Abstract
Two neuromodulatory techniques based on applying direct current (DC) non-invasively through the skin, transcranial cerebellar direct current stimulation (tDCS) and transcutaneous spinal DCS, can induce prolonged functional changes consistent with a direct influence on the human cerebellum and spinal cord. In this article we review the major experimental works on cerebellar tDCS and on spinal tDCS, and their preliminary clinical applications. Cerebellar tDCS modulates cerebellar motor cortical inhibition, gait adaptation, motor behaviour, and cognition (learning, language, memory, attention). Spinal tDCS influences the ascending and descending spinal pathways, and spinal reflex excitability. In the anaesthetised mouse, DC stimulation applied under the skin along the entire spinal cord may affect GABAergic and glutamatergic systems. Preliminary clinical studies in patients with cerebellar disorders, and in animals and patients with spinal cord injuries, have reported beneficial effects. Overall the available data show that cerebellar tDCS and spinal tDCS are two novel approaches for inducing prolonged functional changes and neuroplasticity in the human cerebellum and spinal cord, and both are new tools for experimental and clinical neuroscientists.
Introduction
Two novel and simple methodological approaches described by our group and developed in the past 7 years now suggest that direct current (DC) delivered transcutaneously can modulate functions in the cerebellum (Block & Celnik, 2012; Ferrucci & Priori, 2013; Grimaldi et al. 2013; Tomlinson et al. 2013) and spinal cord (Cogiamanian et al. 2012; Lamy & Boakye, 2013) over a prolonged time. The idea of using transcutaneous DC for modulating function in the human cerebellum and spinal cord arose from the observation that DC delivered through the scalp modulates motor cortex excitability (Priori et al. 1998; Nitsche & Paulus, 2000; Priori, 2003; Ardolino et al. 2005; Brunoni et al. 2012) and hence DC could also modulate other central nervous system (CNS) structures. As happens for cerebral tDCS, novel approaches using DC applied transcutaneously presumably act at least in part by polarising the neuronal membrane and inducing neuroplasticity.
The human cerebellum has unique functions, correlated with motor control, learning, cognition, emotions and behaviours (Strick et al. 2009; Manto & Haines, 2012; Reeber et al. 2013). Though the cerebellum accounts for only approximately 10% of the brain volume, it contains more than half of all neurons. Such a high neuronal concentration may in part account for cerebellar sensitivity to the electric field. The various cerebellar functions arise from its division into three separate portions each having different connections with the rest of the CNS: the vestibulocerebellum (with efferent and afferent connections to the vestibular nuclei), the spinocerebellum (with afferent connections from the spinal cord and efferent connections controlling the medial and lateral descending motor systems), and the cerebrocerebellum (efferent and afferent connections with the cerebral cortex) (Ghez & Thac, 2000). Because no drugs can yet primarily influence cerebellar dysfunction in pathological conditions, cerebellar stimulation offers a promising therapeutic opportunity. Already in their classic experiments conducted in cats in the 1950s Moruzzi and co-workers recognised cerebellar sensitivity to polarising DC (Mollica et al. 1953a,b1953b,c1953c,d1953d; Gauthier et al. 1955; Pompeiano & Cotti, 1959a,b1959b). This sensitivity might well underlie the DC-induced changes in human cerebellar functions that we will review here. Because the cerebellum intervenes in several brain activities, cerebellar stimulation might help improve deficits arising from brain lesions. The cerebellum could therefore be a unique ‘window’ through which cerebellar tDCS could modulate functions residing elsewhere in the brain.
Another fascinating target for novel neuromodulatory approaches is the spinal cord. The spinal cord, besides the reflex centres, contains sensory, motor and associative propriospinal pathways. Increasing evidence implies that because the spinal cord stores some functions that the brain controls under normal conditions, these could be rescued if the cord is diseased or injured and detached from the rest of the CNS (Hubli et al. 2013). Hence spinal neuromodulation could enhance this rescue and facilitate functional recovery in patients. An innovative view comes also from envisaging the spinal cord as a ‘highway’ to the brain: stimulating or modulating the spinal cord might induce diffuse functional changes other than purely motor or sensory functions elsewhere in the CNS, such as for example synchronising the activity in different cortical areas and inducing neuroplasticity. That the spinal cord, like the cerebellum, is sensitive to polarising DC is again hardly surprising given that research conducted more than 50 years ago showed that even low-intensity DC, <0.5–1 mA, can modulate spinal cord function in the cat (Eccles et al. 1962). Although whether results obtained in animals can be translated one-to-one to humans both for cerebellar and spinal tDCS remains unclear owing to various factors (different stimulation intensity and duration, electrode positions and size, and comparative anatomy), these experiments provide a background for the methodologies reviewed here.
Transcranial cerebellar DC stimulation (cerebellar tDCS)
The possibility of non-invasively stimulating the human cerebellum is not new. Already in 1995, Ugawa first described a technique for delivering transcranial magnetic stimulation (TMS) to the cerebellum with single magnetic pulses. He found that a single TMS shock over the cerebellum inhibited the motor response evoked by TMS delivered a few milliseconds later over the contralateral motor cortex; Ugawa and co-workers (1995) called this phenomenon cerebello-brain inhibition (CBI). A few years later, experiments with repetitive transcranial magnetic stimulation (rTMS) over the cerebellum showed that cerebellar rTMS could induce after-effects on cerebellar functions (Theoret et al. 2001). Nonetheless cerebellar tDCS has some practical advantages over rTMS (discussed below).
In this section we will describe cerebellar tDCS designs used so far. For cerebellar DC stimulation (anodal or cathodal) an electrode (measuring about 5 cm × 5 cm) is placed over the cerebellum (the whole or on a part of it) and the reference electrode over the right arm (Ferrucci et al. 2008, 2012, 2013; Pope & Miall, 2012), or the buccinator muscle (Galea et al. 2009, 2012; Boehringer et al. 2012; Hamada et al. 2012; Jayaram et al. 2012; Sadnicka et al. 2013; Shah et al. 2013; Hardwick & Celnik, 2014; Herzfeld et al. 2014; Zuchowski et al. 2014), or contralateral supra-orbital area (Grimaldi & Manto, 2013; Grimaldi et al. 2014), or the motor cortex (Macher et al. 2014). Although no studies have systematically investigated whether placing the reference electrode on the face or arm when applying cerebellar tDCS influences its effects, electrode positioning is probably an important experimental variable. Both electrodes are connected to a stimulator delivering DC for 15–25 min, at an intensity ranging from 1 to 2 mA (Table1). Cerebellar DC stimulation occasionally elicits short-lasting tingling sensations when stimulation begins and ends and sometimes redness under the electrode.
Table 1.
Cerebellar tDCS studies (listed in chronological order)
| Authors | Year | Subjects | Patients | Polarity | Montage | Parameters | Technique | Effects |
|---|---|---|---|---|---|---|---|---|
| Motor functions | ||||||||
| Galea et al. | 2009 | 16 | — | Anodal\cathodal\sham | Active electrode over the right cerebellum Reference on the ipsilateral buccinator muscle |
2 mA; 25 min; AEA = 25 cm2 | Cerebello-brain inhibition | tDCS could modulate in a focal and polarity-specific manner the cerebellar control of the brain |
| Jayaram et al. | 2012 | 17 | — | Anodal\cathodal\sham | Active electrode over the right/left cerebellum Reference on the ipsilateral buccinator muscle |
2 mA; 15 min; AEA = 25 cm2 | Split-belt walking task | A-cerebellar tDCS applied during walking improved locomotor adaptation, whereas C-cerebellar tDCS worsened it |
| Galea et al. | 2012 | 72 | — | Anodal\sham | Active electrode over the right cerebellum Reference on the ipsilateral buccinator muscle |
2 mA; 15 min; AEA = 25 cm2 | Visuomotor adaptation | Cerebellar tDCS caused faster adaptation to the visuomotor transformation |
| Hamada et al. | 2012 | 18 | — | Anodal\cathodal\sham | Active electrode over the right cerebellum Reference on the ipsilateral buccinator muscle |
2 mA; 15 min; AEA = 25 cm2 | Paired associative stimulation | Plasticity induced by PAS25 was blocked by concurrent A- and C-cerebellar tDCS |
| Sadnicka et al. | 2013 | 12 | — | Anodal\cathodal\sham | Active electrode over the right cerebellum Reference on the ipsilateral buccinator muscle |
2 mA; 20 min; AEA = 25 cm2 | Motor surround inhibition | Neither A- nor C-cerebellar tDCS modulated the magnitude of mSI |
| Shah et al. | 2013 | 8 | — | Anodal\cathodal\sham | Active electrode over the non-dominant cerebellum Reference over the ipsilateral buccinator muscle |
1 mA; 15 min; AEA = 8 cm2 | Ankle visuomotor learning | A- and C-cerebellar tDCS improved target-tracking accuracy of the ankle |
| Grimaldi & Manto | 2013 | — | 9 | Anodal\cathodal\sham | Active electrode over the right cerebellum Reference on the contralateral supra-orbital area |
1 mA; 20 min; AEA = 20 cm2 | Stretch reflex responses in upper limb | A-cerebellar tDCS reduced the amplitudes of long-latency stretch reflexes |
| Dutta et al. | 2014 | 12 | — | Anodal\sham | Active electrode over the left cerebellum Reference on the forehead above the right supraorbital ridge |
1 mA; 15 min; AEA = 35 cm2 | Voluntary visually cued muscle activity in the tibialis anterior muscle | A-cerebellar tDCS worsened different aspects of visually cued voluntary contraction of a lower limb muscle |
| Grimaldi et al. | 2014 | 2 | Anodal\sham | Active electrode over the right cerebellum followed by contralateral motor cortex Reference on the contralateral supra-orbital area |
1 mA; 20 min; AEA = 20 cm2 | Evaluation of upper limb tremor and dysmetria | Cerebello-cerebral tDCS improved upper limb tremor and hypermetria | |
| Hardwick & Celnik | 2014 | 33 | — | Anodal\sham | Active electrode over the lateral cerebellum (dominant hand) Reference on the ipsilateral buccinator muscle |
2 mA; 15 min; AEA = 25 cm2 | Center-out reaching task | A-cerebellar tDCS enhanced motor adaptation in older individuals |
| Zuchowski et al. | 2014 | 30 | — | Anodal\sham\cathodal | Active electrode over the right cerebellum Reference on the right buccinator muscle |
2 mA; during the acquisition phase AEA = 35 cm2 | Eyeblink conditioning | A-cerebellar tDCS enhanced acquisition-conditioned eyeblink responses; C-cerebellar tDCS reduced it |
| Herzfeld et al. | 2014 | 50 | — | Anodal\sham\cathodal | Active electrode over the right cerebellum Reference on the right buccinator muscle |
2 mA; 25 min; AEA = 25 cm2 | Force field learning | A-cerebellar tDCS enhanced the error-dependent learning process; C-cerebellar tDCS impaired it |
| Non-motor functions | ||||||||
| Ferrucci et al. | 2008 | 13 | — | Anodal\cathodal\sham | Active electrode over the cerebellum Reference on the right shoulder |
2 mA; 15 min; AEA = 35 cm2 | Sternberg task | A- and C-cerebellar tDCS both impaired the practice-dependent improvement in the reaction times |
| Boehringer et al. | 2012 | 40 | — | Cathodal\sham | Active electrode over the right cerebellum Reference on the buccinator muscle |
2 mA; 25 min; AEA = 25 cm2 | Forward and backward digit spans | C-cerebellar tDCS reduced forward digit spans and blocked the practice-dependent increase in backward digit spans |
| Pope & Miall | 2012 | 22 | — | Anodal\cathodal\sham | Active electrode over the right cerebellum Reference on the right shoulder |
2 mA; 20 min; AEA = 25 cm2 | Paced auditory serial subtraction task and paced auditory serial addition task | Right C-cerebellar tDCS affected working memory and attention differentially depending on task difficulty |
| Ferrucci et al. | 2013 | 21 | — | Anodal\sham | Active electrode over the cerebellum Reference on the right shoulder |
2 mA; 20 min; AEA = 35 cm2 | Serial reaction time task | A-cerebellar tDCS improved procedural learning |
| Ferrucci et al. | 2013 | 21 | — | Anodal\cathodal\sham | Active electrode over the cerebellum Reference on the right shoulder |
2 mA; 20 min; AEA = 35 cm2 | Facial emotion recognition task | A- and C-cerebellar tDCS significantly enhanced the response to negative facial emotions |
| Macher et al. | 2014 | 16 | — | Anodal\cathodal\sham | Active electrode over the right cerebellum Reference on the right buccinator muscle |
2 mA; 25 min; AEA = 25 cm2 | Sternberg task | A-cerebellar tDCS caused an attenuated memory recognition capacity and haemodynamic signals |
| Chen et al. | 2014 | 10 | — | Anodal\cathodal\sham | Active electrode over the right cerebellum Reference on the right buccinator muscle |
2 mA; 25 min; AEA = 25 cm2 | Mismatch negativity | A-cerebellar tDCS increased peak amplitude of somatosensory MMN and C-cerebellar tDCS reduced it |
A-cerebellar tDCS, anodal cerebellar tDCS; AEA, active electrode area; C-cerebellar tDCS, cathodal cerebellar tDCS; mA, milliampere; MMN, mismatch negativity; mSI, motor surround inhibition; PAS25, paired associative stimulation with 25 ms interval.
Motor control and neuroplasticity
The physiological rationale for using cerebellar tDCS to influence motor control depends, in brief, on the observation that Purkinje cell activation inhibits the deep cerebellar nucleus neurons ensuring that they receive the correct amount of inhibition to produce the appropriate motor output and suppress unwanted activity. After research in our laboratory described the technique (Ferrucci et al. 2008), the well-known cerebellar involvement in motor control prompted Galea and co-workers (2009) to study how cerebellar tDCS affects CBI in healthy individuals. CBI is the TMS-elicited motor-evoked potential (MEP) inhibition produced by a single TMS shock over the cerebellum (Ugawa et al. 1995). The main experiment disclosed that cathodal cerebellar tDCS decreased CBI, whereas anodal cerebellar tDCS increased it, and sham stimulation induced no changes. These effects specifically involved the cerebello-cortical connections and no changes were found in other M1 or brainstem excitability variables. These results suggest that cerebellar tDCS can modulate cerebellar control over the motor cortex. Despite the small study sample (individual experiments were conducted in 6–8 subjects, without balancing groups for sex) and the lack of stimulation on a control area (left cerebellum or M1), this is an important study because it reports the first neurophysiological evidence showing the effects of cerebellar tDCS on cerebellar inhibitory output to the motor cortex in healthy subjects.
The functional cerebellum–cerebrum interaction is determinant to plasticity in the somatosensory and motor cortex (Rahmati et al. 2014) and probably also to plasticity in other cortical areas. Cerebellum-dependent brain plasticity is probably also important for complex cognitive tasks with a major timing component. Given that the cerebellum intervenes in neuroplasticity, cerebellar tDCS could induce some effects through neuroplasticity changes and long-term potentiation (LTP). In addition to rTMS and tDCS, another method used to induce neuroplasticity is paired associative stimulation (PAS); this technique entails delivering about 200 repetitive electrical stimuli over the median nerve at the wrist paired with TMS pulses over the spot of the median nerve-innervated small hand muscles at a rate of about 0.2 Hz. The interstimulus interval between the peripheral nerve shock and TMS ranges between 21.5 and 25 ms. After the PAS protocol ends TMS-elicited MEP increases in size in the median nerve-innervated muscles (Stefan et al. 2000). The increased motor cortex excitability is thought to arise from LTP-like neuroplasticity changes (Classen et al. 2004). Assessing whether the cerebellum can influence neuroplasticity changes in the motor cortex, Hamada and co-workers (2012) found that concurrent anodal or cathodal cerebellar tDCS blocked PAS-induced plasticity. They showed that cerebellar tDCS blocks the PAS-induced LTP-like effects specifically only when the interval elapsing between peripheral and motor cortical stimuli is 25 ms, but not when it is 21.5 ms. They therefore speculated that separate mechanisms mediate PAS-induced changes at these two interstimulus intervals and that PAS25-induced changes depend specifically upon the cerebellum. Although delivering stimuli to a cutaneous control area would have ruled out possible subthreshold influences from sensory input on PAS, the study is overall well designed. Besides the conclusion about the different physiological mechanisms underlying PAS, the article bears the important implication that cerebellar stimulation can – at least in part – influence normal LTP-like neuroplasticity in the motor cortex. This effect could be useful in pathological conditions such as dystonia, thought to involve abnormal plasticity.
Surround inhibition (SI) is a neurophysiological mechanism by which the CNS focuses neuronal activation (Beck & Hallett, 2011). SI can be studied in the human motor system by observing thenar muscle TMS-elicited MEP inhibition during a voluntary hypothenar muscle contraction (Sohn & Hallett, 2004). Motor SI (mSI) seems to be based on a GABA-mediated inhibitory mechanism that permits skilled and finger movements. The inhibition appears before the movement execution and reaches its maximum at movement onset (Beck & Hallett, 2010). The cerebellar role in SI is unclear. To assess this issue Sadnicka and co-workers (2013) used cerebellar tDCS to examine the cerebellar role in mSI, but found no evidence that the cerebellar tDCS influences mSI. Although the interval elapsing after cerebellar tDCS ends and the mSI protocol begins is unclear, and might have been important for interpreting the results, these findings suggest that the cerebellum has no role in the SI mechanism. This negative study suggests that the effects of cerebellar stimulation are specific only for certain physiological mechanisms and not for others.
The cerebellum plays a crucial role in locomotion probably by generating patterned limb movements, dynamically controlling posture and balance and adjusting the locomotor output by error feedback learning (Morton & Bastian, 2007; Manto & Haines, 2012). According to this rationale, using cerebellar DC to influence these two functions seemed intriguing. To investigate this possibility, Jayaram and co-workers (2012) used a cerebellum-dependent split-belt walking task to investigate how cerebellar tDCS influences locomotor learning that is controlled by the cerebellum (Morton & Bastian, 2007). In this procedure one leg is set to move faster than the other so that the fast and slow leg steps are asymmetric. Over time, subjects learn to predict and account for the perturbation (Reisman et al. 2005). The investigators studied the laterality in adaptive changes by separately delivering cerebellar tDCS (anodal, cathodal and sham) over the cerebellar hemisphere ipsilateral to fast and slow lower limbs during locomotor adaptation. Anodal cerebellar tDCS applied during walking improved locomotor adaptation, whereas cathodal cerebellar tDCS worsened it but did so only ipsilaterally to the fast leg. Hence the effect was side specific. Even though they provide no information on how long the effect lasts, the results demonstrated that cerebellar tDCS modulates locomotor learning in healthy subjects. This study is important because it provides the first behavioural evidence that cerebellar DC stimulation influences a motor learning task in normal subjects and it opens the avenue to studies in patients with gait abnormalities.
Classic blink reflex conditioning is a simple motor learning form requiring cerebellar integrity. In their study in healthy subjects, Zuchowski et al. (2014) tested the effects of cerebellar tDCS on conditioned eye-blink response acquisition by using a standard delay conditioning protocol. After anodal tDCS, conditioning was significantly enhanced and after cathodal tDCS was significantly reduced compared with sham stimulation. The authors therefore concluded that eye-blink response conditioning is modulated by cerebellar tDCS in a polarity dependent manner. This is an important observation demonstrating that simple motor learning forms depend closely on the human cerebellum and can be bidirectionally (increased or decreased) modulated by cerebellar tDCS.
Another brain function that comes under cerebellar control is visuomotor coordination (Brown et al. 1993). The visuomotor cerebellum comprises the floccular lobe, the paraflocculus, the oculomotor vermis, the uvula-nodulus and the ansiform lobule (Voogd et al. 2012). Prompted by this scientific rationale, in a study designed to address the respective cerebellar and M1 roles during adaptive learning, Galea and co-workers (2009) applied anodal cerebellar and motor cortical tDCS during a visuomotor adaptation Task. The results showed that the error reduction during adaptation was larger after cerebellar tDCS than after M1 tDCS. In contrast, after tDCS over M1, error adaptation was unchanged but the newly learnt visuomotor transformation (the adaptation to an unexpectedly imposed 30 deg counterclockwise screen–cursor displacement) markedly increased. These findings confirm that the cerebellum and M1 have distinct functional roles in acquiring and retaining information during adaptive motor learning. Although assessing the effects of paired cerebellar and motor cortical tDCS in the same subject at the same time would have provided further interesting information, the study clarifies the different roles of the motor cortex and cerebellum in motor adaptation. More recently, Shah and co-workers (2013) assessed the effects induced by cerebellar tDCS vs. motor cortical tDCS on short-term ankle visuomotor learning. Subjects practised a skilled visually controlled ankle motor-tracking task while receiving anodal, cathodal or sham tDCS over the cerebellum, or over the M1. Anodal and cathodal cerebellar tDCS and anodal (but not cathodal) M1 stimulation improved ankle target-tracking accuracy (Fig.1). The lack of polarity specificity reported in this study is not surprising given that anodal and cathodal tDCS can reportedly have polarity-independent effects (Ferrucci et al. 2008). As we explain in previous studies (Ferrucci et al. 2008), a possible reason why cerebellar tDCS lacks polarity specificity comes from general physiological mechanisms (Lorente De Nò, 1947): the loss of function in any excitable tissue can be obtained both with depolarisation and hyperpolarisation. For instance, classic neurophysiological experiments demonstrated that axonal conduction can be blocked, even for several hours, by depolarisation (‘depolarising’ block) and also by hyperpolarisation (‘hyperpolarising’ or ‘anodal’ block), both leading to the same decreased excitability and, ultimately, to a loss of function (Lorente De Nò, 1947). Cerebellar tDCS of both polarities could also interfere with long-term depression (LTD) by altering the membrane potential fine-tuning needed for LTD in the cerebellar cortex. Whatever the mechanism, the study by Shah and co-workers (2013) further supports the conclusion that cerebellar tDCS can improve visuomotor coordination for lower limb movements. Combining cerebellar and motor cortical stimulation might improve visuomotor learning even more effectively.
Figure 1. Effects of cerebellar transcranial direct current stimulation (cerebellar tDCS) on human lower-limb tracking accuracy.
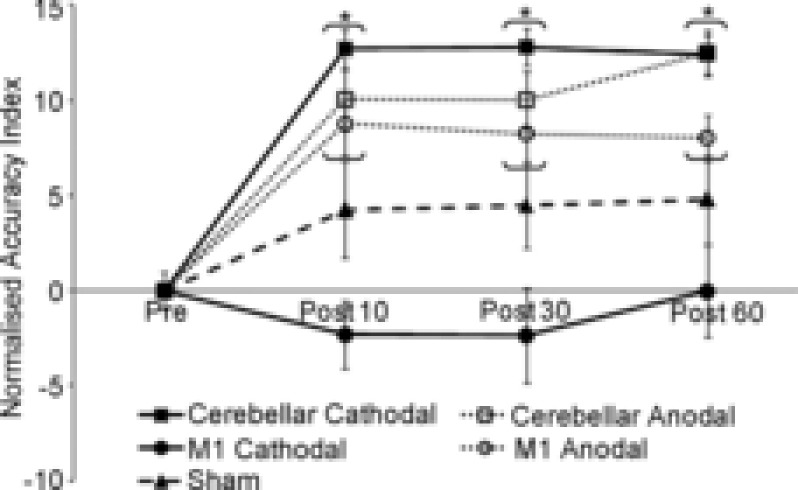
The y-axis depicts the accuracy index normalised to baseline during practice, and the x-axis shows the different time-points: baseline, 10 min after practice (Post 10), 30 min after practice (Post 30) and 60 min after practice (Post 60). The normalised accuracy index improved more after cathodal cerebellar tDCS, anodal cerebellar tDCS and anodal M1 than after sham and cathodal M1 stimulations at all post time-points. Reproduced from Shah et al. (2013), with permission.
Using a relatively similar experimental approach, Dutta and co-workers (2014) assessed whether cerebellar tDCS influenced different types of voluntary visually cued muscle activity in the tibialis anterior muscle. They found that anodal cerebellar tDCS increased the muscle activation latency in ballistic movements and reduced muscle activation learning related to visually tracking sinusoidal movements (0.01 Hz) in the foot. Hence, in essence they found that anodal cerebellar DC stimulation worsened several variables related to voluntary visually cued lower-limb muscle contraction in healthy subjects. The findings reported by Dutta and co-workers (2014) contradict those reported by Shah and co-workers (2013) who used a similar experimental setting for voluntary visually cued muscle activation in the lower limb but at a faster frequency (0.2–0.4 Hz). Also, Shah and co-workers (2013) put the reference electrode over the ipsilateral buccinator muscle, whereas Dutta and co-workers (2014) put it on the contralateral forehead. Despite these methodological differences, overall, the reported results suggest that cerebellar DC stimulation differentially influences visuomotor integration possibly in relation to the frequency of sinusoidal movements.
Current evidence points to the cerebellum as one of the structures that plays a critical role in motor memory acquisition (Criscimagna-Hemminger et al. 2010; Donchin et al. 2012). To investigate how the cerebellum and M1 contribute to human motor memory, Herzfeld and co-workers (2014) compared the effects of cerebellar and M1 tDCS in 50 healthy subjects in a protocol to study motor memory (force field-reaching task). They found that cerebellar anodal stimulation enhanced error-dependent learning, whereas cathodal stimulation impaired it. They also showed that cathodal cerebellar tDCS during acquisition resulted in impaired retention as measured over 24 h. These findings confirmed the critical cerebellar role in motor memory formation and storage.
A key to maintaining health in an older population consists in developing novel strategies against age-related deterioration in motor functions. In healthy older persons, age-related motor deterioration can depend on abnormal motor adaptation, a form of motor learning. Seeking a strategy for improving motor deterioration, Hardwick & Celnik (2014) assessed whether anodal cerebellar tDCS enhances adaptation in older subjects. Subjects had to make a ‘centre-out’ reaching task, adapting to the sudden introduction of a visual cursor rotation. Participants sat in a robotic exoskeleton device with a monitor and vision of the hand and arm was occluded. They moved their arm to shoot a cursor from a start position through a circular target in 1 of 8 potential positions. Older subjects receiving sham tDCS were slower to adapt than younger subjects. But when older participants received anodal cerebellar tDCS they adapted faster, similarly to younger subjects. These findings led Hardwick & Celnik to conclude that anodal cerebellar tDCS improves motor adaptation in older individuals and suggest cerebellar tDCS as a possible novel approach against age-related motor deficits.
In conclusion, overall available data show that cerebellar tDCS modulates several neurophysiological and behavioural motor variables in healthy subjects.
Non-motor functions
How and whether the cerebellum intervenes in non-motor functions remains controversial (Koziol & Lutz, 2013) but the available evidence suggests that cerebellar tDCS is emerging as a valuable tool in cognitive and psychophysiological research. The cerebellum is among the structures activated during a verbal working memory task (Kirschen et al. 2005) and cerebellar damage impairs working memory (Ravizza et al. 2006). Prompted by this rationale, in the study from our laboratory that first described cerebellar tDCS (Ferrucci et al. 2008), we observed that applying DC over the cerebellum influenced proficiency in the Sternberg task (a cognitive task assessing working memory). In the Sternberg task the subject has to remember whether a given number belongs to a sequence of numbers that previously appeared on a computer screen. We found that anodal cerebellar tDCS and cathodal cerebellar tDCS both impaired the practice-dependent improvement whereas tDCS over the dorsolateral prefrontal cortex left it unchanged. This finding showed that the cerebellar tDCS-induced changes are structure specific. Finally, cerebellar tDCS left visual-evoked potentials unchanged, therefore ruling out visual cortex involvement. Substantially confirming our earlier findings (Ferrucci et al. 2008) but using a slightly different methodology, Boehringer and co-workers (2012) showed that cathodal cerebellar tDCS blocked the practice-dependent increase in verbal working memory assessed with the digit span test, a task that requires the subject to remember progressively longer digit sequences read by the experimenter. They also reported that cerebellar tDCS induced no effects on word reading, finger tapping and a visually cued sensorimotor task. Hence, the available data consistently demonstrate that also when used with different experimental approaches, cerebellar tDCS alters the proficiency-related improvement in a working memory task, thus arguing in favour of a crucial cerebellar role in this physiological function and suggesting that cerebellar tDCS can be a valuable tool for manipulating working memory. Pursuing research on the cerebellar role in phonological storage and working memory, in a randomised, crossover and sham-controlled design, Macher and co-workers (2014) combined right cerebellar tDCS with functional magnetic resonance imaging (fMRI) to investigate how the human cerebellum contributes to encoding, maintenance and retrieval of verbal information using a modified Sternberg task. After anodal, but not cathodal tDCS, the authors reported an impaired digit recognition capacity with an attenuated haemodynamic signal from the right cerebellar lobule VIIb, together with weakened functional connectivity between this area and the posterior parietal cortex, during the late encoding phase. These findings suggest that the right cerebellar lobule VIIb interacts with the posterior parietal cortex specifically during the late verbal encoding stages, when verbal information enters phonological storage. Hence they confirm previous results from our laboratory (Ferrucci et al. 2008) and from Boehringer and co-workers (2012), expanding current knowledge to changes induced by cerebellar tDCS on phonological storage. The paper is important because it is the first to report combined findings from cerebellar tDCS and fMRI.
Cognitive tasks known to activate the cerebellum include those requiring attention (Strick et al. 2009). Using cerebellar tDCS to modulate attention seemed an attractive possibility. Hence, to find out more about cerebellar control over working memory and attention, Pope and Miall (2012) used two cognitive tasks involving arithmetic skills of comparable motor difficulty but with different levels of cognitive complexity/load before and after cerebellar tDCS. They found that whereas the difficult task improved only after cathodal cerebellar DC stimulation, the easier task improved after anodal, cathodal or sham cerebellar tDCS. A further experiment in this paper assessed the effect of cerebellar DC stimulation on the ability to generate verbs, a language skill that some researchers attributed to the cerebellum (Fiez et al. 1992). Cathodal cerebellar tDCS selectively facilitated the verb-generation task. Overall this paper has two important implications. First, it shows that cathodal cerebellar tDCS selectively influences cognitive tasks with a high cognitive load related to memory and attention. From a practical point of view the improvement in the arithmetic test might be useful in considering new treatment strategies for dyscalculia. A second important point is that the work reports for the first time that cerebellar tDCS influences language. Although cerebral tDCS is widely used for studies on language in healthy subjects and patients with aphasia (Monti et al. 2013), the observation arising from the study conducted by Pope and Miall suggests testing aphasic patients with tDCS concomitantly applied to cerebral language areas and cerebellum.
Studies in healthy humans and in cerebellar patients underline the important role of the cerebellum in attention and in environmental exploration (Rondi-Reig & Burguiere, 2005; Baillieux et al. 2008; Buckner, 2013; Reeber et al. 2013). The cerebellum has also been implicated in recognising salient changes in the environment by assessing mismatch negativity (MMN) in a group of cerebellar patients (Restuccia et al. 2007). MMN is an event-related potential component yielded by subtracting the EEG recording related to standard stimuli from the EEG recorded during odd stimuli. When Chen and co-workers (2014) assessed the MMN to auditory and sensory stimuli in 10 healthy subjects before and after cerebellar tDCS, they found that whereas anodal polarity increased, cathodal cerebellar tDCS decreased the amplitude of MMN evoked by somatosensory, but not auditory, stimuli. Although spontaneous, non-event-related EEG signal analysis and, again, including stimulation to a control area, would have provided a clearer understanding, the study indicates that by using cerebellar tDCS we can bidirectionally modulate (i.e. increasing or decreasing) the neurophysiological correlate of attention toward salient changes in the environment. This observation in healthy subjects might have implications for cognitive neurorehabilitation and for treating patients with attention deficit disorder.
Another important non-motor function controlled by the cerebellum is procedural learning (Koziol & Lutz, 2013). Functional neuroimaging studies demonstrated cerebellar activation during procedural learning tasks (Molinari et al. 1997; Habas, 2010) and patients with cerebellar disorders often have impaired procedural learning performances. Under this rationale, in a later study we investigated whether cerebellar tDCS influences procedural learning as measured by the serial reaction time task (SRTT) (Ferrucci et al. 2013). The SRTT is more than a simple motor learning task and has both motor and perceptual learning components (Robertson, 2007). During the SRTT the subject is required to mesh a given sequence of buttons on a keyboard after a target appears on a computer screen. Our main finding was that anodal cerebellar tDCS improved subjects’ procedural learning performance. Although this study, suffers from the lack of follow-up and stimulation to a control area, it shows that the technique improves procedural learning. This conclusion prompts further studies in patients with learning disorders.
Several experimental and some clinical observations argue that the cerebellum intervenes in affective control and in forming the associations between sensory stimuli and their emotional value (Strata et al. 2011). For instance, in humans peri-vermian areas are activated for memory of personal emotional episodes and vermian damage influences the retention of fear memory. Hence, continuing research into non-motor cerebellar functions, we assessed whether cerebellar tDCS influences facial emotion recognition (Ferrucci et al. 2012). Anodal and cathodal cerebellar tDCS significantly enhanced only the response to negative facial emotions. Cerebellar tDCS therefore influences the way healthy subjects recognise specific facial expressions thus showing that the cerebellum plays a direct role in recognising negative emotions. These findings suggest that cerebellar tDCS could be useful in patients with psychiatric disorders involving decreased cerebellar activation (Konarski et al. 2005) and abnormal emotional recognition (Baillieux et al. 2008).
Studies in patients
Translating these basic research findings into clinical practice, Grimaldi and Manto (2013) tested anodal cerebellar tDCS in ataxic patients. They studied upper limb stretch reflexes (short-latency stretch reflexes (SLSRs); long-latency stretch reflexes (LLSRs)), a coordination task, and computerised posturography. All these tests are abnormal in patients with cerebellar disorders. Cerebellar tDCS left SLSR amplitudes, coordination task and postural control unchanged, but significantly reduced LLSR amplitudes (Fig.2). The lack of effect upon coordination and posture suggests that cerebellar tDCS has no influence on the cerebello-cerebral networks sub-serving these functions. In a further study, the same authors (Grimaldi et al. 2014) assessed the effects of a novel protocol using transcranial DC stimulation applied in the same session to the motor cortex (cathodal) and cerebellum (anodal) (cerebello-cerebral DC stimulation) on tremor, EMG activity and dysmetria in two patients with spinocerebellar ataxia type 2. The rationale for using this paired cerebro-cerebellar stimulation arises from the observation that cathodal motor cortical DC stimulation improves some features related to a cerebellar deficit (Pozzi et al. 2014) and hence combined stimulation applied to the brain and cerebellum should double the beneficial effects. Yet, Grimaldi and co-workers (2014) found that transcranial cerebello-cerebral DC stimulation reduced tremor, hypermetria and the latency of the antagonist EMG activity. Although the study fails to clarify the individual roles of stimulation over the cerebellum or the motor cortex in inducing the reported clinical improvement, and the results need to be replicated in larger controlled studies, the observation suggests a new therapeutic option for patients with cerebellar tremor. Future clinical research work should systematically assess the patients’ features predicting an optimal response and investigate how to induce a long-lasting clinical effect to durably improve the patient's quality of life.
Figure 2. Effect of cerebellar transcranial direct current stimulation (cerebellar tDCS) on stretch reflexes in an ataxic subject.
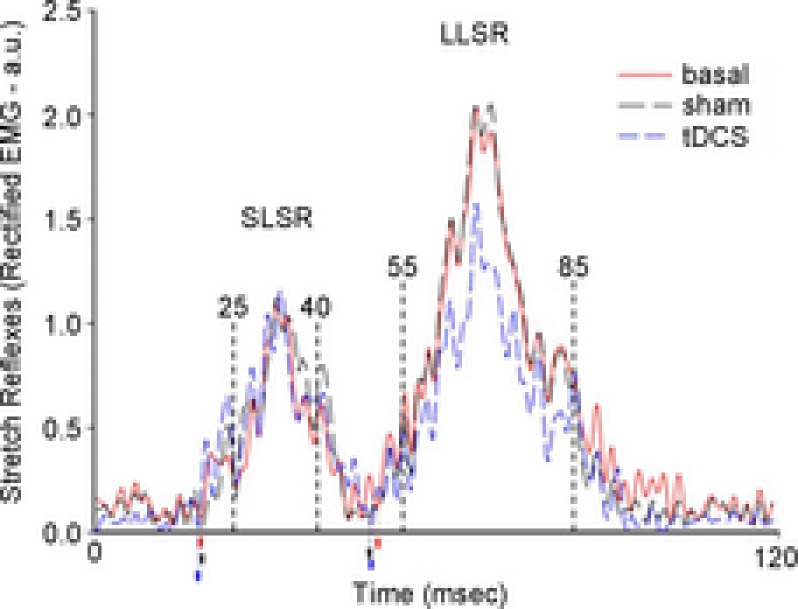
The figure shows rectified and averaged EMG signal from the flexor carpi radialis muscle in a cerebellar patient. Reflexes were elicited by passive wrist extension elicited by a torque motor. Anodal cerebellar tDCS left short-latency stretch reflex (SLSR) amplitudes unchanged but reduced amplitudes for long-latency stretch reflexes (LLSR). Reproduced from Grimaldi & Manto (2013), with permission.
Cerebellar tDCS modelling studies
The effects of transcranial DC stimulation depend on the electric field and current density field distributions produced in the nervous tissue. Their knowledge is therefore important to predict the location and extent of the stimulated region as well as the stimulation intensity in a specific region of the CNS. At present, these distributions are best-estimated by computational models (Peterchev et al. 2012). For transcranial electrical stimulation, the quasi-static regime can be applied, and hence the electric field E can be given by the negative gradient of the electric potential ϕ, whereas the current density J is obtained from the electric field E by means of the relation 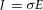 , where σ is the electric conductivity in the tissue. The distribution of the potential ϕ inside the conductive medium (i.e. the head) is obtained by solving the current continuity equation, subjected to the appropriate boundary conditions. In simple cases, the equation can usually be solved analytically but when the volume conductor is geometrically complex numerical methods are needed. Based on this theory, the literature has proposed different computational models for transcranial electrical stimulation in which the head representation ranged in complexity from concentric sphere models to more detailed, simplified geometric representations up to high-resolution, MRI-derived models incorporating complex tissue geometries. Because the tissue properties are poorly known, researchers have used different conductivity values mainly derived from static resistivity measures or extrapolated from 10 Hz data, and have sometimes even included tissue conductivity anisotropy (Bikson et al. 2012; Peterchev et al. 2012; Ruffini et al. 2013). Close to the stimulation site, according to data calculated using realistic head models, the maximum electric field magnitude in the grey matter was about 0.2–1.5 V m−1 for a 1 mA current applied through large electrodes (25–35 cm2) (Ruffini et al. 2013). Overall, modelling studies were useful in interpreting and optimising stimulation outcomes, but an important question is how to validate the electric field calculations experimentally, because empirical data on the current density in the brain during tDCS are largely missing. In early research, Dymond and co-workers (1975) reported electric field values of 0.6–1.6 V m−1 for a current intensity of 1 mA. In their experiment, the stimulation electrodes were placed bilaterally over the frontal pole and the mastoids and the recording electrodes were implanted near the hippocampus. Findings from these models therefore need interpreting with caution. This caveat relates especially to assumptions on tissue conductivities or the accuracy and precision of the segmentation of different tissues from high-resolution anatomical data. Indeed, the number and precision of the tissue obtained may influence predicted current flow. In this context, only two modelling studies have been specifically designed for cerebellar tDCS. Mimicking the electrode montage used by Ferrucci and co-workers (Ferrucci et al. 2008, 2012), a modelling study (Parazzini et al. 2014b) using MRI-derived models for three subjects predicted that using one conventional sponge tDCS electrode placed over the cerebellum and an extra-cephalic electrode over the right arm concentrate the current flow mainly to the cerebellum, with a spread to other structures. This result gave support to previous experimental observations that cerebellar tDCS failed to influence visually evoked potentials (Ferrucci et al. 2008) therefore excluding stimulation to the visual cortex. Additionally, the study showed that individual anatomical variability somehow influences electrical field distributions: the electrical field spreads more toward the brainstem tegmentum in the child model than in adult models (Fig.3). Because experiments with cerebellar tDCS are still lacking in children, nor do we know whether the possible brainstem spread is functionally relevant, the use in paediatric age must be conservatively considered potentially dangerous.
, where σ is the electric conductivity in the tissue. The distribution of the potential ϕ inside the conductive medium (i.e. the head) is obtained by solving the current continuity equation, subjected to the appropriate boundary conditions. In simple cases, the equation can usually be solved analytically but when the volume conductor is geometrically complex numerical methods are needed. Based on this theory, the literature has proposed different computational models for transcranial electrical stimulation in which the head representation ranged in complexity from concentric sphere models to more detailed, simplified geometric representations up to high-resolution, MRI-derived models incorporating complex tissue geometries. Because the tissue properties are poorly known, researchers have used different conductivity values mainly derived from static resistivity measures or extrapolated from 10 Hz data, and have sometimes even included tissue conductivity anisotropy (Bikson et al. 2012; Peterchev et al. 2012; Ruffini et al. 2013). Close to the stimulation site, according to data calculated using realistic head models, the maximum electric field magnitude in the grey matter was about 0.2–1.5 V m−1 for a 1 mA current applied through large electrodes (25–35 cm2) (Ruffini et al. 2013). Overall, modelling studies were useful in interpreting and optimising stimulation outcomes, but an important question is how to validate the electric field calculations experimentally, because empirical data on the current density in the brain during tDCS are largely missing. In early research, Dymond and co-workers (1975) reported electric field values of 0.6–1.6 V m−1 for a current intensity of 1 mA. In their experiment, the stimulation electrodes were placed bilaterally over the frontal pole and the mastoids and the recording electrodes were implanted near the hippocampus. Findings from these models therefore need interpreting with caution. This caveat relates especially to assumptions on tissue conductivities or the accuracy and precision of the segmentation of different tissues from high-resolution anatomical data. Indeed, the number and precision of the tissue obtained may influence predicted current flow. In this context, only two modelling studies have been specifically designed for cerebellar tDCS. Mimicking the electrode montage used by Ferrucci and co-workers (Ferrucci et al. 2008, 2012), a modelling study (Parazzini et al. 2014b) using MRI-derived models for three subjects predicted that using one conventional sponge tDCS electrode placed over the cerebellum and an extra-cephalic electrode over the right arm concentrate the current flow mainly to the cerebellum, with a spread to other structures. This result gave support to previous experimental observations that cerebellar tDCS failed to influence visually evoked potentials (Ferrucci et al. 2008) therefore excluding stimulation to the visual cortex. Additionally, the study showed that individual anatomical variability somehow influences electrical field distributions: the electrical field spreads more toward the brainstem tegmentum in the child model than in adult models (Fig.3). Because experiments with cerebellar tDCS are still lacking in children, nor do we know whether the possible brainstem spread is functionally relevant, the use in paediatric age must be conservatively considered potentially dangerous.
Figure 3. Modelling study on the current density generated by cerebellar transcranial direct current stimulation (cerebellar tDCS) and by transcutaneous spinal cord direct current stimulation (spinal tDCS) in humans.
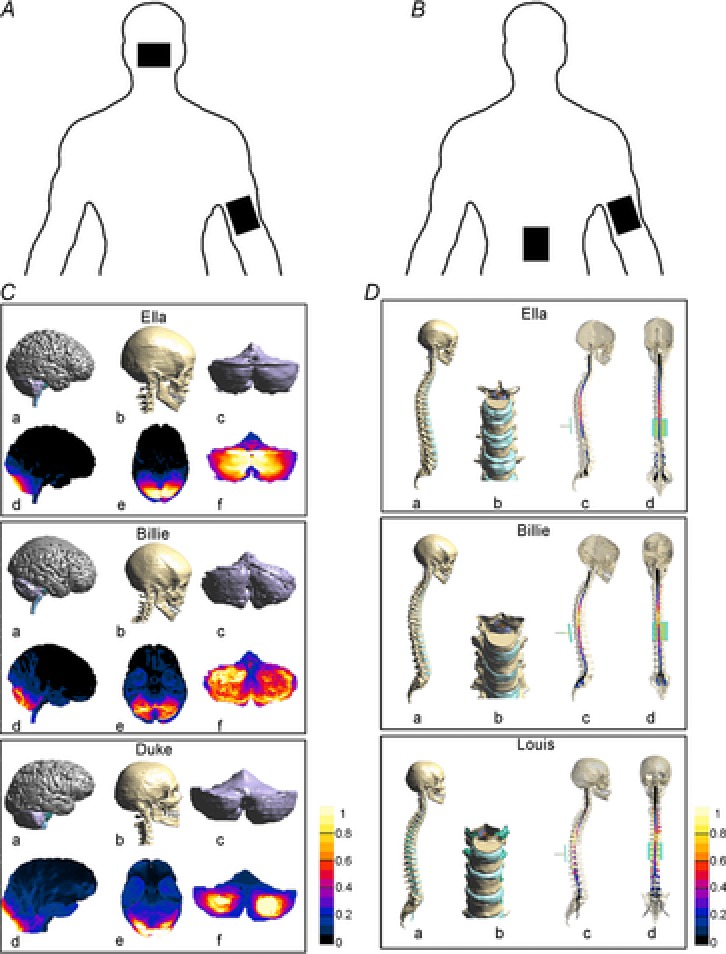
Top panels show a schematic drawing illustrating (viewed from the back) the electrode positions for cerebellar tDCS (A) and spinal tDCS (B). C, Examples of segmented tissues in three human realistic Virtual Family models (Ella, Billie and Duke) undergoing cerebellar tDCS: a, lateral view of cerebellum, pons, midbrain, medulla; b, lateral view of the skull; c, back view of the cerebellum; d and e, lateral and inferior views of normalized current density amplitude field distributions over cortical, subcortical and brainstem regions; f, back view of normalized current density amplitude field distributions over the cerebellum. The values are normalized with respect to the maximum of the current density amplitude in the cerebellum. Note that cerebellar tDCS generates the highest current density in the posterior cerebellum with a slight spread to other structures. Reproduced from Parazzini et al. (2014b2014b), with permission. D, Examples of segmented tissues in three human realistic Virtual Family models undergoing spinal tDCS: a, lateral view of skull, and spine; b, magnified clipped frontal view of the spine around the tenth thoracic vertebra; lateral (c) and frontal (d) views of the normalized current density amplitude field distributions over spinal cord. The values are normalized with respect to the maximum of the current density amplitude in the spinal cord. Note that with the reference electrode over the right arm spinal tDCS acts mainly at thoracic level with minimal current spread. Reproduced from Parazzini et al. (2014a2014a) with permission.
In line with the conclusion by Parazzini and co-workers (2014b2014b), a preliminary modelling study with tDCS electrodes (1 cm in diameter) (Rahman et al. 2014) confirmed that the applied current reaches the cerebellum. Because the electrode montages used by Rahman have never been experimentally tested in humans, their results remains questionable.
Other important variables for cerebellar tDCS that need to be systematically tested in future studies include cerebrospinal fluid current shunting, electrical field funnelling by fissures or holes in the skull, and different conductivities in grey and white matter. Collectively, however, computational models for cerebellar tDCS result in an electric field in the cerebellum with a maximum ranging between 0.2 and 3.5 V m−1 for a 2 mA applied current density. These values, as well as being in the same range as the weak electric field found for cerebral DC stimulation, are of the same order of magnitude as previous experimental results. In experiments using isolated turtle cerebellar cells, Chan and co-workers found that the threshold for modulating both Purkinje and stellate cells was around 15–20 V m−1 (Chan & Nicholson, 1986). In a later study, Chan and co-workers (1988) predicted that Purkinje cells will polarise by 0.2 mV per 1 V m−1 applied electric field. This polarisation, albeit small, can affect the firing rate for large neuronal populations (Frohlich & McCormick, 2010). In mammalian brain, Jefferys (1981) reported a threshold of 5–10 V m−1 for granule cells in the hippocampal slice, whereas in the crayfish stretch receptor Terzuolo and co-workers (1956) reported a 1 V m−1 threshold for modulating active neurons. Several years later, Ghai and co-workers (2000) reported that electric fields as low as 1 V m−1 could modify neuronal activity in hippocampal slices, and Francis and co-workers (2003) provided the experimental evidence that neuronal networks are sensitive to electric fields lower than 1 V m−1. Hence, overall, available experimental data indicate a threshold for neuronal interactions with the electric field between 1 and 20 V m−1 and therefore in the range of those estimated by cerebellar tDCS models.
Cerebellar tDCS mechanisms of action and safety
Because studies on cerebellar tDCS began only recently few data are available about its mechanisms of action. tDCS basically acts at two time-points: first, when the electric field is applied, and second after DC offset.
Although some general principles underlying changes induced by the electric field on the CNS may apply also to the cerebellum, given its intrinsic passive electrical properties this brain area could respond differently to electricity. For instance, permittivity is higher in mouse cerebellum than in the brain or brainstem and conductivity is higher at frequencies below 1000 MHz (Nightingale et al. 1983). Studies on conductivity in the cat cerebellar cortex show also that the various cortical layers differ in isotropy, the granular layer being more isotropic than other layers (Yedlin et al. 1974). All these differences, particularly those related to conductivity, can be important given that tissue dielectric properties play a key role in electric field computation and the current density distributions during transcranial stimulation, as shown by the foregoing equations (see previous section ‘Cerebellar tDCS modelling studies’).
Once the electric field reaches the cerebellum it can induce functional changes. Current experimental knowledge provides no precise information on where cerebellar tDCS-induced changes take place (cerebellar cortex, deep nuclei, white matter). Nor does it specify whether they involve one cerebellar area alone or the whole cerebellum. Cerebellar tDCS could interfere with membrane polarisation in Purkinje cells and in other neurons, fibres (mossy fibres and climbing fibres) and glial cells. DC stimulation applied to the cerebellar cortex in the decerebrated cat influences Purkinje and granular cell activity in a polarity-specific manner: whereas anodal DC (0.1–1 mA) flowing in the dendrite–axonal direction increased tonic neuronal activity, cathodal DC decreased it (Brookhart et al. 1952). Similar more recently reported findings showed that when an electric field is applied across the turtle cerebellum it elicits differential effects in various cerebellar neurones according to its relative orientation (Chan & Nicholson, 1986). Current flowing from the cortical surface to the fourth ventricle predominantly excites Purkinje cells and some stellate cells.
Two main physiological issues about cerebellar tDCS require further research. The first is whether the induced effects are polarity dependent. Whereas some experiments found that anodal and cathodal cerebellar tDCS elicit the same effects (Ferrucci et al. 2008, 2012; Hamada et al. 2012) on a given task or function, others reported polarity-specific changes (Galea et al. 2009; Jayaram et al. 2012; Pope & Miall, 2012; Grimaldi & Manto, 2013). Considering that different cerebellar areas are implicated in various functions, and that neurons in all these areas are variously oriented in relation to the applied electric field, the lack of polarity specificity is hardly surprising because it again underlines that the induced changes in excitability depend on the electric field's direction. And equally important, classic neurophysiological experiments on axonal excitability have shown that both polarities can block action potential propagation (Lorente De Nò, 1947). Overall, current knowledge therefore shows that the human cerebellum responds to cerebellar tDCS in a complex manner, possibly depending on the function studied, the task used, the electric field geometry and orientation, and its strength or duration. Another important point to understand about how or where cerebellar tDCS acts in the brain is that it can induce lateralised effects. Whereas in some experiments from our laboratory we used a stimulating electrode covering the whole of the cerebellum (Ferrucci et al. 2008, 2012, 2013), others placed electrodes to stimulate the hemi-cerebellum and reported highly side-specific cerebellar tDCS-induced changes (Galea et al. 2009; Jayaram et al. 2012).
We also need to know what happens in the cerebellum after current flow ceases. Because transmembrane polarisation lasting only a few minutes induces prolonged spiking activity in Golgi inhibitory cerebellar neurons (Hull et al. 2013), Golgi cell activity could partly explain cerebellar tDCS after-effects. The mechanisms of action underlying cerebellar DC stimulation could also involve ionic gradients in the extracellular space, specific cellular inactivation or activation (including protein synthesis, gene expression and channel-pump inactivation) common to several cell types (including glia, and smooth muscle cells in cerebellar vessels). Other mechanisms could also involve receptors and neurotransmitters. Whereas electrical phenomena probably initiate cerebellar tDCS-induced changes, other mechanisms related partly to non-electrical phenomena could intervene to maintain them. For instance, cerebellar neurotransmitters such as myo-inositol (Goto & Mikoshiba, 2011), GABA and glutamate (Ottersen, 1993), undergo substantial changes in the brain after cerebral DC tDCS (Rango et al. 2008; Stagg et al. 2009), and may do the same in the cerebellum after cerebellar tDCS. Because cerebellar stimulation also modulates dopamine release in the basal ganglia (Nieoullon et al. 1978) this mechanism could also apply for cerebellar tDCS. Hence cerebellar tDCS-induced neurotransmitter changes could help to explain how cerebellar DC stimulation acts.
Theoretical estimations (Parazzini et al. 2014b) and experimental data (Galea et al. 2009) imply that cerebellar tDCS has no significant functional effects on the brainstem in adults. Modelling studies (Parazzini et al. 2014b; Rahman et al. 2014) demonstrate that the electric field centres mainly in the cerebellum and no research groups have reported adverse effects after or during cerebellar tDCS in adults.
Clinical perspectives
Available data provide evidence that cerebellar tDCS modulates human cognitive and motor cerebellar functions in healthy subjects and in the few patients assessed, but further studies need to confirm these findings, find out how cerebellar tDCS works and develop optimum stimulation settings and protocols in patients with neurological and psychiatric disorders. Research findings already provide evidence that cerebellar tDCS can influence motor adaptation, learning, memory and emotional processing in healthy humans and these changes could be clinically important in patients with various disorders involving cerebellar dysfunction, and possibly also as a strategy against motor ageing in the elderly. Future studies in patients should, however, take into account evidence that the cerebellar nuclei can be variably affected by the disease and, among other variables, carefully consider the lesion's location in the cerebellar circuitry, and possible extra-cerebellar lesions.
Transcutaneous spinal cord DC stimulation (spinal tDCS)
Single-pulse TMS to the cervical and lumbar spine has been widely used to assess central and peripheral motor conduction time in clinical neurophysiology by evaluating evoked compound muscle action potentials following motor root activation at the neuroforaminal level (Ugawa et al. 1989; Knikou, 2013). Conversely, few studies have assessed whether repetitive spinal magnetic stimulation can modulate spinal cord functions. A recently published review addressed the effects induced by repetitive spinal TMS on motor control (Beaulieu & Schneider, 2013), whereas few studies have described effects on pain syndromes (Smania et al. 2003; Krause et al. 2005). Spinal tDCS appears nonetheless to have some practical advantages over rTMS.
In applying spinal tDCS, researchers used certain key technical features. For lumbar spinal cord modulation, the active electrode (measuring about 5 cm × 7 cm) was usually placed over the spinous process of the tenth thoracic vertebra and the reference above the right shoulder (Cogiamanian et al. 2008, 2011; Lamy et al. 2012; Lamy & Boakye, 2013). For cervical spinal cord modulation, the active electrode was positioned on the seventh cervical vertebra and the reference on the anterior part of the neck (Lim & Shin, 2011). A probably influential variable that needs to be systematically investigated when applying spinal tDCS is whether the reference electrode is positioned on the arm or elsewhere. Also, stimulation intensity and duration were kept relatively constant across the various studies from different groups: intensities between 2 and 2.5 mA were usually applied for 15/20 min (Table2A). Spinal DC often elicits short-lasting tingling sensations when stimulation begins and ends, and sometimes redness under the electrode.
Table 2.
Spinal tDCS sudies (listed in chronological order and according to the evaluated physiological system)
| A. Studies in humans | ||||||||
|---|---|---|---|---|---|---|---|---|
| Authors | Year | Subjects | Patients | Polarity | Montage | Parameters | Technique | Effects |
| Ascending pathways | ||||||||
| Cogiamanian et al. | 2008 | 12 | — | Anodal\cathodal | Active electrode at Th11 level Reference on right shoulder |
2.5 mA; 15 min; AEA = 35 cm2 | Somatosensory-evoked potentials | A-spinal tDCS impairs conduction along human lemniscal pathway |
| Truini et al. | 2011 | 20 | — | Anodal\cathodal | Active electrode at Th11 level Reference on right shoulder |
2.5 mA; 20 min; AEA = 35 cm2 | Laser-evoked potentials | A-spinal tDCS impairs conduction in the ascending nociceptive spinal pathways |
| Descending pathway | ||||||||
| Lim & Shin | 2011 | 12 | — | Anodal\cathodal\sham | Active electrode at C7 level Reference on the anterior neck |
2 mA; 20 min; AEA = 25 cm2 | Motor-evoked potentials | Spinal tDCS increases corticospinal tract excitability |
| Spinal reflexes | ||||||||
| Winkler et al. | 2010 | 10 | — | Anodal\cathodal\sham | Active electrode at Th11 level Reference on right shoulder |
2.5 mA; 15 min; AEA = 35 cm2 | H-reflex post-activation depression | A-spinal tDCS increases the efficacy of the Ia fibre–motoneuron synapse, whereas C-spinal tDCS reduces it |
| Cogiamanian et al. | 2011 | 11 | — | Anodal\sham | Active electrode at Th11 level Reference on right shoulder |
2 mA; 15 min; AEA = 35 cm2 | Lower limb flexor reflex | A-spinal tDCS elicits long-lasting after-effects on central nociceptive signal transmission |
| Lamy et al. | 2012 | 17 | — | Anodal\cathodal\sham | Active electrode at Th11 level Reference on right shoulder |
2.5 mA; 15 min; AEA = 35 cm2 | H-reflex recruitment curve | Spinal tDCS induces enduring changes in spinal segmental excitability that last for at least 15 min after current offset |
| Lamy & Boakye | 2013 | 17 | — | Anodal | Active electrode at Th11 level Reference on right shoulder |
2.5 mA; 15 min; AEA = 35 cm2 | H-reflex recruitment curve | BDNF Val homozygotes exhibited significantly higher A-spinal tDCS-induced plasticity than BDNF Met carriers |
| Hubli et al. | 2013 | 17 | 17 | Anodal\cathodal\sham | Active electrode at Th11 level Reference on right shoulder |
2.5 mA; 20 min; AEA = 35 cm2 | Spinal reflex (SR) behaviour | A-spinal tDCS and assisted locomotion induces changes in SR behaviour in SCI subjects; C-spinal tDCS and sham leads to a drop in SR amplitudes in healthy subjects |
| B. Studies in animals | ||||||||
| Authors | Year | Sample/Animals | Lesioned | Polarity | Configuration | Parameters | Technique | Effects |
| Ascending pathway | ||||||||
| Aguilar et al. | 2011 | 44 | — | Anodal\cathodal | Active electrode on thoracic spinal cord Reference on anterior abdominal area |
1 mA; 15 min; AEA = 0.79 cm2 | Somatosensory-evoked potentials | A-spinal tDCS increased spontaneous activity in the gracile nucleus while decreasing its local field potential responses to somatosensory stimuli; C-spinal tDCS did the opposite |
| Descending pathways and spinal reflexes | ||||||||
| Ahmed | 2011 | 33 | — | Anodal\cathodal | Active electrode at T10–L1 Reference on lateral abdominal muscles |
From 0.5 mA to 3 mA for 3 min; AEA = 0.79 cm2 | Spontaneous activity recording and repetitive cortical electrical stimulation (rCES) | A-spinal tDCS increased the spike frequency and the amplitude of spontaneous discharges; C-spinal tDCS + rCES increased cortical-elicited muscle twitches |
| Ahmed & Wieraszko | 2012 | 87 | Contusive and hemisectioned mice | Anodal\cathodal | Active electrode at T10–L1 Reference on lateral abdominal muscles |
2 mA; 5 s; AEA = 0.79 cm2 | Cortically elicited muscle actions and low-frequency repetitive cortical stimulation (rCS)Repetitive spinal stimulation (rSS) for in vitro glutamate analog release evaluation | Combination of C-spinal tDCS/rCS enhanced spinal cord responses in control and SCI animals; C-spinal tDCS/rSS released the maximum amount of glutamate |
| Ahmed | 2013B | 116 | Unilateral SCI | Cathodal | Active electrode at T13–L4 (spinal level L3–L6) Reference on abdominal skin flap |
0.8 mA; different durations; AEA = 3.5 cm2 | Spino-Sciatic (SSA) and Cortico-Sciatic (CSA) associative plasticity | C-spinal tDCS + SSA or CSA is able to increase associative plasticity in healthy animals and to improve recovery from unilateral SCI |
| Ahmed | 2013A | 30 | — | Cathodal | Active electrode on lumbar enlargement area Reference on abdominal skin flap |
0.8 mA; 8 s; AEA = 3.5 cm2 | Spinal network and complex multijoint movements | C-spinal tDCS modulates the kinematics of elicited movements and bursting activity in spinal circuitries |
AEA, active electrode area; A-spinal tDCS, anodal spinal tDCS; BDNF, brain-derived neurotrophic factor; C7, seventh cervical vertebra; C-spinal tDCS, cathodal spinal tDCS; CSA, cortico-sciatic associative stimulation; LEPs, laser-evoked potentials; mA, milliampere; rCES, repetitive cortical electrical stimulation; rSS, repetitive spinal stimulation; SCI, spinal cord injury; SR, spinal reflex; SSA, spino-sciatic associative stimulation; Th11, eleventh thoracic vertebra; Val66Met, valine-to-methionine substitution at codon 66.
Studies in healthy humans
Experiments in humans have focused on ascending tracts, descending tracts and spinal reflexes (Table2A). In an earlier study Cogiamanian and co-workers (2008), who first described the technique, assessed the after-effects induced by anodal and cathodal spinal tDCS on somatosensory-evoked potentials (SEPs) before and after applying spinal tDCS. SEPs assess the lemniscal pathways (Cruccu et al. 2008). Anodal spinal tDCS selectively reduced the cervico-medullary SEP amplitudes for at least 20 min after stimulation offset, whereas cathodal tDCS left all SEP components unchanged (Fig.4) thus demonstrating an effect on the lemniscal system. In a similar way, Truini and co-workers (2011) investigated the after-effects of anodal spinal tDCS and cathodal tDCS on laser-evoked potentials (LEPs): the scalp potentials elicited by peripheral laser stimulation reflect mainly peripheral Aδ fibre activation (Truini et al. 2004). These fibres transmit pain information to the brain through the spinothalamic tract. Anodal spinal tDCS reduced LEP amplitudes evoked by foot laser stimulation whereas cathodal tDCS failed to induce attenuation (Fig.5). Hence these two studies show that spinal tDCS modulates at least two ascending sensory pathways in humans, regardless of where the tract lies anatomically on the transverse spinal cord plane: the lemniscal pathway lies posteriorly whereas the spinothalamic tract is ventral and lateral (Bican et al. 2013). This observation therefore implies that spinal DC stimulation can also influence other tracts, including corticospinal fibres. To test the corticospinal hypothesis, Lim and Shin (2011) assessed motor-evoked potentials elicited by TMS to the motor cortex in the upper limb before and after cervical spinal tDCS. They reported that spinal tDCS increased corticospinal excitability in a polarity-independent way, eliciting no effects on the H-reflex (Lim & Shin, 2011). Although interesting, these results are difficult to interpret both because the investigators used a unique experimental set-up (stimulating electrode on C7, reference electrode on the anterior part of the neck) not comparable to the available studies on the thoracic spinal cord and because polarising currents could spread to the brachial plexus. Besides, the different polarity surprisingly has no effect on the direction of the induced effect (excitation or inhibition). A possible explanation for the lack of polarity specificity might reside in the electrode position generating an electric field crossing the spinal cord differing by 90 deg in orientation from the studies by Cogiamanian and co-workers (2008) and Truini and co-workers (2011). As happens within other CNS structures, how the electric field is oriented with respect to cell is critical for cellular polarisation and the associated functional effects.
Figure 4. Effects of transcutaneous spinal cord stimulation (spinal tDCS) on the somatosensory-evoked potentials (SEPs).
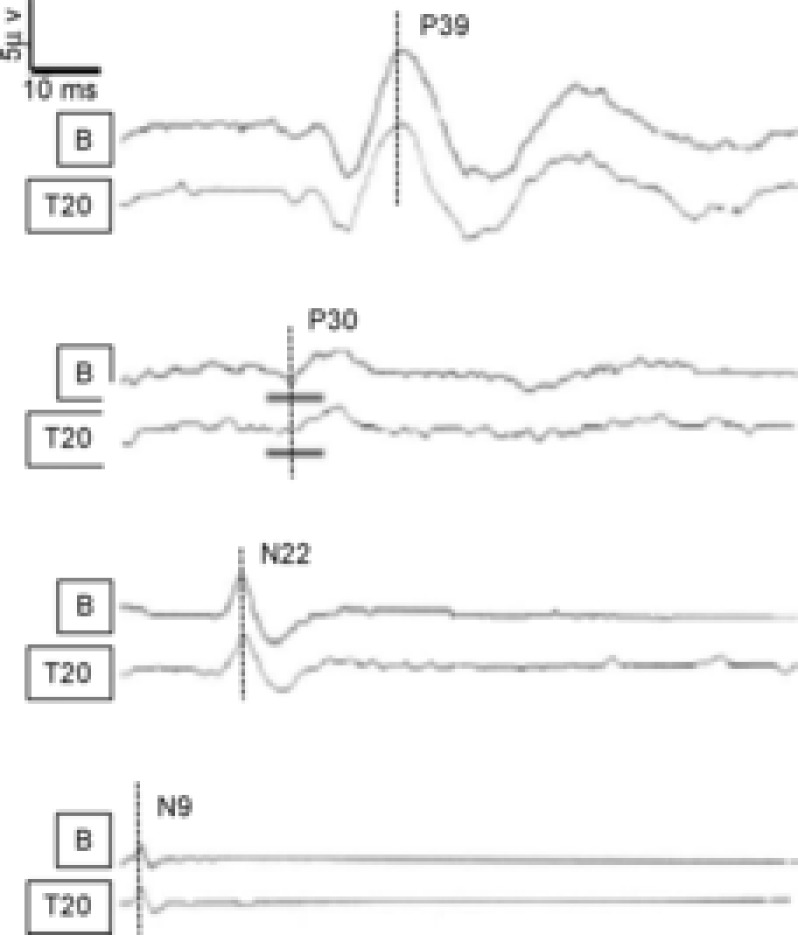
SEPs were obtained after posterior tibial nerve stimulation at the ankles. Activity was measured at the popliteal fossa (popliteal potential, N9), at the first lumbar vertebra referred to the umbilicus (spinal potential, N22), at the sixth cervical vertebra referred to Fpz (cervico-medullary potential, P30) and at Cz referred to the right earlobe (cortical potential, P39). The amplitude and latency for each SEP component were measured at two time points (B, before spinal tDCS; T20, 20 min after spinal tDCS offset). From the top, pairs of traces are P39, P30, N22 and N9. In each pair, the top trace is the baseline recording (B), whereas the bottom trace is recorded 20 min after DC offset (T20). Note that anodal spinal tDCS (2.5 mA, 15 min) decreases the amplitude of the cervico-medullary potential (P30, second pair of traces from the top, grey lines). Conversely spinal tDCS leaves the other potentials unchanged. Modified from Cogiamanian et al. 2008, with permission.
Figure 5. Effects of transcutaneous spinal cord direct current stimulation (spinal tDCS) on laser-evoked potentials (LEPs).
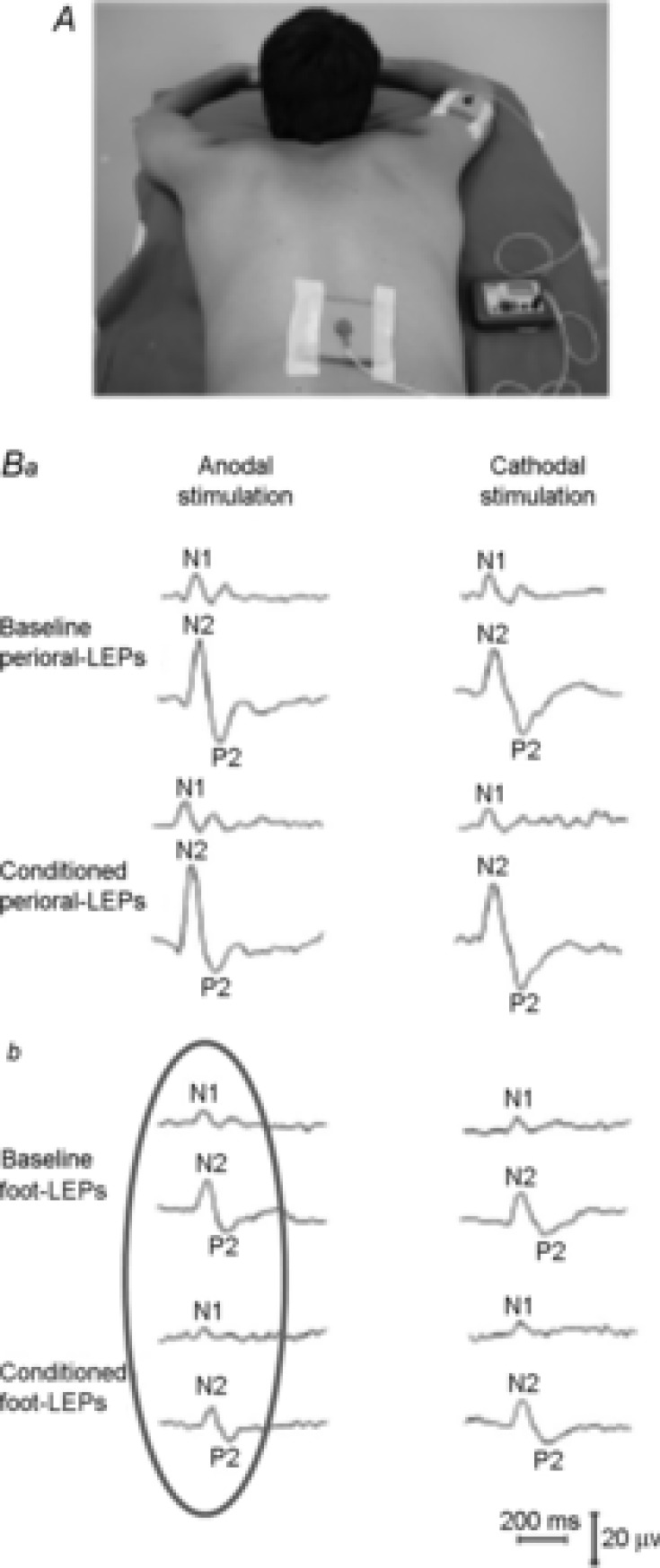
A, spinal tDCS set-up: active electrode on the lower thoracic spinal cord, reference electrode on the right shoulder. B, perioral and foot LEPs in a representative subject. Laser pulses were applied to the right perioral region (Ba) and to the right foot (Bb). LEP N2 and P2 components were recorded from the vertex (Cz with reference on the nose); the N1 component was recorded from the temporal area (T3, reference on Fz). Peak latency and baseline to peak amplitude were measured before and after anodal (on the left) and cathodal (on the right) stimulation. Ba, perioral LEPs before (Baseline) and after (Conditioned) anodal and cathodal DC (2.5 mA, 20 min). Bb, foot LEPs before (Baseline) and after (Conditioned) spinal tDCS. Note that anodal spinal tDCS decreased N1 and N2 amplitude of foot-evoked LEPs (Bb, grey oval) but not of perioral-evoked responses (Ba, left column). Modified from Truini et al. 2011, with permission.
The DC-induced effects on segmental circuitries were mainly assessed on the Ia-motoneuronal connections by recording the H-reflex. Although some studies, not being specifically designed to assess spinal cord modulation at segmental level (Cogiamanian et al. 2011; Lim & Shin, 2011), found no spinal tDCS-induced changes in H-reflex basic features (latency, threshold, Hmax/Mmax ratio), they could not totally exclude a segmental effect. The first study specifically designed to investigate more complex effects at segmental level dates back to 2010. In a protocol designed to investigate spinal DC effects on H-reflex homosynaptic depression (i.e. the progressive H-reflex depression following repetitive H-reflex nerve stimulation at certain frequencies), Winkler and co-workers (2010) showed that even though a simple H-reflex excitability measure (the Hmax/Mmax) remained unchanged, anodal spinal tDCS induced a long-lasting decrease in homosynaptic depression whereas cathodal spinal tDCS increased it. The investigators suggested that the lack of Hmax/Mmax ratio modulation indicates that spinal tDCS had no significant influence on α-motoneuron excitability, and that its effect on homosynaptic depression arises from the spinal tDCS-induced changes on the Ia-motoneuron connections. Whatever the mechanisms, given that homosynaptic depression is decreased in spastic patients (Grey et al. 2008), spinal tDCS might be a promising tool to improve spasticity. Providing more information on how spinal tDCS alters spinal cord plasticity, Lamy and co-workers (2013) showed that anodal spinal tDCS induced a leftward shift in the soleus H-reflex stimulus–response curve (i.e. increased excitability) lasting up to 15 min after stimulation ended, whereas cathodal and sham stimulation left the curve unchanged. A subsequent study (Fig.6) confirmed both findings (Lamy & Boakye, 2013). In accordance with previous observations, Lamy and co-workers confirmed that the Hmax/Mmax ratio was unaffected by spinal tDCS.
Figure 6. Effects of transcutaneous spinal cord direct current stimulation (spinal tDCS) on the H-reflex recruitment curve.
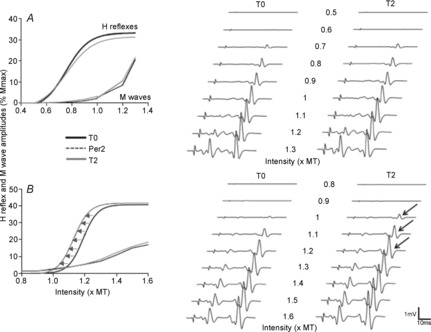
The soleus H-reflex was elicited by stimulating the posterior tibial nerve with pulses lasting 1 ms at a stimulation frequency of 0.33 Hz. The stimulation intensity was progressively increased in 5 to 10% steps of the threshold intensity to evoke an M wave. Five responses were averaged at each stimulus intensity. On the left, the figure shows the differential effect of spinal tDCS on a methionine allele carrier (A) and valine/valine carrier (B). Note that anodal spinal tDCS shifts the curve to the left only in subjects with the valine/valine polymorphism in the BDNF gene (B, leftward shift in the H-reflex recruitment curve, arrows). On the right side, graphic representation showing the effect of anodal spinal tDCS in two representative subjects (raw traces), one from each group (top traces, methionine allele carrier; bottom traces, valine carrier). Black arrows indicate amplitude changes after current offset (T2). Note that in the methionine carrier the H-reflex appeared at the same stimulation intensity (top), whereas in the valine carrier the H-reflex appeared at a lower intensity after spinal tDCS. T0, baseline; Per2, second online recording; T2, 15 min after DC stimulation offset; MT, threshold intensity to evoke an M wave. Modified from Lamy et al. 2012, with permission.
Hence, given that spinal DC leaves the basic H-reflex features (amplitude, area, latency and Hmax/Mmax ratio) unaffected, these measurements are probably unreliable for assessing DC-induced changes in Ia-motoneuron synaptic plasticity. A final interesting observation is that the effect of spinal tDCS on Ia-motoneuronal connections is related also to the stimulated subject's genetic features. Assessing whether the brain-derived neurotrophic factor (BDNF) polymorphism influenced changes in the soleus H-reflex recruitment curve induced by anodal spinal tDCS, Lamy and co-workers (2013) found that the methodology was effective only in BDNF Val homozygotes (Lamy & Boakye, 2013). The study is novel because it correlated the genotype with the response to the electric field and suggests a genetically based interindividual variability in the effect of spinal (and cerebellar?) tDCS.
Continuing neurophysiological studies on reflexes, Cogiamanian and co-workers (2011) evaluated changes in the lower limb polysynaptic flexion reflex (LL-Fr), a response that assesses nociceptive pathways (Cruccu et al. 2004). The Fr late response is a high-threshold nociceptive Aδ fibre-mediated reflex and its threshold corresponds to the pain threshold, whereas the reflex size is related to the pain perception level (Sandrini et al. 2005). Anodal spinal tDCS induced a long-lasting Fr depression and reduced the RIII area (Fig.7) thus demonstrating that spinal tDCS can decrease the nociceptive reflex and suggesting its possible analgesic effect. Nonetheless, although the LL-Fr is a polysynaptic and multisegmental spinal response, supraspinal control exerts an important role in modulating this pain reflex. Thus, a possible descending pathway modulation cannot be excluded. In addition, because withdrawal reflexes are influenced by the cognitive state (Bjerre et al. 2011), further studies should account for a possible correlation between cognitive activity and spinal withdrawal reflex modulation. Overall, the effects induced by spinal tDCS on laser-evoked potentials (Truini et al. 2011) and on the LL-Fr (Cogiamanian et al. 2011) imply that this technique might have a therapeutic role in managing pain.
Figure 7. Effects of anodal transcutaneous spinal cord direct current stimulation (spinal tDCS) on the lower limb flexion reflexes (LL-Fr).
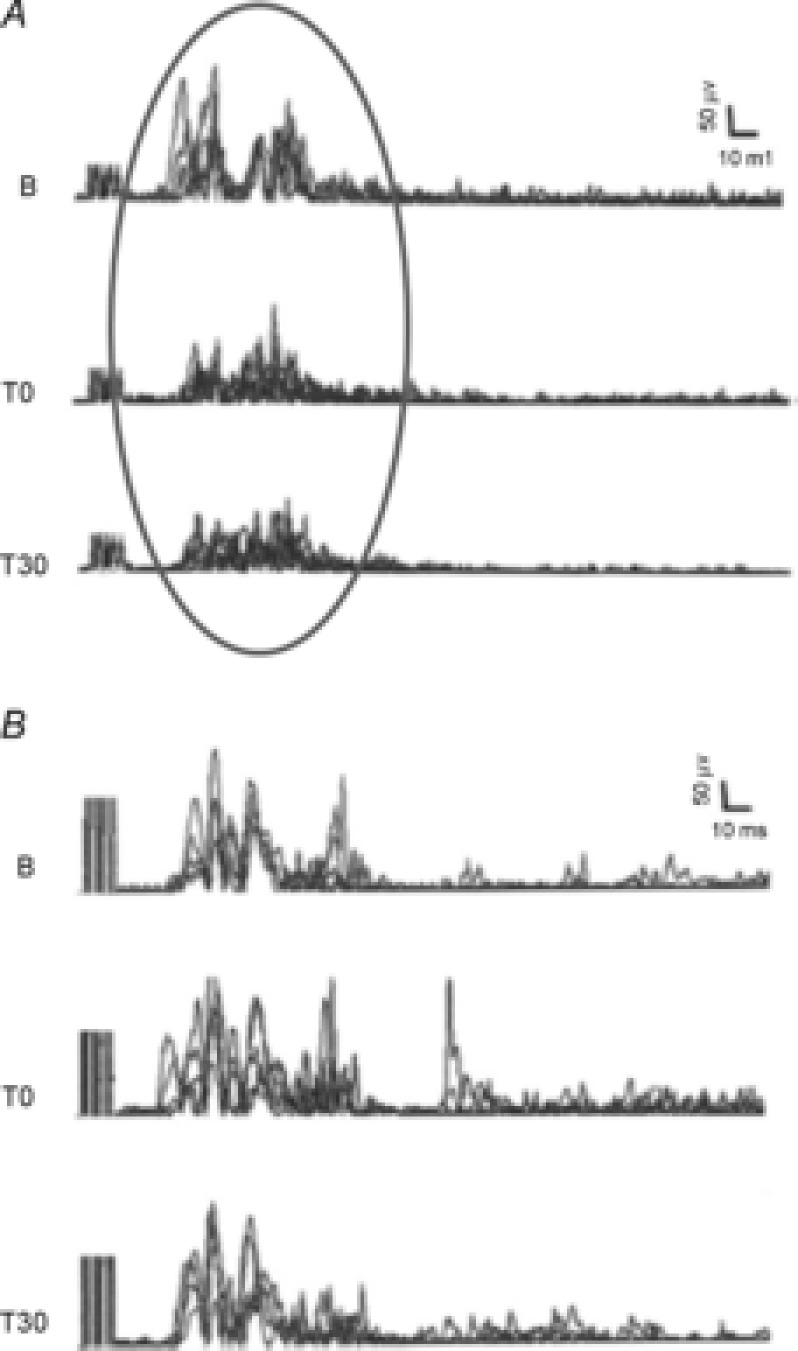
A, trace B (baseline) shows a typical LL-Fr recording from a representative healthy subject. LL-Fr is a polysynaptic spinal reflex elicited by electrical stimulation applied to a sensory nerve. LL-Fr comprises an early response (RIIr) and a late response (RIIIr). RIIIr is a high-threshold nociceptive Aδ fibre-mediated reflex that corresponds to the pain threshold (RIIIr threshold) and pain perception (RIIIr size). LL-Fr was elicited from the sural nerve and responses were recorded from the ipsilateral brevis head of the biceps femoris muscle. The stimulus (5 electrical pulses, pulse duration 1 ms, frequency 200 Hz) was delivered randomly every 5–20 s. The stimulus intensity was set at 120% of RIIIr threshold (average of 5 responses for each leg). RIIIr decreased after anodal spinal tDCS (grey circle) immediately after (T0) and 30 min (T30) after stimulation ended. Sham stimulation left RIII area unchanged (B). Modified from Cogiamanian et al. 2011, with permission.
Studies in animals
In contrast to research on cerebellar DC stimulation, researchers in the past few years have started studying the effects of spinal DC stimulation in healthy animals and animals with spinal cord injury (Table2B).
Aguilar and co-workers (2011) investigated how spinal direct current stimulation delivered at thoracic level influences spontaneous activity and SEPs in the gracile nucleus and primary somatosensory cortex in urethane-anaesthetised rats. The stimulating electrode (total contact surface 0.785 cm2) was placed on the thoracic spinal cord over the dura mater, whereas the return electrode was placed under the skin in the anterior abdominal area. Although this electrode configuration was used to maximise the electrical field across the spinal cord, this orthogonal arrangement could have affected the electric field orientation with respect to the spinal cord, so that the transverse component predominated over the longitudinal one. Intensity was set to 1 mA (current density 1.27 mA cm−2) for 15 min. In the gracile nucleus anodal spinal tDCS increased single-unit activity but decreased the size of the somatosensory-evoked potentials, and cathodal spinal tDCS did the opposite. In addition, anodal tDCS desynchronised activity in the rat somatosensory cortex, whereas cathodal tDCS synchronised it. An important point in interpreting these results is that the invasive electrode arrangement they used generates a far higher current density than that estimated in human spinal cord during non-invasive stimulation (Parazzini et al. 2014a).
After the foregoing study assessing the somatosensory system, to evaluate the corticospinal connections, Ahmed (2011) explored in mice the effects of spinal DC stimulation on motor responses evoked by cortical stimulation in triceps surae muscle. He found that anodal current depressed the triceps surae muscle twitch force for the cortically evoked motor responses with a rebound facilitation persisting 20 min after the current offset, and the opposite with cathodal spinal stimulation. Anodal spinal tDCS increased the latency of the cortically evoked tibial nerve potential during current flow with a rebound latency shortening after current offset, and the opposite with cathodal spinal stimulation. Ahmed concluded that the observed effects could reflect changes induced by the electric field on descending pathways or spinal motoneuronal excitability (Ahmed, 2011). Ahmed also described the effects of anodal and cathodal spinal tDCS matched with repetitive cortical electrical stimulation on the responses elicited by single cortical shocks. He found that anodal spinal tDCS combined with repetitive cortical electrical stimulation induced persistent excitability changes similar to those observed after anodal spinal tDCS alone (Ahmed, 2011). In contrast, concomitant repetitive cortical electrical stimulation boosted the cathodal tDCS effects. He therefore concluded that anodal and cathodal spinal DC stimulation act through different mechanisms. In a further group of experiments in anaesthetised mice (Ahmed, 2013a), Ahmed found that cathodal tDCS enhances the simple and complex behavioural motor responses elicited by electrical stimulation delivered in trains over the motor cortex. Ahmed also found that cathodal spinal DC stimulation increased cortically elicited ankle and multijoint movements. In the same paper he also tested the effects of cathodal spinal DC stimulation on the spinal circuit activity induced by glycine and GABA receptor blockers (picrotoxin at variable concentrations, mixed with strychnine). He reported that cathodal spinal DC stimulation enhanced the receptor-blocker induced bursting spinal activity, thus suggesting that cathodal spinal tDCS increased spinal circuits excitability.
Other experiments aimed also to assess the neurophysiological effects on spinal motoneurones, on segmental Ia-motoneuronal connections, and the neurochemical changes induced by spinal DC stimulation. Ahmed (2011) reported that spontaneous activity in the tibial nerve increased more after anodal than after cathodal spinal tDCS (intensities from 0.5 mA to 3 mA, 30 s steps for 3 min, maximal current density 38.22 A m−2) during current flow but not after its offset. Cathodal DC stimulation increased the H-reflex size (Ahmed & Wieraszko, 2012). Conducting a series of studies on spinal tDCS-induced neurochemical effects, Ahmed (2011) also compared the activity induced in the tibial nerve by anodal and cathodal spinal DC stimulation with the activity induced by injecting GABA and a glycine receptor blocker into the spinal cord in two mice. The activity induced by GABA and glycine blockers resembled the activity induced by cathodal spinal stimulation, thus suggesting that the mechanism underlying the action of cathodal stimulation in mice involves GABA and glycine receptors. Continuing research on the neurochemical effects of spinal DC stimulation Ahmed and Wieraszko (2012) studied whether this technique could induce release of the glutamate-analogue aspartate. Using a special custom-built chamber they evaluated the amount of aspartate released by isolated mouse spinal cord after spinal DC stimulation and found that cathodal spinal DC stimulation increased aspartate, whereas anodal DC reduced aspartate concentrations therefore concluding that the effects of spinal DC stimulation in mice at least partly depend on glutamate changes. In conclusion, neurochemical studies overall suggest that spinal DC stimulation in mice induces its effects partly through the neurotransmitters GABA, glycine and the glutamate analogue aspartate.
Despite their interest, studies in animals have some limitations. First, the anaesthetic drugs used during experiments can influence spinal cord excitability. In particular, ketamine-induced effects on spinal cord vary from animal to animal: for example, ketamine reduces synaptic transmission at excitatory interneuron terminals in the cat spinal cord (Lodge & Anis, 1984), whereas its effect on rat spinal cord seems related to the anaesthesia level (Zandieh et al. 2003). Spinal tDCS can therefore exert different effects on spinal cord functions under different anaesthetic drugs and levels. Second, the results in animals are difficult to compare with those in humans for technical reasons: among other details, electrodes are positioned subcutaneously, hence modifying the electrical field distribution through non-neural tissues and increasing the current density directly applied on the spinal cord. Equally important, the electrode position differs from the configuration used in human experiments (and consequently the electrical field differs in orientation), stimulation duration is often shorter (seconds in mice, minutes in humans) and the applied current density is much higher in animal experiments than in humans. Finally, because animals are smaller than humans, changes induced by spinal DC stimulation could distinctly differ in animals and humans. Thus the effects of spinal tDCS in animals and humans are only partially comparable.
Studies in animals and patients with spinal cord injury
Because spinal cord injury (SCI) is a dramatic emergency that usually defies treatment, any therapeutic option to influence the outcome in patients would be extremely helpful. Under this premise, Ahmed conducted several experiments in spinal cord-injured mice. He found that cathodal spinal tDCS combined with cortico-sciatic stimulation improved walking recovery in unilateral SCI animals (Ahmed, 2013b). The reciprocal potentiating effects of cathodal spinal DC stimulation and repetitive cortical electrical stimulation on motor responses evoked by single cortical shocks are present also in animals with unilateral SCI and spinal cord contusions (Ahmed & Wieraszko, 2012). In these mice the response amplification obtained by combining the two techniques lasted at least 90 min after the stimulation offset, indicating an interesting new direction for research in patients with SCI. Continuing research on a mouse SCI model Ahmed (2013b2013a) assessed whether spinal tDCS increases recovery. To do so he used a complex experimental design based on cathodal spinal tDCS combined either with cortico-sciatic or with spinal sciatic stimulation, so as to increase the cortical- and spinal-evoked motor responses. He found that both paradigms (repetitive cortico-sciatic stimulation/cathodal tDCS and spinal-sciatic stimulation/cathodal spinal tDCS) induced long-term enhancement in cortical and spinal motor outputs in unilateral SCI mice (Ahmed, 2013b). A limitation of the experiments in animals with SCI is that spinal cord lesions evolve over time and spinal circuits change according to the time elapsed after the injury. Spinal tDCS could modulate spinal circuitries in the acute, subacute and chronic phases through different mechanisms. DC could also contribute to facilitate spinal cord healing, as already shown in in vitro experiments (Haan & Song, 2014).
Ahmed's study in SCI mice prompted researchers to ask whether spinal tDCS could have some effect in SCI patients. To answer this question, Hubli and co-workers (2013) applied spinal tDCS in patients undergoing neurorehabilitation after SCI. They evaluated whether spinal tDCS or assisted locomotion using driven gait orthosis (Lokomat, Hocoma AG, Volketswil, Switzerland) enhanced excitability (measured using the spinal reflex) in spinal circuits underlying locomotion in patients with complete SCI (Hubli et al. 2013). In line with the previous observations on spinal reflex modulation in healthy subjects (Winkler et al. 2010), anodal spinal tDCS and assisted locomotion in patients increased SR amplitude, whereas cathodal and sham stimulation left it unchanged. This finding shows that spinal tDCS can also elicit functional effects when the spinal cord is detached from the rest of the CNS. Hence, in patients with complete SCI, spinal direct current stimulation acts through purely spinal mechanisms. These findings open an important avenue of research designed to rescue by spinal tDCS residual spinal functions in SCI patients (Hubli et al. 2013).
Spinal tDCS modelling study
As it did for the cerebellum, a modelling approach could provide determinant information about the effect and mechanism of action underlying spinal tDCS. Continuing their research in the field, Parazzini and co-workers (2014a2014a) conducted the first modelling study aimed at estimating the current density distributions in the spinal cord in various human models (Fig.3). They modelled three electrode montages, with the anode always over the spinal process of the tenth thoracic vertebra and the cathode placed: (1) above the right arm, (2) over the umbilicus, and (3) over the head vertex (in the Cz location according to the 10–20 EEG system). Despite some inter-individual differences due to anatomical variability, within the spinal cord the current density (and the electric field) tended to be primarily directed longitudinally along almost all the vertebral column, particularly with a reference electrode over the right arm and over Cz. On transverse spinal cord sections at thoracic level, the current density distributed uniformly. This finding suggests that the ventral (motor) and dorsal (sensory) axonal tracts undergo identical electric field strength. The reference electrode position had an effect on the spinal cord region where the current density distribution is higher: for instance, the maximum current density at thoracic level was about 0.016 A m−2 with the reference electrode on the right deltoid muscle. Experiments testing the other electrode positions yielded an even higher maximum current density if the reference electrode was placed on the umbilicus or over Cz (0.018 A m−2 at lumbar level and 0.075 A m−2 at cervical level). Across the electrode montages and human models, the maximum electric field ranged between 0.9 and 4.5 V m−1 per 3 mA; these values are of the same order of magnitude as the values found for tDCS. The current density distributions with a reference electrode over the right arm demonstrate that this montage acts mainly at thoracic level with minimal current spread. Conversely, the field distributions with the reference electrode over Cz show that it acts also supraspinally at bulbar level. Spinal tDCS with the reference electrode over Cz could double its physiological effects by doubling the site of action: one located at spinal level and the other at brainstem or cortical level. Hence according to the experimenter's objective, if the aim is to focus on the region for spinal modulation, the reference electrode should be positioned as close as possible to the anode so as to minimise the longitudinal spread of the induced field (as shown for example with the montage on the right arm to focus on the thoracic level). Conversely, moving the electrode to a more distant position will involve larger spinal cord regions and potentially even regions in the brain or brainstem (as shown for example with montage on Cz) (Fig.3).
As expected, owing to the distance between the electrode and the spine, the models show that even if spinal tDCS generates current that reaches the spinal cord, current also disperses in surrounding tissues, mainly the muscles on the back and the nerve roots. This spread is slightly higher in younger. Aside from the limitation discussed before, the results from this modelling study can be useful in translating animal results to humans. First, human studies used lower current density applied on the skin, whereas animal studies mainly used higher current density directly applied over the spinal cord or subcutaneously, thereby substantially differing in current magnitude in the spinal cord. Second, the different body size and electrode arrangements between animals and human studies could change the spatial distribution of the electric field (and current density) in the spinal cord, thereby possibly influencing polarisation. Other important variables for spinal tDCS that need to be systematically tested in future modelling and experimental studies include cerebrospinal fluid current shunting, electric field funnelling by fissures or holes in the spine, and different conductivities in grey and white matter.
Spinal tDCS mechanisms of action and safety
Overall, whereas in humans anodal spinal tDCS suppresses responses mediated by spinal ascending pathways and somehow enhances segmental reflex circuit function, cathodal spinal tDCS tends to enhance responses mediated by spinal ascending pathways and suppresses segmental reflex function. Experiments in animals substantially confirmed that spinal DC stimulation acts on ascending/descending spinal pathways and on segmental reflex responses, suggesting glutamatergic, GABAergic and glycinergic system involvement and effects on spinal plasticity. Given the distance between the spinal cord and the skin surface, the major changes induced by spinal tDCS might seem surprising but current could easily flow within the spinal canal through the intervertebral spaces. As they do for the cerebellum, the spinal DC-induced changes in spinal cord excitability take place at two key time-points, during (online effects) and after spinal tDCS ends (after-effects). The online effects on a neuron/axon exposed to an applied electrical field depend on several features ranging from field properties (intensity, polarity and direction), to neuroanatomical and neurophysiological properties in the targeted spinal structure (Elbasiouny & Mushahwar, 2007). One of the first reports describing how polarising current influences spinal circuitries (Eccles et al. 1962) investigated changes in primary afferent fibre excitability in cats. A dorsal–ventral current hyperpolarised the presynaptic terminals in primary afferent fibres, whereas a ventral–dorsal current did the opposite. Changes in membrane potentials were also associated with changes in trans-synaptic (Eccles et al. 1962) or direct α-motoneuron excitability (Hounsgaard & Kiehn, 1993). The electrical field modulates voltage-sensitive calcium channels in turtle spinal motoneuron dendrites, modifying the total calcium inflow in the motoneuron (Hounsgaard & Kiehn, 1993).
How polarising currents change neuronal membrane excitability depends on how spinal cord fibres are spatially oriented in relation to the electric field (Terzuolo & Bullock, 1956). Hence, a given electrical field polarity can increase spinal tract excitability in the white matter and at the same time decrease excitability in neural elements in the grey matter or vice versa. This mechanism can explain why anodal tDCS inhibits transmission in the ascending spinal pathways (Cogiamanian et al. 2008), whereas it increases H-reflex excitability (Lamy et al. 2012).
Spinal tDCS is a non-invasive technique considered safe in adults. No published studies have reported adverse effects after spinal tDCS. In humans, Cogiamanian and co-workers (2008) failed to report changes in serum neuron-specific enolase (a marker of neuronal damage) before and after stimulation offset. In rats, Ahmed showed that spinal tDCS left spinal cord integrity unchanged (Ahmed, 2011). Current density levels estimated in the spinal cord (see section ‘Spinal tDCS modelling study’) are well below the threshold for neural tissue damage (McCreery et al. 1990). Modelling data (Parazzini et al. 2014a) suggest, however, that current spread towards other anatomical structures is slightly higher in small subjects and children than in adults. Hence spinal tDCS must be used cautiously in children or small subjects.
Clinical perspectives
Available data show that spinal tDCS modulates spinal cord excitability and function, and suggest that the technique might be used to improve outcome in patients with neurological disorders and SCI (Hubli et al. 2013) in different ways. First, SCI can be considered as a disconnection syndrome, in which the segmental circuitries below the lesion are abnormally activated owing to defective supraspinal control. Spinal DC stimulation might therefore help to restore their normal activation by reducing excitability in the hyperactivated circuit and vice versa. A further important point is that electric fields are used in research to promote regeneration and repair interrupted nerve fibres in SCI (Hamid & Hayek, 2008). Hence a technique that could non-invasively generate an electric field able to reach the injured spinal cord in patients might lead to a new therapeutic option aiming to promote axonal regeneration. Finally, in clinical practice patients can easily undergo repetitive spinal tDCS sessions (i.e. daily sessions for more than 2 consecutive days increasing the total charge applied) and outpatients (or their caregivers) can be trained to use this technique at the patient's home.
Concluding remarks
Weak DC delivered transcutaneously in humans over the cerebellum and over the spinal cord for minutes can elicit prolonged changes in neurophysiological and behavioural responses related to cerebellar and spinal functions. The electric field generated by cerebellar tDCS reaches the cerebellum, whereas the electric field for spinal tDCS reaches the spinal cord. The physiological effects elicited by both techniques arise from functional changes in the stimulated structure (cerebellum or spinal cord) though no evidence yet rules out possible (trans-synaptic or antidromic) effects in other brain or brainstem structures triggered by changes in the primary target structure. Several effects probably underlie DC-induced neuroplasticity and possibly neurotransmitter changes could be important. Although no experimental data are available in children, experimental studies failed to report adverse effects in adults.
Preliminary data suggest that both cerebellar tDCS and spinal tDCS can improve some physiological variables in patients and animals with experimental spinal cord injuries. Much work remains to be done to confirm the data, to understand in pathological conditions the possible mechanisms of action underlying these two techniques, also at cellular level, and how to apply them. For example, we need to investigate changes induced by repeated stimulation sessions, compare stimulating electrode montages, examine how body size and age could influence results, study possible interactions with ongoing drug treatments, the possible effects of random noise or alternating current stimulation, and the combined effects of multiple stimulation targets. Concerning multiple targets, a fascinating new direction of research opened by Grimaldi et al. (2014) in patients with cerebellar disorders is multi-target DC stimulation: DC could be used to stimulate the cerebellum, spinal cord and cerebral cortex in the same session or even simultaneously, thus possibly enhancing the induced effects or eliciting still unexplored neuromodulatory responses. Finally, regardless of the stimulation site, research needs to assess whether the electric field modulates inflammatory, reparative or regenerative processes within the CNS. If so, DC electric fields could in theory be used to influence basic disease mechanisms: for instance to modulate inflammatory processes, promote healing and help in regenerating neural elements after CNS injuries (Rueger et al. 2012; Haan & Song, 2014).
Although the cerebellum and spinal cord can be stimulated non-invasively with repetitive magnetic stimulation (Ugawa et al. 1995; Knikou, 2013), neither technique has raised widespread interest for neuromodulation. Cerebral tDCS has several practical advantages over repetitive magnetic stimulation (Priori et al. 2009). These also apply to DC stimulation of the cerebellum and spinal cord: simplicity, low-cost, portability and wearability. Hence, because these two novel neuromodulation techniques are simple to apply, they promise to be increasingly used in the near future and are two novel interesting tools for neuroscientists.
Acknowledgments
The authors wish to thank Mrs Alice Crossman for her linguistic advice and Professor Giovanni Berlucchi for the historical conversation on the experimental work of Professor Giuseppe Moruzzi's group concerning cerebellar stimulation in the cat.
Glossary
- CBI
cerebello-brain inhibition
- cerebellar tDCS
transcranial cerebellar direct current stimulation
- MEP
motor-evoked potential
- PAS
paired associative stimulation
- rTMS
repetitive transcranial magnetic stimulation
- SCI
spinal cord injury
- spinal tDCS
transcutaneous spinal direct current stimulation
- TMS
transcranial magnetic stimulation
Biographies
Alberto Priori is the head of the Clinical Center for Neurostimulation, Neurotechnology and Movement Disorders at the Fondazione IRCCS Ca’ Granda Ospedale Maggiore di Milano and professor of neurology at the University of Milan Italy. He pioneered research on the effect of DC on the human motor cortex in the 1990s and more recently led his research group in studying how constant electric fields influence the human cerebellum and spinal cord.
Matteo Ciocca is a neurologist and clinical research fellow. His research activity assesses the effects of spinal cord DC stimulation in healthy humans.
Marta Parazzini is an engineer. Her research activity deals with the computational modeling of the interactions between electromagnetic field and biological system. She developed the first computational modeling studies specifically designed for cerebellar and spinal cord DC stimulation.
Maurizio Vergari is a neurophysiology technician, who dealt with the technical aspects of cerebellar and spinal cord direct current stimulation since the first experiments.
Roberta Ferrucci is a psychologist, postdoctoral fellow at theUniversity ofMilan. She began her research work by developing the technique for cerebellarDC stimulation and the cognitive and behavioural experimental protocols to assess its effects.
Additional information
Competing interests
A.P., M.V. and R.F. are stakeholders in Newronika s.r.l., a spin-off company formed by the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and Università degli Studi di Milano.
Funding
This review has been partly supported by the University of Milan and by Fondazione IRCCS Ca’ Granda, Milan, Italy.
References
- Aguilar J, Pulecchi F, Dilena R, Oliviero A, Priori A. Foffani G. Spinal direct current stimulation modulates the activity of gracile nucleus and primary somatosensory cortex in anaesthetized rats. J Physiol. 2011;589:4981–4996. doi: 10.1113/jphysiol.2011.214189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z. Trans-spinal direct current stimulation modulates motor cortex-induced muscle contraction in mice. J Appl Physiol (1985) 2011;110:1414–1424. doi: 10.1152/japplphysiol.01390.2010. [DOI] [PubMed] [Google Scholar]
- Ahmed Z. Effects of cathodal trans-spinal direct current stimulation on mouse spinal network and complex multijoint movements. J Neurosci. 2013a;33:14949–14957. doi: 10.1523/JNEUROSCI.2793-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z. Electrophysiological characterization of spino-sciatic and cortico-sciatic associative plasticity: modulation by trans-spinal direct current and effects on recovery after spinal cord injury in mice. J Neurosci. 2013b;33:4935–4946. doi: 10.1523/JNEUROSCI.4930-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z. Wieraszko A. Trans-spinal direct current enhances corticospinal output and stimulation-evoked release of glutamate analog, d-2,3-3H-aspartic acid. J Appl Physiol (1985) 2012;112:1576–1592. doi: 10.1152/japplphysiol.00967.2011. [DOI] [PubMed] [Google Scholar]
- Ardolino G, Bossi B, Barbieri S. Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol. 2005;568:653–663. doi: 10.1113/jphysiol.2005.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Paquier PF, De Deyn PP. Marien P. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110:763–773. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Beaulieu LD. Schneider C. Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. A review. Neurophysiol Clin. 2013;43:251–260. doi: 10.1016/j.neucli.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Beck S. Hallett M. Surround inhibition is modulated by task difficulty. Clin Neurophysiol. 2010;121:98–103. doi: 10.1016/j.clinph.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S. Hallett M. Surround inhibition in the motor system. Exp Brain Res. 2011;210:165–172. doi: 10.1007/s00221-011-2610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bican O, Minagar A. Pruitt AA. The spinal cord: a review of functional neuroanatomy. Neurol Clin. 2013;31:1–18. doi: 10.1016/j.ncl.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Bikson M, Rahman A, Datta A, Fregni F. Merabet L. High-resolution modeling assisted design of customized and individualized transcranial direct current stimulation protocols. Neuromodulation. 2012;15:306–315. doi: 10.1111/j.1525-1403.2012.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerre L, Andersen AT, Hagelskjaer MT, Ge N, Morch CD. Andersen OK. Dynamic tuning of human withdrawal reflex receptive fields during cognitive attention and distraction tasks. Eur J Pain. 2011;15:816–821. doi: 10.1016/j.ejpain.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Block HJ. Celnik P. Can cerebellar transcranial direct current stimulation become a valuable neurorehabilitation intervention. Expert Rev Neurother. 2012;12:1275–1277. doi: 10.1586/ern.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer A, Macher K, Dukart J, Villringer A. Pleger B. Cerebellar transcranial direct current stimulation modulates verbal working memory. Brain Stimul. 2012;6:649–653. doi: 10.1016/j.brs.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Brookhart JM. Blachly PH. Cerebellar unit responses to D.C. polarization. Am J Physiol. 1952;171:711. [Google Scholar]
- Brown SH, Kessler KR, Hefter H, Cooke JD. Freund HJ. Role of the cerebellum in visuomotor coordination. I. Delayed eye and arm initiation in patients with mild cerebellar ataxia. Exp Brain Res. 1993;94:478–488. doi: 10.1007/BF00230206. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS. Fregni F. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Chan CY, Hounsgaard J. Nicholson C. Effects of electric fields on transmembrane potential and excitability of turtle cerebellar Purkinje cells in vitro. J Physiol. 1988;402:751–771. doi: 10.1113/jphysiol.1988.sp017232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY. Nicholson C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol. 1986;371:89–114. doi: 10.1113/jphysiol.1986.sp015963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Hammerer D, D'Ostilio K, Casula EP, Marshall L, Tsai CH, Rothwell JC. Edwards MJ. Bi-directional modulation of somatosensory mismatch negativity with transcranial direct current stimulation: an event related potential study. J Physiol. 2014;592:745–757. doi: 10.1113/jphysiol.2013.260331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Wolters A, Stefan K, Wycislo M, Sandbrink F, Schmidt A. Kunesch E. Paired associative stimulation. Clin Neurophysiol (Suppl) 2004;57:563–569. [PubMed] [Google Scholar]
- Cogiamanian F, Ardolino G, Vergari M, Ferrucci R, Ciocca M, Scelzo E, Barbieri S. Priori A. Transcutaneous spinal direct current stimulation. Front Psychiatry. 2012;3:63. doi: 10.3389/fpsyt.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Pulecchi F, Marceglia S. Priori A. Effect of spinal transcutaneous direct current stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol. 2008;119:2636–2640. doi: 10.1016/j.clinph.2008.07.249. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Schiaffi E, Marceglia S, Ardolino G, Barbieri S. Priori A. Transcutaneous spinal cord direct current stimulation inhibits the lower limb nociceptive flexion reflex in human beings. Pain. 2011;152:370–375. doi: 10.1016/j.pain.2010.10.041. [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Bastian AJ. Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–2284. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F, Rossini PM, Treede RD. Garcia-Larrea L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol. 2008;119:1705–1719. doi: 10.1016/j.clinph.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Anand P, Attal N, Garcia-Larrea L, Haanpaa M, Jorum E, Serra J. Jensen TS. EFNS guidelines on neuropathic pain assessment. Eur J Neurol. 2004;11:153–162. doi: 10.1111/j.1468-1331.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- Donchin O, Rabe K, Diedrichsen J, Lally N, Schoch B, Gizewski ER. Timmann D. Cerebellar regions involved in adaptation to force field and visuomotor perturbation. J Neurophysiol. 2012;107:134–147. doi: 10.1152/jn.00007.2011. [DOI] [PubMed] [Google Scholar]
- Dutta A, Paulus W. Nitsche MA. Facilitating myoelectric-control with transcranial direct current stimulation: a preliminary study in healthy humans. J Neuroeng Rehabil. 2014;11:13. doi: 10.1186/1743-0003-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond AM, Coger RW. Serafetinides EA. Intracerebral current levels in man during electrosleep therapy. Biol Psychiatry. 1975;10:101–104. [PubMed] [Google Scholar]
- Eccles JC, Kostyuk PG. Schmidt RF. The effect of electric polarization of the spinal cord on central afferent fibres and on their excitatory synaptic action. J Physiol. 1962;162:138–150. doi: 10.1113/jphysiol.1962.sp006920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbasiouny SM. Mushahwar VK. Suppressing the excitability of spinal motoneurons by extracellularly applied electrical fields: insights from computer simulations. J Appl Physiol (1985) 2007;103:1824–1836. doi: 10.1152/japplphysiol.00362.2007. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Brunoni AR, Parazzini M, Vergari M, Rossi E, Fumagalli M, Mameli F, Rosa M, Giannicola G, Zago S. Priori A. Modulating human procedural learning by cerebellar transcranial direct current stimulation. Cerebellum. 2013;12:485–492. doi: 10.1007/s12311-012-0436-9. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Giannicola G, Rosa M, Fumagalli M, Boggio PS, Hallett M, Zago S. Priori A. Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cogn Emot. 2012;26:786–799. doi: 10.1080/02699931.2011.619520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci R, Marceglia S, Vergari M, Cogiamanian F, Mrakic-Sposta S, Mameli F, Zago S, Barbieri S. Priori A. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J Cogn Neurosci. 2008;20:1687–1697. doi: 10.1162/jocn.2008.20112. [DOI] [PubMed] [Google Scholar]
- Ferrucci R. Priori A. Transcranial cerebellar direct current stimulation (tcDCS): Motor control, cognition, learning and emotions. Neuroimage. 2013;85:918–923. doi: 10.1016/j.neuroimage.2013.04.122. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE, Cheney MK. Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115:155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- Francis JT, Gluckman BJ. Schiff SJ. Sensitivity of neurons to weak electric fields. J Neurosci. 2003;23:7255–7261. doi: 10.1523/JNEUROSCI.23-19-07255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich F. McCormick DA. Endogenous electric fields may guide neocortical network activity. Neuron. 2010;67:129–143. doi: 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L. Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29:9115–9122. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Xivry JJ. Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex. 2012;21:1761–1770. doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier A, Mollica A. Moruzzi G. [Increased barbiturate resistance of bulbo-reticular responses to localized polarization of the cerebellar cortex] Boll Soc Ital Biol Sper. 1955;31:1217–1218. [PubMed] [Google Scholar]
- Ghai RS, Bikson M. Durand DM. Effects of applied electric fields on low-calcium epileptiform activity in the CA1 region of rat hippocampal slices. J Neurophysiol. 2000;84:274–280. doi: 10.1152/jn.2000.84.1.274. [DOI] [PubMed] [Google Scholar]
- Ghez C. Thach WT, Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4th edn. McGraw-Hill; 2000. The cerebellum; pp. 832–852. [Google Scholar]
- Goto J. Mikoshiba K. Inositol 1,4,5-trisphosphate receptor-mediated calcium release in Purkinje cells: from molecular mechanism to behavior. Cerebellum. 2011;10:820–833. doi: 10.1007/s12311-011-0270-5. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Klinge K, Crone C, Lorentzen J, Biering-Sorensen F, Ravnborg M. Nielsen JB. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp Brain Res. 2008;185:189–197. doi: 10.1007/s00221-007-1142-6. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, Galea JM, Groiss SJ, Hiraoka K, Kassavetis P, Lesage E, Manto M, Miall RC, Priori A, Sadnicka A, Ugawa Y. Ziemann U. Non-invasive cerebellar stimulation – a consensus paper. Cerebellum. 2013;13:121–138. doi: 10.1007/s12311-013-0514-7. [DOI] [PubMed] [Google Scholar]
- Grimaldi G. Manto M. Anodal transcranial direct current stimulation (tDCS) decreases the amplitudes of long-latency stretch reflexes in cerebellar ataxia. Ann Biomed Eng. 2013;41:2437–2447. doi: 10.1007/s10439-013-0846-y. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Oulad Ben Taib N, Manto M. Bodranghien F. Marked reduction of cerebellar deficits in upper limbs following transcranial cerebello-cerebral DC stimulation: tremor reduction and re-programming of the timing of antagonist commands. Front Syst Neurosci. 2014;8:9. doi: 10.3389/fnsys.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan N. Song B. Therapeutic application of electric fields in the injured nervous system. Adv Wound Care (New Rochelle) 2014;3:156–165. doi: 10.1089/wound.2013.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C. Functional imaging of the deep cerebellar nuclei: a review. Cerebellum. 2010;9:22–28. doi: 10.1007/s12311-009-0119-3. [DOI] [PubMed] [Google Scholar]
- Hamada M, Strigaro G, Murase N, Sadnicka A, Galea JM, Edwards MJ. Rothwell JC. Cerebellar modulation of human associative plasticity. J Physiol. 2012;590:2365–2374. doi: 10.1113/jphysiol.2012.230540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid S. Hayek R. Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: an overview. Eur Spine J. 2008;17:1256–1269. doi: 10.1007/s00586-008-0729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM. Celnik PA. Cerebellar direct current stimulation enhances motor learning in older adults. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.03.030. DOI: 10.1016j.neurobiolaging.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld DJ, Pastor D, Haith AM, Rossetti Y, Shadmehr R. O'Shea J. Contributions of the cerebellum and the motor cortex to acquisition and retention of motor memories. Neuroimage. 2014 doi: 10.1016/j.neuroimage.2014.04.076. DOI: 10.1016j.neuroimage.2014.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J. Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol. 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubli M, Dietz V, Schrafl-Altermatt M. Bolliger M. Modulation of spinal neuronal excitability by spinal direct currents and locomotion after spinal cord injury. Clin Neurophysiol. 2013;124:1187–1195. doi: 10.1016/j.clinph.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Hull CA, Chu Y, Thanawala M. Regehr WG. Hyperpolarization induces a long-term increase in the spontaneous firing rate of cerebellar Golgi cells. J Neurosci. 2013;33:5895–5902. doi: 10.1523/JNEUROSCI.4052-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram G, Tang B, Pallegadda R, Vasudevan EV, Celnik P. Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol. 2012;107:2950–2957. doi: 10.1152/jn.00645.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JG. Influence of electric fields on the excitability of granule cells in guinea-pig hippocampal slices. J Physiol. 1981;319:143–152. doi: 10.1113/jphysiol.1981.sp013897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Schraedley-Desmond P. Desmond JE. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. Neuroimage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Knikou M. Neurophysiological characteristics of human leg muscle action potentials evoked by transcutaneous magnetic stimulation of the spine. Bioelectromagnetics. 2013;34:200–210. doi: 10.1002/bem.21768. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Grupp LA. Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders. J Psychiatry Neurosci. 2005;30:178–186. [PMC free article] [PubMed] [Google Scholar]
- Koziol LF. Lutz JT. From movement to thought: the development of executive function. Appl Neuropsychol Child. 2013;2:104–115. doi: 10.1080/21622965.2013.748386. [DOI] [PubMed] [Google Scholar]
- Krause P, Foerderreuther S. Straube A. Effects of conditioning peripheral repetitive magnetic stimulation in patients with complex regional pain syndrome. Neurol Res. 2005;27:412–417. doi: 10.1179/016164105X17224. [DOI] [PubMed] [Google Scholar]
- Lamy JC. Boakye M. BDNF Val66Met polymorphism alters spinal DC stimulation-induced plasticity in humans. J Neurophysiol. 2013;110:109–116. doi: 10.1152/jn.00116.2013. [DOI] [PubMed] [Google Scholar]
- Lamy JC, Ho C, Badel A, Arrigo RT. Boakye M. Modulation of soleus H reflex by spinal DC stimulation in humans. J Neurophysiol. 2012;108:906–914. doi: 10.1152/jn.10898.2011. [DOI] [PubMed] [Google Scholar]
- Lim CY. Shin HI. Noninvasive DC stimulation on neck changes MEP. Neuroreport. 2011;22:819–823. doi: 10.1097/WNR.0b013e32834b939d. [DOI] [PubMed] [Google Scholar]
- Lodge D. Anis NA. Effects of ketamine and three other anaesthetics on spinal reflexes and inhibitions in the cat. Br J Anaesth. 1984;56:1143–1151. doi: 10.1093/bja/56.10.1143. [DOI] [PubMed] [Google Scholar]
- Lorente De Nò R. A Study of Nerve Physiology. New York: Rockefeller Institute for Medical Research; 1947. [PubMed] [Google Scholar]
- McCreery DB, Agnew WF, Yuen TG. Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans Biomed Eng. 1990;37:996–1001. doi: 10.1109/10.102812. [DOI] [PubMed] [Google Scholar]
- Macher K, Bohringer A, Villringer A. Pleger B. Cerebellar-parietal connections underpin phonological storage. J Neurosci. 2014;34:5029–5037. doi: 10.1523/JNEUROSCI.0106-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto M. Haines D. Cerebellar research: two centuries of discoveries. Cerebellum. 2012;11:446–448. doi: 10.1007/s12311-011-0336-4. [DOI] [PubMed] [Google Scholar]
- Molinari M, Leggio MG, Solida A, Ciorra R, Misciagna S, Silveri MC. Petrosini L. Cerebellum and procedural learning: evidence from focal cerebellar lesions. Brain. 1997;120:1753–1762. doi: 10.1093/brain/120.10.1753. [DOI] [PubMed] [Google Scholar]
- Mollica A, Moruzzi G. Naquet R. [Cerebellum, postural tonus, and reticular discharge] J Physiol (Paris) 1953a;45:193. [PubMed] [Google Scholar]
- Mollica A, Moruzzi G. Naquet R. [Discharge of bulbo-reticular impulses and electroencephalographic reactions in awakening induced by positive polarization of the cerebellar cortex] Boll Soc Ital Biol Sper. 1953b;29:435–436. [PubMed] [Google Scholar]
- Mollica A, Moruzzi G. Naquet R. [Effects of positive polarization of the cerebellar cortex on the discharge of bulbo-reticular impulses and on decerebrate rigidity] Boll Soc Ital Biol Sper. 1953c;29:401–402. [PubMed] [Google Scholar]
- Mollica A, Moruzzi G. Naquet R. [Reticular discharges induced by polarization of the cerebellum, their relation with postural tonis and the arousal reaction] Electroencephalogr Clin Neurophysiol. 1953d;5:571–584. doi: 10.1016/0013-4694(53)90034-0. [DOI] [PubMed] [Google Scholar]
- Monti A, Ferrucci R, Fumagalli M, Mameli F, Cogiamanian F, Ardolino G. Priori A. Transcranial direct current stimulation (tDCS) and language. J Neurol Neurosurg Psychiatry. 2013;84:832–842. doi: 10.1136/jnnp-2012-302825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM. Bastian AJ. Mechanisms of cerebellar gait ataxia. Cerebellum. 2007;6:79–86. doi: 10.1080/14734220601187741. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Cheramy A. Glowinski J. Release of dopamine in both caudate nuclei and both substantia nigrae in response to unilateral stimulation of cerebellar nuclei in the cat. Brain Res. 1978;148:143–152. doi: 10.1016/0006-8993(78)90384-0. [DOI] [PubMed] [Google Scholar]
- Nightingale NR, Goodridge VD, Sheppard RJ. Christie JL. The dielectric properties of the cerebellum, cerebrum and brain stem of mouse brain at radiowave and microwave frequencies. Phys Med Biol. 1983;28:897–903. doi: 10.1088/0031-9155/28/8/002. [DOI] [PubMed] [Google Scholar]
- Nitsche MA. Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Neurotransmitters in the cerebellum. Rev Neurol (Paris) 1993;149:629–636. [PubMed] [Google Scholar]
- Parazzini M, Fiocchi S, Liorni I, Rossi E, Cogiamanian F, Vergari M, Priori A. Ravazzani P. Modeling the current density generated by transcutaneous spinal direct current stimulation (tsDCS) Clin Neurophysiol. 2014a doi: 10.1016/j.clinph.2014.02.027. DOI: 10.1016j.clinph.2014.02.027. [DOI] [PubMed] [Google Scholar]
- Parazzini M, Rossi E, Ferrucci R, Liorni I, Priori A. Ravazzani P. Modelling the electric field and the current density generated by cerebellar transcranial DC stimulation in humans. Clin Neurophysiol. 2014b;125:577–584. doi: 10.1016/j.clinph.2013.09.039. [DOI] [PubMed] [Google Scholar]
- Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, Pascual-Leone A. Bikson M. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul. 2012;5:435–453. doi: 10.1016/j.brs.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano O. Cotti E. [Opposite effects exercised by polarization of various vermian cerebellar lamellae on a single deitersian unit] Boll Soc Ital Biol Sper. 1959a;35:387–388. [PubMed] [Google Scholar]
- Pompeiano O. Cotti E. [Topographic localization of the deitersian response to polarization of the vermian cerebellar cortex of the anterior lobe] Boll Soc Ital Biol Sper. 1959b;35:385–386. [PubMed] [Google Scholar]
- Pope PA. Miall RC. Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimul. 2012;5:84–94. doi: 10.1016/j.brs.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi NG, Minafra B, Zangaglia R, De Marzi R, Sandrini G, Priori A. Pacchetti C. Transcranial direct current stimulation (tDCS) of the cortical motor areas in three cases of cerebellar ataxia. Cerebellum. 2014;13:109–112. doi: 10.1007/s12311-013-0524-5. [DOI] [PubMed] [Google Scholar]
- Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol. 2003;114:589–595. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Rona S, Accornero N. Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9:2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- Priori A, Hallett M. Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation. Brain Stimul. 2009;2:241–245. doi: 10.1016/j.brs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Rahman A, Toshev PK. Bikson M. Polarizing cerebellar neurons with transcranial Direct Current Stimulation. Clin Neurophysiol. 2014;125:435–438. doi: 10.1016/j.clinph.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Rahmati N, Owens CB, Bosman LW, Spanke JK, Lindeman S, Gong W, Potters JW, Romano V, Voges K, Moscato L, Koekkoek SK, Negrello M. De Zeeuw CI. Cerebellar potentiation and learning a whisker-based object localization task with a time response window. J Neurosci. 2014;34:1949–1962. doi: 10.1523/JNEUROSCI.2966-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rango M, Cogiamanian F, Marceglia S, Barberis B, Arighi A, Biondetti P. Priori A. Myoinositol content in the human brain is modified by transcranial direct current stimulation in a matter of minutes: a 1H-MRS study. Magn Reson Med. 2008;60:782–789. doi: 10.1002/mrm.21709. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB. Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129:306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- Reeber SL, Otis TS. Sillitoe RV. New roles for the cerebellum in health and disease. Front Syst Neurosci. 2013;7:83. doi: 10.3389/fnsys.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Block HJ. Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored. J Neurophysiol. 2005;94:2403–2415. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- Restuccia D, Della Marca G, Valeriani M, Leggio MG. Molinari M. Cerebellar damage impairs detection of somatosensory input changes. A somatosensory mismatch-negativity study. Brain. 2007;130:276–287. doi: 10.1093/brain/awl236. [DOI] [PubMed] [Google Scholar]
- Robertson EM. The serial reaction time task: implicit motor skill learning. J Neurosci. 2007;27:10073–10075. doi: 10.1523/JNEUROSCI.2747-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondi-Reig L. Burguiere E. Is the cerebellum ready for navigation. Prog Brain Res. 2005;148:199–212. doi: 10.1016/S0079-6123(04)48017-0. [DOI] [PubMed] [Google Scholar]
- Rueger MA, Keuters MH, Walberer M, Braun R, Klein R, Sparing R, Fink GR, Graf R. Schroeter M. Multi-session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PLoS One. 2012;7:e43776. doi: 10.1371/journal.pone.0043776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini G, Wendling F, Merlet I, Molaee-Ardekani B, Mekonnen A, Salvador R, Soria-Frisch A, Grau C, Dunne S. Miranda PC. Transcranial current brain stimulation (tCS): models and technologies. IEEE Trans Neural Syst Rehabil Eng. 2013;21:333–345. doi: 10.1109/TNSRE.2012.2200046. [DOI] [PubMed] [Google Scholar]
- Sadnicka A, Kassavetis P, Saifee TA, Parees I, Rothwell JC. Edwards MJ. Cerebellar transcranial direct current stimulation does not alter motor surround inhibition. Int J Neurosci. 2013;123:425–432. doi: 10.3109/00207454.2012.763165. [DOI] [PubMed] [Google Scholar]
- Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G. Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77:353–395. doi: 10.1016/j.pneurobio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Shah B, Nguyen TT. Madhavan S. Polarity independent effects of cerebellar tDCS on short term ankle visuomotor learning. Brain Stimul. 2013;6:966–968. doi: 10.1016/j.brs.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Smania N, Corato E, Fiaschi A, Pietropoli P, Aglioti SM. Tinazzi M. Therapeutic effects of peripheral repetitive magnetic stimulation on myofascial pain syndrome. Clin Neurophysiol. 2003;114:350–358. doi: 10.1016/s1388-2457(02)00367-x. [DOI] [PubMed] [Google Scholar]
- Sohn YH. Hallett M. Surround inhibition in human motor system. Exp Brain Res. 2004;158:397–404. doi: 10.1007/s00221-004-1909-y. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM. Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R. Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Strata P, Scelfo B. Sacchetti B. Involvement of cerebellum in emotional behavior. Physiol Res. 2011;60(Suppl. 1):S39–48. doi: 10.33549/physiolres.932169. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP. Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Terzuolo CA. Bullock TH. Measurement of imposed voltage gradient adequate to modulate neuronal firing. Proc Natl Acad Sci U S A. 1956;42:687–694. doi: 10.1073/pnas.42.9.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoret H, Haque J. Pascual-Leone A. Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci Lett. 2001;306:29–32. doi: 10.1016/s0304-3940(01)01860-2. [DOI] [PubMed] [Google Scholar]
- Tomlinson SP, Davis NJ. Bracewell RM. Brain stimulation studies of non-motor cerebellar function: a systematic review. Neurosci Biobehav Rev. 2013;37:766–789. doi: 10.1016/j.neubiorev.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Truini A, Romaniello A, Galeotti F, Iannetti GD. Cruccu G. Laser evoked potentials for assessing sensory neuropathy in human patients. Neurosci Lett. 2004;361:25–28. doi: 10.1016/j.neulet.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Truini A, Vergari M, Biasiotta A, La Cesa S, Gabriele M, Di Stefano G, Cambieri C, Cruccu G, Inghilleri M. Priori A. Transcutaneous spinal direct current stimulation inhibits nociceptive spinal pathway conduction and increases pain tolerance in humans. Eur J Pain. 2011;15:1023–1027. doi: 10.1016/j.ejpain.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Genba K, Shimpo T. Mannen T. Physiologic analysis of central motor pathways – simultaneous recording from multiple relaxed muscles. Eur Neurol. 1989;29:135–140. doi: 10.1159/000116396. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R. Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- Voogd J, Schraa-Tam CK, van der Geest JN. De Zeeuw CI. Visuomotor cerebellum in human and nonhuman primates. Cerebellum. 2012;11:392–410. doi: 10.1007/s12311-010-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler T, Hering P. Straube A. Spinal DC stimulation in humans modulates post-activation depression of the H-reflex depending on current polarity. Clin Neurophysiol. 2010;121:957–961. doi: 10.1016/j.clinph.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Yedlin M, Kwan H, Murphy JT, Nguyen-Huu H. Wong YC. Electrical conductivity in cat cerebellar cortex. Exp Neurol. 1974;43:555–569. doi: 10.1016/0014-4886(74)90195-2. [DOI] [PubMed] [Google Scholar]
- Zandieh S, Hopf R, Redl H. Schlag MG. The effect of ketaminexylazine anesthesia on sensory and motor evoked potentials in the rat. Spinal Cord. 2003;41:16–22. doi: 10.1038/sj.sc.3101400. [DOI] [PubMed] [Google Scholar]
- Zuchowski ML, Timmann D. Gerwig M. Acquisition of conditioned eyeblink responses is modulated by cerebellar tDCS. Brain Stimul. 2014 doi: 10.1016/j.brs.2014.03.010. DOI: 10.1016j.brs.2014.03.010. [DOI] [PubMed] [Google Scholar]


