Abstract
Efferent cholinergic neurons project from the brainstem to inhibit sensory hair cells of the vertebrate inner ear. This inhibitory synapse combines the activity of an unusual class of ionotropic cholinergic receptor with that of nearby calcium-dependent potassium channels to shunt and hyperpolarize the hair cell. Postsynaptic calcium signalling is constrained by a thin near-membrane cistern that is co-extensive with the efferent terminal contacts. The postsynaptic cistern may play an essential role in calcium homeostasis, serving as sink or source, depending on ongoing activity and the degree of buffer saturation. Release of calcium from postsynaptic stores leads to a process of retrograde facilitation via the synthesis of nitric oxide in the hair cell. Activity-dependent synaptic modification may contribute to changes in hair cell innervation that occur during development, and in the aged or damaged cochlea.
Introduction and background
Mammalian cochlear hair cells are inhibited by cholinergic neurons projecting from the superior olivary complex in the brainstem (Guinan, 2006). These olivocochlear efferents are activated by sound (Robertson, 1984; Brown, 1989), providing negative feedback that suppresses the response of cochlear afferent neurons (Galambos, 1956; Wiederhold & Kiang, 1970; Winslow & Sachs, 1987). Medial olivocochlear efferents inhibit outer hair cells (OHCs) to improve signal detection in background noise (May & McQuone, 1995; Hienz et al. 1998) and can provide protection from acoustic trauma (Rajan & Johnstone, 1988). In addition to these roles in the mature cochlea, cholinergic efferents temporarily inhibit inner hair cells during the first 2 postnatal weeks in rodents (Glowatzki & Fuchs, 2000; Marcotti et al. 2004), possibly to shape spontaneous afferent activity prior to the onset of hearing (Johnson et al. 2013; Sendin et al. 2014). Intriguingly, this immature innervation pattern reappears in a mouse model of age-related hearing loss (Lauer et al. 2012). Thus, efferent feedback regulates hair cell function moment-by-moment to improve acoustic detection, over the course of days as protection from acoustic trauma, and in as yet undefined ways early and late in life. This multiplicity of roles and timescales implies modulatory mechanisms that extend beyond changes in membrane potential and conductance. Efferent transmission to hair cells is strongly activity dependent (Goutman et al. 2005; Ballestero et al. 2011) resulting in part from a nitric oxide-mediated process of retrograde facilitation (Kong et al. 2012). Studies of postsynaptic calcium signalling and the elaborate synaptic ultrastructure provide a starting point for investigating shorter-term plasticity and longer-term rearrangements during development and ageing.
Inhibitory efferent innervation is common to hair cells in all vertebrates (Manley & Koppl, 1998; Simmons, 2002a). In auditory hair cells of reptiles (turtles; Art et al. 1984), birds (chickens; Fuchs & Murrow, 1992a) and mammals (rodents; Erostegui et al. 1994; Blanchet et al. 1996; Evans, 1996b; Nenov et al. 1996) inhibition is produced by acetylcholine (ACh) opening ionotropic ACh receptors, leading to activation of calcium-dependent potassium channels. Cellular studies have increasingly turned to efferent inhibition of the mammalian cochlea with the obvious motivation to understand the human condition. But equally, the specificity of innervation, its plasticity and modulation during development, and the diversity of functional roles for cochlear efferents make them intrinsically interesting. A cross-section of the mature mammalian organ of Corti illustrates the specificity of these connections (Fig.1).
Figure 1. Cross-section of the organ of Corti (3-week-old rat cochlea – unstained).
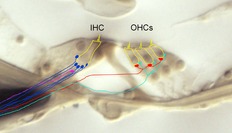
Hair cells are outlined in yellow, stereocilia and innervation schematized. Type I (blue) and type II (turquoise) afferents contact inner and outer hair cells, respectively. Type II afferents travel hundreds of micrometres toward the cochlea base before contacting hair cells (not shown). Medial (red) and lateral (fuchsia) efferents contact outer hair cells and the dendrites of type I afferents, respectively. Only a single lateral efferent is shown for clarity; in reality a rich plexus of endings is formed beneath each inner hair cell.
In the mature cochlea the cholinergic medial olivocochlear efferents (originating in the medial olivary complex) contact OHCs (Guinan, 2006). Lateral olivocochlear efferents (lateral olivary complex origin) contact the dendrites of type I afferents beneath inner hair cells to release ACh, and possibly GABA, dopamine and peptide neurotransmitters as well (Ruel et al. 2007). During cochlear maturation, prior to the onset of hearing, inner hair cells are inhibited temporarily by efferent synaptic contacts (Glowatzki & Fuchs, 2000) similar in action to those found later on OHCs (Oliver et al. 2000; Lioudyno et al. 2004), and thought to be mediated by the same pool of medial olivocochlear neurons (Simmons, 2002b).
Galambos first showed that electrical stimulation of efferent axons suppressed the VIIIth nerve compound action potential evoked by sound (Galambos, 1956). Subsequent single unit recordings confirmed and extended those observations (Wiederhold & Kiang, 1970; Winslow & Sachs, 1987). The first intracellular recordings of hair cell inhibition were obtained from fish lateral line hair cells (Flock & Russell, 1973, 1976). Subsequent studies in the turtle inner ear provided detailed evidence for the effect of efferent inhibition on acoustic receptor potentials (Art et al. 1982, 1984, 1985). Voltage-clamp recording from chicken hair cells helped define the ionic mechanisms and unusual pharmacology of the hair cell's acetylcholine receptor (AChR; Shigemoto & Ohmori, 1991; Fuchs & Murrow, 1992a,b1992b). In all vertebrate hair cells examined to date, two types of ion channel mediate the effects of acetylcholine (Fig.2). The mammalian hair cell's AChR includes α9 and α10 subunits that form a non-selective cation channel with ionic conductance and pharmacology identical to that of the native hair cell AChR (Elgoyhen et al. 1994, 2001). Calcium influx activates nearby small conductance (SK) potassium channels (Erostegui et al. 1994; Blanchet et al. 1996; Evans, 1996a; Nenov et al. 1996; Dulon et al. 1998; Yuhas & Fuchs, 1999; Matthews et al. 2005) and large conductance BK channels in some hair cells (Kong et al. 2005, 2007; Wersinger et al. 2010).
Figure 2. Two-channel mechanism of hair cell inhibition.
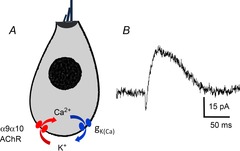
A, the ionotropic α9α10-containing AChR allows calcium entry that opens nearby calcium-activated potassium channels. B, spontaneous synaptic current recorded from rat outer hair cell (membrane potential clamped at –60 mV). Biphasic waveform results from sequential gating of channels as shown in A (unpublished recording by M. Lioudyno).
The cistern as a calcium capacitor
Cholinergic inhibition of hair cells relies on a rise in postsynaptic calcium to activate calcium-dependent potassium channels. This unusual method of inhibition no doubt involves the near-membrane postsynaptic cistern that is co-extensive with the presynaptic efferent terminal (Fig.3; Smith & Sjostrand, 1961; Saito, 1980; Fuchs et al. 2014), an association also described for cholinergic (‘C’, cisternal) synapses in spinal cranial motor neurons (Yamamoto et al. 1991; Nagy et al. 1993). Both in hair cells and motor neurons the synaptic cistern has been proposed to serve as a calcium store, akin to the sarcoplasmic reticulum that supports contraction in muscle, raising the possibility that cholinergic inhibition occurs through some combination of calcium influx and release from internal stores. The participation of a calcium store is supported by the effects of ryanodine and other store-active agents (Sridhar et al. 1997; Evans et al. 2000; Lioudyno et al. 2004). On the other hand, the voltage dependence of ACh-evoked SK current (Martin & Fuchs, 1992) and the brief time course of efferent synaptic potentials (Oliver et al. 2000) are best explained as resulting from calcium influx alone.
Figure 3. Efferent synapses on mouse outer hair cells.
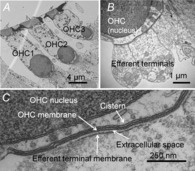
A, cochlear cross-section showing OHCs from three rows (OHC1–3). B, higher power view of multiple efferent terminals on one wild-type OHC. C, high magnification showing parallel membranes that demarcate the synaptic cistern in apposition to an efferent terminal (reproduced from Fuchs et al. 2014, with permission).
Detailed examination of cisternal structure suggests a possible synthesis of these viewpoints. Multiple efferent terminals contact single OHCs (Fig.3; Murthy et al. 2009) to cover several square micrometres of cell surface. The synaptic cistern is co-extensive with the efferent terminals (Fig.4), lying only 14 nm from the postsynaptic membrane (Fuchs et al. 2014) thereby defining a restricted diffusion space where influx through AChRs can raise calcium to high levels. Thus, for brief exposures to ACh the cistern need only serve as a sink, or fixed buffer (Fig.5A) to absorb calcium and enable the rapid decay of ‘quantal’ potassium currents (Glowatzki & Fuchs, 2000; Oliver et al. 2000; Katz et al. 2004; Ballestero et al. 2011). On the other hand, should the cistern become calcium-loaded by sustained influx, uptake would slow, so that SK channel gating would be prolonged, calcium-induced calcium release might occur, and additional calcium-dependent processes could be triggered (Fig.5B), as suggested by previous measurements with fluorescent calcium indicators (Evans et al. 2000). Thus, one might view the cistern as analogous to a capacitor, providing a path for current flow (calcium uptake) until fully charged, at which point current (calcium) flows elsewhere in the circuit (hair cell). In this model, endoplasmic calcium-induced channels (e.g. ryanodine receptors) allow calcium flow into, or out of the store, depending on driving force. Calcium uptake via ATP-dependent pumps also should be involved.
Figure 4. Demarcation and reconstruction of an efferent synapse on a mouse OHC.
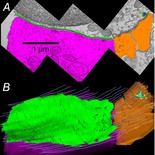
A, single cross-section with the efferent terminal in magenta, the synaptic cistern in green and afferent boutons in umber. B, Z-axis projection (tilted forward ∼30 deg from the plane of section) from a serial reconstruction of 29 sections including that in A (same scale). Same colour scheme as in A with hair cell membrane shown in grey lines. A presynaptic ribbon (turquoise) and associated vesicles (yellow) face the afferent bouton (reproduced from Fuchs et al. 2014, with permission).
Figure 5. The cistern as a calcium capacitor.
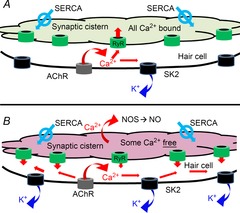
A, Under normal operating conditions (low levels of efferent or hair cell activity) calcium influx through hair cell AChRs (grey) is rapidly absorbed and bound by the synaptic cistern – perhaps through calcium-gated calcium channels such as the ryanodine receptor (green channels). B, overloading of the cistern (e.g. from prolonged efferent activity, or by voltage-gated calcium influx at nearby ribbons, pumped into the cistern by sarco(endo)plasmic reticulum calcium-ATPase (SERCA)) slows the uptake of efferent calcium and leads to calcium-induced calcium release that amplifies and extends activation of SK channels (black channels). The spread of calcium is also thought to stimulate synthesis of nitric oxide (NO) to drive retrograde facilitation.
The effects of calcium store drugs such as ryanodine can be interpreted in light of this model. Such treatments should especially alter postsynaptic currents when the synaptic cistern is calcium-loaded and able to serve as a store. Thus, drugs affecting calcium uptake (inhibitors of endoplasmic calcium pumps such cyclopiazonic acid and thapsigargin) and release (ryanodine and cyclic adenosine phosphoribose (cADPR)) altered the effect of prolonged cholinergic activation in vivo or in excised tissue, presumably because the synaptic cistern was loaded by calcium influx through AChRs (Sridhar et al. 1997; Evans et al. 2000; Lioudyno et al. 2004) or via voltage-gated channels clustered at nearby ribbon synapses (Fig.4B; Frank et al. 2009; Meyer et al. 2009).
This model also predicts that responses to the brief release of ACh should be relatively insensitive to drugs like ryanodine. With some caveats this prediction is partially upheld. The first caveat is that synaptic currents evoked by high potassium or electrical stimulation of efferents were altered in amplitude by ryanodine (Lioudyno et al. 2004; Kong et al. 2012). However, this may be due to changes in efferent release probability by a process of calcium-dependent retrograde facilitation (see section ‘NO-dependent retrograde facilitation’ below). The time course of synaptic currents may be more revealing since this was unaffected by ryanodine at −80 mV (inward potassium currents in 40 mm external potassium; Lioudyno et al. 2004). However, the second caveat is that synaptic waveform was significantly prolonged when studied at −40 mV (Kong et al. 2012). This difference may result from calcium loading via voltage-gated influx at −40 mV, but absent at −80 mV. These observations call for further study of the voltage dependence of calcium store activity in hair cells, as has been described for smooth muscle (Wu et al. 2002). Voltage-gated calcium influx best activates SK channels in turtle hair cells during inhibition of endoplasmic calcium pumps (Tucker & Fettiplace, 1996) supporting their presence in the synaptic cistern. However, neither ryanodine receptors nor SERCA pumps have been immunolocalized to hair cell synaptic cisterns, thus their positions in the model are arbitrary (Fig.5), although calcium-induced calcium release channels probably do face the plasma membrane, by analogy with sarcoplasmic reticulum (Franzini-Armstrong & Protasi, 1997).
Plasticity of efferent transmission
Electrical stimulation of the efferent axons in excised cochlear coils has been used to quantify transmitter release and aspects of synaptic plasticity. The mammalian cochlea provides two opportunities for such experiments: on inner hair cells before the onset of hearing, and on outer hair cells in the mature cochlea. At the inner hair cell contact the resting quantum content was about 1, but with repetitive stimulation rose 2- to 3-fold (Goutman et al. 2005). During prolonged trains of stimulation the summed, facilitated IPSPs could completely prevent action potential firing in these immature inner hair cells. Efferent synaptic transmission onto older OHCs had a smaller resting quantum content and facilitation was more prominent (Ballestero et al. 2011). Efferent transmitter release onto inner hair cells is supported by a combination of P/Q- and N-type voltage-gated calcium channels (VGCCs; Zorrilla de San Martin et al. 2010). In addition, L-type VGCCs and associated BK potassium channels in the efferent terminal act as negative modulators of transmitter release, presumably by abbreviating the presynaptic action potential. Activation of metabotropic GABA receptors (GABAB(1a,2)Rs) down-regulates the amount of ACh released at the efferent synapse by inhibiting P/Q-type VGCCs (Wedemeyer et al. 2013). These are mechanisms of negative feedback, suppressing efferent transmission. The following section describes positive feedback in the form of retrograde facilitation, driven by calcium-dependent production of nitric oxide (NO) in the hair cell. This effect depends on calcium signals associated with the synaptic cistern.
NO-dependent retrograde facilitation
Cholinergic efferent contacts are found on IHCs prior to the onset of hearing at postnatal day 12–14. Efferent release of ACh onto such young IHCs evokes outward SK-mediated currents (inhibitory postsynaptic currents, IPSCs) at −40 mV. When the membrane-impermeant ryanodine receptor agonist, cyclic adenosine phosphoribose (cADPR) was included in the intracellular (pipette) solution, evoked IPSCs were longer-lasting (Kong et al. 2012), supporting the hypothesis that ryanodine receptors are present on the synaptic cistern; their enhanced gating by cADPR prolonged SK channel activity. In addition, and unexpectedly, evoked and spontaneous IPSCs occurred more frequently, as though presynaptic release efficacy had increased. This was confirmed by measuring efferent quantum content during low frequency (1 Hz) evoked release. Treatment with cADPR or ryanodine (1 μm) increased quantum content 5- to 9-fold. Efferent quantum content could also be increased significantly by voltage-gated calcium influx into the hair cell (depolarizing steps interleaved with efferent stimulation). Thus, increases in postsynaptic calcium by voltage-gated influx or enhanced release from internal stores led to an increase in presynaptic quantum content – presumably by way of a retrograde extracellular messenger. Exposure to the nitric oxide (NO) donor 3–morpholinosydnonimine (SIN–1; 100–250 μm) increased the amplitude and frequency of spontaneous IPSCs, nominating NO as a messenger for retrograde facilitation. Exposure to the NO scavenger 2–(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO) effectively prevented the increase in quantum content normally produced by cADPR or hair cell depolarization.
Inner and outer hair cells can produce NO in response to ATP-evoked calcium influx (Shen et al. 2005, 2006), and nitric oxide synthase immunoreactivity has been described throughout cochlear epithelia, including hair cells, and afferent and efferent nerve endings (Heinrich et al. 1997; Riemann & Reuss, 1999). NO stimulates guanylate cyclase to produce cyclic GMP, leading to cGMP-dependent phosphorylation of vesicular release proteins and effects on calcium regulation (Meffert et al. 1996; Garthwaite, 2008). Given the variety of voltage-gated channels present in the efferent terminal (Zorrilla de San Martin et al. 2010) one possibility is that NO alters channel gating through direct nitrosylation (Bredt & Snyder, 1994). BK channel activity has been observed directly in efferent terminals (Wangemann & Takeuchi, 1993); it will be of interest to examine modulation by NO.
Developmental plasticity of efferent innervation
During postnatal maturation of the cochlea, cholinergic efferents inhibit inner hair cells directly (Glowatzki & Fuchs, 2000), perhaps to modulate ongoing spontaneous generation of action potentials (Johnson et al. 2011, 2013; Sendin et al. 2014) that drive activity in associated afferent neurons (Tritsch et al. 2007; Tritsch & Bergles, 2010). These efferent contacts on inner hair cells are temporary, appearing near the day of birth, and have disappeared by the onset of hearing at about postnatal days 12–14 in mice and rats (Fig.6; Katz et al. 2004; Roux et al. 2011). The synaptic mechanism is identical to that found later on OHCs, mediated by α9α10-containing AChRs and associated SK calcium-dependent potassium channels. It is not yet known what mechanisms direct these changes in innervation, but some hints are provided by observations in transgenic mouse models. For example, efferent synapses remain intact and functional up to 4 weeks after birth on IHCs of calcium channel null mice (Brandt et al. 2003), suggesting some interdependence between developmental changes in hair cell excitability and innervation.
Figure 6. Efferent re-arrangements on inner hair cells.
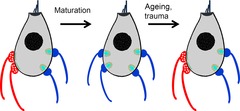
Inner hair cells of the cochlea are temporarily innervated by efferent neurons (red) prior to the onset of hearing (postnatal day 12–14 in rats and mice). Efferent contacts may reappear under conditions that reduce afferent (blue) innervation: age-related hearing loss, acoustic trauma (?), ototoxic damage (?). Thin green lines at efferent contacts represent postsynaptic cisterns. Ribbons (turquoise) surrounded by vesicles (yellow) face afferent boutons.
One consequence of presbycusis (age-related hearing loss) in humans and in mouse models is the loss of afferent contacts onto inner hair cells (Nadol, 1979; Pauler et al. 1986; Spoendlin & Schrott, 1990; Chen et al. 2006; Stamataki et al. 2006). IHCs also lose afferent synaptic contacts after acoustic trauma (Kujawa & Liberman, 2009) or ototoxic insult (Schmiedt et al. 2002). Efferent synaptic contacts return to aged inner hair cells (Lauer et al. 2012) in the C57Bl6 mouse that is a model for age-related hearing loss (Fig.6). Presumptive efferent contacts also have been observed on IHCs following afferent denervation by ouabain (Ruel et al. 2007; Yuan et al. 2013). The factors governing synaptic rearrangements during development, ageing and after trauma are unknown. Certainly there will be a role for growth factors and guidance molecules (Wang & Green, 2011; Brugeaud et al. 2013). Another possibility is activity-dependent governance of efferent synaptic morphology (Murthy et al. 2009) and perhaps competition between afferent and efferent neurites for synaptic territory on the hair cell. Such competition is suggested by the more extensive afferent innervation of OHCs in SK2-null mice that lose efferent contacts (Fuchs et al. 2014).
Speculation
Efferent neurons that inhibit cochlear hair cells exhibit several forms of synaptic plasticity. It is not clear yet what larger role negative feedback plays (Zorrilla de San Martin et al. 2010; Wedemeyer et al. 2013). However, short-term facilitation (Goutman et al. 2005; Ballestero et al. 2011) is required to raise the initially low quantum content, and is consistent with the higher frequency firing required for inhibition in vivo (Gifford & Guinan, 1987). Retrograde facilitation via calcium-dependent NO production is particularly interesting in the context of the developmental and trauma-related changes described above. The synaptic cistern, in addition to its role in regulating on-going synaptic calcium signals, could link global hair cell activity to innervation (Fig.7). Several questions come to mind. What is the functional capacity of efferent synapses on aged or damaged IHCs? Do they recapitulate the cholinergic inhibition seen during development, or take on a novel role? Is the establishment or maintenance of returning efferent synapses activity dependent or NO dependent? Do efferent and afferent synapses compete for territory on the IHC? Answers to these questions will help to identify targets for modulation of synaptic changes wrought by cochlear damage.
Figure 7. Nitric oxide (NO) dependent plasticity.
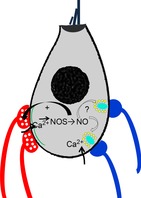
Prior to the onset of hearing, and following cochlear damage, efferent re-innervation of inner hair cells may be modulated by NO-dependent feedback. This can be driven by calcium influx at efferent or afferent specializations. It will be of interest to determine whether NO can also alter afferent synaptic function.
Acknowledgments
With thanks to Gi-jung Im, H. Moskowitz, K. Rohmann and S. Zachary, whose ongoing work and discussion informed this review.
Biography
Paul Fuchs received his doctorate in neuroscience from Stanford University in 1979. After postdoctoral training at Stanford, and Cambridge University, he joined the Physiology faculty at the University of Colorado School ofMedicine in 1984. In 1995 he moved to Johns Hopkins where he is now the John E. Bordley Professor and Vice-chair for research in Otolaryngology–Head and Neck Surgery. Fuchs' research explores signalling in the auditory periphery using intracellular electrical recording, immunohistology and electron microscopy. He and his colleagues have quantified synaptic transmission from sensory hair cells to cochlear afferent neurons as well as the molecular mechanisms of cholinergic inhibition by efferent neurons innervating cochlear hair cells.
Additional information
Competing interests
None declared.
Funding
Work in the author's laboratory is supported by the National Institute for Deafness and other Communication Disorders (NIDCD) R01DC001508, R01DC011741 and by P30 DC005211 to the Centre for Hearing and Balance at Johns Hopkins.
References
- Art JJ, Crawford AC, Fettiplace R. Fuchs PA. Efferent regulation of hair cells in the turtle cochlea. Proc R Soc Lond B Biol Sci. 1982;216:377–384. doi: 10.1098/rspb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Art JJ, Crawford AC, Fettiplace R. Fuchs PA. Efferent modulation of hair cell tuning in the cochlea of the turtle. J Physiol. 1985;360:397–421. doi: 10.1113/jphysiol.1985.sp015624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art JJ, Fettiplace R. Fuchs PA. Synaptic hyperpolarization and inhibition of turtle cochlear hair cells. J Physiol. 1984;356:525–550. doi: 10.1113/jphysiol.1984.sp015481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestero J, Zorrilla de San Martin J, Goutman J, Elgoyhen AB, Fuchs PA. Katz E. Short-term synaptic plasticity regulates the level of olivocochlear inhibition to auditory hair cells. J Neurosci. 2011;31:14763–14774. doi: 10.1523/JNEUROSCI.6788-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet C, Erostegui C, Sugasawa M. Dulon D. Acetylcholine-induced potassium current of guinea pig outer hair cells: its dependence on a calcium influx through nicotinic-like receptors. J Neurosci. 1996;16:2574–2584. doi: 10.1523/JNEUROSCI.16-08-02574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Striessnig J. Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS. Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Brown MC. Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res. 1989;40:93–109. doi: 10.1016/0378-5955(89)90103-2. [DOI] [PubMed] [Google Scholar]
- Brugeaud A, Tong M, Luo L. Edge AS. Inhibition of repulsive guidance molecule, RGMa, increases afferent synapse formation with auditory hair cells. Dev Neurobiol. 2013;74:457–466. doi: 10.1002/dneu.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MA, Webster P, Yang E. Linthicum FH., Jr Presbycusic neuritic degeneration within the osseous spiral lamina. Otol Neurotol. 2006;27:316–322. doi: 10.1097/00129492-200604000-00005. [DOI] [PubMed] [Google Scholar]
- Dulon D, Luo L, Zhang C. Ryan AF. Expression of small-conductance calcium-activated potassium channels (SK) in outer hair cells of the rat cochlea. Eur J Neurosci. 1998;10:907–915. doi: 10.1046/j.1460-9568.1998.00098.x. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE. Heinemann S. α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF. Boulter J. α10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erostegui C, Nenov AP, Norris CH. Bobbin RP. Acetylcholine activates a K+ conductance permeable to Cs+ in guinea pig outer hair cells. Hear Res. 1994;81:119–129. doi: 10.1016/0378-5955(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Evans M( Acetylcholine activates two currents in guinea-pig outer hair cells. J Physiol. 1996a;491:563–578. doi: 10.1113/jphysiol.1996.sp021240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M, Lagostena L, Darbon P. Mammano F( Cholinergic control of membrane conductance and intracellular free Ca2+ in outer hair cells of the guinea pig cochlea. Cell Calcium. 2000;28:195–203. doi: 10.1054/ceca.2000.0145. [DOI] [PubMed] [Google Scholar]
- Evans MG. Acetylcholine activates two currents in guinea-pig outer hair cells. J Physiol. 1996b;491:563–578. doi: 10.1113/jphysiol.1996.sp021240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A. Russell I. Efferent nerve fibres: postsynaptic action on hair cells. Nat New Biol. 1973;243:89–91. [PubMed] [Google Scholar]
- Flock A. Russell I. Inhibition by efferent nerve fibres: action on hair cells and afferent synaptic transmission in the lateral line canal organ of the burbot Lota lota. J Physiol. 1976;257:45–62. doi: 10.1113/jphysiol.1976.sp011355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank T, Khimich D, Neef A. Moser T. Mechanisms contributing to synaptic Ca2+ signals and their heterogeneity in hair cells. Proc Natl Acad Sci U S A. 2009;106:4483–4488. doi: 10.1073/pnas.0813213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Lehar M. Hiel H. Ultrastructure of cisternal synapses on outer hair cells of the mouse cochlea. J Comp Neurol. 2014;522:717–729. doi: 10.1002/cne.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA. Murrow BW. Cholinergic inhibition of short (outer) hair cells of the chick's cochlea. J Neurosci. 1992a;12:800–809. doi: 10.1523/JNEUROSCI.12-03-00800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA. Murrow BW. A novel cholinergic receptor mediates inhibition of chick cochlear hair cells. Proc Biol Sci. 1992b;248:35–40. doi: 10.1098/rspb.1992.0039. [DOI] [PubMed] [Google Scholar]
- Galambos R( Suppression of auditory nerve activity by stimulation of efferent fibers to the cochlea. J Neurophysiol. 1956;19:424–437. doi: 10.1152/jn.1956.19.5.424. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford ML. Guinan JJ., Jr Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear Res. 1987;29:179–194. doi: 10.1016/0378-5955(87)90166-3. [DOI] [PubMed] [Google Scholar]
- Glowatzki E. Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science. 2000;288:2366–2368. doi: 10.1126/science.288.5475.2366. [DOI] [PubMed] [Google Scholar]
- Goutman JD, Fuchs PA. Glowatzki E. Facilitating efferent inhibition of inner hair cells in the cochlea of the neonatal rat. J Physiol. 2005;566:49–59. doi: 10.1113/jphysiol.2005.087460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006;27:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Heinrich UR, Maurer J, Gosepath K. Mann W. Electron microscopic localization of nitric oxide I synthase in the organ of Corti of the guinea pig. Eur Arch Otorhinolaryngol. 1997;254:396–400. doi: 10.1007/BF01642558. [DOI] [PubMed] [Google Scholar]
- Hienz RD, Stiles P. May BJ. Effects of bilateral olivocochlear lesions on vowel formant discrimination in cats. Hear Res. 1998;116:10–20. doi: 10.1016/s0378-5955(97)00197-4. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Eckrich T, Kuhn S, Zampini V, Franz C, Ranatunga KM, Roberts TP, Masetto S, Knipper M, Kros CJ. Marcotti W. Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells. Nat Neurosci. 2011;14:711–717. doi: 10.1038/nn.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Wedemeyer C, Vetter DE, Adachi R, Holley MC, Elgoyhen AB. Marcotti W. Cholinergic efferent synaptic transmission regulates the maturation of auditory hair cell ribbon synapses. Open Biol. 2013;3:130163. doi: 10.1098/rsob.130163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E, Elgoyhen AB, Gomez-Casati ME, Knipper M, Vetter DE, Fuchs PA. Glowatzki E. Developmental regulation of nicotinic synapses on cochlear inner hair cells. J Neurosci. 2004;24:7814–7820. doi: 10.1523/JNEUROSCI.2102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong JH, Zachary S, Rohmann KN. Fuchs PA. Retrograde facilitation of efferent synapses on cochlear hair cells. J Assoc Res Otolaryngol. 2012;14:17–27. doi: 10.1007/s10162-012-0361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong WJ, Guo CK, Zhang S, Hao J, Wang YJ. Li ZW. The properties of ACh-induced BK currents in guinea pig type II vestibular hair cells. Hear Res. 2005;209:1–9. doi: 10.1016/j.heares.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Guo CK, Zhang XW, Chen X, Zhang S, Li GQ, Li ZW. Van Cauwenberge P. The coupling of acetylcholine-induced BK channel and calcium channel in guinea pig saccular type II vestibular hair cells. Brain Res. 2007;1129:110–115. doi: 10.1016/j.brainres.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Kujawa SG. Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Fuchs PA, Ryugo DK. Francis HW. Efferent synapses return to inner hair cells in the aging cochlea. Neurobiol Aging. 2012;33:2892–2902. doi: 10.1016/j.neurobiolaging.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioudyno M, Hiel H, Kong JH, Katz E, Waldman E, Parameshwaran-Iyer S, Glowatzki E. Fuchs PA. A “synaptoplasmic cistern” mediates rapid inhibition of cochlear hair cells. J Neurosci. 2004;24:11160–11164. doi: 10.1523/JNEUROSCI.3674-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. Koppl C. Phylogenetic development of the cochlea and its innervation. Curr Opin Neurobiol. 1998;8:468–474. doi: 10.1016/s0959-4388(98)80033-0. [DOI] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL. Kros CJ. A transiently expressed SK current sustains and modulates action potential activity in immature mouse inner hair cells. J Physiol. 2004;560:691–708. doi: 10.1113/jphysiol.2004.072868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR. Fuchs PA. The dependence of calcium-activated potassium currents on membrane potential. Proc R Soc Lond B Biol Sci. 1992;250:71–76. doi: 10.1098/rspb.1992.0132. [DOI] [PubMed] [Google Scholar]
- Matthews TM, Duncan RK, Zidanic M, Michael TH. Fuchs PA. Cloning and characterization of SK2 channel from chicken short hair cells. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:491–503. doi: 10.1007/s00359-005-0601-4. [DOI] [PubMed] [Google Scholar]
- May BJ. McQuone SJ. Effects of bilateral olivocochlear lesions on pure-tone intensity discrimination in cats. Aud Neurosci. 1995;1:385–400. [PMC free article] [PubMed] [Google Scholar]
- Meffert MK, Calakos NC, Scheller RH. Schulman H. Nitric oxide modulates synaptic vesicle docking fusion reactions. Neuron. 1996;16:1229–1236. doi: 10.1016/s0896-6273(00)80149-x. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Frank T, Khimich D, Hoch G, Riedel D, Chapochnikov NM, Yarin YM, Harke B, Hell SW, Egner A. Moser T. Tuning of synapse number, structure and function in the cochlea. Nat Neurosci. 2009;12:444–453. doi: 10.1038/nn.2293. [DOI] [PubMed] [Google Scholar]
- Murthy V, Taranda J, Elgoyhen AB. Vetter DE. Activity of nAChRs containing α9 subunits modulates synapse stabilization via bidirectional signaling programs. Dev Neurobiol. 2009;69:931–949. doi: 10.1002/dneu.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB., Jr Electron microscopic findings in presbycusic degeneration of the basal turn of the human cochlea. Otolaryngol Head Neck Surg. 1979;87:818–836. doi: 10.1177/019459987908700617. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Yamamoto T. Jordan LM. Evidence for the cholinergic nature of C-terminals associated with subsurface cisterns in alpha-motoneurons of rat. Synapse. 1993;15:17–32. doi: 10.1002/syn.890150103. [DOI] [PubMed] [Google Scholar]
- Nenov AP, Norris C. Bobbin RP. Acetylcholine response in guinea pig outer hair cells. II. Activation of a small conductance Ca2+-activated K+ channel. Hear Res. 1996;101:149–172. doi: 10.1016/s0378-5955(96)00143-8. [DOI] [PubMed] [Google Scholar]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP. Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron. 2000;26:595–601. doi: 10.1016/s0896-6273(00)81197-6. [DOI] [PubMed] [Google Scholar]
- Pauler M, Schuknecht HF. Thornton AR. Correlative studies of cochlear neuronal loss with speech discrimination and pure-tone thresholds. Arch Otorhinolaryngol. 1986;243:200–206. doi: 10.1007/BF00470622. [DOI] [PubMed] [Google Scholar]
- Rajan R. Johnstone BM. Electrical stimulation of cochlear efferents at the round window reduces auditory desensitization in guinea pigs. I. Dependence on electrical stimulation parameters. Hear Res. 1988;36:53–73. doi: 10.1016/0378-5955(88)90137-2. [DOI] [PubMed] [Google Scholar]
- Riemann R. Reuss S. Nitric oxide synthase in identified olivocochlear projection neurons in rat and guinea pig. Hear Res. 1999;135:181–189. doi: 10.1016/s0378-5955(99)00113-6. [DOI] [PubMed] [Google Scholar]
- Robertson D. Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hear Res. 1984;15:113–121. doi: 10.1016/0378-5955(84)90042-x. [DOI] [PubMed] [Google Scholar]
- Roux I, Wersinger E, McIntosh JM, Fuchs PA. Glowatzki E. Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea. J Neurosci. 2011;31:15092–15101. doi: 10.1523/JNEUROSCI.2743-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel J, Wang J, Rebillard G, Eybalin M, Lloyd R, Pujol R. Puel JL. Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear Res. 2007;227:19–27. doi: 10.1016/j.heares.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Saito K. Fine structure of the sensory epithelium of the guinea pig organ of Corti: afferent and efferent synapses of hair cells. J Ultrastruct Res. 1980;71:222–232. doi: 10.1016/s0022-5320(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Okamura HO, Lang H. Schulte BA. Ouabain application to the round window of the gerbil cochlea: a model of auditory neuropathy and apoptosis. J Assoc Res Otolaryngol. 2002;3:223–233. doi: 10.1007/s1016200220017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendin G, Bourien J, Rassendren F, Puel JL. Nouvian R. Spatiotemporal pattern of action potential firing in developing inner hair cells of the mouse cochlea. Proc Natl Acad Sci U S A. 2014;111:1999–2004. doi: 10.1073/pnas.1319615111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Harada N, Nakazawa H, Kaneko T, Izumikawa M. Yamashita T. Role of nitric oxide on ATP-induced Ca2+ signaling in outer hair cells of the guinea pig cochlea. Brain Res. 2006;1081:101–112. doi: 10.1016/j.brainres.2005.12.129. [DOI] [PubMed] [Google Scholar]
- Shen J, Harada N, Nakazawa H. Yamashita T. Involvement of the nitric oxide-cyclic GMP pathway and neuronal nitric oxide synthase in ATP-induced Ca2+ signalling in cochlear inner hair cells. Eur J Neurosci. 2005;21:2912–2922. doi: 10.1111/j.1460-9568.2005.04135.x. [DOI] [PubMed] [Google Scholar]
- Shigemoto T. Ohmori H. Muscarinic receptor hyperpolarizes cochlear hair cells of chick by activating Ca2+-activated K+ channels. J Physiol. 1991;442:669–690. doi: 10.1113/jphysiol.1991.sp018814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DD. Development of the inner ear efferent system across vertebrate species. J Neurobiol. 2002a;53:228–250. doi: 10.1002/neu.10130. [DOI] [PubMed] [Google Scholar]
- Simmons DD. Development of the inner ear efferent system across vertebrate species. J Neurobiol. 2002b;53:228–250. doi: 10.1002/neu.10130. [DOI] [PubMed] [Google Scholar]
- Smith CA. Sjostrand FS. A synaptic structure in the hair cells of the guinea pig cochlea. J Ultrastruct Res. 1961;5:182–192. doi: 10.1016/s0022-5320(61)80025-7. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Schrott A. Quantitative evaluation of the human cochlear nerve. Acta Otolaryngol Suppl. 1990;470:61–69. doi: 10.3109/00016488909138358. discussion 69–70. [DOI] [PubMed] [Google Scholar]
- Sridhar TS, Brown MC. Sewell WF. Unique postsynaptic signaling at the hair cell efferent synapse permits calcium to evoke changes on two time scales. J Neurosci. 1997;17:428–437. doi: 10.1523/JNEUROSCI.17-01-00428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamataki S, Francis HW, Lehar M, May BJ. Ryugo DK. Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hear Res. 2006;221:104–118. doi: 10.1016/j.heares.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Tritsch NX. Bergles DE. Developmental regulation of spontaneous activity in the mammalian cochlea. J Neurosci. 2010;30:1539–1550. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E. Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- Tucker TR. Fettiplace R. Monitoring calcium in turtle hair cells with a calcium-activated potassium channel. J Physiol. 1996;494:613–626. doi: 10.1113/jphysiol.1996.sp021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. Green SH. Functional role of neurotrophin–3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J Neurosci. 2011;31:7938–7949. doi: 10.1523/JNEUROSCI.1434-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P. Takeuchi S. Maxi-K+ channel in single isolated cochlear efferent nerve terminals. Hear Res. 1993;66:123–129. doi: 10.1016/0378-5955(93)90133-l. [DOI] [PubMed] [Google Scholar]
- Wedemeyer C, Zorrilla de San Martin J, Ballestero J, Gomez-Casati ME, Torbidoni AV, Fuchs PA, Bettler B, Elgoyhen AB. Katz E. Activation of presynaptic GABAB(1a,2) receptors inhibits synaptic transmission at mammalian inhibitory cholinergic olivocochlear–hair cell synapses. J Neurosci. 2013;33:15477–15487. doi: 10.1523/JNEUROSCI.2554-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger E, McLean WJ, Fuchs PA. Pyott SJ. BK channels mediate cholinergic inhibition of high frequency cochlear hair cells. PloS One. 2010;5:e13836. doi: 10.1371/journal.pone.0013836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold ML. Kiang NY. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am. 1970;48:950–965. doi: 10.1121/1.1912234. [DOI] [PubMed] [Google Scholar]
- Winslow RL. Sachs MB. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J Neurophysiol. 1987;57:1002–1021. doi: 10.1152/jn.1987.57.4.1002. [DOI] [PubMed] [Google Scholar]
- Wu C, Sui G. Fry CH. The role of the L-type Ca2+ channel in refilling functional intracellular Ca2+ stores in guinea-pig detrusor smooth muscle. J Physiol. 2002;538:357–369. doi: 10.1113/jphysiol.2001.013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Hertzberg EL. Nagy JI. Subsurface cisterns in alpha-motoneurons of the rat and cat: immunohistochemical detection with antibodies against connexin32. Synapse. 1991;8:119–136. doi: 10.1002/syn.890080206. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Shi F, Yin Y, Tong M, Lang H, Polley DB, Liberman MC. Edge AS. Ouabain-induced cochlear nerve degeneration: synaptic loss and plasticity in a mouse model of auditory neuropathy. J Assoc Res Otolaryngol. 2013;15:31–43. doi: 10.1007/s10162-013-0419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhas WA. Fuchs PA. Apamin-sensitive, small-conductance, calcium-activated potassium channels mediate cholinergic inhibition of chick auditory hair cells. J Comp Physiol A. 1999;185:455–462. doi: 10.1007/s003590050406. [DOI] [PubMed] [Google Scholar]
- Zorrilla de San Martin J, Pyott S, Ballestero J. Katz E. Ca2+ and Ca2+-activated K+ channels that support and modulate transmitter release at the olivocochlear efferent–inner hair cell synapse. J Neurosci. 2010;30:12157–12167. doi: 10.1523/JNEUROSCI.2541-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


