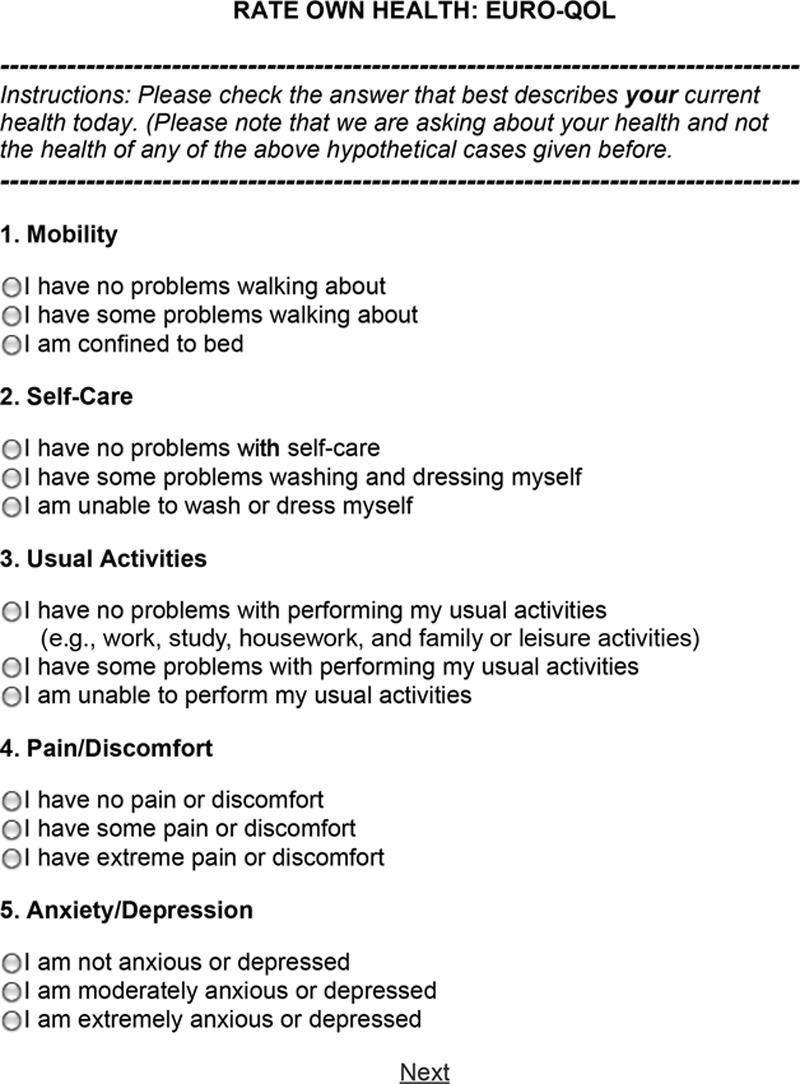
Example of EuroQol survey. Five subdivisions of life quality are assessed including (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort, and (5) anxiety/depression.
HEALTH OUTCOMES AND TIME
There are quality of life measures that integrate both health outcomes and time. These tools allow the development of a numeric value by the incorporation of quantity and quality of life. Examples of such tests are quality-adjusted life years (QALY), potential years of life lost, disability-adjusted life years, health-adjusted life expectancy, and years of healthy life.18 Data from such studies can be extrapolated to educate decision makers and consequently influence allocation of health care resources to improve population health. The assumption made with these tools is that community preferences can represent individual preferences.
OTHER TOOLS: PROMIS AND BREAST-Q
PROMIS is a National Institutes of Health–funded project and can be found at http://www.nihpromise.org. It was shown to be a highly reliable and precise measure of patient-reported health status for physical, mental, and social well-being.35,36 PROMIS instruments are developed using mixed qualitative and quantitative methods engaged to complement one another in an iterative process. The goal of PROMIS is to provide clinicians and researchers access to efficient, valid, and responsive self-reported measures of health. This method of study includes symptoms, function, and well-being. PROMIS measures can be used as endpoints in clinical trials of effectiveness of treatment. These data can be used to design treatment plans and the management of chronic disease. Current available measures of PROMIS include physical activity, experiences of stress, family belonging, subjective well-being, and pain. PROMIS fits in this fifth subcategory “other tools” as it is a modular tool that can measure many outcome endpoints including functional status and effectiveness of treatment. It would not be sufficient to include it in only 1 of the 4 outcome categories.
Similar to PROMIS but specific to breast surgery, the BREAST-Q was developed. It is also a modular tool that measures more than one outcomes endpoint including functional subscales and satisfaction subscales and thus better fitting in this fifth category of “other tools.” Its primary focus was to study of the impact and effectiveness of breast surgery from the patient’s perspective.37 The survey is subdivided into 4 modules of breast surgery: (1) augmentation; (2) reduction (or mastopexy); (3) reconstruction; and (4) mastectomy-only patients. Each module is based on 6 outcome measures of patient satisfaction and HR-QOL in breast surgery: (1) satisfaction with breasts; (2) satisfaction with overall outcome; (3) psychosocial well-being; (4) sexual well-being; (5) physical well-being; and (6) satisfaction with care.
The BREAST-Q quantifies patient satisfaction and HR-QOL experienced by women undergoing breast surgery. The outcomes of such a survey may provide further insight into cost-effectiveness analysis, patient and surgeon education, and surgical advocacy. It allows the perioperative objective measure of patient satisfaction and thus has the potential to help surgeons improve and modify their performance accordingly.
WHICH OUTCOME TOOLS TO USE IN PLASTIC SURGERY?
To determine which outcome measures provide the greatest yield, it is first important to establish the endpoints in questions. After the endpoints have been established, investigators can fall back on the 4 categorical endpoint outcome tools that have been validated: (1) health perception, (2) functional measures, (3) preference-based measures, and (4) patient satisfaction. Because each outcome measure has inherent bias, it can be advantageous to use at least 3 different tools to complement one another. Important examples of objective measures and endpoints in plastic surgery include sustainability of aesthetic results, preservation of function, pain and/or psychological relief.
OUTCOME RESEARCH FUNDING
Patient-Centered Outcomes Research Institute was specifically created to support outcome research. Its mission is to provide the science and evidence to practitioners and patients to help make informed decisions in terms of prevention, treatment, and care. Its aims are to assess the value of health care options that is determined by patients and their caregivers. One of the primary aims of this research is to answer what patients are to expect given their own personal characteristics, conditions, and preferences. The Patient-Centered Outcomes Research Institute is funded by a trust fund authorized by the US Congress as part of the Patient Protection and Affordable Care Act of 2012.
TRACKING OPERATIONS AND OUTCOMES FOR PLASTIC SURGEONS
Tracking Operations and Outcomes for Plastic Surgeons (TOPS) is another tool specifically made for plastic surgeons. This is a registry instrument and not by itself an outcome tool. Data derived from TOPS can be used to develop important outcome measures specifically for plastic surgeons.
This system was launched in 2002 and can be found at https://tops.plasticsurgery.org/. TOPS is national electronic database of plastic surgery procedures provided free of charge by the American Society of Plastic Surgeons. It is Health Insurance Portability and Accountability Act of 1996 (HIPAA) compliant, secure, and confidential. TOPS provides plastic surgeons with a means to enter clinical and demographic data into a confidential database. With the support of as many plastic surgeons, a great database of procedures and treatments can be collected. This database can lead to the development of evidence-based practice parameters and monitoring of clinical outcomes and emerging trends. This very important database can provide evidence-based monitoring of outcomes in our field.
COST-EFFECTIVENESS ANALYSIS
Cost-effectiveness analysis is an important outcomes tool used to establish priorities for funding health care programs.38 The clinical outcomes and costs associated with any one intervention must be weighed against alternative strategies for treating the same patients. Additionally, distribution may be driven by political objectives. Using this tool, policymakers are able to gain a better understanding of how to allocate resources among competing uses. Furthermore, the incremental cost per incremental unit of clinical outcome may be determined.38 In this way, the incremental cost-effectiveness ratios of various treatment modalities can be compared and set to funding priorities. The result is an objective assessment of cost which can optimize the net health benefit for a target population from a fixed budget.38 Although clinicians are more concerned with the effectiveness of a treatment regimen rather than the benefit attained from spending resources, in time, they will likely take into consideration the consequences of such decisions and policies. Moreover, they are obliged to take in active role in aiding those directly responsible for these choices.38 Plastic surgeons have a responsibility to provide high-quality economic evaluations that support the cost-effectiveness of the procedures they perform.39 As a specialty, plastic surgery is at the forefront regarding introduction of innovative techniques and technologies. A majority of which are not only more effective but also more costly, necessitating the need for cost evaluation.
Economic evaluation encompasses 4 basic concepts: cost-utility analysis, cost analysis, cost-benefit, and cost effectiveness.40 The most appropriate type of analysis for most plastic surgery interventions may be cost-utility analyses primarily because these methods use QALY units that integrate both the quantity and quality of life gained by a proposed intervention.41 Cost analysis alone may not useful because it may not account for variations in the efficacy of an intervention and focus only on cost. Thus, it is considered a partial economic evaluation. Cost benefit entails converting outcomes into monetary values, which can be complicated when attempting to monetize patients’ time and burden of disease. Furthermore, as a result of equity issues, this type of analysis is considered discriminatory against those economically disadvantaged.40 Economic evaluations have been performed in the field of plastic surgery.42–45 Thoma et al45 compared the costs of endoscopic versus open carpel tunnel release reporting an additional $124,311/QALY when the endoscopic approach was performed, indicating that this technique should not be adopted. When a comparison of costs and health utilities for unilateral hand transplantation, unilateral prosthesis, bilateral hand transplantation, and bilateral prostheses was performed by Chung et al,42 the authors reported that although bilateral hand transplantation was preferred to prosthesis in terms of quality of life, it posed an additional $381,961/QALY. Other studies have shown that deep inferior epigastric perforator flaps are more cost effective than transverse rectus abdominis myocutaneous (TRAM) flaps and free TRAMs are more cost effective than unipedicled TRAMs.43,44 Since most plastic surgeons have not been educated in standard health research methodology, collaborating with a health economist may be useful to ensure proper cost-utility analysis.39 It is important to adopt these analyses not only for sensible allocation of resources but also to be considered substantial, reliable, and relevant information by government, professional organizations, and third-party payers.39
CONCLUSIONS
Outcomes research helps provide evidence for superior treatments by demonstrating the relationship between clinical care and their end results. This scientific evidence illustrates the treatment end results that factor the experience of patients and the impact on society. It helps recognize the shortcoming in clinical practice and provide rationale for the development of gold standard care, consequently influencing cost-effectiveness. Moreover, vital information can be used for plastic surgery care and funding allocation and improve patient informed consent while allowing clinicians to conform to gold standard care. Furthermore, outcome studies can objectify subjective health states and outcomes in plastic surgery. These data are integral in providing metric values for comparison with other health states and specialties. Future direction in outcomes research in plastic surgery should focus on the development of tools directed to answer specific endpoints such as the BREAST-Q. PROMIS has a great potential to be further developed for specific health states within plastic surgery. TOPS is a very important resource that all plastic surgeons should support. These cumulative tools should continue to be developed so that the field may be universally improved and standardization of measures be made potentially for patient care.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Wennberg J, Gittelsohn A. Small area variations in health care delivery. Science. 1973;182:1102–1108. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 2.McPherson K, Wennberg JE, Hovind OB, et al. Small-area variations in the use of common surgical procedures: an international comparison of New England, England, and Norway. N Engl J Med. 1982;307:1310–1314. doi: 10.1056/NEJM198211183072104. [DOI] [PubMed] [Google Scholar]
- 3.Chassin MR, Kosecoff J, Park RE, et al. Does inappropriate use explain geographic variations in the use of health care services? A study of three procedures. JAMA. 1987;258:2533–2537. [PubMed] [Google Scholar]
- 4.Clancy CM, Eisenberg JM. Outcomes research: measuring the end results of health care. Science. 1998;282:245–246. doi: 10.1126/science.282.5387.245. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes R. Managing healthcare costs within an integrated framework. Healthc Pap. 2002;3:70–76; discussion 87–94. doi: 10.12927/hcpap..16913. [DOI] [PubMed] [Google Scholar]
- 6.Goode CJ, Tanaka DJ, Krugman M, et al. Outcomes from use of an evidence-based practice guideline. Nurs Econ. 2000;18:202–207. [PubMed] [Google Scholar]
- 7.Understanding costs and cost-effectiveness in critical care: report from the second American Thoracic Society workshop on outcomes research. Am J Respir Crit Care Med. 2002;165:540–550. doi: 10.1164/ajrccm.165.4.16541. [DOI] [PubMed] [Google Scholar]
- 8.Hartwig GB, Leatherman NE, McNeil WP, et al. Exercise performance in debrancher deficiency myopathy. Trans Am Neurol Assoc. 1979;104:248–252. [PubMed] [Google Scholar]
- 9.Kaplan GA, Camacho T. Perceived health and mortality: a nine-year follow-up of the human population laboratory cohort. Am J Epidemiol. 1983;117:292–304. doi: 10.1093/oxfordjournals.aje.a113541. [DOI] [PubMed] [Google Scholar]
- 10.Wennberg JE, Barry MJ. Outcomes research. Science. 1994;264:758–759. doi: 10.1126/science.7513442. [DOI] [PubMed] [Google Scholar]
- 11.Kerrigan CL, Collins ED, Striplin D, et al. The health burden of breast hypertrophy. Plast Reconstr Surg. 2001;108:1591–1599. doi: 10.1097/00006534-200111000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE., Jr. The status of health assessment 1994. Annu Rev Public Health. 1995;16:327–354. doi: 10.1146/annurev.pu.16.050195.001551. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 14.Froberg DG, Kane RL. Methodology for measuring health-state preferences–II: Scaling methods. J Clin Epidemiol. 1989;42:459–471. doi: 10.1016/0895-4356(89)90136-4. [DOI] [PubMed] [Google Scholar]
- 15.Llewellyn-Thomas H, Sutherland HJ, Tibshirani R, et al. Describing health states. Methodologic issues in obtaining values for health states. Med Care. 1984;22:543–552. [PubMed] [Google Scholar]
- 16.Read JL, Quinn RJ, Berwick DM, et al. Preferences for health outcomes. Comparison of assessment methods. Med Decis Making. 1984;4:315–329. doi: 10.1177/0272989X8400400307. [DOI] [PubMed] [Google Scholar]
- 17.Torrance GW, Feeny D. Utilities and quality-adjusted life years. Int J Technol Assess Health Care. 1989;5:559–575. doi: 10.1017/s0266462300008461. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296:716–721. doi: 10.1056/NEJM197703312961304. [DOI] [PubMed] [Google Scholar]
- 19.Kerrigan CL, Collins ED, Kneeland TS, et al. Measuring health state preferences in women with breast hypertrophy. Plast Reconstr Surg. 2000;106:280–288. doi: 10.1097/00006534-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Chang WT, Collins ED, Kerrigan CL. An Internet-based utility assessment of breast hypertrophy. Plast Reconstr Surg. 2001;108:370–377. doi: 10.1097/00006534-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50:235–242. doi: 10.1038/ki.1996.307. [DOI] [PubMed] [Google Scholar]
- 22.van Osch SM, Stiggelbout AM. The construction of standard gamble utilities. Health Econ. 2008;17:31–40. doi: 10.1002/hec.1235. [DOI] [PubMed] [Google Scholar]
- 23.Stevens KJ, McCabe CJ, Brazier JE. Mapping between Visual Analogue Scale and Standard Gamble data; results from the UK Health Utilities Index 2 valuation survey. Health Econ. 2006;15:527–533. doi: 10.1002/hec.1076. [DOI] [PubMed] [Google Scholar]
- 24.Boyd NF, Sutherland HJ, Heasman KZ, et al. Whose utilities for decision analysis? Med Decis Making. 1990;10:58–67. doi: 10.1177/0272989X9001000109. [DOI] [PubMed] [Google Scholar]
- 25.McNeil BJ, Pauker SG, Sox HC, Jr, et al. On the elicitation of preferences for alternative therapies. N Engl J Med. 1982;306:1259–1262. doi: 10.1056/NEJM198205273062103. [DOI] [PubMed] [Google Scholar]
- 26.Barker JH, Furr A, Cunningham M, et al. Investigation of risk acceptance in facial transplantation. Plast Reconstr Surg. 2006;118:663–670. doi: 10.1097/01.prs.0000233202.98336.8c. [DOI] [PubMed] [Google Scholar]
- 27.Sinno HH, Thibaudeau S, Duggal A, et al. Utility scores for facial disfigurement requiring facial transplantation [outcomes article]. Plast Reconstr Surg. 2010;126:443–449. doi: 10.1097/PRS.0b013e3181e094fa. [DOI] [PubMed] [Google Scholar]
- 28.Sinno H, Tahiri Y, Thibaudeau S, et al. Cleft lip and palate: an objective measure outcome study. Plast Reconstr Surg. 2012;130:408–414. doi: 10.1097/PRS.0b013e3182589d4b. [DOI] [PubMed] [Google Scholar]
- 29.Torrance GW. Utility approach to measuring health-related quality of life. J Chronic Dis. 1987;40:593–603. doi: 10.1016/0021-9681(87)90019-1. [DOI] [PubMed] [Google Scholar]
- 30.Brooks RG, Jendteg S, Lindgren B, et al. EuroQol: health-related quality of life measurement. Results of the Swedish questionnaire exercise. Health Policy. 1991;18:37–48. doi: 10.1016/0168-8510(91)90142-k. [DOI] [PubMed] [Google Scholar]
- 31.Sinno H, Thibaudeau S, Tahiri Y, et al. Utility assessment of body contouring after massive weight loss. Aesthetic Plast Surg. 2011;35:724–730. doi: 10.1007/s00266-011-9676-1. [DOI] [PubMed] [Google Scholar]
- 32.Sinno H, Izadpanah A, Thibaudeau S, et al. The impact of living with a functional and aesthetic nasal deformity after primary rhinoplasty: a utility outcomes score assessment. Ann Plast Surg. 2012;69:431–434. doi: 10.1097/SAP.0b013e3182480384. [DOI] [PubMed] [Google Scholar]
- 33.Sinno H, Thibaudeau S, Izadpanah A, et al. Utility outcome scores for unilateral facial paralysis. Ann Plast Surg. 2012;69:435–438. doi: 10.1097/SAP.0b013e318246e698. [DOI] [PubMed] [Google Scholar]
- 34.Sinno HH, Ibrahim AM, Izadpanah A, et al. Utility outcome assessment of the aging neck following massive weight loss. Otolaryngol Head Neck Surg. 2012;147:26–32. doi: 10.1177/0194599812439028. [DOI] [PubMed] [Google Scholar]
- 35.Morita Y, Iseki M, Ifuku M, et al. [Development and evaluation of a patient-reported outcome measure of pain-related sleep disturbances for pain clinic patients]. Masui. 2012;61:130–137. [PubMed] [Google Scholar]
- 36.Rothrock NE, Kaiser KA, Cella D. Developing a valid patient-reported outcome measure. Clin Pharmacol Ther. 2011;90:737–742. doi: 10.1038/clpt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124:345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 38.Detsky AS, Naglie IG. A clinician’s guide to cost-effectiveness analysis. Ann Intern Med. 1990;113:147–154. doi: 10.7326/0003-4819-113-2-147. [DOI] [PubMed] [Google Scholar]
- 39.Thoma A, Ignacy TA, Ziolkowski N, et al. The performance and publication of cost-utility analyses in plastic surgery: making our specialty relevant. Can J Plast Surg. 2012;20:187–193. doi: 10.1177/229255031202000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. New York: Oxford University Press; 2005. [Google Scholar]
- 41.Gold MR, Seigel JE, Russell LB, et al. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 42.Chung KC, Oda T, Saddawi-Konefka D, et al. An economic analysis of hand transplantation in the United States. Plast Reconstr Surg. 2010;125:589–598. doi: 10.1097/PRS.0b013e3181c82eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thoma A, Khuthaila D, Rockwell G, et al. Cost-utility analysis comparing free and pedicled TRAM flap for breast reconstruction. Microsurgery. 2003;23:287–295. doi: 10.1002/micr.10138. [DOI] [PubMed] [Google Scholar]
- 44.Thoma A, Veltri K, Khuthaila D, et al. Comparison of the deep inferior epigastric perforator flap and free transverse rectus abdominis myocutaneous flap in postmastectomy reconstruction: a cost-effectiveness analysis. Plast Reconstr Surg. 2004;113:1650–1661. doi: 10.1097/01.prs.0000117196.61020.fd. [DOI] [PubMed] [Google Scholar]
- 45.Thoma A, Wong VH, Sprague S, et al. A cost-utility analysis of open and endoscopic carpal tunnel release. Can J Plast Surg. 2006;14:15–20. doi: 10.1177/229255030601400101. [DOI] [PMC free article] [PubMed] [Google Scholar]


