Abstract
In this symposium review we discuss the role of neurotransmitters as paracrine signals that regulate pancreatic islet function. A large number of neurotransmitters and their receptors has been identified in the islet, but relatively little is known about their involvement in islet biology. Interestingly, neurotransmitters initially thought to be present in autonomic axons innervating the islet are also present in endocrine cells of the human islet. These neurotransmitters can thus be released as paracrine signals to help control hormone release. Here we propose that the role of neurotransmitters may extend beyond controlling endocrine cell function to work as signals modulating vascular flow and immune responses within the islet.
Introduction
The pancreatic islet of Langerhans is an endocrine micro-organ that secretes the hormones insulin and glucagon to prevent life-threatening fluctuations in blood glucose. The human pancreas contains ∼1,000,000 islets that work in a concerted manner to produce bursts of hormone secretion at 5 min intervals (Tengholm & Gylfe, 2009). To generate this secretory pattern, the activity of the insulin-secreting β cells must be synchronized within the islet and across islets. At the same time, the secretory activity of other islet endocrine cells such as the glucagon-secreting α cell, which has opposing effects on glucose homeostasis, needs to be coordinated with that of the β cell. As endocrine cells communicate with each other within the islet they may simultaneously send signals that adjust blood flow to efficiently deliver islet hormones into the circulation and eventually to the liver, where they help regulate glucose output to maintain glucose homeostasis (Conn et al. 1998). Anatomical and functional defects disrupting these coordinated, rhythmic activities probably diminish the efficiency of the islet to a point where they probably contribute to the pathogenesis of diabetes. A loss of pulsatile insulin secretion is indeed characteristic of patients with type 2 diabetes (Tengholm & Gylfe, 2009).
Most of the regulation of hormone secretion is accomplished at the level of the individual islet. While influenced by circulating nutrients and hormones and by nervous input, each islet is a functional unit that has all the necessary elements to produce adequate responses to changes in glucose concentration. Any given islet is a good representative of all the other ones and includes all pancreatic endocrine cell types, a unique vasculature, and a set of resident macrophages (Fig.1). Islets are self-reliant units whose hormonal responses are small versions of the whole pancreatic output. It is within the structural and functional frame of the islet that the individual endocrine cells coordinate their activities to generate appropriate hormonal responses. It may at first seem counterintuitive that β and α cells, which can be defined as antagonists, are located in the same micro-organ. When isolated, however, β and α cells do not respond appropriately, suggesting that the close association with other endocrine cells within the islet is required to optimize hormone secretion (Wojtusciszyn et al. 2008). Paracrine signalling within the islet seems so entrenched that there is a cell population, the δ cells, dedicated to modulate the activity of the neighbouring β and α cells (Taborsky et al. 1979; Samols & Stagner, 1990).
Figure 1. The cell composition of the human pancreatic islet.
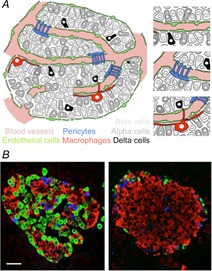
A, schematic representation of possible cell–cell interactions within a human islet. Different endocrine cells can communicate via cell contacts, via the interstitium, or via the bloodstream (top, right). Vascular cells can also be targets for paracrine modulation (centre, right). Immune cells (macrophages) are located along blood vessels and may sense both humoral and local signals (bottom, right). Not depicted are endocrine cells expressing pancreatic polypeptide or ghrelin. B, confocal images of pancreatic sections showing a human (left) and a mouse islet (right) immunostained for insulin (red), glucagon (green) and somatostatin (blue). In the human islet, most β cells face cells of other cell types, thus increasing the possibilities for paracrine signalling. Scale bar represents 20 μm in both panels.
Paracrine interactions within the islet may actually be more important than previously thought. It is likely that the roles α and δ cells play in shaping β cell function may have been underestimated because our model of islet biology is mostly derived from studies performed on rodent islets. The rodent islet cytoarchitecture differs from that of the human islet in that most β cells only contact other β cells within the core of the islet, far away from α and δ cells located in the periphery of the islet (Fig.1B). In rodent islets blood reportedly flows first through β cell-rich regions, implying that β cells are not exposed to signals derived from other endocrine cells (Stagner & Samols, 1992). These cellular arrangements restrict paracrine interactions in the rodent islet mainly to those in which β cells play a dominant role. In the human islet, by contrast, most β cells are surrounded by α and δ cells and there is no particular cellular arrangement along blood vessels (Brissova et al. 2005; Cabrera et al. 2006). Thus, in the human islet the different endocrine cells are close enough to influence each other through direct contacts, via the interstitium, or using the vascular route.
The theme of this presentation is that paracrine signalling within the islet serves to orchestrate hormonal secretion and promote islet health and survival. Because endocrine cells also release potent vasoactive and immune modulatory substances, we will further discuss the possibility that paracrine signalling may extend beyond regulating hormone secretion to control vascular and immune functions within the islet.
The neuroscience of the pancreatic islet
The list of neurotransmitters and neuropeptides that have been suggested to play a role as paracrine signals within the islet is extensive. Most of the small neurotransmitter molecules expressed in the brain have been reported to be present in the islet. To add to the complexity, the hormones released by α and β cells also act locally on neighbouring cells. The abundance of signalling molecules and their receptors is overwhelming: a recent study lists more than 100 ligands for G-protein-coupled receptors present in human islets (Amisten et al. 2013). Investigators in the field of islet biology are struggling to put an order to the intricacy of possible paracrine interactions. For some of these molecules, the different signalling components, including release mechanisms and receptors on putative target cells, have been demonstrated in the islet. This may establish these molecules as bona fide paracrine signals within the islet, but a major remaining challenge is to determine the physiological context in which the signalling pathway is operating.
What are these physiological contexts? The regulation of hormone secretion is one setting in which paracrine signalling is thought to be essential. Many candidate paracrine molecules have been shown to modulate hormone secretion. Prominent examples are GABA and somatostatin, which are produced and released by β and δ cells, respectively. Receptors for somatostatin have been mapped to the different endocrine cells and their activation inhibits all endocrine cells within the islet (Taborsky et al. 1979). Although somatostatin is considered a negative regulator of insulin and glucagon secretion, we still know little about how the somatostatin-secreting δ cell is regulated. Without knowing what controls the δ cell, however, it is difficult to understand the biological meaning of this paracrine pathway, and thus we can only speculate about the functional role of somatostatin in the islet. GABA has been proposed as a paracrine signal released from β cells to inhibit α cells, which may help explain how an increase in glucose concentration leads to increases in insulin secretion while simultaneously inhibiting glucagon secretion (Rorsman et al. 1989). However, as long as we do not know under what circumstances and how GABA is released from β cells, the functional significance of the GABA paracrine pathway will remain elusive.
Whereas most studies are aimed at understanding paracrine regulation of hormone secretory responses, other physiological roles for paracrine signals seem to have been neglected. Anti-apoptotic and proliferative effects on islet cells have been described for insulin and serotonin, but there is a whole spectrum of physiological effects that has not been explored. For instance, ATP is released from β cells to modulate hormone secretion (Jacques-Silva et al. 2010), but ATP is also known for its effects on vascular and immune cells (Burnstock, 2006; 2009; Eltzschig et al. 2012). The islet does not only contain endocrine cells, but also vascular cells and immune cells such as resident macrophages that could be targets for ATP and other paracrine molecules (Figs1 and 2).
Figure 2. Resident macrophages of the pancreatic islet.
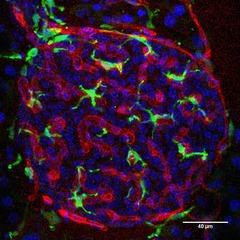
Confocal image of a pancreatic section showing a mouse islet immunostained for the endothelial marker CD31 (red) and the macrophage marker Iba1 (green). The islet contains numerous resident macrophages that are closely associated with blood vessels. Scale bar, 40 μm.
The roles endogenous ATP and other signalling molecules play in vascular and immune responses have barely been investigated, mainly because of technical limitations. Local vascular and immune responses are dynamic processes that have to be monitored in situ and in real time, and thus far there are few approaches allowing imaging of pancreatic islets in vivo. A major problem is that islets are a minor fraction of the pancreas (1–2%) and are deeply embedded within this already inaccessible organ, making it very difficult to image islets in vivo. To overcome these difficulties we are transplanting islets into the anterior chamber of the mouse eye (Speier et al. 2008; Caicedo et al. 2009). Once they engraft on the iris, islets are vascularized and reinnervated, and their structure and function can be monitored non-invasively and longitudinally. We expect this technical approach to help us elucidate the role of paracrine signalling in adjusting blood flow and in suppressing immune responses. An interesting hypothesis we would like to test is whether β and α cells secrete vasoactive substances together with their main hormones to promote their release into the bloodstream.
According to a recent study, the human islet expresses 293 out of 384 known functional, non-odorant G-protein-coupled receptors (Amisten et al. 2013). This list is extensive, and it does not include ligand-gated channels also expressed in the islet. Many of the ligands are conventional neurotransmitters thought to be released from autonomic axons innervating the islet. Indeed, rodent islets are strongly innervated by parasympathetic and sympathetic axons that probably co-release a variety of neurotransmitters and neuropeptides together with acetylcholine and noradrenaline. In the human islet, however, autonomic innervation is sparse and almost half of the ligands for the locally expressed G-protein-coupled receptors are present in islet cells (Gilon & Henquin, 2001; Rodriguez-Diaz et al. 2011a). In other words, the rodent islet may rely more on nervous input whereas the human islet makes more extensive use of paracrine interactions to control hormone secretion. This trend is exemplified by acetylcholine: a dense plexus of cholinergic axons innervates the mouse islet, but few if any axons contain acetylcholine in the human islet. Instead, acetylcholine is released from α cells as a paracrine signal in the human islet (Rodriguez-Diaz et al. 2011a, b2011b).
Beating to the rhythm of neurotransmitter release
Acetylcholine has been known for decades to amplify insulin secretion (Gilon & Henquin, 2001). The consensus is that it is released from parasympathetic axons innervating the islet. As with other inner organs, the nervous input would couple the function of the islet to the behavioural status of the organism. When we examined the autonomic innervation of the human islet, however, we found that the cholinergic markers were not labelling axons but endocrine cells (Rodriguez-Diaz et al. 2011a,b2011b). Immunostaining for vesicular acetylcholine transporter and choline acetyltransferase was mostly confined to α cells. RT-PCR studies on isolated, denervated islets confirmed that these markers were not contributed by autonomic axons but expressed in islet cells. Interestingly, within α cells, vesicular acetylcholine transporter did not seem to localize to glucagon granules, suggesting that acetylcholine is packed in vesicles that can be secreted independently of glucagon (Fig.3).
Figure 3. Acetylcholine and glucagon are present in different compartments in the human alpha cell.
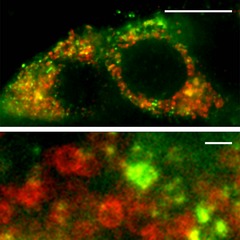
High-resolution confocal images using stimulated emission depletion (STED) microscopy showing human α cells immunostained for glucagon (red) and vesicular acetylcholine transporter (green). Glucagon and vesicular acetylcholine transporter immunoreactivities label distinct compartments. Scale bars: 5 μm (top) and 200 nm (bottom).
The next challenge was to demonstrate that acetylcholine was released from human islet cells. We detected acetylcholine by monitoring Ca2+ responses in biosensor cells expressing the muscarinic receptor M3. Biosensor cells placed in apposition to human islets recorded acetylcholine release in response to treatments known to specifically stimulate α cells. By contrast, we could not detect acetylcholine release from mouse islets. These experiments demonstrated that human α cells secrete acetylcholine.
We next examined the effects α cell-derived acetylcholine could have on islet function (Rodriguez-Diaz et al. 2011b). Studies had shown that exposure to acetylcholine sensitizes the β cell to subsequent stimuli. To test the hypothesis that α cells release acetylcholine to prime neighbouring β cells, we subjected isolated human islets to an experimental protocol in which we stimulated α and β cells intermittently while modulating cholinergic signalling (Rodriguez-Diaz et al. 2011b). We found that endogenous acetylcholine sensitized the β cell to subsequent increases in glucose concentration. Based on these results we proposed that in the human islet acetylcholine serves as a paracrine signal to keep the β cell responsive to future challenges, thus limiting glucose fluctuations.
The actions of acetylcholine, however, may be more complex than we assume. We do not yet know the exact circumstances under which acetylcholine is released in vivo, and acetylcholine may activate other endocrine cells such as the δ cell. If so, this would affect the hormonal output of the whole islet. Furthermore, acetylcholine is known to have trophic effects and thus could promote cell survival in the islet. We must not neglect the effects acetylcholine has on vascular and immune cells. It is plausible that acetylcholine is released simultaneously with glucagon to change vascular permeability and promote the delivery of glucagon into the circulation.
So far we have discussed paracrine signalling as a regulated process in which a cell secretes a modulatory molecule in response to a specific stimulus. However, our recent work on pancreatic islets suggests that there are additional possibilities. While investigating GABA secretion from the human islet we found that most of the GABA in the islet is released constitutively in robust pulses from β cells (Fig.4). This secretory pattern could not be altered with treatments known to activate or inhibit endocrine cells in the islet, but instead depended directly on intracellular GABA metabolism. Importantly, the GABA pulses imposed a rhythm on insulin secretion, suggesting that the background GABA secretion may help shape insulin pulses. We are still determining the influence this paracrine signalling pathway has on islet function, but it is already clear that it is highly unconventional in that it is periodic and does not depend on stimulus-dependent exocytosis.
Figure 4. Paracrine GABA is released from islets in regular pulses.
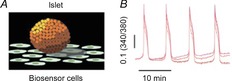
A, schematic diagram of the biosensor cell approach to detect GABA secretion from pancreatic islets. Responses in the GABA biosensor cells were recorded by loading cells with Fura-2 and imaging cytoplasmic [Ca2+]. B, pulsatile GABA release from a human islet maintained in 3 mm glucose. Fura-2 ratio traces (340/380) from 4 biosensor cells located under an islet, as shown in A (representative of >40 human islet preparations).
This brief introduction to the physiology of the pancreatic islet shows that the more we learn about intra-islet communication the less we seem to know. Every finding opens new possibilities. The functional characterization of the different putative paracrine interactions seems to lag behind the massive identification of ligands and their receptors in omics studies, but so far there is no substitute for the careful dissection of the different elements constituting a paracrine loop. Without it, molecules are lost in space and time.
Acknowledgments
We are grateful to Joana Almaça for providing the image in Fig.2.
Biographies
Rayner Rodriguez-Diaz did his PhD with Per-Olof Berggren and Alejandro Caicedo after leaving his native Cuba with an almost finished PhD in virology. He is currently using his diverse background to study the role inflammation and viruses play in the pathogenesis of diabetes.
Danusa Menegaz started her research work in Brazil and did her PhD between the Universidade Federal de Santa Catarina, Brazil, and the University of California, Riverside. After a postdoctoral fellowship working as a patch-clamp electrophysiologist at the Department of Physiology and Biophysics at the University of Miami, she joined Alejandro Caicedo's group in 2011. Having wandered through different senses (hearing, taste and vision), countries (Colombia, Germany, France) and training (Stephen D. Roper, Per-Olof Berggren). 
Alejandro Caicedo established his laboratory at the Department of Medicine at the University of Miami Miller School of Medicine in Miami. We share a common interest in understanding the physiology of the human pancreatic islet, with a particular focus on innervation and paracrine signaling. In our research we apply methods that include in vivo imaging of islet transplanted into the anterior chamber of the eye and detection of paracrine signal release from pancreatic islets with biosensor cells.
Additional information
Competing interests
None declared.
Funding
Funded by NIH grants R56DK084321 and R01DK084321.
References
- Amisten S, Salehi A, Rorsman P, Jones PM. Persaud SJ. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol Ther. 2013;139:359–391. doi: 10.1016/j.pharmthera.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Brissova M, Fowler M, Nicholson W, Chu A, Hirshberg B, Harlan D. Powers A. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- Cabrera O, Berman D, Kenyon N, Ricordi C, Berggrern P. Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Rodriguez-Diaz R, Molano R, Ricordi C, Berggren P. Pillegi A. Noninvasive, live imaging studies of human islet cell biology in the anterior chamber of the eye. Xenotransplantation. 2009;16:415–416. [Google Scholar]
- Conn PM, Goodman HM. Kostyo JL. The Endocrine System. New York: Published for the American Physiological Society by Oxford University Press; 1998. [Google Scholar]
- Eltzschig HK, Sitkovsky MV. Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilon P. Henquin J. Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocr Rev. 2001;22:565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- Jacques-Silva M, Correa-Medina M, Cabrera O, Rodriguez-Diaz R, Makeeva N, Fachado A, Diez J, Berman D, Kenyon N, Ricordi C, Pileggi A, Molano R, Berggren P. Caicedo A. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic β cell. Proc Natl Acad Sci USA. 2010;107:6465–6470. doi: 10.1073/pnas.0908935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO. Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011a;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO. Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med. 2011b;17:888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P, Berggren PO, Bokvist K, Ericson H, Möhler H, Ostenson CG. Smith PA. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341:233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- Samols E. Stagner JI. Islet somatostatin–microvascular, paracrine, and pulsatile regulation. Metabolism. 1990;39:55–60. doi: 10.1016/0026-0495(90)90212-u. [DOI] [PubMed] [Google Scholar]
- Speier S, Nyqvist D, Cabrera O, Yu J, Molano R, Pileggi A, Moede T, Kohler M, Wilbertz J, Leibiger B, Ricordi C, Leibiger I, Caicedo A. Berggren P. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagner JI. Samols E. The vascular order of islet cellular perfusion in the human pancreas. Diabetes. 1992;41:93–97. doi: 10.2337/diab.41.1.93. [DOI] [PubMed] [Google Scholar]
- Taborsky GJ, Jr, Smith PH. Porte D., Jr Differential effects of somatostatin analogues on alpha- and beta-cells of the pancreas. Am J Physiol Endocrinol Metab. 1979;236:E123–E128. doi: 10.1152/ajpendo.1979.236.2.E123. [DOI] [PubMed] [Google Scholar]
- Tengholm A. Gylfe E. Oscillatory control of insulin secretion. Mol Cell Endocrinol. 2009;297:58–72. doi: 10.1016/j.mce.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Wojtusciszyn A, Armanet M, Morel P, Berney T. Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia. 2008;51:1843–1852. doi: 10.1007/s00125-008-1103-z. [DOI] [PubMed] [Google Scholar]


